Abstract
Introduction
Atrial fibrillation (AF) is a frequently encountered complication after coronary artery bypass grafting (CABG), but its underlying mechanisms are still unclear. The natriuretic peptides have been reported as markers for predicting the occurrence of postoperative AF. This study evaluates whether the ScaI ANP gene polymorphisms predict the occurrence of postoperative AF.
Material and methods
A prospective study of 203 consecutive patients with coronary artery disease undergoing elective CABG was undertaken for atrial natriuretic peptide (ANP) ScaI gene polymorphism. Several perioperative data were analysed. Postoperative AF was defined as lasting for at least 15 min, confirmed by 12-lead ECG and occurring within 6 postoperative days. The ScaI polymorphism of the ANP gene was determined by polymerase chain reaction (PCR). Size-dependent separation of the PCR products on a polyacrylamide gel was followed by staining with ethidium bromide.
Results
The total frequency of AF was 19.7%. The frequencies of ScaI ANP gene polymorphisms were as follows: A1A1 4.90%, A1A2 59.60% and A2A2 35.46%. In order to assess the hypothesis that the A2 allele is a marker of increased risk of postoperative atrial fibrillation, the odds ratio (OR) was calculated: A2 vs. non-A2, OR = 0.98 (0.23–4.1), p = 0.97, which was not significant. The odds ratios for A2A2 and A1A1 were not significant either: A2A2 vs. non-A2A2, OR = 1.11 (0.54–2.29), p = 0.76, and A1A1 vs. non-A1A1, OR = 1.17 (0.23–5.92), p = 0.84.
Conclusions
ANP genotype did not predispose to the incidence of “new-onset” AF.
Keywords: coronary artery disease, gene polymorphisms, coronary artery bypass grafting, ScaI ANP, atrial fibrillation
Introduction
Atrial fibrillation (AF) is a common complication after coronary artery bypass grafting (CABG) [1–3]. Arrhythmia is considered to be one reason for increased peri-operative morbidity, prolonged hospital stay and increased costs [1, 4]. The pathogenesis of postoperative AF is still not well established. The best documented risk factors are increased age, congestive heart failure and peri-operative ischaemia [1, 2, 4–6]. Studies suggest that an important role is played by a peri-operatively induced systemic inflammatory response in the pathogenesis of AF [7]. The recently published data showed that perioperative treatment with atorvastatin is useful to decrease the incidence of AF in patients undergoing isolated heart valve surgery [8]. Moreover, a genetic predisposition to postoperative AF has been proved [9]. Among various pathophysiological factors predisposing to development of paroxysmal AF, interesting ones seem to be increased P-wave duration and dispersion that are observed in patients with diabetic autonomic neuropathy [10]. More recently, a simple risk score for prediction of postoperative AF has been validated [11]. In this large observational study several clinical features, i.e. age, chronic obstructive pulmonary disease, need of preoperative intra-aortic balloon pump, left ventricular ejection fraction less than 30%, renal failure and any valve surgery were identified as independent predictors of postoperative AF. Although there are many well-identified clinical and biochemical risk factors, there is still a lack of sufficient data on genetic predictors of postoperative arrhythmia.
It has been demonstrated that atrial natriuretic peptide (ANP) is a hormone which plays an important role in congestive heart failure (CHF) and hypertension [12, 13]. The ANP has natriuretic, diuretic and vasodilatative properties. Increased atrial-wall tension as a result of increased intravascular volume stimulates release of ANP [14]. This phenomenon occurs in patients with heart failure but also during cardiac surgery. There is strong evidence that low-dose continuous infusion of human ANP during cardiac surgery leads to a reduction in central venous pressure, the systemic vascular resistance index and the pulmonary vascular resistance index. Additionally, a reduction in levels of renin, angiotensin-II, aldosterone, and pleural effusion has been observed in comparison with patients who did not receive ANP infusion [15]. As pleural effusion and fluid overload of the atrium are considered to be partially responsible for postoperative atrial fibrillation, these observations may suggest the potential role of ANP in the pathogenesis of the arrhythmia. On the other hand, a relationship between ANP gene polymorphism and a history of supraventricular tachycardia has been observed in patients with dilated cardiomyopathy [16].
The aim of the study was to explore whether the A2 allele is a marker of increased risk of postoperative atrial fibrillation.
Material and methods
Study population
The 203 white patients included in the study, 152 male and 51 female, were qualified for elective coronary artery surgery. Written informed consent was obtained from each person. All consecutive patients were inhabitants of the Pomeranian region and were operated on at the Department of Cardiac Surgery at the Medical University of Gdansk. All the patients were interviewed by one physician before the operation and data were recorded on the following: angina status according to the Canadian Cardiovascular Society (CCS), history of myocardial infarction, arterial hypertension, diabetes and previous arrhythmias, including chronic and paroxysmal AF. Patients who had either persistent or permanent AF, atrial flutter or atrial tachycardia on a standard 12-lead ECG obtained on the day of the operation were excluded from the study. Twenty-four of the included patients had reduced ejection fraction heart failure (REF-HF) and 5 of them had EF less than 30%. Ninety-five patients were operated on with standard CABG using extracorporeal circulation and 108 patients underwent off-pump coronary artery bypass surgery (OPCABG) without cardiopulmonary bypass, on a beating heart. The risk of peri-operative mortality was estimated according to EuroSCORE [17]. The study protocol was approved by the local ethics committee.
Genetic analysis
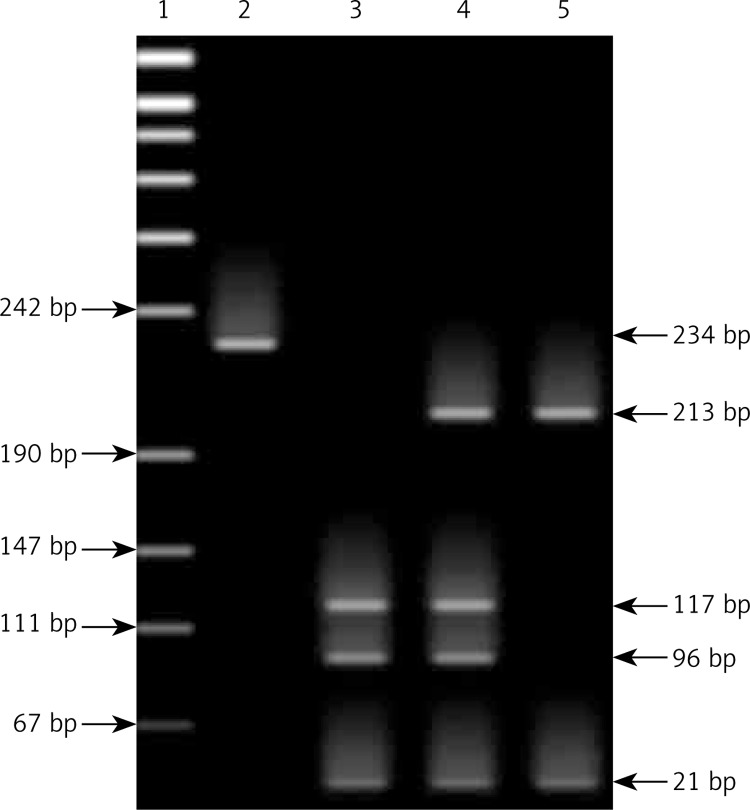
Genomic DNA was isolated from whole blood taken from a patient on the day before surgery using the Blood DNA Prep Plus spin-column system according to the protocol provided by the manufacturer (A&A Biotechnology, Gdansk, Poland). The ScaI site polymorphism of the ANP gene was determined by polymerase chain reaction (PCR) by standard techniques [18]. A fragment of 234 base pairs (bp) was amplified in the region encompassing exon 3 of the ANP gene, with the sense primer (5′-GGT GGG AAG CAG GTG GTC AGT ACT CAA GTT CAG AGG ATG GGC-3′) and antisense primer (5′-CAC AAC TCC ATG GCA ACA AGA TGA CAC AAA TGC-3′). To detect T2238→C transition, restriction enzyme digestion with ScaI was performed according to the protocol provided by the manufacturer (Promega, Madison, Wis). The size-dependent separation of the PCR products on a 12% polyacrylamide gel was followed by staining with ethidium bromide. In the presence of the polymorphic site for the A2 allele 3 fragments were generated corresponding to sizes 117, 96 and 21 bp. For the A1 allele a fragment of 234 bp was split into 213 and 21 bp products [19], as shown in Figure 1.
Figure 1.
Genotyping by polyacrylamide gel electrophoresis of PCR–RFLP. A fragment of the ANP gene was amplified and products were digested with ScaI restriction enzyme, subsequently run on 12% polyacrylamide gel using 1% solution ethidium bromide to visualize the fragments
1 – Marker, 2 – ANP amplified product, 3 – the homozygous A1 allele appeared as 213 and 21-bp bands, 4 – the homozygous A2 allele appeared as bands of 117, 96 and 21 bp, 5 – the heterozygotes A1A2 exhibited all bands (213, 117, 96 and 21 bp).
Arrhythmia analysis
Atrial fibrillation was assessed using a continuous ECG monitor system (Hewlett Packard, USA) with the possibility of rhythm disturbance analysis during the patient’s stay in the intensive care unit (ICU). Subsequently, during the first 24 h after leaving the ICU, each patient was monitored using the Space Lab system (Space Lab, USA). A standard 12-lead ECG was performed once a day and continually in any case of clinical manifestations of arrhythmia. The analysis took into account every incident of AF lasting longer than 15 min or requiring medical treatment because of the patient’s instability. The period during which the patients were observed was restricted to 6 postoperative days.
Statistical analysis
Values are expressed as mean and standard deviations. The EuroSCORE data are median and range and were compared using the Kruskal-Wallis test. The χ2 test was used for analysis of the categorical variables, and Student’s t-test was used for continuous variables with normal distribution. On the other hand, the Mann-Whitney U test was used for non-normally distributed continuous variables. When more than two groups were compared, an ANOVA test was used. The relationship between ScaI ANP gene variants and the incidence of “new-onset” AF was assessed and the odds ratios were calculated using a logistic regression model.
P-values of less than 0.05 were considered to be significant. Statistical analysis was performed using Statistica 10.0 (StatSoft).
Results
Allele distribution in this group of 203 patients is presented in Table I. There was dominance of A1A2 heterozygotes.
Table I.
ScaI ANP genotype frequency in 203 patients with ischaemic heart disease admitted for CABG
| ScaI ANP genotype | Number of patients (%) |
|---|---|
| A2A2 | 72 (35.5) |
| A1A2 | 121 (59.6) |
| A1A1 | 10 (4.9) |
CABG – coronary artery bypass grafting, ANP – atrial natriuretic peptide.
The clinical profile of the 203 patients with ischaemic heart disease admitted for CABG and OPCABG is presented according to ScaI ANP genotype in Table II. In general, there were no significant differences between the patients’ parameters. There were no significant differences in mean age, body mass index (BMI), left ventricle ejection fraction (LVEF) or left atrial size (LA) between the A2A2 and A1A2 groups. There was a significant difference in the length of time from the first angina pectoris episode to the time of cardiac surgery between patients with A1A1 and A1A2, 10.9 years versus 8 years respectively. Patients with the A1A2 genotype had a higher level of plasma triglycerides compared to those with the A2A2 genotype, 185.7 and 150.7 mg/dl respectively.
Table II.
Clinical profile of 203 patients with ischaemic heart disease admitted for CABG and OPCABG according to ScaI ANP genotype. The analysis was made using the ANOVA test for continuous variables and χ2 test for categorical data
| Parameter | ScaI ANP genotype | P-value | ||
|---|---|---|---|---|
| A1A2 | A2A2 | A1A1 | ||
| Number of patients | 121 | 72 | 10 | |
| Age [years] | 62 ±9 | 64 ±8.9 | 63 ±7.19 | 0.7 |
| BMI | 27.9 ±4.1 | 25.7 ±3.9 | 28 ±2.9 | 0.5 |
| EuroSCORE | 3 (0; 9)* | 3 (0; 10)* | 4 (0; 6)* | 0.6 |
| Duration of angina [years] | 8 ±7.5** | 8.8 ±7.4 | 10.9 ±9.7** | 0.004 (0.4) |
| NYHA | 1 ±0.73 | 1 ±0.76 | 1 ±0.69 | 0.8 |
| Total cholesterol [mg%] | 225.9 ±51.2 | 214 ±48 | 216 ±51 | 0.9 |
| LDL cholesterol [mg%] | 140.9 ±43.3 | 132 ±41 | 144 ±57.8 | 0.5 |
| HDL cholesterol [mg%] | 43.2 ±12.5 | 42 ±12 | 45 ±13 | 0.9 |
| Triglycerides [mg%] | 185.7 ±138.4** | 150.7 ±66.4** | 136 ±56 | 0.04 (0.1) |
| Creatinine [mg%] | 1.14 ±0.2 | 1.1 ±0.2 | 1.19 ±0.2 | 0.7 |
| EF | 53.6 ±10.2 | 52.8 ±11.3 | 47.5 ±9.5 | 0.9 |
| LA | 4 ±0.5 | 4 ±0.5 | 4.15 ±0.4 | 0.6 |
| LVESd | 3.5 ±0.8 | 3.6 ±0.7 | 4.1 ±1.2 | 0.3 |
| LVEDd | 5.2 ±0.8 | 5.2 ±0.6 | 5.6 ±0.8 | 0.9 |
| Male/female | 87/34 | 55/17 | 10/0 | 0.49 |
| Myocardial infarction history | 81 | 47 | 8 | 0.81 |
| Diabetes | 32 | 16 | 1 | 0.49 |
| Hypertension | 82 | 48 | 4 | 0.9 |
| Paroxysmal atrial fibrillation | 8 | 3 | 0 | 0.7 |
Values are expressed as median and range and were compared using the Kruskal-Wallis test,
p < 0.05. CABG – coronary artery bypass grafting, OPCABG – off-pump coronary artery bypass grafting, LA – left atrial diameter, LVESd – left ventricle systolic diameter, LVEDd – left ventricle end-diastolic diameter, ANP – atrial natriuretic peptide, BMI – body mass index, EF – ejection fraction.
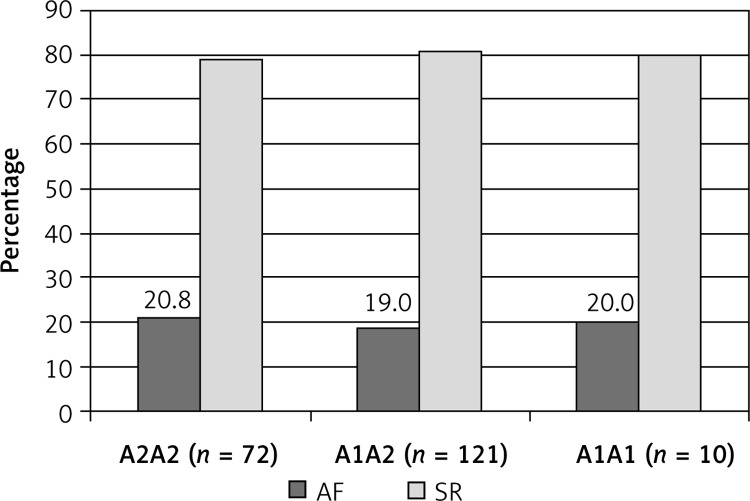
Two hundred of the patients completed the 6 days follow-up. A total of 4 patients died peri-operatively, 2 with the A2A2 and 2 with the A1A2 genotype. Three deaths occurred in the first 24 h after surgery and 1 patient died on the 10th postoperative day. All deaths were due to perioperative low output syndrome and none was caused directly by atrial fibrillation. A total of 40 (19.7%) patients developed AF postoperatively. The distribution of AF in particular allele groups is presented in Figure 2.
Figure 2.
Incidence of atrial fibrillation after 203 CABG procedures
AF – atrial fibrillation, SR – sinus rhythm, CABG – coronary artery bypass grafting.
ANP genotype did not predispose to the occurrence of “new-onset” AF, as shown in Table III.
Table III.
Relationship between ScaI ANP gene variants and the incidence of “new-onset” AF in 192 patients (after excluding patients with a history of paroxysmal AF before the operation). The analysis was made using a logistic regression model
| OR for “new-onset” atrial fibrillation A1A2 vs. A2A2 and A1A1 | OR = 0.65 (0.12–3.34) | p = 0.6 |
| OR for “new-onset” atrial fibrillation A2A2 vs. non A2A2 | OR = 1.35 (0.59–3.07) | p = 0.46 |
| OR for “new-onset” atrial fibrillation A1A1 vs. non A1A1 | OR = 1.8 (0.34–9.5) | p = 0.48 |
AF – atrial fibrillation, ANP – atrial natriuretic peptide.
The distribution between particular genotypes of other clinical data obtained during the intra- and postoperative periods is presented in Table IV. There were no significant differences in postoperative complications between any particular groups.
Table IV.
Intra- and postoperative data in 203 CABG patients according the ScaI ANP genotype. The analysis was made using the ANOVA test for continuous variables and χ2 test for categorical data
| Parameter | ScaI ANP genotype | P-value | ||
|---|---|---|---|---|
| A1A2 | A2A2 | A1A1 | ||
| Number of patients | 121 | 72 | 10 | |
| Neuro-mental complications | 5 (4.1%) | 3 (4.1%) | 0 | 0.7 |
| Excessive bleeding | 7 (5.8%) | 2 (2.7%) | 0 | 0.4 |
| Peri-operative death | 2 (1.6%) | 2 (2.7%) | 0 | 0.9 |
| CABG/OPCABG | 59/62 | 29/43 | 7/3 | 0.2 |
| 1st day drainage [ml] | 562 ±300.5 | 554 ±258 | 586.25 ±269.2 | 0.8 |
| CPK-MB | 33.2 ±86.7 | 21 ±35.3 | 15.6 ±16.9 | 0.7 |
| CPB time [min] | 90.47 ±31.5 | 92.11 ±35.4 | 71.5 ±17.7 | 0.4 |
| Duration of aortic cross clamp [min] | 47.5 ±18.6 | 50 ±24 | 39.8 ±11.3 | 0.9 |
| Number of distal anastomoses | 2.63 ±0.9 | 2.69 ±0.9 | 3.1 ±0.9 | 0.2 |
CABG – coronary artery bypass grafting, OPCABG – off-pump coronary artery bypass grafting, ANP – atrial natriuretic peptide, CPB – cardiopulmonary bypass.
The limited number of the patients with the A1A1 genotype might have distorted our results. Therefore, as a next step, the patients carrying the A1A1 and A1A2 genotypes were analysed jointly to represent the A1-allele-positive participants, and these were contrasted with the A2A2 genotype patients.
From the 3rd to the 6th day after surgery the frequency of the AF episodes decreased and the frequency of AF was not noticeably higher among the A2A2 patients (χ2; p = 0.1).
A comparison of the distribution of patients according to NYHA grade revealed that the patients with the A2A2 genotype were not significantly shifted towards the higher NYHA values in relation to those with A1A2 and A1A1.
Discussion
According to our knowledge, this study is the first to test an association between ScaI ANP gene polymorphism with the clinical peri-operative state in patients undergoing cardiac surgery. A previous study performed by a research group from our university showed that the A2A2 allele is associated with a higher incidence of histories of non-fatal myocardial infarction and more extensive CAD [20]. More recently Rubattu et al. demonstrated that ANP polymorphism is associated with higher risk for an acute coronary syndrome recurrence in patients with CAD [21]. Moreover, a recently published meta-analysis indicated that ANP 2238 T/C polymorphism is related to increased risk of ischaemic stroke [22].
The interesting observation from our study is the distribution of the ScaI ANP genotype and allele frequency in the study group. We observed a relatively large group of the A2A2 genotype (35.5% and 27.5% respectively) in comparison with a previous study [20]. This may be a reflection of a selection bias in the population that included only patients undergoing CABG.
An elevated level of ANP was tested with respect to AF prediction after CABG in two other studies, which, however, did not show a significant correlation with postoperative arrhythmia [23, 24].
Loke et al. pointed out that in the healthy population, plasma levels of N-ANP, brain natriuretic peptide (BNP) and N-BNP were variably influenced by clinical covariates [25].
While all 3 peptides were higher in women, only N-ANP and N-BNP were influenced by age. Levels of all peptides were inversely correlated with heart rate. When an immunoluminometric assay is used, plasma BNP is not influenced by age, in contrast to N-ANP and N-BNP. In constructing normal ranges for diagnostic use, covariates such as age and gender must be considered, in addition to the format of the assay being used [25]. Histological examination revealed that the collagen volume in the left atrial tissue was higher in AF than it was in SR and inversely correlated with plasma ANP. In addition, the messenger RNA expression of ANP and collagen types I and III was lower in AF than in sinus rhythm [26]. The spectrum of histological alterations is not completely known. Patients with long-standing AF and rheumatic heart disease had a very high prevalence of atrial amyloidosis. Amyloid deposition was more frequent in the left atrial appendage than in the right and correlates with AF duration and female gender. Amyloid deposition could constitute an additional histological feature in the structural remodelling of the atria during long-standing AF [27].
Atrial fibrillation was described as an independent determinant of higher N-ANP levels, and its association with LV dysfunction is blurred. Conversely, BNP was not independently associated with AF and was strongly determined by LV dysfunction, for which it was an independent marker [28]. Chello et al. suggested that plasma levels of ANP and BNP might be used in routine clinical practice to support echocardiography in detecting recovery of LV function after coronary surgery [29].
As the hormonal environment is known to be very unstable during the early postoperative period, we hypothesised that analysis of the pre-operative ANP genotype, which is constant, may support a new predictor of postoperative AF. In our study the overall incidence of AF after cardiac surgery was comparable in the A2A2 and A1A2 groups. On the other hand, the B-type natriuretic peptide has been reported as predicting the occurrence of postoperative AF. A pre-operatively elevated plasma BNP level was a strong and independent predictor of postoperative AF. This finding has important implications for identifying patients at higher risk of postoperative AF who could be considered for prophylactic anti-arrhythmic or β-blocker therapy [30]. On the other hand, Masson et al., in their more recently published study, found that both pre- and postoperative plasma levels of NT-proBNP were not independently associated with postoperative AF [31].
In conclusion, the obvious limitation of our study is the lack of statistical significance, which may be due to the relatively small population. Further analysis is required, especially in order to assess the incidence of postoperative AF in patients with the A1A1 genotype. The small number of patients with that genotype in our study group suggests that the A2 allele is particularly expressed in patients with coronary artery disease. There is no evidence of an association between the genotype and new onset AF.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Aranki SF, Shaw DP, Adams DH, et al. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on the hospital resources. Circulation. 1996;94:390–7. doi: 10.1161/01.cir.94.3.390. [DOI] [PubMed] [Google Scholar]
- 2.Creswell LL, Schuessler RB, Rosenbloom M, Cox JL. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. 1993;56:539–49. doi: 10.1016/0003-4975(93)90894-n. [DOI] [PubMed] [Google Scholar]
- 3.Mathew JP, Parks R, Savino JS, Friedman AS, Koch C, Mangano DT. Atrial fibrillation following coronary artery bypass graft surgery: predictors, outcomes, and resource utilization. MultiCenter Study of Perioperative Ischemia Research Group. JAMA. 1996;276:300–6. [PubMed] [Google Scholar]
- 4.Almassi GH, Schowalter T, Nikolosi AC, et al. Atrial fibrillation after cardiac surgery – a major morbid event? Ann Surg. 1997;226:501–13. doi: 10.1097/00000658-199710000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Shanafey S, Dodds L, Langille D, Ali I, Henteleff H, Dobson R. Nodal vessels disease as a risk factor for atrial fibrillation after coronary artery bypass graft surgery. Eur J Cardiothorac Surg. 2001;19:821–826. doi: 10.1016/s1010-7940(01)00725-4. [DOI] [PubMed] [Google Scholar]
- 6.Kolvekar S, D’Souza A, Akhtar P, Reek C, Garratt C, Spyt T. Role of atrial ischaemia in development in atrial fibrillation following coronary artery bypass surgery. Eur J Cardiothorac Surg. 1997;11:70–5. doi: 10.1016/s1010-7940(96)01095-0. [DOI] [PubMed] [Google Scholar]
- 7.Bruins P, te Velthuis H, Yazdanbakhsh AP, et al. Activation of the complement system during and after cardiopulmonary bypass surgery. Circulation. 1997;96:3542–8. doi: 10.1161/01.cir.96.10.3542. [DOI] [PubMed] [Google Scholar]
- 8.Dehghani MR, Kasianzadeh M, Rezaei Y, Sepehrvand N. Atorvastatin reduces the incidence of postoperative atrial fibrillation in statin-naive patients undergoing isolated heart valve surgery: a double-blind, placebo-controlled randomized trial. J Cardiovasc Pharmacol Ther. 2015;20:465–72. doi: 10.1177/1074248414564869. [DOI] [PubMed] [Google Scholar]
- 9.Gaudino M, Andreotti F, Zamparelli R, et al. The -174G/C interleukin-6 polymorphism influences postoperative interleukin-6 levels and postoperative atrial fibrillation. Is atrial fibrillation an inflammatory complication? Circulation. 2003;108(Suppl. II):195–9. doi: 10.1161/01.cir.0000087441.48566.0d. [DOI] [PubMed] [Google Scholar]
- 10.Bissinger A, Grycewicz T, Grabowicz W, Lubinski A. The effect of diabetic autonomic neuropathy on P-wave duration, dispersion and atrial fibrillation. Arch Med Sci. 2011;7:806–12. doi: 10.5114/aoms.2011.25555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mariscalco G, Biancari F, Zanobini M, et al. Bedside tool for predicting the risk of postoperative atrial fibrillation after cardiac surgery: the POAF score. J Am Heart Assoc. 2014;3:e000752. doi: 10.1161/JAHA.113.000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levin E, Gardner D, Samson W, et al. Natriuretic peptides. N Engl J Med. 1998;339:321–8. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 13.Kato N, Sugiyama T, Morita H, et al. Genetic analysis of the atrial natriuretic peptide gene in essential hypertension. Clin Sci. 2000;98:251–8. [PubMed] [Google Scholar]
- 14.Lang RE, Tholken H, Ganten D, et al. Atrial natriuretic factor-circulating hormone stimulated by volume loading. Nature. 1985;314:2642–7. doi: 10.1038/314264a0. [DOI] [PubMed] [Google Scholar]
- 15.Sezai A, Shiono M, Orime Y, et al. Low-dose continuous infusion of human atrial natriuretic peptide during and after cardiac surgery. Ann Thorac Surg. 2000;69:732–8. doi: 10.1016/s0003-4975(99)01305-3. [DOI] [PubMed] [Google Scholar]
- 16.Richter D, Soccio M, Needham E, et al. Investigation of the atrial natriuretic peptide gene in patients with idiopathic dilated cardiomyopathy. Eur Heart J. 1997;18(Suppl):911. [abstract] [Google Scholar]
- 17.Roques F, Nashef SAM, Michel P, et al. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg. 1999;15:816–23. doi: 10.1016/s1010-7940(99)00106-2. [DOI] [PubMed] [Google Scholar]
- 18.Ramasawmy R, Kotea N, Lu C, et al. Investigation of the polymorphic ScaI site by a PCR-based assay at the human natriuretic peptides (hANP) gene locus. Hum Genet. 1992;90:323–4. doi: 10.1007/BF00220093. [DOI] [PubMed] [Google Scholar]
- 19.Nannipieri M, Manganiello M, Pezzatini A, et al. Polymorphism in the hANP (Human Atrial Natriuretic Peptide) gene, albuminuraia, and hypertension. Hypertension. 2001;6:1416–22. doi: 10.1161/01.hyp.37.6.1416. [DOI] [PubMed] [Google Scholar]
- 20.Gruchała M, Ciećwierz D, Wasag B, et al. Association of the ScaI atrial natriuretic peptide gene polymorphism with nonfatal myocardial infarction and extent of coronary artery disease. Am Heart J. 2003;145:125–31. doi: 10.1067/mhj.2003.52. [DOI] [PubMed] [Google Scholar]
- 21.Rubattu S, De Giusti M, Farcomeni A, et al. T2238C ANP gene variant and risk of recurrent acute coronary syndromes in an Italian cohort of ischemic heart disease patients. J Cardiovasc Med. 2016;17:601–7. doi: 10.2459/JCM.0000000000000195. [DOI] [PubMed] [Google Scholar]
- 22.Xing DG, Zhang DY, Wang ZF, Ding DL, Wang J, Wang YJ. Correlations of ANP genetic polymorphisms and serum levels with ischemic stroke risk: a meta-analysis. Genet Test Mol Biomarkers. 2014;18:349–56. doi: 10.1089/gtmb.2013.0498. [DOI] [PubMed] [Google Scholar]
- 23.Hakala T, Hedman A, Turpeinen A, et al. Prediction of atrial fibrillation after coronary artery bypass grafting by measuring atrial peptide levels and preoperative atrial dimensions. Eur J Cardiothorac Surg. 2002;22:939–43. doi: 10.1016/s1010-7940(02)00565-1. [DOI] [PubMed] [Google Scholar]
- 24.Jideus L, Blomstrom P, Nilsson L, et al. Tachyarrhythmias and triggering factors for atrial fibrillation after coronary artery bypass operation. Ann Thorac Surg. 2000;69:1064–9. doi: 10.1016/s0003-4975(99)01431-9. [DOI] [PubMed] [Google Scholar]
- 25.Loke I, Squire IB, Davies JE, Ng LL. Reference ranges for natriuretic peptides for diagnostic use are dependent on age, gender and heart rate. Eur J Heart Fail. 2003;5:599–606. doi: 10.1016/s1388-9842(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 26.Yoshihara F, Nishikimi T, Sasako Y, et al. Plasma atrial natriuretic peptide concentration inversely correlates with left atrial collagen volume fraction in patients with atrial fibrillation. J Am Coll Cardiol. 2002;39:288–94. doi: 10.1016/s0735-1097(01)01719-3. [DOI] [PubMed] [Google Scholar]
- 27.Leone O, Boriani G, Chiappini B, et al. Amyloid deposition as a cause of atrial remodelling in persistent valvular atrial fibrillation. Eur Heart J. 2004;25:1237–41. doi: 10.1016/j.ehj.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Rossi A, Enriquez-Sarano M, Burnett JC, Jr, Lerman A, Abel MD, Seward JB. Natriuretic peptide levels in atrial fibrillation: a prospective hormonal and Doppler-echocardiographic study. J Am Coll Cardiol. 2000;35:1256–62. doi: 10.1016/s0735-1097(00)00515-5. [DOI] [PubMed] [Google Scholar]
- 29.Chello M, Mastroroberto P, Perticone F, et al. Plasma levels of atrial and brain natriuretic peptides as indicators of recovery of left ventricular systolic function after coronary artery bypass. Eur J Cardiothorac Surg. 2001;20:140–6. doi: 10.1016/s1010-7940(01)00754-0. [DOI] [PubMed] [Google Scholar]
- 30.Wazni OM, Martin DO, Marrouche NF, et al. Plasma B-type natriuretic peptide levels predict postoperative atrial fibrillation in patients undergoing cardiac surgery. Circulation. 2004;110:124–7. doi: 10.1161/01.CIR.0000134481.24511.BC. [DOI] [PubMed] [Google Scholar]
- 31.Masson S, Wu JH, Simon C, et al. Circulating cardiac biomarkers and postoperative atrial fibrillation in the OPERA trial. Eur J Clin Invest. 2015;45:170–8. doi: 10.1111/eci.12393. [DOI] [PubMed] [Google Scholar]