Abstract
An excised tissue system consisting of corolla rib segments was developed to study the relationship between senescence and ethylene production in morning-glory flowers (Ipomoea tricolor). Such segments, isolated 1 or 2 days (day −1 or day −2) before flower opening (day 0) passed through the same developmental phases as did the corresponding tissues of the intact organ. When excised on day −1 and incubated overnight, the rib segments turned from purple to blue and changed from a slightly curled to a flat configuration. On day 0, these segments rolled up during the afternoon and turned purple again, as did the ribs of an intact corolla; the rolling up coincided with an increased rate of ethylene production. Premature rolling up and associated ethylene evolution were induced by ethylene or propylene treatment. When segments were excised on day −2 and incubated overnight, there were no changes in color or shape; during day −1, no spontaneous rolling up and little ethylene evolution occurred. Application of ethylene or propylene to these immature segments elicited rolling up but did not stimulate endogenous ethylene production.
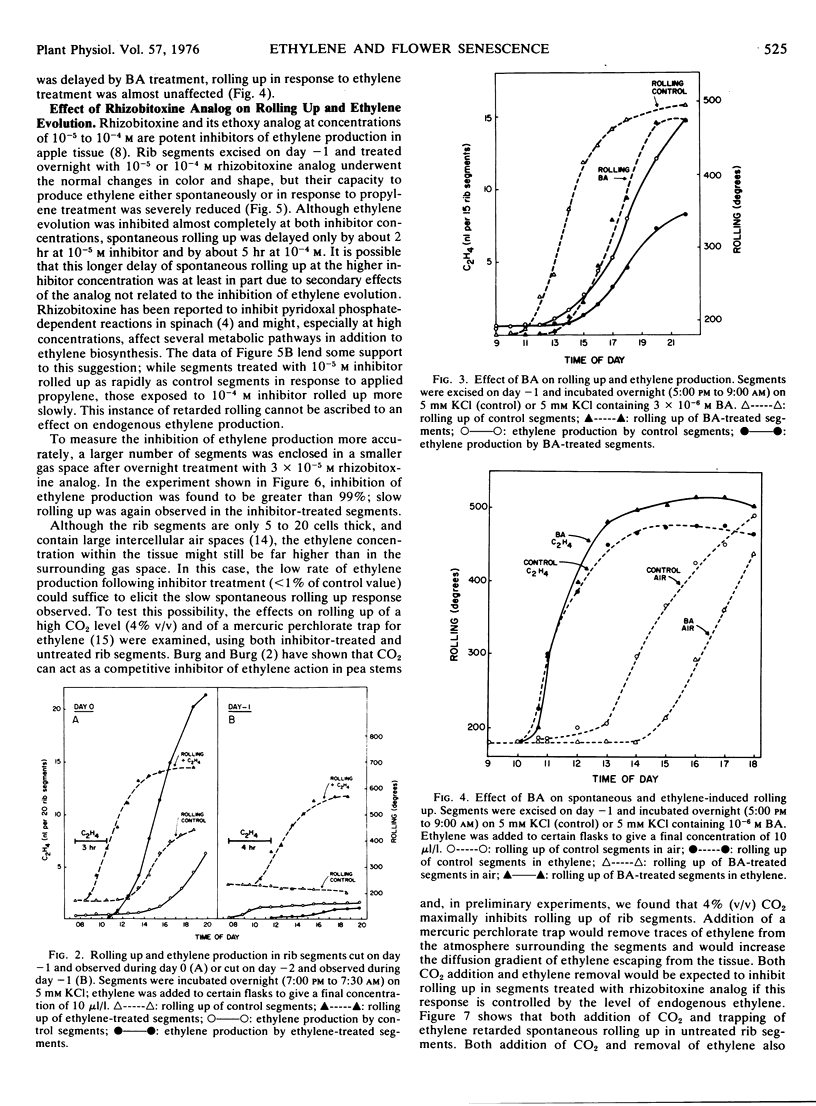
Overnight treatment of segments cut on day −1 with 10−6m benzyladenine markedly retarded spontaneous rolling up and ethylene evolution, although the response to applied ethylene was only slightly slowed. Overnight treatment of segments cut on day −1 with the ethoxy analog of rhizobitoxine (10−5 to 10−4m) resulted in almost complete (>99%) inhibition of both spontaneous and propylene-induced ethylene evolution. Although spontaneous rolling up was delayed, it was not abolished, and ethylene-induced rolling up was almost unaffected.
These data indicate that an ethylene-generating system develops as an integral part of the aging process in flower tissue. Ethylene hastens aging of the flower, but may not play an obligatory role in flower senescence.
Full text
PDF

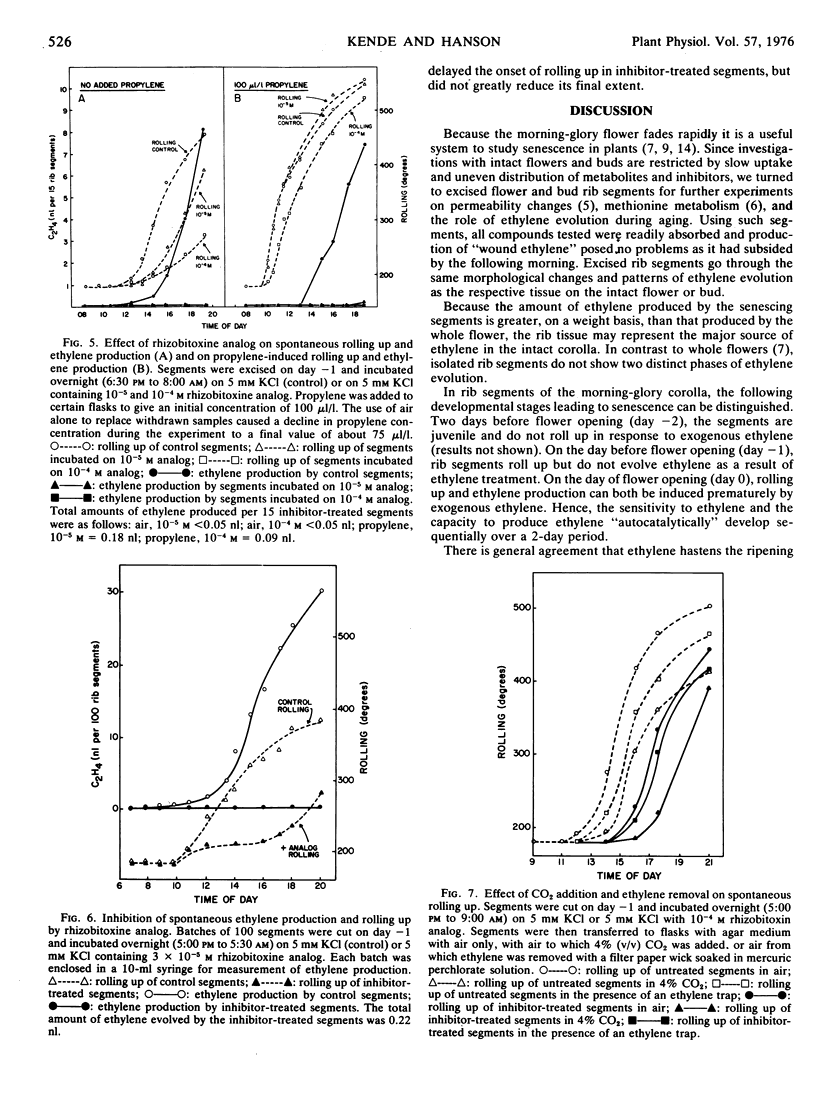

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burg S. P., Burg E. A. Molecular requirements for the biological activity of ethylene. Plant Physiol. 1967 Jan;42(1):144–152. doi: 10.1104/pp.42.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Dijkman M. J. Ethylene and auxin participation in pollen induced fading of vanda orchid blossoms. Plant Physiol. 1967 Nov;42(11):1648–1650. doi: 10.1104/pp.42.11.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanelli J., Owens L. D., Mudd S. H. Mechanism of inhibition of spinach beta-cystathionase by rhizobitoxine. Biochim Biophys Acta. 1971 Mar 10;227(3):671–684. doi: 10.1016/0005-2744(71)90016-7. [DOI] [PubMed] [Google Scholar]
- Hanson A. D., Kende H. Ethylene-enhanced Ion and Sucrose Efflux in Morning Glory Flower Tissue. Plant Physiol. 1975 Apr;55(4):663–669. doi: 10.1104/pp.55.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson A. D., Kende H. Methionine metabolism and ethylene biosynthesis in senescent flower tissue of morning-glory. Plant Physiol. 1976 Apr;57(4):528–537. doi: 10.1104/pp.57.4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlasson W. B., Dostal H. C., Tigchelaar E. C. Comparison of Propylene-induced Responses of Immature Fruit of Normal and rin Mutant Tomatoes. Plant Physiol. 1975 Feb;55(2):218–222. doi: 10.1104/pp.55.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]


