Abstract
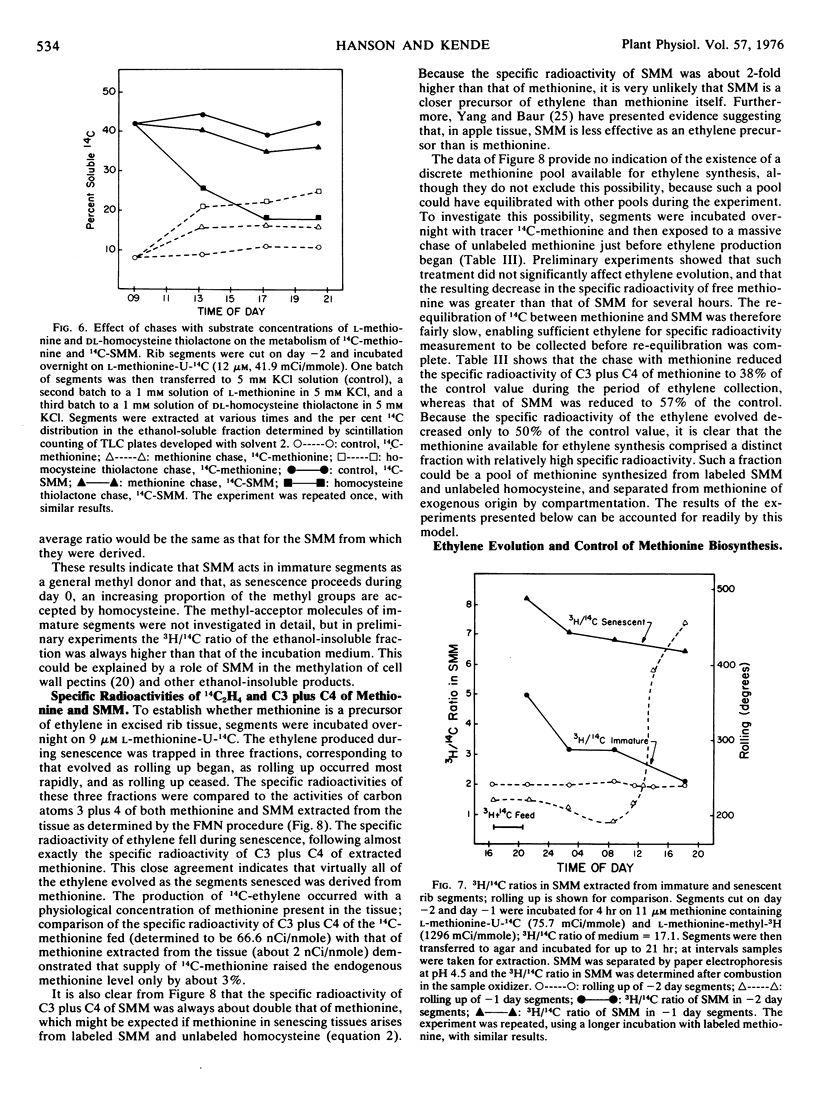
In immature rib segments prepared from morning-glory (Ipomoea tricolor) flower buds, the major soluble metabolite formed from tracer amounts of l-methionine-U-14C was S-methylmethionine (SMM). In segments of senescing ribs, 14C was progressively lost from SMM and appeared in free methionine. Immature segments contained about 4 nmoles of free methionine and about 16 nmoles of SMM per 30 segments. As the segments senesced, the methionine content increased about 10-fold while the SMM content remained unchanged; during this time about 0.8 nmole of ethylene was produced per 30 segments. Tracer experiments with l-methionine-U-14C, l-methionine-methyl-3H, and l-homocysteine thiolactone-35S indicated that SMM was capable of acting as a methyl donor, and that in senescent segments the methyl group was utilized for methionine production with homocysteine serving as methyl acceptor. Of the 2 molecules of methionine produced in this reaction, 1 was re-methylated to SMM, and the other contributed to the observed rise in the content of free methionine.
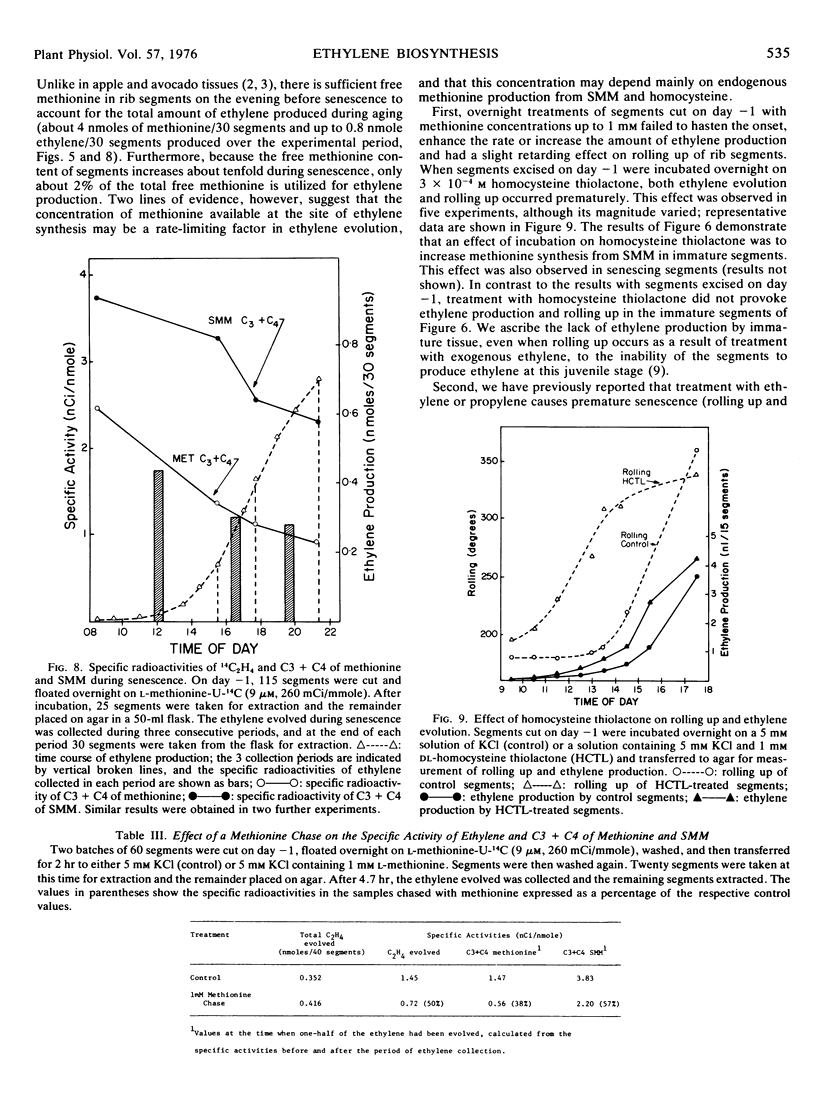
Internal pools of methionine and SMM were prelabeled (but not significantly expanded) by overnight incubation on 10 μm l-methionine-U-14C. The specific radioactivity of the ethylene subsequently evolved during the senescence of the segments closely paralleled the specific radioactivity of carbon atoms 3 plus 4 of free methionine extracted from the tissue, demonstrating that methionine was the major precursor of ethylene in this system. The specific radioactivity of carbon atoms 3 plus 4 of extracted SMM was about twice that of the free methionine.
Based on these results, a scheme for methionine biosynthesis in senescent rib tissue is presented. The operation of this pathway in the control of ethylene production is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baur A. H., Yang S. F., Pratt H. K. Ethylene biosynthesis in fruit tissues. Plant Physiol. 1971 May;47(5):696–699. doi: 10.1104/pp.47.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Dijkman M. J. Ethylene and auxin participation in pollen induced fading of vanda orchid blossoms. Plant Physiol. 1967 Nov;42(11):1648–1650. doi: 10.1104/pp.42.11.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENE R. C., DAVIS N. B. Biosynthesis of S-methylmethionine in the jack bean. Biochim Biophys Acta. 1960 Sep 23;43:360–362. doi: 10.1016/0006-3002(60)90457-1. [DOI] [PubMed] [Google Scholar]
- Hanson A. D., Kende H. Ethylene-enhanced Ion and Sucrose Efflux in Morning Glory Flower Tissue. Plant Physiol. 1975 Apr;55(4):663–669. doi: 10.1104/pp.55.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H., Hanson A. D. Relationship between Ethylene Evolution and Senescence in Morning-Glory Flower Tissue. Plant Physiol. 1976 Apr;57(4):523–527. doi: 10.1104/pp.57.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAVINE T. F., FLOYD N. F., CAMMAROTI M. S. The formation of sulfonium salts from alcohols and methionine in sulfuric acid. J Biol Chem. 1954 Mar;207(1):107–117. [PubMed] [Google Scholar]
- Lamport D. T. The isolation and partial characterization of hydroxyproline-rich glycopeptides obtained by enzymic degradation of primary cell walls. Biochemistry. 1969 Mar;8(3):1155–1163. doi: 10.1021/bi00831a049. [DOI] [PubMed] [Google Scholar]
- Lieberman M., Kunishi A. Stimulation of ethylene production in apple tissue slices by methionine. Plant Physiol. 1966 Mar;41(3):376–382. doi: 10.1104/pp.41.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapson L. W., March J. F., Rhodes M. J., Wooltorton L. S. A comparative study of the ability of methionine or linolenic acid to act as precursors of ethylene in plant tissues. Biochem J. 1970 Apr;117(3):473–479. doi: 10.1042/bj1170473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATO C. S., BYERRUM R. U., ALBERSHEIM P., BONNER J. Metabolism of methionine and pectin esterification in a plant tissue. J Biol Chem. 1958 Jul;233(1):128–131. [PubMed] [Google Scholar]
- TURNER J. E., SHAPIRO S. K. S-Methylmethionine- and S-adenosylmethionine-homocysteine transmethylase in higher plant seeds. Biochim Biophys Acta. 1961 Aug 19;51:581–584. doi: 10.1016/0006-3002(61)90617-5. [DOI] [PubMed] [Google Scholar]
- Yang S. F., Ku H. S., Pratt H. K. Photochemical production of ethylene from methionine and its analogues in the presence of flavin mononucleotide. J Biol Chem. 1967 Nov 25;242(22):5274–5280. [PubMed] [Google Scholar]


