Abstract
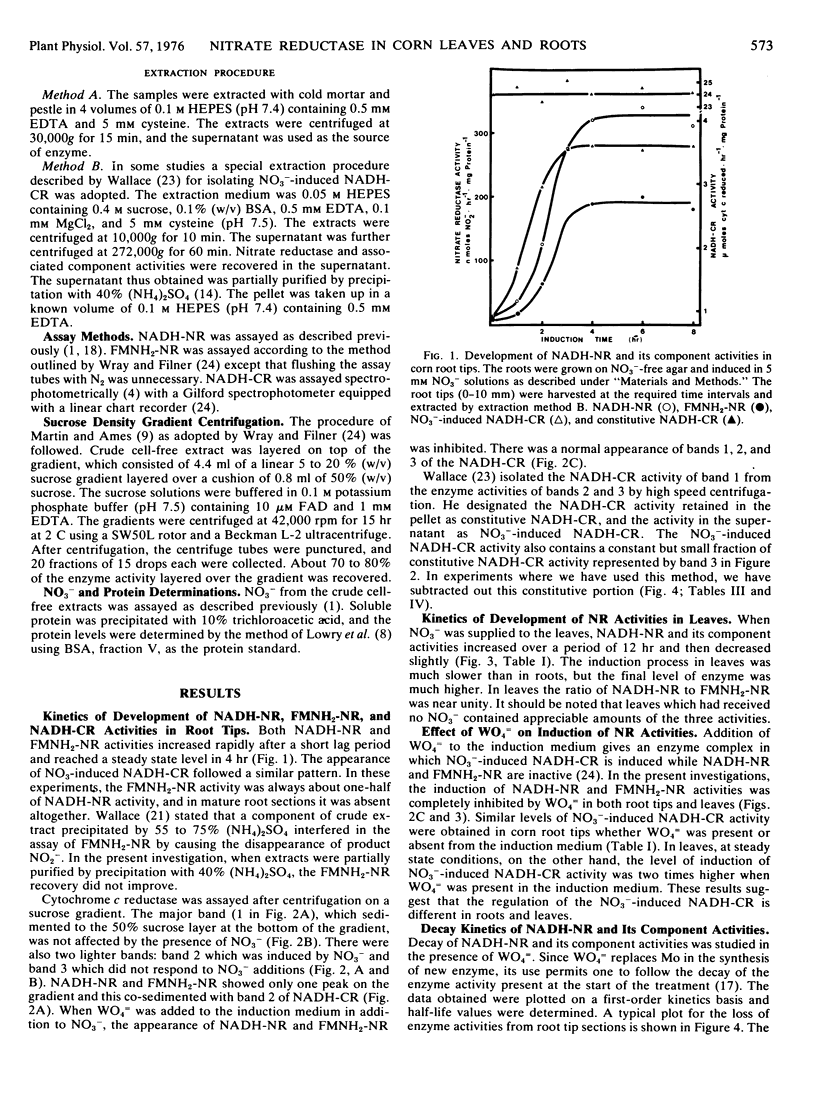
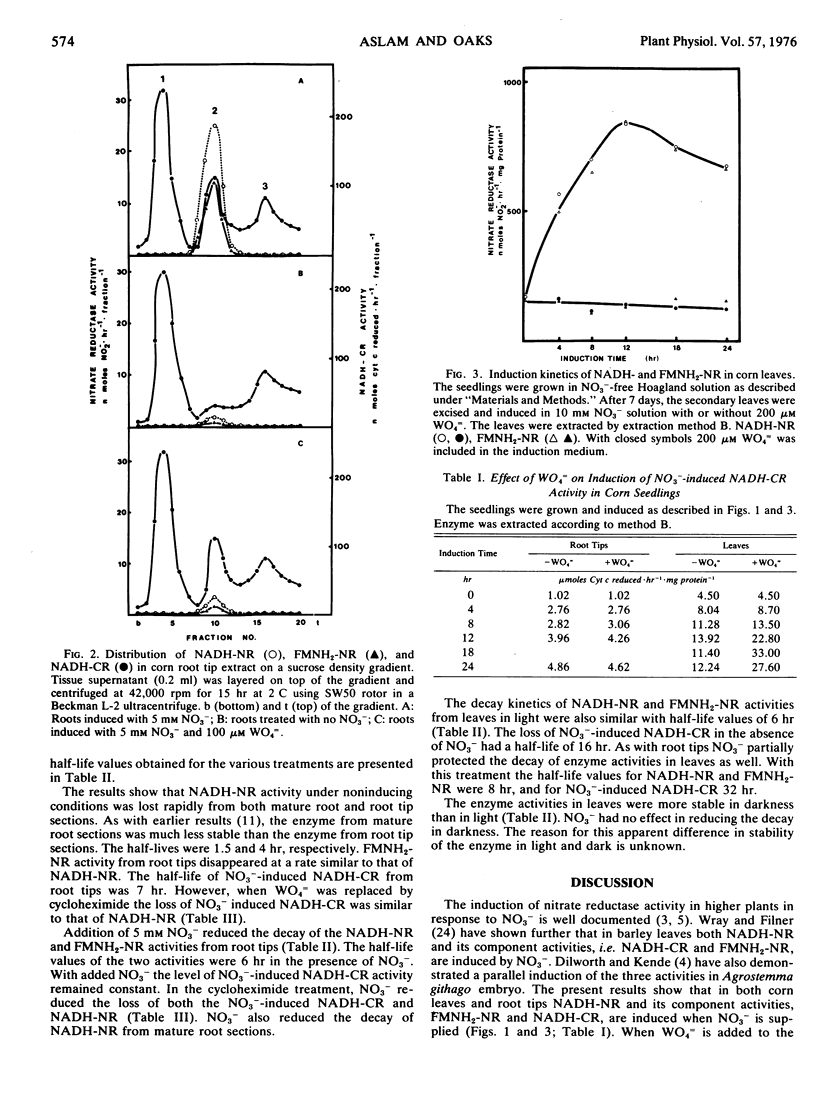
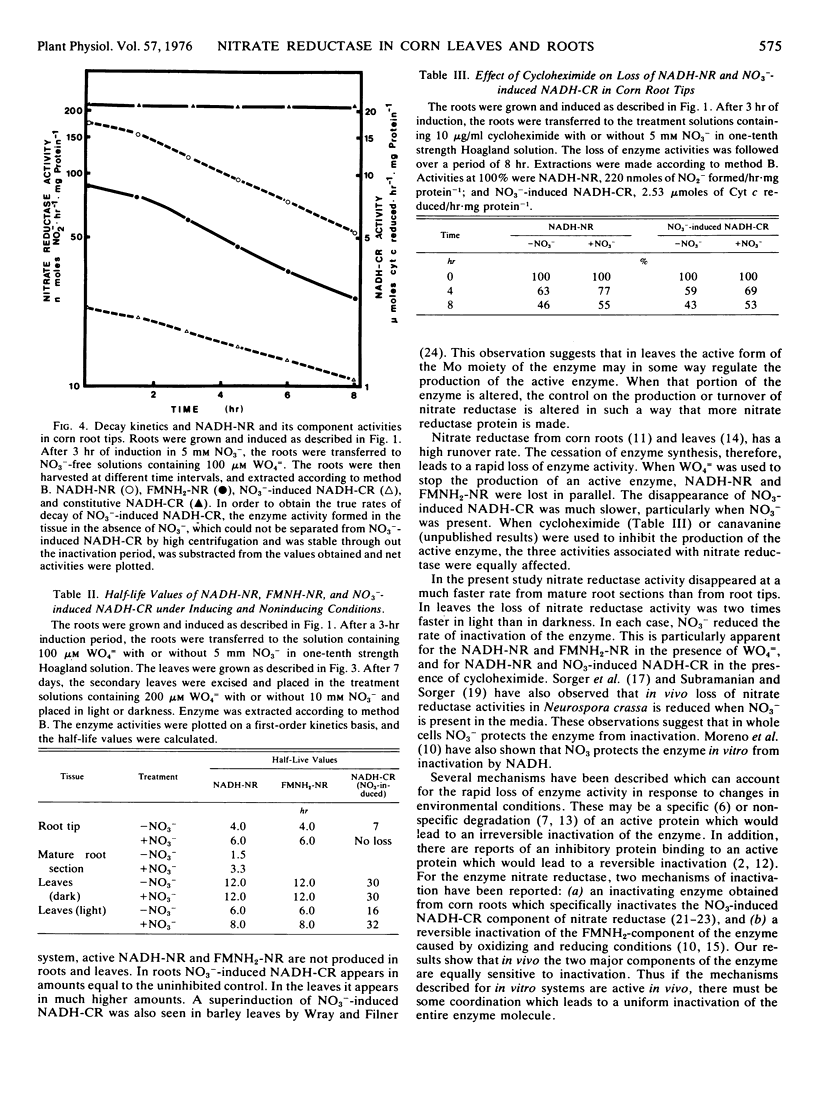
A comparison of induction and inactivation of nitrate reductase and two of its component activities, namely FMNH2-nitrate reductase and NO3−-induced NADH-cytochrome c reductase, was made in roots and leaves of corn (Zea mays L. var. W64A × 182E). The three activities were induced in parallel in both tissues when NO3− was supplied. WO4= suppressed the induction of NADH- and FMNH2-nitrate reductase activities in root tips and leaves. The NO3−-induced NADH-cytochrome c reductase activity showed a normal increase in roots treated with WO4=. In leaves, on the other hand, there was a marked superinduction of the NO3−-induced NADH-cytochrome c reductase in the presence of WO4=.
The half-life values of NADH-nitrate reductase and FMNH2-nitrate reductase measured by removing NO3− and adding WO4= to the medium, were 4 hours in root tips and 6 hours in excised leaves. Addition of NO3− in the induction medium together with WO4= gave partial protection of NADH-nitrate reductase and FMNH2-nitrate reductase activities in both root tips and leaves with a t0.5 of 6 and 8 hours, respectively. NO3− also reduced the loss of nitrate reductase activity from mature root sections. In the presence of cycloheximide, both NADH-nitrate reductase and NO3−-induced NADH-cytochrome c reductase activities were lost at similar rates in root tips. NO3− protected the loss of NO3−-induced NADH-cytochrome c reductase to the same extent as that of NADH-nitrate reductase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aslam M., Oaks A. Effect of glucose on the induction of nitrate reductase in corn roots. Plant Physiol. 1975 Nov;56(5):634–639. doi: 10.1104/pp.56.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechet J., Wiame J. M. Indication of a specific regulatory binding protein for ornithinetranscarbamylase in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1965 Nov 8;21(3):226–234. doi: 10.1016/0006-291x(65)90276-7. [DOI] [PubMed] [Google Scholar]
- Dilworth M. F., Kende H. Comparative Studies on Nitrate Reductase in Agrostemma githago Induced by Nitrate and Benzyladenine. Plant Physiol. 1974 Dec;54(6):821–825. doi: 10.1104/pp.54.6.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filner P., Varner J. E., Wray J. L. Environmental or developmental changes cause many enzyme activities of higher plants to rise or fall. Science. 1969 Jul 25;165(3891):358–367. doi: 10.1126/science.165.3891.358. [DOI] [PubMed] [Google Scholar]
- Katsunuma T., Schött E., Elsässer S., Holzer H. Purification and properties of tryptophan-synthase-inactivating enzymes from yeast. Eur J Biochem. 1972 Jun 9;27(3):520–526. doi: 10.1111/j.1432-1033.1972.tb01868.x. [DOI] [PubMed] [Google Scholar]
- Kenney F. T. Turnover of rat liver tyrosine transaminase: stabilization after inhibition of protein synthesis. Science. 1967 Apr 28;156(3774):525–528. doi: 10.1126/science.156.3774.525. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Moreno C. G., Aparicio P. J., Palacián E., Losada M. Interconversion of the active and inactive forms of Chlorella nitrate reductase. FEBS Lett. 1972 Oct 1;26(1):11–14. doi: 10.1016/0014-5793(72)80530-1. [DOI] [PubMed] [Google Scholar]
- Oaks A., Wallace W., Stevens D. Synthesis and turnover of nitrate reductase in corn roots. Plant Physiol. 1972 Dec;50(6):649–654. doi: 10.1104/pp.50.6.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressey R. Separation and properties of potato invertase and invertase inhibitor. Arch Biochem Biophys. 1966 Mar;113(3):667–674. doi: 10.1016/0003-9861(66)90246-3. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., Sweeney E. W., Berlin C. M. An analysis of the kinetics of rat liver tryptophan pyrrolase induction: the significance of both enzyme synthesis and degradation. Biochem Biophys Res Commun. 1964 Mar 26;15(3):214–219. doi: 10.1016/0006-291x(64)90148-2. [DOI] [PubMed] [Google Scholar]
- Schrader L. E., Ritenour G. L., Eilrich G. L., Hageman R. H. Some characteristics of nitrate reductase from higher plants. Plant Physiol. 1968 Jun;43(6):930–940. doi: 10.1104/pp.43.6.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomonson L. P., Jetschmann K., Vennesland B. Reversible inactivation of the nitrate reductase of Chlorella vulgaris Beijerinck. Biochim Biophys Acta. 1973 May 5;309(1):32–43. doi: 10.1016/0005-2744(73)90314-8. [DOI] [PubMed] [Google Scholar]
- Sorger G. J., Debanne M. T., Davies J. Effect of nitrate on the synthesis and decay of nitrate reductase of Neurospora. Biochem J. 1974 Jun;140(3):395–403. doi: 10.1042/bj1400395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger G. J. Nitrate reductase electron transport systems in mutant and in wild-type strains of Neurospora. Biochim Biophys Acta. 1966 Jun 15;118(3):484–494. doi: 10.1016/s0926-6593(66)80091-7. [DOI] [PubMed] [Google Scholar]
- Subramanian K. N., Sorger G. J. Regulation of nitrate reductase in Neurospora crassa: stability in vivo. J Bacteriol. 1972 May;110(2):538–546. doi: 10.1128/jb.110.2.538-546.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis R. L., Jordan W. R., Huffaker R. C. Evidence for an Inactivating System of Nitrate Reductase in Hordeum vulgare L. during Darkness That Requires Protein Synthesis. Plant Physiol. 1969 Aug;44(8):1150–1156. doi: 10.1104/pp.44.8.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace W. A nitrate reductase inactivating enzyme from the maize root. Plant Physiol. 1973 Sep;52(3):197–201. doi: 10.1104/pp.52.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace W. Effects of a nitrate reductase inactivating enzyme and NAD(P)H on the nitrate reductase from higher plants and Neurospora. Biochim Biophys Acta. 1975 Feb 19;377(2):239–250. doi: 10.1016/0005-2744(75)90306-x. [DOI] [PubMed] [Google Scholar]
- Wallace W. Purification and properties of a nitrate reductase-inactivating enzyme. Biochim Biophys Acta. 1974 Mar 21;341(1):265–276. doi: 10.1016/0005-2744(74)90087-4. [DOI] [PubMed] [Google Scholar]
- Wray J. L., Filner P. Structural and functional relationships of enzyme activities induced by nitrate in barley. Biochem J. 1970 Oct;119(4):715–725. doi: 10.1042/bj1190715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielke H. R., Filner P. Synthesis and turnover of nitrate reductase induced by nitrate in cultured tobacco cells. J Biol Chem. 1971 Mar 25;246(6):1772–1779. [PubMed] [Google Scholar]


