Abstract
AIM:
Prostate cancer (PCa) is the second most common cancers in men worldwide. Its incidence can be influenced by several risk factors including genetic susceptibility. Therefore the search for the expression of a certain gene (ERG) and its rearrangement could give us clues for proper identification of PCa. And the study of ERG expression and its comparison to FISH in Egyptian patients can show whether ERG immunophenotype could be used instead of FISH, as it is cheaper.
MATERIALS AND METHODS:
This study was performed on 85 cases of PCa, showing 30 cases with HGPIN and 30 cases of prostatic hyperplasia. All were immunohistochemistry stained using ERG monoclonal rabbit antihuman antibody was used (clone: EP111). FISH analysis was performed in 38 biopsies of PCa cases to detect TMRPSS2-ERG rearrangement using the FISH ZytoLight TriCheck Probe (SPEC TMRPSS2-ERG).
RESULTS:
ERG expression was found in 26% of PCa cases and 20% of HGPIN cases. FISH analysis showed fusion of 21 cases of PCa (out of 22 cases showing ERG immunoexpression).
CONCLUSION:
Our findings emphasise that only malignant and pre-malignant cells and not benign cells from the prostate stain positive. ERG expression may offer a simpler, accurate and less costly alternative for evaluation of ERG fusion status in PCa.
Keywords: Prostatic carcinoma, ERG Immunoexpression, TMRPSS2-ERG, Fluorescence in situ hybridization (FISH), High grade prostatic intraepithelial neoplasia
Introduction
Prostatic carcinoma (PCa) is a heterogeneous disease process with a varied spectrum of light microscopic morphologic as well as biological features [1-2]. Identification of prostate malignant acini can sometimes present a diagnostic challenge for pathologists since PCa can mimic benign prostate glands [3] and the architectural or cytologic clues for the diagnosis of carcinoma may not always be seen in small foci of suspicious glands. Histopathological diagnosis of PCa can be established by transrectal ultrasound-guided (TRUS) biopsy [4] after an abnormal finding on digital rectal examination or finding an augmentation in prostate-specific antigen (PSA) level [5].
Prostatic intraepithelial neoplasia (PIN) consists of pre-existing prostatic ducts and acini lined by cytologically atypical cells. HGPIN is considered the precancerous lesion of PCa [6, 7]. There is a strong association between HGPIN and PCa. The incidence of HGPIN in radical prostatectomy specimens for PCa is remarkably high, approximately 85–100% of specimens [6].
About 4% of contemporary prostate needle biopsies contain collections of small acini that are suspicious for cancer and are reported as atypical small acinar proliferation (ASAP) suspicious for but not diagnostic of malignancy. Prostate cancer has been identified in subsequent biopsies in the majority of cases of ASAP, indicating that this finding is a significant predictor of cancer [8]. Histopathological diagnosis of small focus carcinomas in prostatic needle biopsies is often assisted by IHC [9].
One of the earliest methods of detection of this cancer is PSA, which has been used to screen since the late 1980s [10]. The early disease diagnosis in the Western world is due to the improvement in and widespread availability of the measurement of PSA, including PSA density, PSA volume and adjusted age-specific PSA ranges [5]. The free to total (f/t) PSA ratio was shown, and this ratio is usually lower in patients with PCa than in those with BPH [11]. PCa patients with higher Gleason score correlated with increased total PSA and decreased free PSA [12].
ERG (v-ets erythroblastosis virus E26 oncogene homolog [avian], chromosome 21q22.3) is only seen in prostate cancer. It was first reported as an overexpression of the oncogene ERG at the transcript level in 50% of clinically localised and metastatic PCa samples [13]. Numerous studies showed that the basis for this overexpression was a result of ERG fusion with the promoter region of the androgen-induced TMRPSS2-ERG gene, the most common variant [14-17] HGPIN showing TMRPSS2-ERG fusion is associated with co-existing TMPRSS2-ERG PCa. The detection of isolated TMRPSS2-ERG fusion in HGPIN would improve the positive prognostic value of finding TMRPSS2-ERGfusion PCa in subsequent biopsies [18].
ERG’s oncogenic potential is known because of its involvement in Ewig’s sarcoma and leukaemia [17]. Additionally, ERG is a highly specific endothelial marker. It is expressed in small-caliber vessels adjacent to PCa using IHC. Hence, it can be used as an internal staining control, regardless of ERG rearrangement status in cancer [20].
The aim of the study was to determine the frequency of ERG expression in PCa of Egyptian patients and in HGPIN lesions juxtaposed to positively expressed PCa cases as well as to assess the concordance between ERG immunoexpression and TMRPSS2-ERG fusion status.
Patients and Methods
The present study was conducted on 115 prostatic cases including 85 patients with PCa, 30 of them containing PIN in the adjacent area of the tumour and 30 patients diagnosed with prostatic hyperplasia. Ten biopsies from patients with hemangioma served as a control group: they were five males and five females. According to the procedure, 1) there were 28 cases of TRUS cores, each case consisting of 6 tissue core samples, in total = 28 cases x 6 cores = 168 cores.
We chose the cases of most presentable areas and cores were representing a composite score, 2) 24 TURP, eight blocks from each case or 24 x 8 = 192 blocks. We chose blocks containing tumours, 3) 33 prostatectomies: we processed 20 blocks from both lobes = 33x20 = 660 blocks, and we chose blocks showing most tumour areas representing a composite score. Total blocks=162+192+660=1114 blocks, also 30 TRUS cases with benign prostatic hyperplasia, 30x6 cores=180 block. We reconstructed 15 tissue microarrays.
Histopathological study
Sections were cut from the paraffin block on the microtome, 4-micron thickness, and were stained with Haematoxylin and Eosin for histopathological diagnosis. Scoring was assessed for pathological evaluation of cases using Gleason scoring system. The PCa cases were sorted into three groups according to the current grading criteria of the ISUP 2014 modified GS (ISUP-GS) [23]: Group 1: GS≤ 6 (including Grade group 1) included 34 cases, Group 2: GS= 7 (including Grade Group 2 & 3) included 25 cases, and Group 3: GS ≥ 8 (including Grade group 4&5) included 26 cases.
Immunohistochemical (IHC) technique
ERG expression was evaluated using a commercial rabbit anti-ERG monoclonal antibody (cloneEP111; Dako, Denmark A/S). Deparaffinization, hydration of the slides, and blocking with pre-antibody solution (20 min) where performed in Dako PT Link (Code PT100/PT101). Then, a protocol template was created. The staining steps and incubation times were pre-programmed into the Autostainer Link software (Dako Autostainer). These were diluting anti-ERG primary antibody (1:50 for 20 min at room temp); applying Poly-HRP anti-rabbit IgG (20 min); applying DAB (20 min, Sigma Fast DAB tablets, Sigma-Aldrich, St. Louis MO); counter staining with EnVision FLEX hematoxylin (5 min); and dehydration, clearing, mounting, and covering.
Interpretation of immunostaining
ERG expression is normally observed in lymphocytes and endothelium. These cells were used as positive internal controls for the ERG staining but were not included in the evaluation of ERG fusion status, and only nuclear staining was considered to be positive.
The prostatic sections were examined using a Zeiss light microscope (Oberkochen, Germany). The number of positively stained cells with the highest expression recorded within ten successive fields (x400) was counted per section.
Each case was independently assessed by two pathologists (HO and KH) using an H-score system obtained by multiplying the intensity of the stain (0: no staining; 1: weak staining; 2: moderate staining; 3: intense staining) by the percentage (0–100) of the cell showing that staining intensity (H-score range 0–300). Any nuclear staining positivity (H-score >0) was considered as indicative of ERG expression [22].
Fluorescence in situ hybridization (FISH) technique
The formation of TMRPSS2-ERG fusions leads to disruption of the ERG gene locus. Of the 115 prostatic tissue specimens, 38 specimens were tested with the FISH approach including different Gleason scores using a previously described triple-color FISH assay (Zytolight SPEC TMPRSS2/ERG Tricheck probe), that detects the hybridization signals of the labelled ERG gene (21q22.2) which appear green and orange, whereas the hybridization signals of the labelled TMPRSS2 gene (21q22.3) appear blue [23].
Sections were heated for 10 min at 70°C, pretreated by dewaxing and proteolysis. Preparation of slides was carried out according to manual. TMPRSS2/ERG probe was added, and slides were denatured for 10 min at 75°C. Then slides were transferred to a humidity chamber and hybridised at 37°C overnight. Cell nuclei were stained with DAPI/Dura Tech-Solution.
Interpretation of FISH
Evaluation of the ERG rearrangement is carried out by fluorescence microscopy (Olympus X51) [using four filters red (FITC), green, blue and DAPI] using a × 100 oil immersion objective lens. The microscope is attached to high-resolution video camera (Jale) and monitor. We captured and interpreted photos using hardware (Cytovision 2.3, USA). In nuclei without TMRPSS2-ERG rearrangements, two green/orange fusion signals and two blue signals appeared. An ERG translocation without the involvement of TMPRSS2 was indicated by the split of one green/orange fusion signal, resulting in a separated green signal, near one blue signal, and a separated orange signal. On 21q22.13-q22.3 locus affected by a 21q22.2 deletion resulting in the TMPRSS2-ERG, fusion is indicated by one separate orange signal co-localizing with one blue signal and the loss of one green signal.
Statistical analyses
SPSS software version 18 was used for data management and analysis. We did median and interquartile range and compared the medians using Mann-Whitney U-test. Qualitative data were presented as frequencies and percentages. Tests were considered statistically significant when P< 0.05.
Results
ERG protein expression was analysed by IHC in 115 patients who underwent TRUS, TURP, RP, open prostatectomy and radical cystoprostatectomy at Theodor Bilharz Research Institute in 2014 and 2015. 85 cases were histopathologically diagnosed as PCa, 30 cases of HGPIN and ERG positivity was correlated to age, PSA, and histological parameters. Patients’ mean age was 71 years (range 50–88), GS in PCa cases was Group 1<7 in 34 (40.1%) including Grade group 1, Group 2 = 7 in 25 (29.4%) including Grade Group 2 and 3, and Group 3>7 in 26 (30.5%) cases. It was expanded by additional FISH analyses of 40 cases; 38 diagnosed as PCa and two benign cases.
ERG expression by IHC
The mean age of ERG positive cases is 67 years, which is slightly lower than the ERG negative cases that are 73 years old with no statistical significance. Median total PSA (tPSA) shows a higher level in Group 2 & 3 (PCa with GS>7) than Group 1 (PCa with GS<7) with no statistical significance (Table 1).
Table 1.
PSA in correlation to different Grade Groups
| Median P-value | Interquartile Range /Median | |
|---|---|---|
| Free PSA: | ||
| Group 1 | 14.28 | 16.75 (1-64) |
| Group 2 | 10 | 14 (0.8-800) |
| Group 3 | 20 | 31.03 (0.8-500) |
| Total PSA: | ||
| Group 1 | 35 | 75.88 (5-300) |
| Group 2 | 60 | 123 (4.5-5000) |
| Group 3 | 101 | 216.75 (4-2700) |
| PSA ratio | ||
| Group 1 | 0.25 | 0.10 (0.4-0.98) |
| Group 2 | 0.27 | 0.45 (0.2-0.9) |
| Group 3 | 0.25 | 0.24 (0.4-0.95) |
Group 1: GS≤ 6 (including Grade group 1) included 34 cases; Group 2: GS= 7 (including Grade Group 2&3) included 25 cases; Group 3: GS ≥ 8 (including Grade group 4&5) included 26 cases; Using Mann-Whiteny U-test, we found no statistical difference between different groups.
Interquartile Range /Median tPSA in ERG positive cases was 126.95 (16-300), and ERG negative cases were 00.00 (Table 2).
Table 2.
Total PSA in correlation to ERG expression by H Score
| ERG expression | Total PSA | No. of cases | Interquartile Range /Median |
|---|---|---|---|
| ERG + | ≤10 | 2 | 00.00 |
| >10 | 20 | 126.95 (16-300) | |
| ERG - | ≤ 10 | 6 | 00.00 |
| > 10 | 55 | 00.00 |
ERG overexpression was found in 22 (26.0%) out of the 85 PCa cases; 2 cases show mild intensity, 6 cases show moderate intensity, and 14 cases show severe intensity. GS groups were sorted into low (GS<7), intermediate (GS =7) and high (GS>7), revealing a higher prevalence with the higher intensity of GS = 7 tumours (50%) in the ERG + group. ERG was positive in 6 of 34 specimens (17.6%) with GS<7, in 11 of 25 specimens (44%) with GS=7, and in 5 of 26 specimens (19.2%) with GS>7.
All benign prostatic hyperplasia cases are negative for ERG expression while ERG immunoexpression was found in 6 (20%) out of the 30 HGPIN cases present in the same specimen with ERG + PCa cases. HGPIN showed six positive cases; 5 cases (83.3%) with mild intensity and 1 case (16.7%) with moderate intensity of ERG expressed HGPIN cases (Figs. 1 & 2).
Figure 1.
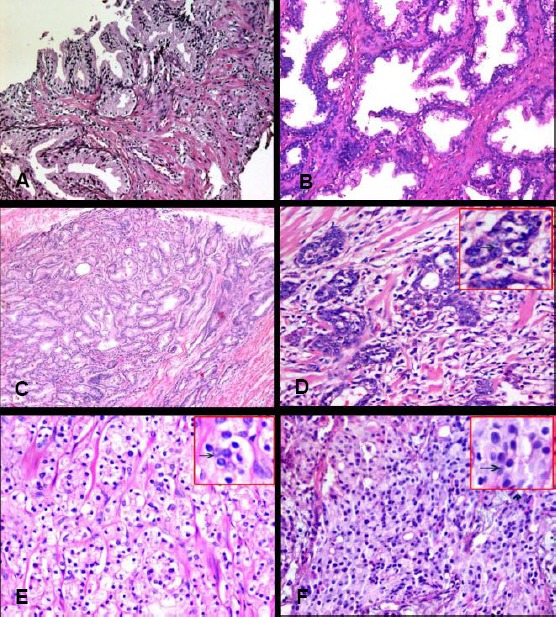
(A) A case of prostatic carcinoma with Gleason score 3+3 showing malignant glands infiltrating in-between hyperplastic acini (Hx&E) (X10). (B) High grade PIN (Hx&E) (x20). (C) A case of prostatic adenocarcinoma showing separated glands lined with single epithelial layer, with Gleason score 3+3, radical prostatectomy (Hx&E) (x10). (D) A case of prostatic adenocarcinoma with Gleason score 3+4, showing separated and fused glands (Hx&E) (X40). (E) A case of prostatic adenocarcinoma with Gleason score 4+3, TURP (Hx&E) (X10) Fig (F): A case of prostatic adenocarcinoma with Gleason score 4+4, TURP (Hx&E) (40 HPF)
Figure 2.
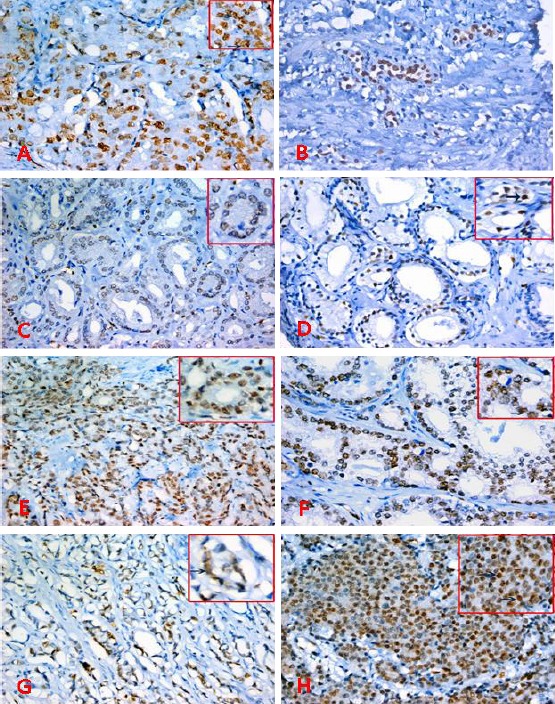
Prostatic sections stained with ERG immunohistochemistry, x200 &400, (A&B) HGPIN with positive nuclear staining for ERG, (C&D) PCs, Gleason score 3+3 with mild-moderate staining for ERG, (E&F) PCs, Gleason score 3+4 with moderate – marked staining for ERG, (G) PCs, Gleason score 4+4 with moderate staining for ERG, (H) PCs, Gleason score 4+5 with marked staining for ERG.
H score was increased in group 2 compared to group 1 with no statistical significance (Table 3).
Table 3.
H score in correlation to different Grade Groups
| ERG H-SCORE | ||
|---|---|---|
| Grading Groups | Interquartile Range/Median | |
| Group 1 (n=34) | 124.4 (16-300) | |
| Group 2 (n=25) | 138.4 (52-300) | |
| Group 3 (n=26) | 207.9(14.4-300) | |
Group 1: GS≤ 6 (including Grade group 1) included 34 cases; Group 2: GS= 7 (including Grade Group 2&3) included 25 cases; Group 3: GS ≥ 8 (including Grade group 4&5) included 26 cases; Using Mann-Whitney U-test, we found no statistical difference between different groups.
FISH procedure was conducted on 38 cases diagnosed as prostatic carcinoma. FISH analysis showed TMPRSS2-ERG fusion of 21 cases specimen selected of PCa (out of 22 cases that showed ERG immunoexpression).
The gene fusion occurs as a result of either a chromosomal translocation or an interstitial deletion, where 13 cases show interstitial deletion, and 8 cases show chromosomal translocation. Therefore about 61.9% of cases show deletion and 38.1% show translocation (Table 4; Fig. 3).
Table 4.
H score positive IHC cases in correlation to FISH positive cases
| ERG by IHC | TMRPSS2- ERG FISH | Total | |
|---|---|---|---|
| Number of negative cases | Number of positive cases | ||
| Negative | 16 | 0 | 16 |
| Positive | 1 | 21 | 22 |
| Total | 38 | ||
ERG overexpression was found in 22 (26.0%) out of 85 PCa cases. TMPRSS2-ERG fusion in the specimen selected, 21 cases of the prostatic carcinoma (out of 38 cases) show fusion. The gene fusion occurs as a result of either a chromosomal translocation or an interstitial deletion, where 13 cases show interstitial deletion, and 8 cases show chromosomal translocation. Therefore about 61.9% of cases show deletion and 38.1% show translocation.
Figure 3.
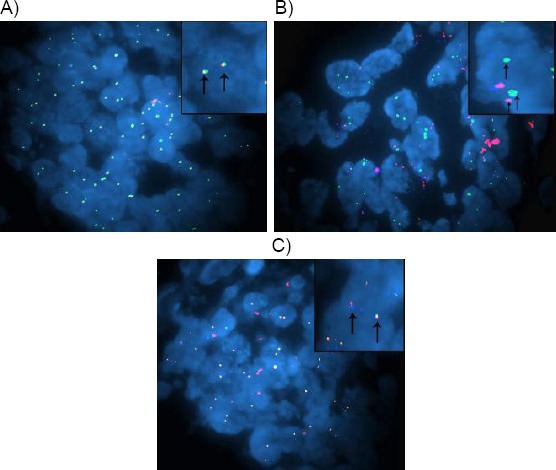
(A) case of prostatic adenocarcinoma, Gleason score 3+3, showing malignant cells with normal expression of TMRPSS2-ERG as merged picture or triple Bandpass filter set (orange, green and blue) of SPEC TMRPSS2-ERG TriCheck Probe (FISH, magnification × 1000), (B) case of prostatic adenocarcinoma, Gleason score 4+4, showing rearranged SPEC TMRPSS2-ERG as fusion associated deletion in malignant cells with merged picture or triple Bandpass filter set (fused orange and blue, missing green) (arrows) (FISH, SPEC TMRPSS2-ERG TriCheck Probe, magnification × 1000), (C) case of prostatic adenocarcinoma, Gleason score 5+4, showing rearranged SPEC ERG translocation not affecting TMPRSS2 in malignant cells with merged picture or triple Bandpass filter set (fused orange and blue, presence of green but away) (arrows) (FISH, SPEC TMRPSS2-ERG TriCheck Probe, magnification × 1000)
We found a strong association between ERG immunohistochemical overexpression and TMRPSS2-ERG rearrangement detected by FISH with 95.5% concordance, 100% sensitivity and 94.4% specificity.
Discussion
Globally, prostate cancer is reported to be the sixth most common type of cancer, and the second most common in men, especially in Europe, North America, and parts of Africa [24-25]. Studies are underway to evaluate new tests for PCa that could distinguish more aggressive cancers from those less likely to be lethal, to identify men at higher risk of developing prostate cancer, and to enable more efficient use of PSA testing [26]. Diagnosis, treatment and recommendations of PCa are based on biopsy assessments. Hence, to affect therapeutic decision making, a biomarker must be proved in biopsies [23].
In this study, no association between the immunoexpression of ERG and the clinical parameters including age, and PSA level was found (no statistical significance). This is in agreement with the study by Rubio-Briones et al. which showed no association of ERG status and preoperative PSA [27]. On the other hand, a previous PSA screening study reported a higher frequency of ERG+ cancer in low PSA patients, however, ‘low PSA’ was defined as ≤10 ng /ml[28]. However, two potential limitations in our current study are the small sample size and the inability to separate age and PSA entirely. Age-adjusted PSA cut points ensure that a young man with a lower PSA is more likely to get a biopsy than an older man with the same PSA level.
Data presented in this study demonstrated that ERG was overexpressed in 26% of the PCa group whereas most of the studies that have been done in Western countries demonstrated that ERG gene is overexpressed in approximately 50% in patients with PCa [28-31]. However, our results are higher than those for Asian patients. For example, Chinese prostate cancer patients showed ERG overexpression in 14.9% only [37]. Japan and Korea also show similar low frequency [32-34].
This study showed ERG expression in 20% of the HGPIN cases (HGPIN in association with PCa in the same cases). This is in agreement with other studies demonstrating that ERG was expressed in 27% of HGPIN and that ERG-positive HGPIN is strongly associated with positive ERG expression PCa in the specimen [35-36]; this indicates that ERG fusions appear early in the development of the disease.
Yu and his co-workers found that TMPRSS2-ERG plays a central role as a “malignant regulatory switch” that shuts down androgen signalling inhibiting normal prostate differentiation [37]. Our study showed that ERG is expressed in the PCa cases with Gleason score (GS) equal to 6 or higher. This is in agreement with other studies that support the role for TMRPSS2-ERG fusion in the initiation of carcinogenesis, as it is found in early lesions and typically homogenously maintained within high-grade tumours [38-41]. Previous papers have reported an association with low-grade prostate tumours [42-44]; whereas other studies support an association with high-grade ones [45].
ERG expression showed no correlation to different GS groups with any statistical significance. Our results, in agreement with many studies, did not find any association between the rearrangement and Gleason grade [47-51]. Conversely, previous studies showed that higher GS were associated with an increased frequency of TMRPSS2-ERG fusion event; ERG expression is inversely related to GS [51-53]. A recent study reported that the expression levels of TMRPSS2-ERG and ERG mRNA are related to more aggressive tumours [54]. However, there is controversy about the role of TMRPSS2-ERG in the development and progression of PCa [51-55].
The standard assessment for TMRPSS2-ERG is FISH [23]. In our research study, we have compared the result of ERG immunoexpression with TMRPSS2-ERG fusion status that revealed high sensitivity (100%) and high specificity (94.4%) with a concordance of 95.5%. This is in agreement with Berg and his colleagues’ study that showed a sensitivity of 100% and a specificity of 95.5% for an ERG rearrangement of 100%. This is in agreement with Jiang and his colleagues’ study that showed correlation with 100% sensitivity and 88.9% specificity [56]. Furthermore, many former studies have displayed high sensitivities (95.7%–99.1%) and specificities (92.3–99.4%) [57-58]. Consequently, our research applied ERG protein overexpression to determine the presence or absence of TMRPSS2-ERG rearrangement. We established that detection of ERG expression by IHC technique could be used as a surrogate marker for ERG rearrangement, in agreement with Svensson et al. and Berg and his co-workers [23, 57].
In conclusion, the prevalence of ERG protein expression is 26% in Egyptian patients with PCa. ERG immunoexpression was present in 20% of HGPIN lesions exclusively when juxtaposed to fusion positive PCa, implying that this is an early event in the process of PCa formation. The standard immunohistochemistry has high accuracy for defining ERG fusion status in men with PCa. Consequently, ERG expression may offer a simpler, accurate and less costly alternative for evaluation of ERG fusion status in PCa. The association between ERG expression and prostate cancer based on the Egyptian population should be further investigated.
Footnotes
Funding: This work was financed by TBRI internal project No. 107T. Principle investigator: Prof. Dr Ahmed Abdel Hadi.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Ferlay J, Soerjomataram, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns. In: GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Epstein JI, Algaba F, Allsbrook WC, Bastacky S, Boccon-Glbod L, De Marzo A. Acinar adenocarcinoma. World Health Organization, International Agency for Research on Cancer. In: Eble JN, et al., editors. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Urinary System and Male Genital Organ. Lyon: IARC Pres; 2004. pp. 162–192. [Google Scholar]
- 3.Donovan MJ, Cordon-Cardo C. Predicting high-risk disease using tissue biomarkers. Curr Opin Urol. 2013;23(3):245–5. doi: 10.1097/MOU.0b013e32835f89cc. https://doi.org/10.1097/mou.0b013e32835f89cc . [DOI] [PubMed] [Google Scholar]
- 4.Ghei M, Pericleous S, Kumar A, Miller R, Nathan, Maraj B, et al. Finger-guided transrectal biopsy of the prostate: a modified, safer technique. Ann R Coll Surg Engl. 2005;87(5):386–7. PMid:16402467. PMCid:PMC1963966. [PMC free article] [PubMed] [Google Scholar]
- 5.Greene KL, Albertsen PC, Babaian RJ, Carter HB, Gann PHH, Han M, et al. Prostate specific antigen best practice statement. J Urol. 2009;182:2232–2241. doi: 10.1016/j.juro.2009.07.093. https://doi.org/10.1016/j.juro.2009.07.093 . PMid:19781717. [DOI] [PubMed] [Google Scholar]
- 6.Ayala AG, Ro JY. Prostatic intraepithelial neoplasia: recent advances. Arch Pathol Lab Med. 2007;131:1257–6. doi: 10.5858/2007-131-1257-PINRA. PMid:17683188. [DOI] [PubMed] [Google Scholar]
- 7.Epstein JI. Precursor lesions to prostatic adenocarcinoma. Virchows Arch. 2009;454:1–16. doi: 10.1007/s00428-008-0707-5. https://doi.org/10.1007/s00428-008-0707-5 . PMid:19048290. [DOI] [PubMed] [Google Scholar]
- 8.Isabelle M, Hillel K, David B. Atypical Small Acinar Proliferation in the Prostate. AJSP. 2008;13(4):129–134. [Google Scholar]
- 9.Helin H, Lundin M, Laakso M, Lundin J, Helin J, Isola J. Virtual Microscopy in Prostate Histopathology: Simultaneous Viewing of Biopsies Stained Sequentially With Hematoxylin and Eosin, and α-Methylacyl-Coenzyme A Racemase/p63 Immunohistochemistry. Journal of Urology. 2006;175(2):495–499. doi: 10.1016/S0022-5347(05)00164-3. https://doi.org/10.1016/S0022-5347(05)00164-3 . [DOI] [PubMed] [Google Scholar]
- 10.Kitagawa Y, Mizokami A, Namiki M. Trends of clinical symptoms and prognosis of middle-aged prostate cancer patients after instigation of prostate specific antigen-based population screening. Prostate Int. 2013;1:65–68. doi: 10.12954/PI.12017. https://doi.org/10.12954/PI.12017 . PMid:24223404. PMCid:PMC3814114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stenman UH, Hakama M, Knekt P, Aromaa A, Teppo L, et al. Serum concentrations of prostate specific antigen and its complex with a-1-antichymotrypsin before diagnosis of prostatic cancer. Lancet. 1994;344:1594–1598. doi: 10.1016/s0140-6736(94)90405-7. https://doi.org/10.1016/S0140-6736(94)90405-7 . [DOI] [PubMed] [Google Scholar]
- 12.Ceylan C, Gazel E, Keleş İ, Doluoğlu Ö, Yığman M. Can the Free/Total PSA Ratio Predict the Gleason Score Before Prostate Biopsy? Curr Urol. 2016;9(1):24–7. doi: 10.1159/000442846. https://doi.org/10.1159/000442846 . PMid:26989367. PMCid:PMC4789969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanaja DK, Cheville JC, Iturria SJ, Young CY. Transcriptional silencing of zinc finger protein 185 identified by expression profiling is associated with prostate cancer progression. Cancer Res. 2003;63(14):3877–3882. PMid:12873976. [PubMed] [Google Scholar]
- 14.Mosquera JM, Mehra R, Regan MM, Perner S, Genega EM, Bueti G, et al. Prevalence of TMPRSS2-ERG fusion prostate cancer among men undergoing prostate biopsy in the United States. Clin Cancer Res. 2009;15(14):4706–11. doi: 10.1158/1078-0432.CCR-08-2927. https://doi.org/10.1158/1078-0432.CCR-08-2927 . PMid:19584163. PMCid:PMC3717524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomlins SA, Bjartell A, Chinnaiyan AM, Jenster G, Nam RK, Rubin MA, et al. ETS gene fusions in prostate cancer: from discovery to daily clinical practice. Eur Urol. 2009;56(2):275–286. doi: 10.1016/j.eururo.2009.04.036. https://doi.org/10.1016/j.eururo.2009.04.036 . PMid:19409690. [DOI] [PubMed] [Google Scholar]
- 16.Kumar-Sinha C, Tomlins SA, Chinnaiyan AM. Recurrent gene fusions in prostate cancer. Nat Rev Cancer. 2008;8(7):497–511. doi: 10.1038/nrc2402. https://doi.org/10.1038/nrc2402 . PMid:18563191. PMCid:PMC2711688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. https://doi.org/10.1126/science.1117679 . PMid:16254181. [DOI] [PubMed] [Google Scholar]
- 18.Mosquera JM, Perner S, Genega EM, Sanda M, Hofer MD, Mertz KD, et al. Characterization of TMPRSS2-ERG fusion high-grade prostatic intraepithelial neoplasia and potential clinical implications. Clin Cancer Res. 2008;14(11):3380–5. doi: 10.1158/1078-0432.CCR-07-5194. https://doi.org/10.1158/1078-0432.CCR-07-5194 . PMid:18519767. PMCid:PMC3717517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen S, Deniz K, Sung YS, Zhang L, Dry S, Antonescu CR. Ewing sarcoma with ERG gene rearrangements: A molecular study focusing on the prevalence of FUS-ERG and common pitfalls in detecting EWSR1-ERG fusions by FISH. Genes Chromosomes Cancer. 2016;55(4):340–9. doi: 10.1002/gcc.22336. https://doi.org/10.1002/gcc.22336 . PMid:26690869. PMCid:PMC5006947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gavrilov D, Kenzior O, Evans M, Calaluce R, Folk WR. Expression of urokinase plasminogen activator and receptor in conjunction with the ets family and AP-1 complex transcription factors in high grade prostate cancers. Eur J Cancer. 2001;37(8):1033–1040. doi: 10.1016/s0959-8049(01)00077-6. https://doi.org/10.1016/S0959-8049(01)00077-6 . [DOI] [PubMed] [Google Scholar]
- 21.Epstein JI, Zelefsky MJ, Sjoberg DD, Nelson JB, Egevad L, Magi-Galluzzi C, Vickers AJ, Parwani AV, Reuter VE, Fine SW, Eastham JA, Wiklund P, Han M, Reddy CA, Ciezki JP, Nyberg T, Klein EA. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur Urol. 2016;69(3):428–35. doi: 10.1016/j.eururo.2015.06.046. https://doi.org/10.1016/j.eururo.2015.06.046 . PMid:26166626. PMCid:PMC5002992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaux A, Albadine R, Toubaji A, Hicks J, Meeker A, Platz EA, et al. Immunohistochemistry for ERG expression as a surrogate for TMPRSS2-ERG fusion detection in prostatic adenocarcinomas. Am J Surg Pathol. 2011;35:1014–1020. doi: 10.1097/PAS.0b013e31821e8761. https://doi.org/10.1097/PAS.0b013e31821e8761 . PMid:21677539. PMCid:PMC3505676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berg KD, Brasso K, Thomsen FB, Roder MA, Holten-Rossing H, Toft BG, et al. J Clin Pathol. 2015;0:1–7. doi: 10.1136/jclinpath-2015-202894. [DOI] [PubMed] [Google Scholar]
- 24.Hilal L, Shahait M, Mukherji D, Charafeddine M, Farhat Z, Temraz S, et al. Prostate cancer in the Arab world: A view from the inside Clin Genitourin Cancer. 2015;13(6):505–11. doi: 10.1016/j.clgc.2015.05.010. https://doi.org/10.1016/j.clgc.2015.05.010 . PMid:26149392. [DOI] [PubMed] [Google Scholar]
- 25.Gronberg H. Prostate cancer epidemiology. Lancet. 2003;361:859–864. doi: 10.1016/S0140-6736(03)12713-4. https://doi.org/10.1016/S0140-6736(03)12713-4 . [DOI] [PubMed] [Google Scholar]
- 26.Cuzick J, Thorat MA, Andriole G, Brawley O, Brown P, Culig Z, et al. Prevention and early detection of prostate cancer. Lancet Oncol. 2014;15:e484–e492. doi: 10.1016/S1470-2045(14)70211-6. https://doi.org/10.1016/S1470-2045(14)70211-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubio-Briones J, Fernandez-Serra A, Calatrava A, García-Casado Z, Rubio L, Bonillo MA, et al. Clinical implications of TMPRSS2-ERG gene fusion expression in patients with prostate cancer treated with radical prostatectomy. J Urol. 2010;183(5):2054–61. doi: 10.1016/j.juro.2009.12.096. https://doi.org/10.1016/j.juro.2009.12.096 . PMid:20303538. [DOI] [PubMed] [Google Scholar]
- 28.Hoogland AM, Jenster G, van Weerden WM, Trapman J, Kwast Tvd, Roobol MJ, et al. ERG immunohistochemistry is not predictive for PSA recurrence, local recurrence or overall survival after radical prostatectomy for prostate cancer. Mod Pathol. 2012;25(3):471–479. doi: 10.1038/modpathol.2011.176. https://doi.org/10.1038/modpathol.2011.176 . PMid:22080055. [DOI] [PubMed] [Google Scholar]
- 29.Schaefer G, Mosquera J-M, Ramoner R, Park K, Romianel A, Steiner E, et al. Distinct ERG rearrangement prevalence in prostate cancer: higher frequency in young age and in low PSA prostate cancer. Prostate Cancer and Prostatic Disease. 2013;16:132–138. doi: 10.1038/pcan.2013.4. https://doi.org/10.1038/pcan.2013.4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomlins SA, Palanisamy N, Siddiqi J, Chinnaiyan AM, Kunju LP. Antibody-Based Detection of ERG Rearrangements in Prostate Core Biopsies, Including Diagnostically Challenging Cases. ERG Staining in Prostate Core Biopsies. Arch Pathol Lab Med. 2012;136:935–946. doi: 10.5858/arpa.2011-0424-OA. https://doi.org/10.5858/arpa.2011-0424-OA . PMid:22849743. PMCid:PMC3667408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitelman F, Johansson B, Mertens F. The impact of translocations and gene fusions on cancer causation. Nat Rev Cancer. 2007;7(4):233–45. doi: 10.1038/nrc2091. https://doi.org/10.1038/nrc2091 . PMid:17361217. [DOI] [PubMed] [Google Scholar]
- 32.Furusato B, van Leenders GJ, Trapman J, Kimura T, Egawa S, Furusato M, et al. Immunohistochemical ets-related gene detection in a Japanese prostate cancer cohort: diagnostic use in Japanese prostate cancer patients. Pathol Int. 2011;61(7):409–14. doi: 10.1111/j.1440-1827.2011.02675.x. https://doi.org/10.1111/j.1440-1827.2011.02675.x . PMid:21707844. [DOI] [PubMed] [Google Scholar]
- 33.Lee K, Chae JY, Kwak C, Ku JH, Moon KJ. TMPRSS2-ERG gene fusion and clinicopathologic characteristics of Korean prostate cancer patients. Urology. 2010;76(5):1268.e7–13. doi: 10.1016/j.urology.2010.06.010. https://doi.org/10.1016/j.urology.2010.06.010 . PMid:20800881. [DOI] [PubMed] [Google Scholar]
- 34.Miyagi Y, Sasaki T, Fujinami K, et al. ETS family-associated gene fusions in Japanese prostate cancer: analysis of 194 radical prostatectomy samples. Mod Pathol. 2010;23(11):1492–8. doi: 10.1038/modpathol.2010.149. https://doi.org/10.1038/modpathol.2010.149 . PMid:20693979. [DOI] [PubMed] [Google Scholar]
- 35.Carver BS, Tran J, Chen Z, Carracedo-Perez A, Alimonti A, Nardella C, et al. ETS rearrangements and prostate cancer initiation. Nature. 2009;457(7231):E1. doi: 10.1038/nature07738. discussion E2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han B, Mehra R, Lonigro RJ, Wang L, Suleman K, Menon A, et al. Fluorescence in situ hybridization study shows association of PTEN deletion with ERG rearrangement during prostate cancer progression. Mod Pathol. 2009;22(8):1083–93. doi: 10.1038/modpathol.2009.69. https://doi.org/10.1038/modpathol.2009.69 . PMid:19407851. PMCid:PMC2760294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu J, Yu J, Mani RS, Cao Q, Brenner CJ, Cao X, et al. An Integrated Network of Androgen Receptor, Polycomb, and TMPRSS2-ERG Gene Fusions in Prostate Cancer Progression. Cancer cells. 2010;17(5):443–454. doi: 10.1016/j.ccr.2010.03.018. https://doi.org/10.1016/j.ccr.2010.03.018 . PMid:20478527. PMCid:PMC2874722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williamson SR, Zhang S, Yao JL, et al. ERG-TMPRSS2 rearrangement is shared by concurrent prostatic adenocarcinoma and prostatic small cell carcinoma and absent in small cell carcinoma of the urinary bladder: evidence supporting monoclonal origin. Mod Pathol. 2011;24(8):1120–7. doi: 10.1038/modpathol.2011.56. https://doi.org/10.1038/modpathol.2011.56 . PMid:21499238. PMCid:PMC3441178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang S, Pavlovitz B, Tull J, Wang Y, Deng FM, Fuller C. Detection of TMPRSS2 gene deletions and translocations in carcinoma, intraepithelial neoplasia, and normal epithelium of the prostate by direct fluorescence in situ hybridization. Diagn Mol Pathol. 2010;19(3):151–6. doi: 10.1097/PDM.0b013e3181bb216a. https://doi.org/10.1097/PDM.0b013e3181bb216a . PMid:20736744. [DOI] [PubMed] [Google Scholar]
- 40.Dai MJ, Chen LL, Zheng YB, Chen W, Tao ZH, Weng ZL, et al. Frequency and transcript variant analysis of gene fusions between TMPRSS2 and ETS transcription factor genes in prostate cancer. Zhonghua Yi Xue Za Zhi. 2008;88(10):669–73. PMid:18642766. [PubMed] [Google Scholar]
- 41.Mehra R, Han B, Tomlins SA, Wang L, Menon A, Wasco MJ, et al. Heterogeneity of TMPRSS2 gene rearrangements in multifocal prostate adenocarcinoma: molecular evidence for an independent group of diseases. Cancer Res. 2007;67(17):7991–5. doi: 10.1158/0008-5472.CAN-07-2043. https://doi.org/10.1158/0008-5472.CAN-07-2043 . PMid:17804708. [DOI] [PubMed] [Google Scholar]
- 42.Gopalan A, Leversha M A, Dudas M E, Maschino A C, Chang J, et al. TMPRSS2–ERG rearrangement in dominant anterior prostatic tumours: incidence and correlation with ERG immunohistochemistry. Histopathology. 2013;63:279–286. doi: 10.1111/his.12153. https://doi.org/10.1111/his.12153 . PMid:23701505. PMCid:PMC3723763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fine SW, Gopalan A, Leversha MA, Al-Ahmadie HA, Tickoo SK, Zhou Q, et al. TMPRSS2-ERG gene fusion is associated with low Gleason scores and not with high-grade morphological features. Mod Pathol. 2010;23(10):1325–33. doi: 10.1038/modpathol.2010.120. https://doi.org/10.1038/modpathol.2010.120 . PMid:20562851. PMCid:PMC3413944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bismar TA, Dolph M, Teng L-H, Liu S, Donnelly B. ERG protein expression reflects hormonal treatment response and is associated with Gleason score and prostate cancer specific mortality. Eur J Cancer. 2012;48(4):538–546. doi: 10.1016/j.ejca.2012.01.001. https://doi.org/10.1016/j.ejca.2012.01.001 . PMid:22300588. [DOI] [PubMed] [Google Scholar]
- 45.Hagglof C, Hammarsten P, Str€omvall K, Egevad L, Josefsson A, Stattin P, Granfors T, et al. TMPRSS2-ERG expression predicts prostate cancer survival and associates with stromal biomarkers. PLoS ONE. 2014;9(2):e86824. doi: 10.1371/journal.pone.0086824. https://doi.org/10.1371/journal.pone.0086824 . PMid:24505269. PMCid:PMC3914792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qi M, Yang X, Zhang F, Lin T, Sun X, et al. ERG rearrangement is associated with prostate cancer-related death in Chinese prostate cancer patients. PLoS One. 2014;9(2):e84959. doi: 10.1371/journal.pone.0084959. https://doi.org/10.1371/journal.pone.0084959 . PMid:24516518. PMCid:PMC3917829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Muga S, Herandez S, Salido M, Lorenzo M, Agell A, Juanpere-Rodero, et al. CXCR4 mRNA overexpression in high grade prostate tumors: Lack of association with TMPRSS2-ERG rearrangement. Cancer Biomark. 2012;12(1):21–30. doi: 10.3233/CBM-2012-00288. https://doi.org/10.3233/CBM-2012-00288 . PMid:23321466. [DOI] [PubMed] [Google Scholar]
- 48.Lapointe J, Li C, Giacomini CP, Salari K, Huang S, Wang P, et al. Genomic profiling reveals alternative genetic pathways of prostate tumorigenesis. Cancer Res. 2007;67(18):8504–10. doi: 10.1158/0008-5472.CAN-07-0673. https://doi.org/10.1158/0008-5472.CAN-07-0673 . PMid:17875689. [DOI] [PubMed] [Google Scholar]
- 49.FitzGerald LM, Agalliu I, Johnson K, Miller MA, Kwon EM, Hurtado-coll A, et al. Association of TMPRSS2-ERG gene fusion with clinical characteristics and outcomes: results from a population-based study of prostate cancer. BMC Cancer. 2008;11:8–230. doi: 10.1186/1471-2407-8-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saramaki O, Harjula A, Martikainen P, Vessella RL, Tammela TL, Visakorpi T. TMPRSS2:ERG fusion identifies a subgroup of prostate cancers with a favorable prognosis. Clin Cancer Res. 2008;14(11):3395–400. doi: 10.1158/1078-0432.CCR-07-2051. https://doi.org/10.1158/1078-0432.CCR-07-2051 . PMid:18519769. [DOI] [PubMed] [Google Scholar]
- 51.Perner S, Demichelis F, Beroukhim R, Schmidt FH, Mosquera JM, Setlur S, et al. TMPRSS2:ERG fusion-associated deletions provide insight into the heterogeneity of prostate cancer. Cancer Res. 2006;66(17):8337–8341. doi: 10.1158/0008-5472.CAN-06-1482. https://doi.org/10.1158/0008-5472.CAN-06-1482 . PMid:16951139. [DOI] [PubMed] [Google Scholar]
- 52.Rouzier C, Haudebourg J, Carpentier X, Valério L, Amiel J, Michiels JF, et al. Detection of the TMPRSS2-ETS fusion gene in prostate carcinomas: retrospective analysis of 55 formalin-fixed and paraffin-embedded samples with clinical data. Cancer Genet Cytogenet. 2008;183(1):21–7. doi: 10.1016/j.cancergencyto.2008.01.021. https://doi.org/10.1016/j.cancergencyto.2008.01.021 . PMid:18474293. [DOI] [PubMed] [Google Scholar]
- 53.Lee SL, Yu D, Wang C, Saba R, Liu S, Trpkov K, et al. ERG Expression in Prostate Needle Biopsy: Potential Diagnostic and Prognostic Implications. Appl Immunohistochem Mol Morphol. 2015;23(7):499–505. doi: 10.1097/PAI.0000000000000119. https://doi.org/10.1097/PAI.0000000000000119 . PMid:25517865. [DOI] [PubMed] [Google Scholar]
- 54.Font-Tello A, Juanpere N, de Muga S, Lorenzo M, Lorente JA, Fumado L, et al. Association of erg and tmprss2-erg with grade, stage, and prognosis of prostate cancer is dependent on their expression levels. Prostate. 2015;75(11):1216–26. doi: 10.1002/pros.23004. https://doi.org/10.1002/pros.23004 . PMid:25939480. [DOI] [PubMed] [Google Scholar]
- 55.Demichelis F, Fall K, Perner S, Andren O, Schmidt F, Setlur SR, et al. TMPRSS2:ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene. 2007;26:4596–4599. doi: 10.1038/sj.onc.1210237. https://doi.org/10.1038/sj.onc.1210237 . PMid:17237811. [DOI] [PubMed] [Google Scholar]
- 56.Jiang H, Mao X, Huang X, Zhao J, Wang L, Xu J, Zhang H, Lu Y, Yu Y. TMPRSS2:ERG fusion gene occurs less frequently in Chinese patients with prostate cancer. Tumour Biol. 2016;37(9):12397–12402. doi: 10.1007/s13277-016-5116-9. Erratum in: Tumour Biol. 2016;37(10):14331. https://doi.org/10.1007/s13277-016-5116-9 . PMid:27320318. [DOI] [PubMed] [Google Scholar]
- 57.Svensson MA, Perner S, Ohlson AL, et al. A comparative study of ERG status assessment on DNA, mRNA, and protein levels using unique samples from a Swedish biopsy cohort. Appl Immunohistochem Mol Morphol. 2014;22:136–41. doi: 10.1097/PDM.0b013e31829e0484. https://doi.org/10.1097/PDM.0b013e31829e0484 . PMid:24517914. [DOI] [PubMed] [Google Scholar]
- 58.Braun M, Goltz D, Shaikhibrahim Z, Vogel W, Scheble V, Sotlar K, et al. ERG protein expression and genomic rearrangement status in primary and metastatic prostate cancer—a comparative study of two monoclonal antibodies. Prostate Cancer Prostatic Dis. 2012;15:165–9. doi: 10.1038/pcan.2011.67. https://doi.org/10.1038/pcan.2011.67 . PMid:22231490. [DOI] [PubMed] [Google Scholar]


