Abstract
BACKGROUND:
Bladder cancer represents the fifth most common malignancy worldwide and a major cause of cancer-related morbidity and death. Incidence and mortality rates have remained relatively constant over the past four decades. Urothelial bladder cancers have identified multiple risk factors.
AIM:
We aimed at evaluating the expression of the FGFR3 protein and gene amplification in the urothelial cells of neoplastic and non-neoplastic urothelial lesions of the urinary bladder, and correlation with tumour grade, stage and associated bilharziasis.
MATERIAL AND METHODS:
One hundred and five different urinary bladder lesions were studied, including 15 cystitis cases (9 bilharzial and 6 non-bilharzial cystitides), 75 urothelial carcinoma cases (18 bilharzial associated and 57 non-bilharzial associated) and 15 squamous cell carcinoma associated with bilharziasis, beside 5 control cases. Data concerning age, sex, tumour grade, stage, and associated bilharziasis were obtained. Each case was studied for FGFR3 expression, and FISH technique was applied on forty malignant cases that show high protein expression.
RESULTS:
The highest incidence of cystitis was in the fourth decade while of bladder cancer was in the seventh decade. Tumour grade was correlated significantly with tumour stage. FGFR3 correlates significantly with tumour grade, stage and with a bilharzial infestation. FGFR3 gene amplification was reported mainly in low grade and NNMBIC tumours.
CONCLUSIONS:
FGFR3 overexpression in malignant cases was significantly higher than in chronic cystitis. FGFR3 gene amplification was reported mainly in low grade and NNMBIC tumours. FGFR3 may be further studied as a subject for target therapy of bladder cancer.
Keywords: FGFR3, IHC, FISH, urothelial bladder lesions, cystitis, carcinoma
Introduction
In Egypt, bladder cancer accounts for about 30% of all cancers, with many pathogenic factors most commonly schistosomal infestation, which is an endemic disease in Nile River area [1]. In areas where schistosomiasis is endemic there is a high incidence of squamous cell carcinoma of the urinary bladder. Schistosomiasis causes chronic granulomatous cystitis leading to squamous metaplasia of transitional epithelium, and subsequently development of squamous cell carcinoma [2]. Transitional cell carcinoma is the most common type of bladder cancer. It arises primarily from transitional cells of bladder mucosal epithelium and may be present as non-invasive papillary or non-papillary tumours [3].
The interaction between transitional cell carcinoma cells and the adjacent or underlying bladder stroma may be an important determinant in the progression of superficial to invasive disease [4]. Fibroblast growth factors (FGFs) orchestrate a variety of cellular functions by binding to their trans-membrane tyrosine-kinase receptors (FGFRs) and activating downstream signalling pathways, including RAS/MAPK, PLCγ1, PI3K, and STATs [5]. Fibroblast growth factor receptor three signalling is altered in a high proportion of bladder tumours. Activating mutations and overexpression of FGFR3 are common in urothelial tumours with low malignant potential and low-stage and -grade urothelial carcinomas and are associated with a lower risk of progression and better survival in some subgroups [5]. FGFR3 mutations are linked to favourable (low grade/stage) pTa bladder cancer [6]. Fibroblast Growth Factor receptor 3 (FGFR3) mutations are most common in low-risk NMIBC (non-muscle invasive bladder cancer), a subset of MIBC (muscle invasive bladder cancer) that carries FGFR3 mutations shows particularly poor prognosis [7]. Foth et al., [7] stated that FGFR3 activation leads to urothelial cell abnormalities in the absence of Pten gene. The fgfr3 expression is present in both primary and metastatic urothelial cancer, and the majority of metastases retain immmunohisto-chemical expression of FGFR3 if it was expressed in the primary tumour [8]. Subsequent larger studies established that FGFR3 mutations occur in around 50% of both lower and upper urinary tract tumours and these cluster in three distinct hotspots in exons 7, 10, and 15 [6,9]. FGFR3 mutations are thought to induce a conformational change in the kinase domain resulting in ligand-independent receptor activation and signalling; they have also been shown to alter FGFR3 cellular localisation, inducing aberrant signalling from the endoplasmic reticulum [10].
FGFR3 mutation status was the strongest predictor of recurrence when compared with stage and grade. This is the first mutation in bladder cancer that selectively identifies patients with favourable disease characteristics [11]. While FGFR3 mutation or copy number gain is a rare event, studies of these tumours may shed light on how FGFR3 signalling contributes to urothelial carcinoma growth and proliferation [8]. Furthermore, based on preclinical studies that suggest the FGFR pathway, and specifically FGFR3, may be a valid therapeutic target, we believe this area of investigation warrants further exploration [8]. In vitro and in vivo studies have shown that FGFR inhibition has cytotoxic and cytostatic effects in FGFR-dependent bladder cancer cells and FGFR-targeted agents are currently being investigated in clinical studies for the treatment of urothelial carcinoma [5]. Overall, FGFRs hold promise as therapeutic targets, diagnostic and prognostic markers, and screening tools for early detection and clinical management of urothelial carcinoma [5].
In our study, we are aiming to evaluate the association of FGFR3 expression and gene mutation and its risk in the development of urothelial carcinoma (bilharzial and non-bilharzial), the effect on grading and staging of the tumour and the benefit of using this marker as a therapeutic target and diagnostic tool in bladder cancer patients.
Patients and Methods
The material of this study included 110 formalin fixed paraffin embedded (FFPE) biopsies. The cases were included according to the following criteria:
Inclusion criteria: Adult Cases with bladder cancer; with different grades and stages; with or without bilharzial cystitis; nature of the specimen: TUR-T or radical cystectomy;
Exclusion criteria: receiving chemotherapy and radiotherapy; children.
Groups
According to [12-13], the 110 cases were categorised into the following groups:
Group (1): Control; 5 cases. These cases were (4 males and 1 female with age range 20–46 y) with normal, healthy urothelium, and served as controls undergoing cystoscopy for other reasons than lower urinary tract symptoms, such as ureteroscopy or ureteric catheter or double J-insertion and biopsies were taken upon their consent.
Group (2): Cystitis: a) Bilharzial cystitis, 9 cases; b) Non-Bilharzial cystitis, 6 cases.
Group (3): Urothelial (Transitional cell) carcinoma: n= 75 cases. A) Bilharzial Transitional cell carcinoma (Bil.TCC), 18 cases; b) Non Bilharzial Transitional cell carcinoma (TCC), 57 cases.
Group (4): Squamous cell carcinoma: 15 cases. A) Bilharzial Squamous cell carcinoma (Bil SqCC), 15 cases; Obtained from the archives of Pathology Departments of Theodor Bilharz Research Institute (TBRI).
Histopathological grading and staging
According to Histopathological grading and staging, bladder cancer was categorized into:
a- Histopathological grading: 1- Low grade transitional cell carcinoma (n = 28); 2- High grade transitional cell carcinoma (n = 47); 3- High grade squamous cell carcinoma (n = 15).
b- Histopathological staging: 1- NMIBC (superficial tumors transitional cell carcinoma (n = 45); 2- MIBC transitional cell carcinoma (n = 30); 3- SCC cases were MIBC (n = 15).
The personal and clinical data
For each case: A) The personal and clinical data were obtained from the patient’s records (including age and sex); B) Four-μm sections were prepared from the paraffin block and stained as follows: Hematoxylin and Eosin stain; Immunohistochemical stain against FGFR3; and Fluorescent in Situ Hybridization for FGFR3 (for malignant cases only).
Photomicrography
Photomicrographs included in this study were captured by AxioCam MRc5 camera mounted to Zeiss Scope A1 Microscope.
Immunohistochemical procedure
Immunohistochemistry for FGFR3 was performed on sections cut from the paraffin blocks with a commercially available rabbit monoclonal Anti-FGFR3 antibody (sc-13121, Santa Cruz, USA). Slides were sectioned at 4μm onto positively charged slides (Superfrost Plus, Menzel-Glaser, Germany) and the slides were stained on an automated platform the (Dako Autostainer Link 48). Heat-induced antigen retrieval was used for 30 min at 97°C in the high-PH EnVision™ FLEX Target Retrieval Solution, and the primary antibody was used at a dilution of 1 in 100. The detailed histopathological assessment was done regarding confirmation of diagnosis and grading of malignant cases.
Immunohistochemical assessment was done according to [9]. Cells with membrane cytoplasmic staining were considered positive: The intensity and extent of cells stained positively for FGFR3 was evaluated by assessing 10 high-power microscopic fields by two independent pathologists (OH &TA): *Score 0: All tumor cells are negative; *Score +1: Weak but detectable positivity in some or all cells; *Score +2: moderate but extensive positivity; and *Score +3: strong positivity (regardless of extent).
Fluorescence in situ hybridization (FISH) technique
The probe contains green-labeled polynucleotides (Zygreen) which target FGFR3 gene and orange-labeled polynucleotides which target sequences of chromosome 4 in the chromosomal region 4p11. The code number of the used probe is (ZytoLight® SPEC FGFR3/4p11 Dual Color Probe, Previously: ZytoLight SPEC FGFR3/CEN 4 Dual Color Probe).
Sections were de-waxed, then incubated for 10 minutes at 70% on a hot plate, followed by incubation for 2x 10 minutes in xylene then rehydrated in 100%, 100%, 90%, and 70% ethanol, each for 5 minutes and lastly washed 2x2 minutes in distilled water. Sections were incubated for 15 minutes in pre-warmed Heat Pretreatment Solution Citric at 98, then transferred immediately to distilled water, washed for 2x2 minutes and water was drained off. Pepsin solution was applied to tissue sections that were incubated for 10 minutes at 37 in a humidity chamber. After that, sections were washed for 5 minutes in Wash Buffer SSC and 1 minute in distilled water. The following steps were then successively done: Dehydration in 70%, 90%, and 100% ethanol, each for 1 minute. Air dry sections then, pipette 10μl Zytolight FISH probe each onto individual samples. Cover the samples with a coverslip (with hot glue from an adhesive pistol). Denature the slides at 75 ºC for 10 minutes on a hot plate. Transfer the sections to a humidity chamber and hybridise overnight at 37ºC. Remove the glue carefully and remove the coverslip by submerging in 1x Wash Buffer for 2x5 minutes at 37 ºC for 1-3 minutes. Incubate the slides in 70%.90%, and 100% each for one minute.
Air dry samples protected from light. Cell nuclei were stained by pipetting 30μl DAP/Dura-Tect-solution onto the sections. Avoiding trapped bubbles cover the samples with a coverslip. Carefully remove excess DAP/Dura-1-solution by gently pressing the slide between filter papers. Store the slides in the dark. For longer storage periods; this should take place at 2-8C. Evaluation of the sample material; is carried out by Fluorescence microscopy.
Interpretation of FISH
Evaluation of the FGFR3 gene is carried out by fluorescence microscopy (Olympus X51) [using three filters red (FITC), green, and DAPI] using a × 1000 oil immersion objective lens. The microscope is attached to a high-resolution video camera (Jale) and monitor. We captured and interpreted photos using hardware (Cytovision 2.3, USA).
SPEC FGFR3 Dual Color Probe hybridized to normal interphase as indicated by two orange/green fusion signals per nucleus no amplification of Fibroblast growth factor receptor 3 (FGFR3), SPEC FGFR3 Dual Color Probe hybridized with interphase cells showing an over expression of chromosome 4 as indicated by multiple green more than orange signals in the nuclei according to [14].
Statistical analysis
SPSS software version 18 was used for data management and analysis. Quantitative data were presented as mean ± SD. Qualitative data were presented as frequencies and percentages. To study the relationship between variables, Spearman’s correlation coefficient was calculated. Tests were considered statistically significant when P< 0.05.
Results
Sex distribution in different lesions studied
Urothelial lesions were more predominated in males; as 12 out of 15 cases (80%) with chronic cystitis, 11 out of 15 cases with squamous cell carcinoma (73%) and 49 out of 75 cases with transitional cell carcinoma (65%) were males. The highest frequency of urothelial carcinomas was in the seventh decade (51.2%) (Table 1).
Table 1.
Age distribution among patients studied
| Decade | Cystitis | Malignant | Total No | % | ||
|---|---|---|---|---|---|---|
| No | % | No | % | |||
| 21-30 | 4 | 40% | 0 | 0 | 4 | 7.2% |
| 31-40 | 5 | 50% | 1 | 2.3% | 6 | 10.9% |
| 41-50 | 1 | 10% | 4 | 8.9% | 5 | 9.1% |
| 51-60 | 0 | 0 | 15 | 33.3% | 15 | 27.3% |
| 61-70 | 0 | 0 | 23 | 51.2% | 23 | 41.8% |
| 71-80 | 0 | 0 | 2 | 4.5% | 2 | 3.7% |
| Total | 10 | 100% | 45 | 100% | 55 | 100% |
Extent and Intensity of FGFR3 expression in studied cases by IHC
FGFR3 immunoexpression was demonstrated as membrane-cytoplasmic staining of urothelial cells. In control, all cases were negative for FGFR3 immunostaining. In the chronic cystitis group, 10 cases out of 15 cases (66.6%) were positive for FGFR3 immunostaining; eight cases with mild intensity and two cases
In the malignant group; 61 out of 90 cases (67.8%) were positive for FGFR3 immunostaining; thirty-three cases were of mild intensity, 19 cases were of moderate intensity, and 9 cases were of marked intensity. There was a significant difference between malignant group and control group at p-value <0.01 (Table 2; Fig. 1: A-D).
Table 2.
Extent and Intensity of FGFR3 expression in studied cases
| Item | Positive cases | Mean % of positive cells ± SD | Intensity of FGER immunostaining | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Marked | |||||||
| No | % | No | % | No | % | No | % | ||
| Control n=5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chronic cystitis n=15 | 10 | 66.6% | 22 ± 30.8 | 8 | 53% | 2 | 13% | 0 | 0 |
| Non – Bilharzial cystitis, n=6 | 4 | 66.6% | 8.4 ± 7.6 | 4 | 66.6% | 0 | 0 | 0 | 0 |
| Bilharzial cystitis n=9 | 6 | 66.6% | 31.2 ± 37.3(b) | 4 | 44.5% | 2 | 22.5% | 0 | 0 |
| Malignant n=90 | 61 | 67.8% | 38.5 ± 31.8(a) | 33 | 36% | 19 | 21% | 9 | 10% |
| Non – Bilharzial carcinomas n=57 | 43 | 75.4% | 43.6 ± 30.4 | 21 | 36.8% | 14 | 24.5% | 8 | 14% |
| Bilharzial carcinomas n=33 | 18 | 54.5% | 30.9 ± 32.8 | 12 | 36.3% | 5 | 15.2% | 1 | 3.1% |
a) p-value < 0.01 compared to chronic cystitis cases; b) p-value < 0.01 compared to non-bilharzial carcinoma cases.
Figure 1.
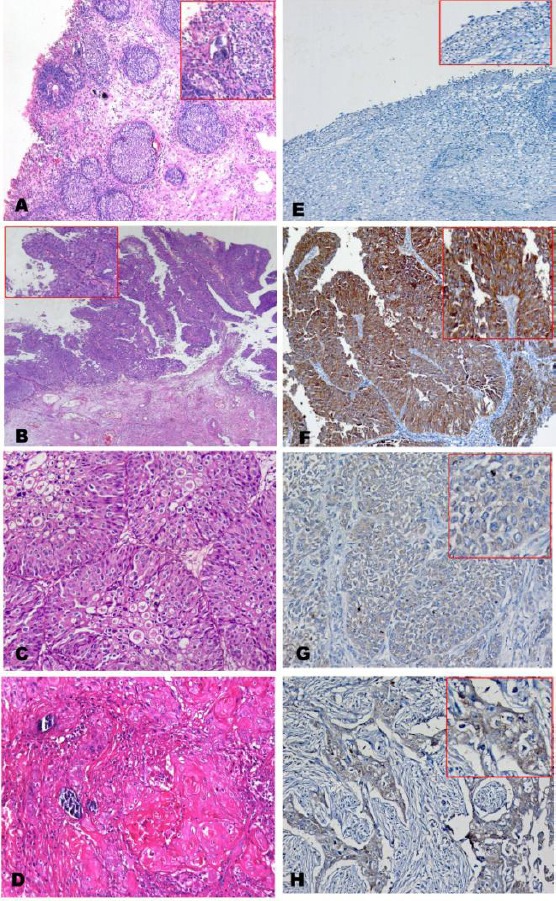
(A) Case of bilharzial cystitis with proliferative changes(Brunn’s nests) and calcified bilharzial ova (H&E, X 100); (B) Case of Papillary urothelial carcinoma low grade, not invading lamina (Ta) (H&Ex50); (C) Case of urothelial carcinoma high grade, invading lamina and muscle layer (T2) associated with bilharziasis (H&E x200); (D) Case of Squamous cell carcinoma with calcified bilharzial ova, high grade, invading lamina and muscle layer (T2) (H&E x200); (E) Case of cystitis, negative for FGFR3 immunostaining (IHC, DAB, x100); (F) Case of papillary urothelial carcinoma low grade, not infiltrating lamina Ta, strongly positive for FGFR3 immunostaining. (IHC, DAB, x100); (G) Case of a case of urothelial carcinoma high grade, infiltrating lamina and muscle layer T2, positive cytoplasmic for FGFR3 immunostaining, mild intensity (IHC, DAB, x200); (H) Case of squamous cell carcinoma high-grade, malignant cells show positive cytoplasmic for FGFR3 immunostaining, mild intensity. (IHC, DAB, x200)
Seventy-two percent of TCC cases were positive for FGFR3 by immunostaining with mild, moderate, and marked staining (38.6%, 21.4% and 12%) respectively. Seven cases out of 15 squamous cell carcinoma cases (46.7%) were positive for FGFR3 expression with mild and moderate immunostaining in 26.7% and 20% respectively (Table 3). There was a significant inverse correlation between the score of expression and both the stage and the grade of malignancy (p<0.05 and p<0.01 respectively) (Table 4; Fig. 1: E-H).
Table 3.
Extent and intensity of FGFR3 Expression in different malignant cases about tumour grades and stages
| Carcinoma | Number of positive cases | Mean % of positive cells ± S.D. | Intensity of FGFR3 immunostaining | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Marked | |||||||
| No | % | No | % | No | % | ||||
| Transitional cell carcinoma n=75 | 54 | 72% | 40.5±30 | 29 | 38.6% | 16 | 21.4% | 9 | 12% |
| Superficial Tumours (NMIBC)TCC n=45 | 40 | 88.8% | 52.4±26.2 | 18 | 40% | 15 | 33.3% | 7 | 15.6% |
| Muscle invasive in TCC n=30 | 14 | 46.7% | 22.6±28.5(a) | 11 | 78.5% | 1 | 7.2% | 2 | 14.2% |
| Low grade Tumors TCC n=28 | 26 | 92.8% | 58.5±22 | 9 | 32.2% | 11 | 39.2% | 6 | 21.4% |
| High Grade Tumors in TCC n=47 | 28 | 59.5% | 29.7±30.2(b) | 20 | 71.4% | 5 | 17.8% | 3 | 10.7% |
| Squamous cell carcinoma n=15 | 7 | 46.7% | 31.3±36 | 4 | 26.7% | 3 | 20% | 0 | 0 |
| Superficial Tumors (NMIBC) in scc n=0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Muscle invasive Tumors in scc n=15 | 7 | 46.7% | 31.3±36.8 | 4 | 26.7% | 3 | 20% | 0 | 0 |
| Low Grade SCC N=0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| High-Grade SCC N=15 | 7 | 46.7% | 31.3±36.8 | 4 | 26.7% | 3 | 20% | 0 | 0 |
(a)A significant difference between NMIBC and Invasive bladder cancer of TCC type; (b) Significant difference between low-grade tumours and high-grade tumours at p-value <0.05
Table 4.
Correlation between FGFR3 parameters and grade and stage of malignancy:
| Grade | Stage | Score | Positive | |||
|---|---|---|---|---|---|---|
| Spearman’s rho | Grade | Correlation Coefficient | 1.000 | 0.479** | -0.416** | -0.064 |
| Sig. (2-tailed) | . | 0.001 | 0.003 | 0.656 | ||
| N | 50 | 46 | 50 | 50 | ||
| Stage | Correlation Coefficient | 0.479** | 1.000 | -0.233 | -0.035 | |
| Sig. (2-tailed) | 0.001 | . | 0.047* | 0.798 | ||
| N | 46 | 55 | 55 | 55 | ||
| Score | Correlation Coefficient | -0.416** | -0.233 | 1.000 | 0.378** | |
| Sig. (2-tailed) | 0.003 | 0.047* | . | 0.002 | ||
| N | 50 | 55 | 62 | 62 | ||
| Positive | Correlation Coefficient | -0.064 | -0.035 | 0.378** | 1.000 | |
| Sig. (2-tailed) | 0.656 | 0.798 | 0.002 | . | ||
| N | 50 | 55 | 62 | 62 | ||
Correlation is significant at the 0.05 level (2-tailed);
Correlation is significant at the 0.01 level (2-tailed).
FGFR3 expression by FISH
The fish technique was applied to forty cases in a malignant group with overexpression of the FGFR3 protein. 36 malignant cases out of 40 cases show amplified FGFR3 gene. Positive malignant cases show a significant difference compared to negative cases at p-value <0.01. Most of the positive cases were of low grade (55.5%) and showed a significant difference at p-value < 0.01 compared to high grade. In NMIBC group (77.7%) there was a statistical difference at p-value 0.01 compared to MIBC group.
compared to low grade.
A total of 88.2% of TCC cases showed FGFR3 gene amplification; mostly were of low grade (70%) and showed a significant difference at p-value < 0.01 compared to high grade. In NMIBC group (80%) there is a statistical difference of FGFR3 gene amplification at p-value 0.01 compared to MIBC group.
While all cases of SCC have FGFR3 gene amplification, most of the positive cases were of high grade and belong to the MIBC group (83.3%) with a significant difference at p-value < 0.01 compared to high grade and NMIBC group. Almost all cases of SCC and TCC associated with bilharziasis report FGFR3 gene amplification with a significant difference between TCC on bilharzial compared to TCC bilharzial groups at P value < 0.05 (Table 5).
Table 5.
FGFR3 gene amplification by FISH technique in different studied groups
| Group | Positive | Negative | P value | ||
|---|---|---|---|---|---|
| No | % | No | % | ||
| Malignant cases n=40 | 36 | 90% | 4 | 10% | p<0.01 |
| Grade | |||||
| Low | 20 | 55.5% | 2 | 50% | p<0.05 |
| High | 16 | 45.5% | 2 | 50% | |
| Stage | |||||
| NMIBC | 28 | 77.7% | 1 | 25% | p<0.01 |
| MIBC | 8 | 22.2% | 3 | 75% | |
| TCC n=34 | 30 | 88.2% | 4 | 11.8% | p<0.01 |
| TCC bIlharzial n=7 | 7 | 100% | 0 | 0 | |
| TCC non Bilharzial n=27 | 23 | 85.2%* | 4 | 14.8% | p<0.05 |
| SCC n= 6 | 6 | 100% | 0 | 0 | p<0.01 |
| TCC | |||||
| Low grade | 21 | 70% | 2 | 50% | p<0.01 |
| High grade | 9 | 30% | 2 | 50% | |
| SCC n=6 | |||||
| Low grade | 1 | 16.7% | 0 | 0 | p<0.01 |
| High grade | 5 | 83.3% | |||
| TCC n=34 | |||||
| NMIBC | 24 | 80% | 3 | 75% | |
| MIBC | 6 | 20% | 1 | 25% | p<0.01 |
| Scc n=6 | |||||
| NMIBC | 1 | 16.7% | 0 | 0 | p<0.01 |
| MIBC | 5 | 83.3% | 0 | 0 | |
There are 36 malignant cases out of 40 cases show amplified FGFER3 gene, most of the positive cases were of low grade (55%) and were in NMIBC group (72.5%). There were 88.2% of TCC cases show FGFR3 gene amplification most were of LOW grade (61.8%) and were in NMIBC group (82.4%) while all cases of SCC have FGFR3 gene amplification most of the positive cases were of high grade (83.3%) and MIBC (82.4%).
Amplified FGFR3 gene was documented in 90% of malignant cases with mild FGFR3 staining intensity and 84.6% of positive malignant cases with moderate staining intensity showing a significant difference compared to the negative malignant cases with moderate intensity at p-value < 0.01.
Amplified FGFR3 gene was reported in 85.7% of low-grade tumours with marked staining intensity showing a significant difference compared to the high-grade tumours at p-value < 0.01. Amplified FGFR3 gene was also reported in 92.3% in NMIBC with moderate staining intensity showing a significant difference compared to MIBC group at P value < 0.01 (Table 6; Fig. 2: A-D).
Table 6.
FGFR3gene amplification by FISH technique in malignant cases with different staining intensities
| FGFR3 Intensity | Negative | Positive | NMIBC | MIBC | Low Grade | High Grade | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | % | No | % | No | % | No | % | No | % | No | % | |
| Mild n=20 | 2 | 10% | 18 | 90% | 10 | 50% | 10 | 50% | 8 | 40% | 12 | 60% |
| Moderate n=13 | 2 | 15.4% | 11 | 84.6%** | 12 | 92.3% | 1 | 7.7%* | 9 | 69.2% | 4 | 30.8%* |
| Marked n=7 | 0 | 0 | 7 | 100% | 6 | 85.7% | 1 | 14.3% | 6 | 85.7% | 1 | 14.3%* |
p < 0.01compared Mild intensity.
p < 0.05compared Marked intensity.
Figure 2.
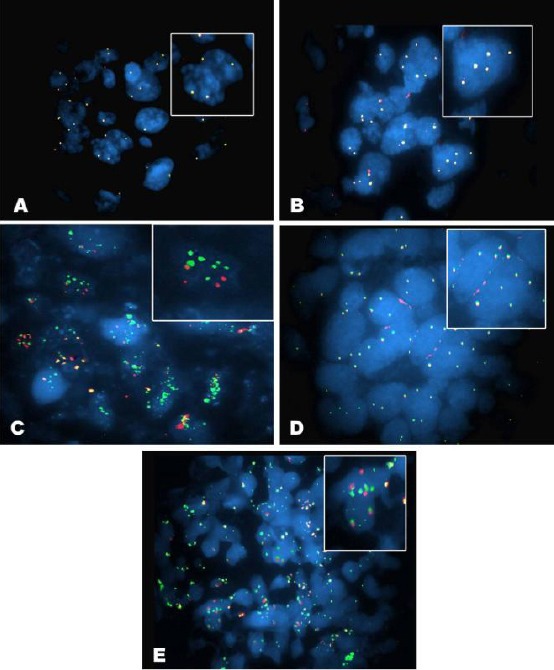
(A) SPEC FGFR3 Dual Color Probe hybridized to normal interphase as indicated by two orange/green fusion signals per nucleus from a case of TCC Ta GI showing malignant cells with normal expression, no amplification of Fibroblast growth factor receptor 3 (FGFR3) as two FGFR3 signals (green) and two chromosome 4 signal (orange/red) (red arrows) (FISH, magnification ×1000); (B) Urinary bladder tissue from a case of SCC, SPEC FGFR3 Dual Color Probe hybridized with interphase cells showing polysomy of chromosome 4 as indicated by multiple green and orange signals in the nuclei (magnification ×1000); (C) Urinary bladder tissue from a case of SCC, SPEC FGFR3 Dual Color Probe hybridized with interphase cells showing an amplification of the FGFR3 gene (green signals) (magnification×1000); (D) Urinary bladder tissue from a case of TCC T1 GII, SPEC FGFR3 Dual Color Probe hybridized with interphase cells showing polysomy of chromosome 4 as indicated by multiple green and orange signals in the nuclei (magnification×1000); (E) Urinary bladder tissue from a case of TCC T1 GII, SPEC FGFR3 Dual Color Probe hybridized with interphase cells showing an amplification of the FGFR3 gene (green signals) (magnification×1000)
Discussion
Schistosomiasis is not known to be associated with any malignant disease other than bladder cancer. In Egypt, the frequency rate of the bladder cancer has declined significantly during the last 25 years. This drop is mainly related to the control of schistosomiasis [15]. In the present work, the incidence of bilharzial infestation in malignant studied cases was about 40%, and this was close to what was mentioned by Abdulamir et al. [16]. Also, all cases of SCC were associated with bilharzial affection and this in agreement with [17] who stated that Schistosoma infection-induced urinary bladder carcinogenesis and also in agreement with Khaled et al. [15] who stated that bladder cancer associated with schistosomal infection has a high occurrence of SCC type.
We found that FGFR3 expression was negative in control group and this finding is compatible Gust et al. [18]. There was no evidence of such staining in normal urothelium. We also found that 72 % of malignant cases were positive for FGFR3 immunostaining, which is compatible with Junker et al. [19] who stated that expression of FGFR3 was found in approximately 70% of both low and high-grade tumours, as well as equally distributed between invasive and non-invasive urothelial carcinoma.
In our study, 88% of urothelial carcinoma cases positive for FGFR3 immunostaining were superficial non-invasive tumours. A recent study done on 255 primary urothelial carcinomas reported that 63.5 % of superficial carcinoma were positive for FGFR3 protein expression by immunophenotyping, while only 33% of invasive muscle tumours were positive [20].
About 92 % of low-grade urothelial cancers of our cases were positive for FGFR3 immunostaining, and this is compatible with Poyet at al. [20] who found that 69.4% of low-grade urothelial carcinoma were positive to FGFR3, while only 26.7% of high-grade tumours were positive for FGFR3. We also reported that all cases of SCC were of high grade and with muscle invasion and this was in agreement with Abdulamir et al. [16] and Salim et al. [17]. About half of the SCC cases in the present study were positive for FGFR3 immunostaining, and this was also explained by Salim et al. [17] who stated that SCC showed a predisposition to a high level of FGFR3 protein expression and mutation. The fish technique was applied to forty malignant cases with FGFR3 protein overexpression to detect gene amplification of FGFR3. We found that 36 cases out of 40 cases of malignancy (90%) were positive, which is close to Tomlinson et al. [9] who mentioned that an association between expression level and mutation status. Fifty-five percent of positive cases were of low grade, and 77.7% were in NMIBC group. This result is close to what was reported by Junker et al. [19] who stated that 63% of NMBIC group and 59% were at low-grade show FGFR3 gene amplification.
We found positivity for FGFR3gene amplification in almost all bilharziasis associated malignant cases (SCC and TCC). All cases of bilharziasis associated SCC cases showed FGFR3 gene amplification. They were mostly of high grade and were in MIBC group. This was previously mentioned by Khaled et al. [15] who explained that by chromosomal alterations that characterise schistosomal bladder cancer cases. FGFR3 point mutations, overexpression of FGFR3 protein has been described. Although this overexpression was associated strongly with the presence of FGFR3 mutations (up to 85% of FGFR3-mutated tumours show strong FGFR3 protein expression), FGFR3 overexpression was also found in 40% of tumours with wild-type FGFR3, particularly in those with an invasive phenotype [9, 21].
We detected FGFR3 gene amplification in 88.2% of the TCC cases; about 70% were of low grade, and 80% were in NMIBC group. Amplified FGFR3 gene was documented in 90% of malignant cases with mild FGFR3 staining intensity; in 84.6% with moderate staining intensity and in all cases with marked staining intensity. Similar results were also achieved by Tomilinson et al. [9] who stated that there is an association between expression level and mutation status also Fischbach et al. [22] found in their study all FGFR3amplified samples showed concomitant FGFR3 mutations and FGFR3 protein overexpression.
In conclusion, our findings suggest that invasive tumours may develop either from carcinoma in situ or Ta cases having multiple genetic alterations together including gene amplifications in bilharzial associated carcinomas. Estimation of FGFR3 expression could serve as a prognostic indicator in the follow-up of cancer bladder patients along surgical or therapy managements, especially in bilharzial associated malignant cases. Expression of FGFR3 could also be utilised for molecular targeted therapy in urinary bladder cancer, so, we need further studies to target these molecular markers to outline an effective treatment strategy for the bladder cancer shortly.
Footnotes
Funding: This work was financed by TBRI internal project No. 115T. Principle Investigator: Prof. Dr Olfat Hammam.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Ashley SF, Soliman SA, Khaled H, Zaghloul SM, Mousumi B. The changing patterns of bladder cancer in Egypt over the past 26 years. Cancer causes Control. 2008;19:421. doi: 10.1007/s10552-007-9104-7. https://doi.org/10.1007/s10552-007-9104-7 . PMid:18188671. PMCid:PMC4274945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rambau PF, Chalya PL, Jackson k. Schistosomiasis and urinary bladder cancer in North Western Tanzania: a retrospective review of 185 patients. Infectious Agents and Cancer. 2013. pp. 8–19. https://doi.org/10.1186/1750-9378-8-19 . PMid:23705833. PMCid:PMC3665673. [DOI] [PMC free article] [PubMed]
- 3.Michaud DS, Clinton SK, Rimm EB. Risk of bladder cancer by geographic region in a U.S. cohort of male health professionals. Epidemiology. 2001;12:719. doi: 10.1097/00001648-200111000-00022. https://doi.org/10.1097/00001648-200111000-00022 . PMid:11679802. [DOI] [PubMed] [Google Scholar]
- 4.Pritchett TR, Wang JK, Jones PA. Mesenchymal-epithelial interactions between normal and transformed human bladder cancer cells. Cancer Res. 1989;49:2750. PMid:2713858. [PubMed] [Google Scholar]
- 5.di Martino E, Tomlinson DC, Knowles MA. A Decade of FGF Receptor Research in Bladder Cancer: Past, Present, and Future Challenges Advances in Urology. 2012;2012 doi: 10.1155/2012/429213. Article ID 429213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Rhijn BW, van der Kwast TH, Liu L, Fleshner NE, Bostrom PJ, Vis AN, Alkhateeb SS, Bangma CH, Jewett MA, Zwarthoff EC, Zlotta AR, Bapat B. The FGFR3 mutation is related to favorable pT1 bladder cancer. J Urol. 2012;187(1):310–4. doi: 10.1016/j.juro.2011.09.008. PMid:22099989. [DOI] [PubMed] [Google Scholar]
- 7.Foth M, Ahmad I, van Rhijn BWG, van der Kwast T, Andre M, Bergman AM, King L, Ridgway R, Leung HY, Fraser S, Sansom OG, Iwata T. Fibroblast Growth Factor Receptor 3 activation plays a causative role in urothelial cancer pathogenesis in cooperation with Pten loss in mice. J Pathol. 2014;233(2):148–158. doi: 10.1002/path.4334. https://doi.org/10.1002/path.4334 . PMid:24519156. PMCid:PMC4612374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guancial EA, Werner L, Bellmunt J, Bamias A, Choueiri TK, Ross R, Schutz FA, Park RS, O’Brien RG, Hirsch MS, Justine A Barletta JA, Berman DM, Lis R, Loda M, Stack EC, 8 Levi A Garraway LA, Riester M, Michor F, Kantoff PW, Rosenberg JE. FGFR3 expression in primary and metastatic urothelial carcinoma of the bladder. Cancer Med. 2014;3(4):835–844. doi: 10.1002/cam4.262. https://doi.org/10.1002/cam4.262 . PMid:24846059. PMCid:PMC4303151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomlinson DC, Baldo O, Harnden P, Knowles MA. FGFR3 protein expression and its relationship to mutation status and prognostic variables in bladder cancer. J Pathol. 2007;213(1):91–8. doi: 10.1002/path.2207. https://doi.org/10.1002/path.2207 . PMid:17668422. PMCid:PMC2443273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lievens PM, Zanolli E, Garofalo S, Liboi E. Cell adaptation to activated FGFR3 includes Sprouty4 up regulation to inhibit the receptor-mediated ERKs activation from the endoplasmic reticulum. FEBS Lett. 2009;583(19):3254–8. doi: 10.1016/j.febslet.2009.09.021. https://doi.org/10.1016/j.febslet.2009.09.021 . PMid:19761767. [DOI] [PubMed] [Google Scholar]
- 11.van Rhijn, Lurkin I, Radvanyi F, Kirkels WJ, van der Kwast TH, Zwarthoff CE. The Fibroblast Growth Factor Receptor 3 (FGFR3) Mutation Is a Strong Indicator of Superficial Bladder Cancer with Low Recurrence Rate. Cancer Res. 2001;61:1265–1268. PMid:11245416. [PubMed] [Google Scholar]
- 12.Eble JN, Sauter G, Epstein JI, Sesterhenn IA Pathology and genetics of tumors of the urinary system and male genital organs. World Health Organization Classification of Tumors. Lyon, France: IARC Press; 2004. PMCid:PMC1618623. [Google Scholar]
- 13.AJCC CANCER STAGING MANUAL Seventh Edition American Joint Committee on Cancer Executive Office, 633 North Saint Clair Street Chicago, IL 60611-3211. 2010:503–504. [Google Scholar]
- 14.Arao T, Ueshima K, Matsumoto K, Nagai T, Kimura H, Hagiwara S, Sakurai T, Haji S, Kanazawa A, Hidaka H, Iso Y, Kubota K, Shimada M, Utsunomiya T, Hirooka M, Hiasa Y, Toyoki Y, Hakamada K, Yasui K, Kumada T, Toyoda H, Sato S, Hisai H, Kuzuya T, Tsuchiya K, Izumi N, Arii S, Nishio K, Kudo M. FGF3/FGF4 amplification and multiple lung metastases in responders to sorafenib in hepatocellular carcinoma. Hepatology. 2013;57(4):1407–15. doi: 10.1002/hep.25956. https://doi.org/10.1002/hep.25956 . PMid:22890726. [DOI] [PubMed] [Google Scholar]
- 15.Khaled H. “Schistosomiasis and Cancer in Egypt: Review”. Journal of Advanced Research. 2013;4(5):461–466. doi: 10.1016/j.jare.2013.06.007. https://doi.org/10.1016/j.jare.2013.06.007 . PMid:25685453. PMCid:PMC4293882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdulamir AS, Hafidh RR, Kadhim HS, Abubakar F. Tumor markers of bladder cancer: the schistosomal bladder tumors versus non-schistosomal bladder tumors. J Exp Clin Cancer Res. 2009;28(1):27. doi: 10.1186/1756-9966-28-27. https://doi.org/10.1186/1756-9966-28-27 . PMid:19243595. PMCid:PMC2650688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salim EI, Morimura K, Menesi A, El-Lity M, Fukushima S, Wanibuchi H. Elevated oxidative stress and DNA damage and repair levels in urinary bladder carcinomas associated with schistosomiasis. International Journal of Cancer. 2008;123(3) doi: 10.1002/ijc.23547. https://doi.org/10.1002/ijc.23547 . PMid:18478569. [DOI] [PubMed] [Google Scholar]
- 18.Gust KM, McConkey DJ, Awrey S, Hegarty PK, Qing J, Bondaruk J, Ashkenazi A, Czerniak B, Dinney CP, Black PC. Fibroblast Growth Factor Receptor 3 Is a Rational Therapeutic Target in Bladder Cancer Mol Cancer Ther. 2013;12(7) doi: 10.1158/1535-7163.MCT-12-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Junker K, van Oers GMM, Zwarthoff EC, Kania I, Schubert J, Hartmann A. Fibroblast Growth Factor Receptor 3 Mutations in Bladder Tumors Correlate with Low Frequency of Chromosome Alterations. Neoplasia. 2008;10(1):1–7. doi: 10.1593/neo.07178. https://doi.org/10.1593/neo.07178 . PMid:18231634. PMCid:PMC2213896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poyet C, Buser L, Roudnicky F, Detmar M, Hermanns 1, Doris Mannhard T, Höhn A, Rüschoff J, Zhong Q, Sulser T, Moch H, Wild PJ. Connexin 43 expression predicts poor progression-free survival in patients with non-muscle invasive urothelial bladder cancer. J Clin Pathol. 2015:2015–202898. doi: 10.1136/jclinpath-2015-202898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bodoor K, Ghabkari A, Jaradat Z, et al. FGFR3 mutational status and protein expression in patients with bladder cancer in a Jordanian population. Cancer Epidemiol. 2010;34:724–732. doi: 10.1016/j.canep.2010.05.003. https://doi.org/10.1016/j.canep.2010.05.003 . PMid:20542753. [DOI] [PubMed] [Google Scholar]
- 22.Fischbach A, Rogler A, Erber R, Stoehr R, Poulsom R, Heidenreich A, Schneevoigt BS, Hauke S, Hartmann A, Knuechel R, Veeck J, Gaisa NT. Fibroblast growth factor receptor (FGFR) gene amplifications are rare events in bladder cancer. Histopathology. 2015;66(5):639–49. doi: 10.1111/his.12473. https://doi.org/10.1111/his.12473 . PMid:24898159. [DOI] [PubMed] [Google Scholar]


