Abstract
AIM:
The aim of the study is to evaluate the changes of the kinetic parameters of gait in patients with supratentorial unilateral stroke in the chronic period (SUSChP).
MATERIAL AND METHODS:
The study was conducted with 67 patients with SUSChP (56 patients included in the experimental group - 32 men and 24 women, with duration of disease 7.8 ± 2.0 months, and 11 patients in the control group - 9 men and 2 women, with duration of disease 7.3 ± 1.5 months). To evaluate the changes in the gait were followed cadence of 6 m and 10 m and the speed of movement which are the most informative kinetic parameters. Patients in the experimental group were treated with a specialised 10-day KT, which later continued to be performed as an adapted exercise program at home for one month.
RESULTS:
After applying specialised kinesitherapeutic methodology (SKTM), the highest trend towards improvement in the kinetic parameters of gait was established after the 1st month with a level of significance during treatment p < 0.001.
CONCLUSION:
The enclosed SKTM in the experimental group continued later as an adapted exercise program at home, significantly improving the kinetic parameters of gait in patients with SUSChP, compared with the usual kinesi-therapeutic methodology applied in the control group.
Keywords: Kinetic parameters, Gait, Chronic period, Supratentorial unilateral stroke
Introduction
Normal human gait is a natural movement defined as bipedal and biphasic forward centre of gravity with minimal energy expenditure while walking. This is the result of dynamic interactions between central program and feedback mechanisms [1].
Difficulties in walking in stroke patients are due to many factors, such as reduced muscle strength imbalance in weight distribution, impaired proprioception, increased tendon reflexes, spasticity and impairment in motor control [2].
Typical gait disturbances that occur after stroke for impairments in the middle cerebral artery with consecutive one-sided weakness and spasticity are: reduced flexion of the knee in swing phase and phase of support, hyperextension of the knee (dynamic recurvate) phase of support and excessive plantar flexion of the ankle (equines) in swing phase and/or the phase of support. Each of these impairments has a potential negative effect and increases the need for energy for walking. For reduced flexion of the knee in the swing phase, sometimes are necessary compensatory mechanisms such circumduction hip, lifting hip and contralateral footing with an excessive elevation of the pelvis. Inadequate knee flexion in supporting phase led to a relatively higher position of the centre of gravity of the body. Similarly hyperextension of the knee can change the center of gravity in the support phase and cause damage to the knee because overstretching of ligaments and capsular structures: the vertical component of the reaction force of the support (external structure) and muscle strength (internal structure) appear together to cause a moment of extension in the knee. Equinus foot in the swing phase may be due to the weakness of the dorsal flexor ankle, plantar flexor spasticity and plantar flexion contracture of the ankle. In the supporting phase, the last reasons can cause hyperextension of the knee by preventing the gradual dorsal flexion of the ankle, which usually begins during the reaction of the load and remains in final supporting phase [3-5].
After stroke more time is spent in the supporting phase of the unaffected side, and this is the main reason for the reduced speed of gait. The continuous supporting phase of the intact leg side is due to the slow movement of the affected foot. Specifically, in patients with stroke, the swing phase of the affected limb lasts longer due to delayed initiation and reduced speed of flexion of the hip [6]. The difficulties of the affected limb in swing phase are associated with reduced flexion knee limited by the spasticity of the m. quadriceps femoris [4, 7].
The majority of the recovery of the gait following a stroke occurs in a stereotyped manner. It is associated with the stored generators central programs in the spinal cord that operate under the influence of template supraspinal signals by seeking maximum to preserve the basic structure of gait and its programming. This is possible due to uncorrupted connections to the brainstem and unaffected hemisphere [6].
The central mechanisms for functional recovery are not fully understood. In the early period of brain damage processes of restitution, adaptable reorganisation and compensatory strategies are undergoing. In the late period (after 6 months) occurs organising new neural network which overlaps damaged premorbid network [8]. Recovery depends on the severity, the extent of damage to the brain tissue and the applied kinesitherapy [5].
The gait of patients with chronic post-stroke hemiparesis is realised by using compensatory strategies that are alternatively replacement behaviour of the motor deficit. They appear as the adaptive and optimal realisation of the final motor response and can be evaluated and documented by analysing the human step [9-17].
The step of the patients with unilateral stroke reflects bilateral changes in motor control in which the participation of nonparetic side is greater and correlates with the extent of brain reorganisation [11, 12, 14, 17].
Despite the continuous development of neurorehabilitation, many patients with stroke have permanent disabilities in walking that affect their quality of life and their ability to participate in the activities of daily life [18, 19]. In patients with chronic post-stroke hemiparesis short 3 weeks neurorehabilitation improve temporal, spatial and stepping indicators gait - increases the normal speed of movement, reduces the duration of the phases of gait and increases its length predominantly nonparetic side, without affecting the asymmetry of the kinetic and stepper indicators and their coefficient of variation [6].
The aim of this study was to trace the early (10th day) and late (1st month) effect of the specialised kinesitherapeutic methodology (SKTM) on the kinetic parameters of gait. It is based on contemporary principles of neurorehabilitation adapted for home use with the individual needs of patients with supratentorial unilateral stroke in the chronic period (SUSChP).
Material and Methods
Methodology of the research
The research includes a study of 67 patients with SUSChP (56 patients included in the experimental group - 32 men and 24 women, with duration of disease 7.8 ± 2.0 months, and 11 patients in the control group - 9 men and 2 women, with duration of disease 7.3 ± 1.5 months).
The clinical characteristics of the patients are given in Table 1. To determine the severity of the paresis, a modified scale Chedoke-McMaster was used, according to which patients with 4th and 5th stages are with moderate disease, while patients of 6th and 7th the stage have mild impairment [20, 21]. On this basis, the patients are divided into two subgroups (moderate and mild impairment).
Table 1.
Clinical characteristics of contingent baseline
| Parameters | Patients | Moderate degree | Mild degree |
|---|---|---|---|
| Experimental group | N = 56 | N = 33 | N = 23 |
| Age | 63.2 ± 8.8 | 63.9 ± 7.1 | 62.3 ± 10.9 |
| Sex (men/women) | 32/24 | 22/11 | 10/13 |
| Limitation periods (months) | 7.8 ± 2.0 | 8.3 ± 2.2 | 7.2 ± 1.5 |
| Localization (left/right) | 26/30 | 16/17 | 10/13 |
| Control group | N = 11 | N = 5 | N = 6 |
| Age | 63.3 ± 6.0 | 63.6 ± 5.3 | 63.1 ± 7.1 |
| Sex (men/women) | 9/2 | 5/0 | 4/2 |
| Limitation periods (months) | 7.3 ± 1.5 | 7.6 ± 1.8 | 7.0 ± 1.2 |
| Localization (left/right) | 5/6 | 2/3 | 3/3 |
Mean ± SD - mean and standard deviation; EG - the experimental group; KG - control group. The significance of the intra-group changes is defined by the binominal test. Intergroup significance of sex and localisation is determined by U-criteria of Mann-Whitney for independent samples, while for age and limitation period, a Student t-test for independent samples is attached.
The existence of homogeneity in the study did not include patients with acute stroke and brain haemorrhage spent, as well as with the case of bilateral or severe paresis. After the selection of patients additionally were excluded from the study patients who have refused to participate in the study for various reasons (greater distance they need to travel, business and family commitments) and those who were prescribed a change of drug therapy during the applied treatment. All patients were able to move independently or with assistance without serious problems in communication and previously prescribed medication therapy by neurologists, including antiplatelet and antihypertensive drugs.
Changes in gait before and after the treatment were recorded; the cadence of gait and maximum travel speed was estimated. To determine the cadence, the number of steps to cover 6 m and 10 m was measured. The patient was asked to walk with the preferred speed [22]. The speed of the gait is defined in m /min, dividing the distance undergone (m) by the time it took the individual to walk that distance.
All parameters were evaluated three times - at the beginning of the study, the 10th day and 1st month after the start of kinesitherapy.
Methods of kinesitherapy
The experimental group comprised 56 patients (32 men and 24 women) with duration of disease 7.8 ± 2.0 months who received a specialised kinesitherapeutic methodology (SKTM) for 10 days and then continues to run at home as adjusted program of exercises over a period of 3 weeks [23].
The control group (CG) consisted of 11 patients (9 men and 2 women) with duration of the disease 7.3 ± 1.5 months treated in a routine manner by a conventional 10-day kinesitherapeutic methodology. They were held only trace control without meeting kinesitherapeutic program after 10 days of treatment.
The attached two kinesitherapeutical methods are different in treatment duration, structure and incorporated applied kinesitherapy (postural movements, walking, active movements of the upper limbs and transfers). In SKTM, principles of modern neurorehabilitation and motor learning as opposed to usual kinesitherapy.
It is based on the fundamental principles of modern neurorehabilitation, namely: to be individualized, intensive and specifically - tailored and focused on the individual needs of the patient; be realized with the active participation of the patient and his family, with prolonged use so as to ensure that care tailored to the needs of the patient throughout his life to achieve recovery and relief of late complications of the disease [8].
Specialised kinesitherapeutic methodology conforms to the principles of motor learning. They are specificity of the task, active participation of the patient repetition adaptation of complexity, feedback variability “contextual interference” [24].
Statistics
Used is a suite of statistical programs for quantitative processing of data received. Attached is variation (Student-Fisher t-test), alternative and correlation analysis for objectifying the changes of the applied treatment.
When comparing the non-parametric parameters in the course of treatment, Wilcoxon test was used, to determine the significance of differences between groups was given U-criterion of Mann-Whitney.
Paired Samples Test was applied to compare the parametric performance. An alternative analysis is used to determine the significance in the percentage of patients.
The correlation analysis Spearman is used to search for a link between changes in various indicators.
Results
Comparative assessment of monitored indicators objectifying changes in gait in patients with experimental and control groups is presented in Table 2.
Table 2.
Evaluation of cadence and speed of gait in the monitored patients during treatment
| Parameters | Groups | At the beginning EG (n = 56) CG (n = 11) x̄ ± SD | 10th day EG (n = 56) CG (n = 11) x̄ ± SD | 1st month EG (n = 56) CG (n = 11) x̄ ± SD |
|---|---|---|---|---|
| 6 m (number of steps) | EG | 9.6 ± 3.7 | 8.1 ± 3.0*** | 7.3 ± 2.3*** |
| CG | 7.4 ± 1.0 | 6.6 ± 0.7** | 7.0 ± 0.6* | |
| P | 0.057 | 0.122 | 0.694 | |
| 10 m (number of steps) | EG | 16.4 ± 6.3 | 14.0 ± 5.3*** | 12.2 ± 4.1*** |
| CG | 13.1 ± 1.7 | 11.7 ± 1.9** | 12.5 ± 1.6* | |
| P | 0.097 | 0.149 | 0.856 | |
| Speed of gait (m/min) | EG | 31.3 ± 16.6 | 39.4 ± 16.6*** | 46.1 ± 16.2*** |
| CG | 36.1 ± 14.3 | 42.1 ± 11.8** | 41.5 ± 11.6** | |
| P | 0.371 | 0.605 | 0.380 |
x̄ ± SD – mean and standard deviation,
p < 0.001,
p < 0.01,
p <0.05 - significant change compared to baseline in the course of treatment assessed by Wilcoxon Test; P - the significance of the change between the two groups as measured by U-criteria of Mann-Whitney Test. The reduced number of steps and the increased speeds mean an improvement in the kinematic parameters of gait.
The difference between net and baseline at the studied patients from both groups is presented in Figure 1.
Figure 1.
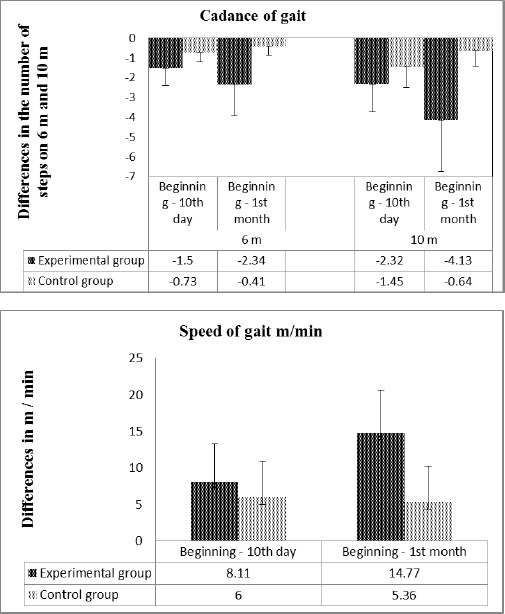
Changes in gait cadence of 6 m and 10 m, represented as the difference between the results obtained and output values of the two groups studied (top). Changes in the speed of movement represented as the difference between the results obtained and the baseline in both treatment groups (bottom)
After the applied kinesitherapy, a significant improvement in gait is seen in all patients. Compared to baseline a significant reduction in the number of steps is present when walking 6 m and 10 m, and an increase of the speed of gait throughout the follow-up period.
In the monitored patient’s experimental group a significant reduction is found in the number of steps when walking 6 m and 10 m (from 9.6 to 8.1 - 6 m, and from 16.4 to 14.0 - 10 m walking) on the 10th day. In the first month after the start of treatment in cadence changes are expressed in the fact that the number of steps decreases to 7.3 at 6 m and 12.2 m at 10 m walking, with a significance level of p < 0.001.
Data speed of gait in the experimental group is similar. The initial velocity of the gait is 31.3 m/min that after day 10 tends to increase up to 39.4 m/min and is a most pronounced increase in the 1st month (46.1 m/min), with a level of significance p < 0.001.
The output data of the control group did not differ significantly from EG. After attached kinesitherapeutic common methodology with 10-day duration, a trend is established of significantly reducing the number of steps from 7.4 to 6.6 feet of 6 m, and from 13.1 to 11.7 feet of 10 m. The first month as a result of the lack of performance of the targeted exercise, as compared to day 10 the number of steps increases is established whereby at 6 m is 7 m and 10 m is 12.5 steps, i.e. there is a tendency to return to baseline.
The speed of gait in the control group is an initial value of 36.1 m/min, which after day 10 is tending to increase up to 42.1 m/min and the 1st month insignificant decreased to 41.5 m/min. Although in absolute terms the change is more pronounced for the experimental group, there was no significant difference from the application of the two methodologies kinesitherapeutic at the end of the detected period.
Changes in the EG in the kinematic parameters of gait, depending on the severity of the impairments and the importance between subgroups studied are presented in Table 3.
Table 3.
Changes in cadence and speed of gait according to the severity of involvement in the experimental group during treatment
| Parameters | Subgroups | At the beginning Moderate (n = 33) Mild (n = 23)x̄±SD | 10th day Moderate (n = 33) Mild (n = 23)x̄±SD | 1st month Moderate (n = 33) Mild (n = 23)x̄±SD |
|---|---|---|---|---|
| 6 m (number of steps) | Moderate | 11.2 ± 3.8 | 9.4 ± 3.2*** | 8.2 ± 2.5*** |
| Mild | 7.2 ± 2.0 | 6.2 ± 1.2*** | 5.9 ± 1.1*** | |
| P | 0.000 | 0.000 | 0.000 | |
| 10 m (number of steps) | Moderate | 19.1 ± 6.3 | 16.5 ± 5.4*** | 14.0 ± 4.4*** |
| Mild | 12.4 ± 3.6 | 10.7 ± 2.5*** | 9.7 ± 1.9*** | |
| P | 0.000 | 0.000 | 0.000 | |
| Speed of gait (m/min) | Moderate | 22.5 ± 14.4 | 30.0 ± 15.1*** | 36.2 ± 13.8*** |
| Mild | 44.0 ± 9.9 | 52.9 ± 5.9*** | 60.2 ± 5.5*** | |
| P | 0.000 | 0.000 | 0.000 |
x̄±SD – mean and standard deviation,
p < 0.001,**p < 0.01,*p < 0.05 - significant change compared to baseline in the course of treatment assessed by Wilcoxon Test; P <0.001 - the significance of the change between the two subgroups as measured by U-criteria of Mann-Whitney Test. The reduced number of steps and speed increase mean an improvement in the kinematic parameters of gait.
It was found that in the EG at the beginning of the treatment performed between two subgroups with moderate and mild effect there is a significant difference (P < 0.001). Compared with the supplied 10-day treatment, improvement in all patients is significant and most pronounced in the 1st month of the study. In absolute terms, the positive effect of the applied SKTM is more pronounced in patients with mild severity of impairment, although the significance of the changes between the two groups was maintained until the end of treatment.
In control patients, significant differences were established between patients with mild and moderate severity during the treatment only for the speed of gait (Table 4). These positive changes are associated with a significant improvement in patients with moderate severity of impairment throughout the follow-up period.
Table 4.
Changes in cadence and speed of gait according to the severity of involvement in the control group during treatment
| Parameters | Subgroups | At the beginning Moderate (n = 5) Mild (n = 6)x̄± SD | 10th day Moderate (n = 5) Mild (n = 6)x̄± SD | 1st month Moderate (n = 5) Mild (n = 6)x̄± SD |
|---|---|---|---|---|
| 6 m (number of steps) | Moderate | 7.9 ± 0.6 | 7.0 ± 0.6 | 7.3 ± 0.2 |
| Mild | 7.0 ± 1.1 | 6.4 ± 0.7** | 6.7 ± 0.7* | |
| P | 0.126 | 0.247 | 0.177 | |
| 10 m (number of steps) | Moderate | 14.1 ± 1.2 | 12.6 ± 1.3** | 13.3 ± 0.9* |
| Mild | 12.4 ± 2.0 | 10.9 ± 2.2* | 11.8 ± 1.8 | |
| P | 0.145 | 0.171 | 0.148 | |
| Speed of gait (m/min) | Moderate | 24.4 ± 11.3 | 31.8 ± 9.2** | 31.4 ± 9.1** |
| Mild | 46.0 ± 7.1 | 50.8 ± 3.5 | 50.0 ± 3.6 | |
| P | 0.004 | 0.001 | 0.001 |
˙±SD – mean and standard deviation,
p < 0.01,
p < 0.05 - significant change compared to baseline in the course of treatment assessed by Wilcoxon Test; P <0.01 - the significance of the change between the two subgroups as measured by U-criteria of Mann-Whitney Test. The reduced number of steps and speed increase mean an improvement in the kinematic parameters of gait.
There were no significant correlations between changes in gait and the age, sex of the patient, duration of the disease and the localisation of the pathological process.
Discussion
The survey results show a positive effect of applied kinesitherapeutical methods on gait in both groups, although in absolute terms it is for specialised methodology.
A distinct positive change in absolute values in the experimental group on day 10 and primarily the 1st month on gait, probably are due to the included movements of lower limbs, control of the trunk and pelvis and swimming which normalize movements of the lower limb, the healthy and the affected side of the body as well as the consistency of motor response. They stimulate a response in the quadriceps femoris muscle and facilitate walking. Of essential importance are the walking and dosed swimming involved in the methodology, leading to normalisation of the control body and upper extremities, improved dynamic control and ease of movement. Similar are claims of other authors who establish a positive influence on the ability and walking speed [25-27].
The positive effect on the recovery of gait in both groups linked to the fact that walking can be exercised continuously in daily life in patients for whom this activity is possible.
Improvement in gait in patients with unilateral, chronic stroke is established by a research of E. Titianova (2007) after a 3-week specialized program for neurorehabilitation, including over 20 min walking with electromechanical simulator for 25 min kinesitherapy (immediately after the simulator) 30 min kinesitherapy (in the afternoon aimed at training gait) and 30 min, group kinesitherapy to train the balance, swimming, sitting and relaxation. The improvement is significant regarding the kinetics of gait but does not change the central programming stepper footprint regarding chronic neurological deficit. These data are associated with the results obtained in this study, which also records improvement in walking speed related to better daily opportunities [6].
A possible explanation of one-month positive effect on gait in patients studied is probably due to those targeted functional activities included in SKTM which seek active participation by the patient to gain experience and to seize the opportunities of the processes of neuroplasticity recovery. The patient must learn strategies for solving specific mobility problems, optimal orientation of the body, a good starting position, the ability to perform a sequence of movements to enable the use of skills that are adaptable in everyday life [23, 28].
A distinct improvement in gait in the experimental group is associated with the 1-month application of the presented methodology. In patients with chronic post-stroke hemiparesis application of 3-week specialised neurorehabilitation improves spatial and temporal parameters of gait causing a significant increase in normal speed, the length of step and stride as a result of a significant reduction in their length [6, 14]. For a successful kinesitherapy is an essential optimal balance between duration of treatment and clinical recovery of the patient [8, 29, 30].
In conclusion, the applied SKTM significantly improves gait and increases walking speed as the observed beneficial effects accumulate throughout the follow-up period and with a maximum expression in the 1st month of treatment. Unlike the patients in the control group, SKTM showed progressive improvement throughout the follow-up period.
Footnotes
Funding: This research did not receive any financial support.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Duysens J, Verheyden G, Massaad F, Meyns P, Smits-Engelsman B, Jonkers U. Rehabilitation of gait and balance after CNS injury. In: Dietz V, Ward N, editors. Oxford Textbook of Neurorehabilitation. Oxford University Press; 2015. pp. 211–227. [Google Scholar]
- 2.Brandstater M, de Bruin H, Gowland C, Clark B. Hemiplegic gait: analysis of temporal variables. Arch Phys Med Rehabil. 1983;64:583–587. PMid:6661021. [PubMed] [Google Scholar]
- 3.Kerrigan D, Deming L, Holden M. Knee recurvatum in gait: a study of associated knee biomechanics. Arch Phys Med Rehabil. 1996;77:645–650. doi: 10.1016/s0003-9993(96)90002-7. https://doi.org/10.1016/S0003-9993(96)90002-7 . [DOI] [PubMed] [Google Scholar]
- 4.Kerrigan D, Schaufele M, Wen M. Gait analysis. In: Delisa J, Gans B, editors. Rehabilitation medicine principles and practice. 3rd ed. Philadelphia: Lippincott-Raven; 1998. pp. 167–187. [Google Scholar]
- 5.Pis V, Bouer B, Kadian V. The gait in humans. In: Physical Medicine and Rehabilitation - Principles and Practice. Vol. 1. Skopje: Delisa J. Tabernakul; 2012. pp. 155–168. [Google Scholar]
- 6.Titianova E Indicators for bilateral altered motor control of gait in chronic hemiparesis after supratentorial stroke. Dissertation for awarding the degree of “Doctor of Science”. Sofia: Specialized Scientific Council of Neurology and Psychiatry at BAK; 2007. [Google Scholar]
- 7.Kramers de Quervain I, Sheldon S, Leurgans S, Pease W, Mcallister D, Columbus Ohio. Gait Pattern in the Early Recovery Period after Stroke. The Journal of Bone and Joint Surgery, Incorporated. 1996;78-A(10):1506–1514. doi: 10.2106/00004623-199610000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Lubenova D, Titianova E Neurorehabilitation. In: Textbook on nervous diseases - General Neurology. Under the editing Titianova E. Sofia: University Publishing House “St Kliment Ohridski”; 2015. pp. 249–259. [Google Scholar]
- 9.Titianova E, Tarkka I. Assymetry in walking performance and postural sway in patients with chronic unilateral infarction. Journal of Rehabilitation Research and Development. 1995;32:236–244. PMid:8592295. [PubMed] [Google Scholar]
- 10.Titianova E, Pitknen K, Pääkkönen A, Sivenius J, Tarkka I. Gait characteristics and functional ambulation profile in patients with chronic unilateral stroke. American Journal of Physical Medicine & Rehabilitation. 2003;82:778–823. doi: 10.1097/01.PHM.0000087490.74582.E0. https://doi.org/10.1097/01.PHM.0000087490.74582.E0 . PMid:14508410. [DOI] [PubMed] [Google Scholar]
- 11.Titianova E, Mateev P, Peurala S, Sivenius J, Tarkka I. Footprint peak time analysis and Functional Ambulation Profile reflect the potential for hemiparetic gait recovery. Brain Injury. 2005;19:623–631. doi: 10.1080/02699050400013634. https://doi.org/10.1080/02699050400013634 . PMid:16175816. [DOI] [PubMed] [Google Scholar]
- 12.Titianova E, Peurala S, Pitkanen K, Tarkka I. Gait reveals bilateral adaptation in motor control in patients with chronic unilateral stroke. Aging Clinical and Experimental Research. 2008;20:131–138. doi: 10.1007/BF03324759. https://doi.org/10.1007/BF03324759 . PMid:18431080. [DOI] [PubMed] [Google Scholar]
- 13.Mateev P, Tarkka I, Titianova E. Gait measurements and motor recovery after stroke. Pliska Studia Matematica Bulgarica. 2004;16:121–128. [Google Scholar]
- 14.Titianova E. Reorganization of motor control after unilateral stroke. Neurosonology and cerebral hemodynamics. 2007;3(1):42–47. [Google Scholar]
- 15.Titianova E, Tarka I. Effect of neurorehabilitation on gait in patients with chronic post-stroke hemiparesis. Bulgarian Neurology. 2008;8(2):57–64. [Google Scholar]
- 16.Peurala S, Titianova E, Mateev P, Pitkänen K, Sivenius J, Tarkka I. Gait characteristics after gait-oriented rehabilitation in chronic stroke. Restorative Neurology and Neuroscience. 2005;23:57–65. PMid:15990412. [PubMed] [Google Scholar]
- 17.Titianova E, Lubenova D Hemiparetic gait after stroke. Modern research methods and neurorehabilitation. Sofia: KOTI EOOD; 2016. [Google Scholar]
- 18.Carod-Artal F, Gonzalez-Gutie J, Herrero J, Horan T, De Seijas E. Functional recovery and instrumental activities of daily living: follow-up 1-year after treatment in a stroke unit. Brain Inj. 2002;16(3):207–216. doi: 10.1080/02699050110103337. https://doi.org/10.1080/02699050110103337 . PMid:11874614. [DOI] [PubMed] [Google Scholar]
- 19.Robinson C, Shumway-Cook A, Matsuda P, Ciol M. Understanding physical factors associated with participation in community ambulation following stroke. Disabl Rehabil. 2011;33(12):1033–1042. doi: 10.3109/09638288.2010.520803. https://doi.org/10.3109/09638288.2010.520803 . PMid:20923316. [DOI] [PubMed] [Google Scholar]
- 20.Cowland C, Stratford P, Ward M. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke. 1993;24(1):58–63. doi: 10.1161/01.str.24.1.58. https://doi.org/10.1161/01.STR.24.1.58 . [DOI] [PubMed] [Google Scholar]
- 21.Wade D. Measurement in neurological rehabilitation. Oxford: University Press; 1992. [PubMed] [Google Scholar]
- 22.Bronstein A, Brand T, Wollacott M. Clinical disorders of balance, posture and gait. London: Amold; 1996. pp. 79–84. [Google Scholar]
- 23.Vasileva D, Lubenova D. Adapted program for independent home rehabilitation in patients with stroke in the chronic period. Sport and Science. 2014;58(3):61–72. [Google Scholar]
- 24.Krakauer J. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006;19(1):84–90. doi: 10.1097/01.wco.0000200544.29915.cc. https://doi.org/10.1097/01.wco.0000200544.29915.cc . PMid:16415682. [DOI] [PubMed] [Google Scholar]
- 25.Wang R, Chen H, Chen C, Yang Y. Efficacy of Bobath versus orthopaedic approach on impairment and function at different motor recovery stages after stroke: a randomized controlled study. Clin Rehabil. 2005;19:155–164. doi: 10.1191/0269215505cr850oa. https://doi.org/10.1191/0269215505cr850oa . PMid:15759530. [DOI] [PubMed] [Google Scholar]
- 26.Gelber D, Josefczy P, Herrman D, Good D, Verhulst S. Comparison of two therapy approaches in the rehabilitation of the pure motor hemiparetic stroke patient. J Neurol Rehabil. 1995;9:191–196. https://doi.org/10.1177/154596839500900401 . [Google Scholar]
- 27.Kollen B, Lennon S, Lyons B. The Effectiveness of the Bobath Concept in Stroke Rehabilitation: What is the Evidence? Stroke. 2009;40(4):89–97. doi: 10.1161/STROKEAHA.108.533828. https://doi.org/10.1161/STROKEAHA.108.533828 . PMid:19182079. [DOI] [PubMed] [Google Scholar]
- 28.Vasileva D, Lubenova D, Mihova M, Dimitrova A, Grigorova Pertova K. Influence of kinesitherapy on gait in patients with ischemic stroke in the chronic period. Open Access Maced J Med Sci. 2015;3(4):619–623. doi: 10.3889/oamjms.2015.107. https://doi.org/10.3889/oamjms.2015.107 . PMid:27275297. PMCid:PMC4877897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lubenova D, Titianova E. Principles of modern neurorehabilitation. Neurosonology and cerebral hemodynamics. 2012;8(1):45–55. [Google Scholar]
- 30.Lubenova D. Hemiparetic gait after stroke. In: Modern research methods and neurorehabilitation. Titianova E, Lubenova D, editors. Sofia: KOTI EOOD; 2016. pp. 89–135. [Google Scholar]


