Abstract
BACKGROUND:
Gait disorders or postural instability has been done before. However, lack of reviews has addressed the relation between gait and postural stability in Parkinson’s disease (PD).
AIM:
The aim was to evaluate the relation between gait parameters and postural stability in early and late stages of PD.
MATERIALS AND METHODS:
The forty-one idiopathic PD patients were divided into two groups into a group (A) considered as early PD and group (B) considered as late ambulant PD. They were evaluated for postural stability by computerised dynamic posturography (CDP) device and gait analysis using an 8 m-camera Vicon 612 data capturing system set.
RESULTS:
There was a statistically significant improvement of composite equilibrium score, the composite latency of motor response, walking speed and cadence after treatment as compared to before training (p < 0.05) in early PD. However, in the late PD, there was a non-significant change of previous parameters after treatment as compared to before training (p > 0.05). There was a significant correlation between UPDRS motor part score, walking speed and composite equilibrium score after training in early PD (p > 0.05).
CONCLUSIONS:
Both gait analysis and CDP are important quantitative assessment tools of gait and posture instability.
Keywords: Parkinson’s disease (PD), Computerized Dynamic Posturography (CDP), kinetic and kinematic gait and Rehabilitation
Introduction
There is a lack of studies comparing postural instability and gait impairment of early and late idiopathic Parkinson‘s disease (PD) patients with healthy elder subjects. Thus, our aim was to evaluate the correlation of postural stability and gait parameters by using computerised dynamic posturography and an 8 m-camera Vicon 612 data capturing system set in early and late stages PD as well as visual feedback-based balance training effects.
Parkinson‘s disease (PD) is a typical disorder of the basal ganglia. It is associated with characteristic changes in resting tremor, muscle rigidity, bradykinesia, and postural instability and the gait instability risk as well as due to decreased stimulation of the motor cortex by the basal ganglia and The PD gait is mainly characterized by a reduction of gait speed and stride length leading to falls [1].
Gait disorder is one of the cardinal features of Parkinson‘s disease. Understanding how gait is influenced by PD is perhaps the most important task [2]. Gait analysis plays an important role in maintaining human mobility and is a valuable tool for obtaining quantitative information on motor deficits in PD [3]. Moreover, postural instability is a major problem in PD; it increases the frequency of fall episodes and their consequences in PD [4].
A most of rating scales are used for the evaluation of motor impairment and disability in patients with PD including Hoehn and Yahr scale and the Unified Parkinson‘s Disease Rating Scale (UPDRS) in PD. The Hoehn and Yahr scale defines broad categories of motor function and disease progression in PD. The UPDRS is the most well-established scale for assessing disability and impairment [5, 6]. Computerising dynamic posturography measures postural instability assists in the analysis of the functional aspects of the body imbalance, treatment and prognosis of PD [7].
The aim of this study was to evaluate the relation between gait parameters and postural stability in early and late stages of PD by using computerised dynamic posturography and gait analysis using an 8 m-camera Vicon 612 data capturing system set. Also, to evaluate visual feedback-based balance training effects in early and late stages of PD. Moreover, we explored the correlation between postural stability and gait parameters in PD patients.
Patient and Methods
Patients
The forty-one idiopathic Parkinson‘s disease (IPD) patients were divided into two groups according to a modified Hoehn and Yahr Staging Scale (mHY). The group A (n = 20) was early-stages Parkinson‘s disease (early PD) with mH&Y &Y about 1.52 ± 0.44 and the group B (n = 21) was late-stages ambulant Parkinson‘s disease (late PD) with mH&Y about 3.23 ±0.56 [8, 9]. In twenty early-stages PD patients were without a history of falling, or other balance problems were included. Group C (n = 18) is eighteen healthy elderly subjects’ matched age and sex as a control group. Those patients were chosen from outpatient clinics, neurological department.
The Modified Hoehn and Yahr staging scale (mHY) is a commonly used system for describing the progress of PD. It included stages 1 through 5 with the addition of stages 1.5 and 2.5 to help describe the intermediate course of the disease [8, 9]. The Unified PD Rating Scale (UPDRS) was done in early and late PD [9].
Parkinson‘s disease (PD) was diagnosed on clinical criteria; there is no definitive test for diagnosis. The diagnosis was based on the criteria of the UK Parkinson‘s Disease Society Brain Bank, which requires the presence of bradykinesia and at least one of the three cardinal signs of Parkinson‘s disease: tremor at rest, muscle rigidity, and postural instability [10, 11].
Inclusion criteria of our patients included the patients with the ability to ambulate 25 feet independently and willingness to participate in this study. The exclusion criteria included the external and middle ear disorders; those with psychiatric disorders; major surgery; or who had severe visual impairment; orthopaedic disorders resulting in limited movement or who have received body balance rehabilitation in the last six months.
All patients gave their informed verbal voluntary consent to use the recorded data in their follow up sheets according to the protocol approved by the local ethics committee and by the ethical standards of the Helsinki Declaration.
Methods
The Functional Independence Measure (FIM) is an instrument that aims to describe activities of daily living (ADL) and levels of dependency/ independency. It is designed 18 items including 13 physical items and 5 cognitive/social items. Each item is rated on a seven-point scale, from total assistance to complete independence. Total scores range from18 to 126, with 126 indicating complete independence [12]. The Berg Balance Scale (BBS) also was used to assess balance. Thirty-eight component balance tests were originally selected and then refined to 14 items, each scored from 0 to 4, making a possible total score between 0 and 56, with a higher score indicating better balance [13].
Gait analysis (a video motion analysis system) was conducted using an 8 M-camera Vicon 612 data capturing system set at 120 Hz, and 3 force plates mounted midway on an 8-m walkway. Retro-reflective markers were placed on the specific anatomic points of the subjects‘lower limbs, enabling 3-dimensional analysis during the gait cycle. These points were the anterior superior iliac spines, sacrum, mid thighs, lateral malleoli, a dorsolateral aspect of the foot between the second and third metatarsal heads, and on the calcaneus. Workstation and Polygon software were used to manually define gait cycle events and to process kinematic and kinetic data [14]. Assessment and training for postural stability wsere done by using computerised dynamic posturography (CDP) device. The SMART Balance Master was used for assessment by the sensory organisation test (SOT) and motor control test (MCT). (The SMART Balance Master, NeuroCom International, Inc., Clackamas, OR, USA) [15].
The sensory organisation test (SOT) is included six test conditions which lasting 20 seconds each and repeated three times to get stable values. The first three conditions include SOT 1 (eyes open), SOT 2 (eyes closed), SOT 3 (sway-referenced vision with standing on a fixed platform called static posturography. The second three conditions include SOT 4 (eyes open), SOT 5 (eyes closed), SOT 6 (sway-referenced vision with standing on a moving platform called dynamic posturography. Composite equilibrium score (CES%) was calculated that describes the overall level of performance under the six conditions. Scores range from 0 to 100, with 0 representing a fall and 100 representing perfect stability [15].
Motor control test (MCT) assesses the ability of the motor control system to recover from an unpredictable forward or backwards perturbation. In this test, sequences of small, medium, and large platform translations in forward and backwards elicit automatic postural responses that are quantified in term of response latency (the time delay between the perturbation and the initiation of a change in force for each leg in msec). The composite latencies of the motor responses were calculated and describe the overall level of performance during all the MCT trials [15].
All patients received complex training course for three months including visual feedback balance training (twice weekly) by using CDP and conventional physical therapy (three times weekly). All patients were evaluated by CDP before and three months after the training program.
Statistical analysis
We used SPSS (Statistical Package from Social Science Program) version 15.0 for data processing (SPSS Inc, Chicago, IL, USA). Quantitative data were presented as mean (± SD). Statistical evaluation of the data was performed with one–way analysis of variance (ANOVA) test for multiple comparisons between early PD, late PD groups and control group. Findings with an error probability value of less than 0.05 were considered as statistically significant, that of less than 0.001 were considered as highly significant. Correlation between variables was done, and Pearson correlation coefficient was calculated. All tests were 2-tailed and considered statistically significant at p < 0.05.
Results
We found postural instability in early PD and late PD groups as shown Table 1 and Figures 1 & 2. We found a significant decline of mean (± SD) of FIM score, BBS score, composite equilibrium score (CES%) and a significant increasing of mean (± SD) of composite latency of motor response (msec) in early and late PD as compared with results of control group (p < 0.05). Moreover, there was a statistically significant decline of the mean (± SD) of UPDRS - Motor part score, FIM score, BBS score and CES%, and significant rise of the mean (± SD) of composite latency of motor response in late PD as compared with results of early PD.
Table 1.
Baseline Demographic, clinical and posture characteristics by sensory organisation test and motor control test in PD patients and control group
| Parameters (Mean ± SD) | Group C, the control subjects (18) | Group A, Early PD (20) | Group B, late ambulant PD (21) | |
|---|---|---|---|---|
| Comparison | - | A and control | B and control | A and B |
| Age (years) | 56.39 ± 6.29 | 61.10 ± 4.94 | 62.61 ± 4.52 | (P > 0.05) NS |
| Sex (male/female) | 13/5 | 14/6 | 15/6 | - |
| BMI, kg/m^2 | 22.35 ± 2.18 | 21.32 ± 2.34 | 22.39 ± 1.99 | (P > 0.05) NS |
| Disease duration (years) | - | 3.70 ±1.50 | 10.63 ± 3.16 | - |
| mH&Y score | - | 1.52 ±0.44 | 3.23 ± 0.56 | - |
| UPDRS - motor part score | - | 36.01 ± 5.55 | 28.26 ± 8.36 | - |
| FIM score | 108.61 ± 8.18 | 75.50±7.05*(*P <0.05) | 48.23 ±10.96*(*P <0.05) | (*P <0.05) |
| BBS score | 45.83 ± 6.84 | 40.15 ± 5.31*(*P <0.05) | 39.57 ± 6.39 *(*P <0.05) | (P > 0.05) NS |
| Composite equilibrium score (CES %) | 73.16 ± 6.65 | 54.25± 9.55*(*P <0.05) | 43.38± 7.92*(*P <0.05) | (*P <0.05) |
| Composite latency of motor response (msec) | 131.16 ± 9.79 | 163.25 ± 10.89*(*P <0.05) | 201.28 ± 2.86*(*P <0.05) | (*P <0.05) |
Body Mass Index (BMI); Modified Hoehn and Yahr (mH&Y); Unified Parkinson‘s Disease Rating Scale (UPDRS); functional independence measure (FIM); Berge balance scale (BBS); composite equilibrium score (CES%) of sensory organization test (SOT); composite latency of motor response (msec) of motor control test (MCT); Non-Significant (NS) P > 0.05; Significant
P < 0.05; highly significant ** P < 0.001; (ANOVA).
Figure 1.
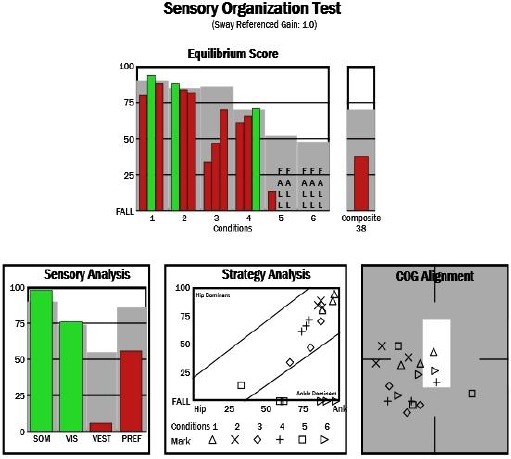
Composite equilibrium score of SOT parameters with Composite Equilibrium Score (CES) about 35 % for Parkinson diseases (PD) patient
Figure 2.
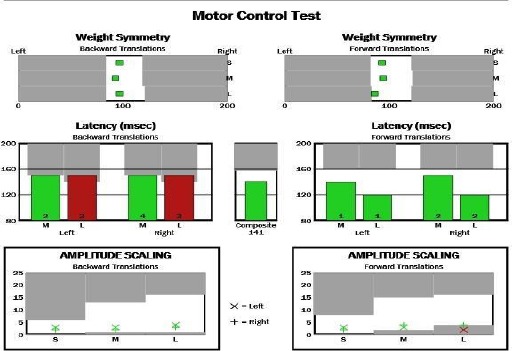
Composite latency of motor response of motor control test (MCT) for Parkinson diseases (PD) patient
We found slow walking speed, lower cadence, stride length reduction, a decline of a range of ROM of hip flexion on the swing, a hip extension on stance and knee flexion on swing and ankle planter flexion of PD gait as shown Table 2 and Figures 3 and 4.
Table 2.
Comparison of kinetic and kinematic gait parameters in PD patients and the control group
| Parameters (Mean ±SD) | Group C, the control subjects | Group A Early PD | Group B, late ambulant PD | |
|---|---|---|---|---|
| Comparison | - | A and control | B and control | A and B |
| 1- Kinetic parameters and Gait cycle | ||||
| Walking speed (m/s) | 0.85 ± 0.09 | 0.98 ± 0.08*(*p<0.05) | 1.13 ± 0.08*(*p<0.05) | (*P <0.05) |
| Cadence(steps/min) | 108.83 ± 9.33 | 92.50 ± 5.05*(*p<0.05) | 80.76 ± 5.69*(*p<0.05) | (*P <0.05) |
| Stride length(m) | 1.36 ± 0.06 | 1.29 ± 0.08*(*p<0.05) | 1.27 ± 0.11(*p<0.05) | (P > 0.05) NS |
| Stride time(s) | 1.21 ± 0.12 | 1.24 ± 0.05(p > 0.05) NS | 1.29 ± 0.10(p > 0.05) NS | (P > 0.05) NS |
| 2- Kinematic parameters(Joint rotation angles) | ||||
| Maximum hip extension on stance(degrees) | –15.47 ± 2.69 | –7.60 ± 1.84*(*p<0.05) | –5.56 ± 1.23*(*p<0.05) | (*P <0.05) |
| Maximum hip flexion on swing(degrees) | 29.81 ± 5.75 | 14.95 ± 3.40*(*p<0.05) | 10.89 ± 2.90*(*p<0.05) | (*P <0.05) |
| Maximum knee flexion on swing(degrees) | 46.74 ± 3.50 | 39.50 ± 5.91*(*p<0.05) | 33.30 ± 4.62*(*p<0.05) | (*P <0.05) |
| Plantar flexion Ankle(degrees) | –0.42 ± 0.07 | 0.42 ± 0.05(p > 0.05) NS | –0.39 ± 0.07(p > 0.05) NS | (P > 0.05) NS |
Figure 3.
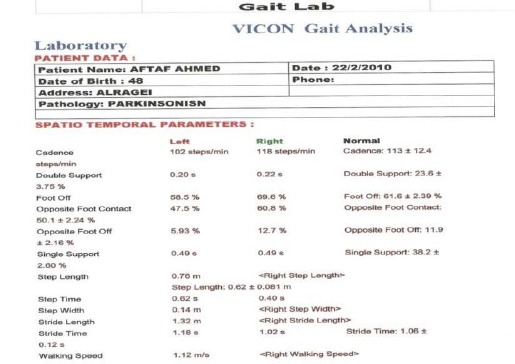
Kinetics: gait cycle parameters for Parkinson diseases (PD) patient
Figure 4.
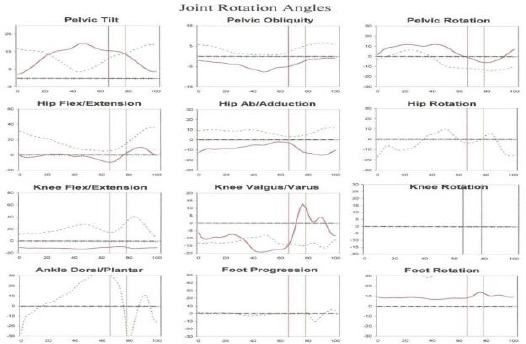
Kinematics (joint rotation angles): Average curves of joint rotation angles for Parkinson diseases (PD) patient (less dark line) and control subject (dark line) with low angle of hip joints
There was the significantly slow walking speed, lower cadence and reduction of stride length (p < 0.05) and is accompanied by a longer duration of stride time. While, there was the significant decline in a range of motion of joints of maximum hip flexion on the swing, a maximum hip extension on stance and maximum knee flexion on the swing of PD gait (p < 0.05), and non-significantly change of ankle planter flexion(p > 0.05).
Also, we found improvement in UPDRS motor part score, FIM score, BBS score, composite equilibrium score, the composite latency of motor response, walking speed, cadence and stride length after visual feedback-based balance training only in early PD as shown Table 3.
Table 3.
Comparison of UPDRS - Motor part score, FIM score, BBS score, posture and gait parameters before and after training in early and late PD
| Group A, Early PD | Group B, late ambulant PD | |||
|---|---|---|---|---|
| Before training | After Training | Before training | After Training | |
| UPDRS - motor part score | 36.01 ± 5.55 | 40.31 ± 4.10*(p < 0.001) | 28.26 ± 8.36 | 28.66 ± 7.52(P >0.05) NS |
| FIM score | 75.50 ± 7.05 | 80.85 ± 6.99* (p<0.05) | 48.23 ± 10.96 | 49.23 ± 10.34(P >0.05) NS |
| BBS score | 39.40 ± 3.25 | 43.28 ± 6.73* (p<0.05) | 39.57 ± 6.39 | 38.00 ± 4.73 (P >0.05) NS |
| Composite equilibrium score (CES %) | 54.25 ± 9.55 | 63.90 ± 8.54* (p<0.001) | 43.38 ± 7.92 | 44.42 ± 8.34(P >0.05) NS |
| Composite latency of motor response (msec) | 163.25 ± 10.89 | 152.25 ± 19.17* (p<0.05) | 201.28 ± 2.86 | 200.19 ± 4.28(P >0.05) NS |
| Walking speed (m/s) | 0.98 ± 0.08 | 1.23 ± 0.10* (p<0.05) | 1.13 ± 0.08 | 1.13 ± 0.06 (P > 0.05) NS |
| Cadence (steps/min) | 92.50 ± 5.05 | 99.87 ± 6.86* (p<0.05) | 80.76 ± 5.69 | 82.14 ± 5.78 (P >0.05) NS |
| Stride length (m) | 1.29 ± 0.08 | 1.24 ± 0.09* p<0.05) | 1.27 ± 0.11 | 1.27 ± 0.12 (P >0.05) NS |
| Stride time (s) | 1.24 ± 0.05 | 1.21 ± 0.05 (p>0.05) NS | 1.29 ± 0.10 | 1.31 ± 0.07 (P >0.05) NS |
Unified Parkinson‘s Disease Rating Scale (UPDRS); functional independence measure(FIM); Berge balance scale (BBS); composite equilibrium score (CES%); composite latency of motor response (msec); Non-Significant (NS) P > 0.05; Significant
P < 0.05; highly significant **P < 0.001.
There was a statistical significant improvement of UPDRS motor part score, FIM score, BBS score, composite equilibrium score, composite latency of motor response, walking speed, cadence and stride length in early PD (p < 0.05), non-significant improvement of mean (± SD) of stride time (p > 0.05) after treatment as compared to results before training in early PD. However, there was a non-significant change of previous parameters after treatment as compared to results before training (p > 0.05) in the late PD.
We found that gait velocity and step length, composite equilibrium score and composite latency of motor response were the only parameters which correlated with an improvement on the UPDRS motor part after visual feedback-based balance training in early PD as shown in Table 4 and Figure 5.
Table 4.
Linear regression correlation of the UPDRS motor part and walking speed, step length, composite equilibrium score and composite latency of motor response after visual feedback-based balance training in early PD patients
| Correlation | UPDRS motor part after training in early PD |
|---|---|
| Walking speed | r = 0.686**(P < 0.001) |
| Step length | r = 0.875**(P < 0.001) |
| Composite equilibrium score | r = 0.431*(P < 0.05) |
| Composite latency of motor response | r = 0.827**(P < 0.001) |
**, Correlation is significant at the 0.01 level (2-tailed); Non-Significant (NS) P > 0.05; significant
P < 0.05; highly significant P < 0.001.
Figure 5.
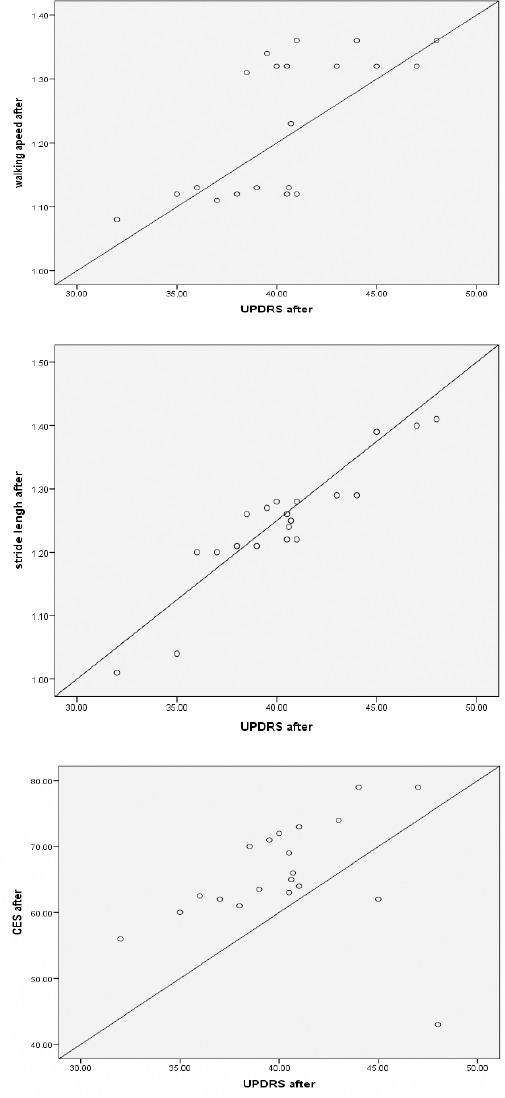
Correlation between the UPDRS motor part and walking speed (r = 0.686, p < 0.001) after visual feedback-based balance training for early Parkinson diseases (PD) patient (top). Correlation between the UPDRS motor part and step length (r = 0.875, p < 0.001) after visual feedback-based balance training for early Parkinson diseases (PD) patient (middle). Correlation between the UPDRS motor part and Composite equilibrium score (CES) (r = 0.431, p < 0.05) after visual feedback-based balance training for early Parkinson diseases (PD) patient (bottom)
This suggests that particularly highly mobile PD patients benefit from visual feedback-based balance training in early PD. After visual feedback-based balance training in patients with early PD, there was a significant correlation between the UPDRS motor part after training and walking speed (r = 0.686, p < 0.001), step length (r = 0.875, p < 0.001), composite equilibrium score (r = 0.431, p < 0.05) and composite latency of motor response (r = 0.827, p < 0.001).
Discussion
There is a lack of reviews to evaluate the effectiveness of visual feedback-based balance training in patients with Parkinson‘s disease (PD). We believe that a specific early rehabilitation treatment in patients with PD aimed at altered balance control mechanism. We aim to evaluate visual feedback-based balance training effects on posture stability and gait in early and late stages of PD.
In the present study, we found postural instability in early PD and late PD groups with a significant decline of composite equilibrium score and UPDRS - Motor part score in early PD and late PD groups as compared with control group (p < 0.05).
Our findings are supported by other publications; some authors reported that decline of UPDRS with motor impairment in PD [16]. Others found that of the UPDRS impairment is associated with gait impairment in PD [6]. Others found postural instability with the frequency of fall episodes in PD [4]. The postural instability may induce difficulties with transfers, gait disorders and falls in PD [17]. Also, postural instability is one of the hallmarks of PD and major risk factors of falling in PD [18]. Others suggested that posturography may identify early signs of postural instability and treatment of Parkinson‘s disease [19].
The pathophysiology of postural instability is not yet fully understood in PD. Several mechanisms have been explained the postural instability in PD, including abnormal postural reflexes, gait abnormalities and impaired proprioception [19]. Also, others have been explained the postural instability by impaired proprioception in late PD [20]. Basal ganglia neurones may have many proprioceptive receptive deficits which may explain the impaired postural in late PD [21]. Also, the afferent proprioceptive information to the basal ganglia may be normal, but these signals are perhaps abnormally within the basal ganglia due to defective higher level integration in PD [22]. Moreover, several other factors may contribute to postural instability in PD patients such as their ability to integrate visual, vestibular, and proprioceptive inputs as well as orthostatic hypotension or age-related sensory changes [18].
In the present study, we found slow walking speed, lower cadence, stride length reduction, a decline of a range of ROM of hip flexion on the swing, a hip extension on stance and knee flexion on swing and ankle planter flexion of PD gait. Our findings are supported by previous studies; some author reported there was a decrease of stride length and velocity. The velocity reduction is related to stride length shortening, but may not be related to cadence [23]. Also, other authors found the decrease in gait velocity and stride length shortening in PD [24]. The velocity decrease seems to be related to cadence in initial stages of PD [25]. The stride velocity and length and cadence may produce a slower gait in PD [26]. The reduction of stride length is considered the most prominent feature of PD gait and is accompanied by lower walking speed [27]. The PD results showed a significant reduction in step length and walking velocity compared with controls [28].
Our finding was in agreement with other studies in the kinematics, Morris et al. found reduced ROM values for all major joints of the lower extremity in the PD [27]. Other data show a hip extension deficit, decreased knee extension during single stance support, and reduced ankle plantar flexion at the toe-off. Importantly, all these changes can be explained by or contribute to a shortened stride length [29]. The subjects with PD had limited hip flexion, inadequate knee extension, or absent heel strike. At the ankle joint, the dorsiflexion moment was reduced in the PD [28]. In disagreement with our results, some author did not find any differences in the knee and hip kinematic variables of PD patients on therapy comparing with a control group in their recent extensive description of PD gait [24].
Several mechanisms have been explained gait impairments in PD including alterations at the peduncle pontine area, the substantia nigra, and the temporal neocortex and the prefrontal cortex are as in the advanced stage of PD [30]. Gait impairments probably result from the progressive loss of dopamine producing cells in the substantia nigra of basal ganglia [24].
Also, we found improvement in UPDRS motor part score, FIM score, BBS score, composite equilibrium score, the composite latency of motor response, walking speed, cadence and stride length after visual feedback-based balance training only in early PD. Other results were in agreement with our study, Others suggest that early rehabilitation treatment aimed to improve the balance control by several mechanisms have to include (a) stretching exercises to minimize the stiffness of the ankle, (b) a weight-shift training program, to improve balance control, (c) a proprioceptive-motor training, and (d) a balance training based on visual and auditory feedback [31]. Also, others found that gait training with visual cues for one month was successful in improvement in gait speed and step length and increasing the stability of the motor system [32].
We found that gait velocity and step length and composite equilibrium score were the parameters which correlated with an improvement on the UPDRS motor part after visual feedback-based balance training in early PD. This suggests that particularly highly mobile PD patients benefit from visual feedback-based balance training in early PD. Our finding agreed with other studies, others found that gait velocity and step length were the parameters which correlated with an improvement on the UPDRS motor part after Nordic walking (also called pole walking) in PD [33]. Gait velocity correlates with the UPDRS motor part in PD [34]. A positive correlation was observed between gait speed and UPDRS score in response to the intervention treatment [35]. The stride length presented significant correlation with motor UPDRS and timed up & goes and the velocity had correlation with Timed up & goes [23]. In contrast, no correlation was found between stride length or walking velocity and power generation at the ankle, knee, and hip joints in the PD group [28].
Finally, several hypotheses have been explained the improvements of gait by visual feedback-based balance training. The first possible set of explanations is related to the visual information provided by training and some conditions visual information may improve locomotion and relieve freezing [36]. Another explanation could be that the distance from the front of the visual feedback training is used as a static visual cue, provides constant the position of the body and can be guided externally rather than internally in PD gait [37].
We conclude that both gait analysis and computerised dynamic posturography are important quantitative assessment tools of gait and posture instability as well as visual feedback-based balance training program can improve gait parameters and postural stability in early PD.
The balance dysfunction and gait impairment can be discovered before its appearance by using a CDP and gait analysis that these data may allow developing a specific rehabilitative treatment to prevent or delay their onset in early PD. Visual feedback- based balance training is simple, safe, and effective as a complementary treatment for gait disorders in PD. However, there were some limitations in our review and insufficient data for the long follow-up effect of visual feedback-based balance training for PD.
Abbreviation: Body Mass Index (BMI); Modified Hoehn and Yahr (mH&Y); Unified Parkinson‘s Disease Rating Scale (UPDRS); functional independence measure(FIM); Berge balance scale (BBS); composite equilibrium score (CES%); sensory organization test (SOT); motor control test (MCT).
Acknowledgment
We would like to thank all the patients that participated in this study. We are grateful to our colleagues in neurology and physical medicine and rehabilitation departments for their assistance during this study and review of the manuscript.
Footnotes
Funding: This research did not receive any financial support.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Morris ME. Movement disorders in people with Parkinson disease: a model for physical therapy. Phys Ther. 2000;80(6):578–597. PMid:10842411. [PubMed] [Google Scholar]
- 2.Chen P-H, Wang R-L, Liou D-J, Shaw J-S. Gait Disorders in Parkinson’s disease: Assessment and Management. International Journal of Gerontology. 2013;7:189e193. [Google Scholar]
- 3.Zeng W, Liu F, Wang Q, Wang Y, Ma L, Zhang Y. Parkinson’s disease classification using gait analysis via deterministic learning. Neurosci Lett. 2016;633:268–278. doi: 10.1016/j.neulet.2016.09.043. https://doi.org/10.1016/j.neulet.2016.09.043 . PMid:27693437. [DOI] [PubMed] [Google Scholar]
- 4.Mak MK, Pang MY. Balance confidence and functional mobilityare independently associated with falls in people with Parkin-son’s disease. J Neurol. 2009;256(5):742–9. doi: 10.1007/s00415-009-5007-8. https://doi.org/10.1007/s00415-009-5007-8 . PMid:19240961. [DOI] [PubMed] [Google Scholar]
- 5.Ebersbach G, Baas H, Csoti I, Müngersdorf M, Deuschl G. Scales in Parkinson’s disease. J Neurol. 2006;253(Suppl 4):IV32–5. doi: 10.1007/s00415-006-4008-0. https://doi.org/10.1007/s00415-006-4008-0 . PMid:16944355. [DOI] [PubMed] [Google Scholar]
- 6.Goetz CG, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stebbins GT, Stern MB, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov Disord. 2007;22(1):41–7. doi: 10.1002/mds.21198. https://doi.org/10.1002/mds.21198 . PMid:17115387. [DOI] [PubMed] [Google Scholar]
- 7.Valkovic P, Abrahámová D, Hlavacka F, Benetin J. Static post-urography and infraclinical postural instability in early-stageParkinson’s disease. Mov Disord. 2009;15(24(11)):1713–4. doi: 10.1002/mds.22396. [DOI] [PubMed] [Google Scholar]
- 8.Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology. 1967;17(5):427–42. doi: 10.1212/wnl.17.5.427. https://doi.org/10.1212/WNL.17.5.427 . [DOI] [PubMed] [Google Scholar]
- 9.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–70. doi: 10.1002/mds.22340. https://doi.org/10.1002/mds.22340 . PMid:19025984. [DOI] [PubMed] [Google Scholar]
- 10.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. doi: 10.1136/jnnp.55.3.181. https://doi.org/10.1136/jnnp.55.3.181 . PMid:1564476. PMCid:PMC1014720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56(1):33–9. doi: 10.1001/archneur.56.1.33. https://doi.org/10.1001/archneur.56.1.33 . PMid:9923759. [DOI] [PubMed] [Google Scholar]
- 12.Grimby G, Gudjonsson G, Rodhe M, Sunnerhagen KS, Sundh V, Ostensson ML. The functional independence measure in Sweden: experience for outcome measurement in rehabilitation medicine. Scand J Rehabil Med. 1996;28(2):51–62. PMid:8815989. [PubMed] [Google Scholar]
- 13.Berg K, Wood-Dauphine S, Williams JI, Gayton D. Measuring balance in the elderly: preliminary development of an instrument. Physiother Canada. 1989;41:304–311. https://doi.org/10.3138/ptc.41.6.304 . [Google Scholar]
- 14.Nieuwboer A, Dom R, De Weerdt W, Desloovere K, Fieuws S, Broens-Kausik E. Abnormalities of the spatiotemporal characteristics of gait at the onset of freezing in Parkinson’s disease. Mov Disord. 2001;16:1066–75. doi: 10.1002/mds.1206. https://doi.org/10.1002/mds.1206 . PMid:11748737. [DOI] [PubMed] [Google Scholar]
- 15.Chaudhry H, Findley T, Quigley KS, Bukiet B, Ji Z, Sims T, Maney M. Measures of postural stability. J Rehabil Res Dev. 2004;41(5):713–20. doi: 10.1682/jrrd.2003.09.0140. https://doi.org/10.1682/JRRD.2003.09.0140 . PMid:15558401. [DOI] [PubMed] [Google Scholar]
- 16.Mak MK, Pang MY. Fear of falling is independently associated with recurrent falls in patients with Parkinson’s disease: a 1-year prospective study. J Neurol. 2009;256(10):1689–95. doi: 10.1007/s00415-009-5184-5. https://doi.org/10.1007/s00415-009-5184-5 . PMid:19479166. [DOI] [PubMed] [Google Scholar]
- 17.Bloem BR, Grimbergen YA, Cramer M, Willemsen M, Zwinderman AH. Prospective assessment of falls in Parkinson’s disease. J Neurol. 2001;248(11):950–8. doi: 10.1007/s004150170047. https://doi.org/10.1007/s004150170047 . PMid:11757958. [DOI] [PubMed] [Google Scholar]
- 18.Benatru I, Vaugoyeau M, Azulay J-P. Postural disorders in Parkinson’s disease. Clinical Neurophysiology. 2008;38:459–465. doi: 10.1016/j.neucli.2008.07.006. https://doi.org/10.1016/j.neucli.2008.07.006 . PMid:19026965. [DOI] [PubMed] [Google Scholar]
- 19.Bloem BR, Beckley DJ, Remler MP, Roos RA, van Dijk JG. Postural reflexes in Parkinson’s disease during ’resist’ and ’yield’ tasks. J Neurol Sci. 1995;129:109–119. doi: 10.1016/0022-510x(94)00253-k. https://doi.org/10.1016/0022-510X(94)00253-K . [DOI] [PubMed] [Google Scholar]
- 20.Lee AJ, Lin WH, Huang CH. Impaired proprioception and poor static postural control in subjects with functional instability of the ankle. Journal of Exercise Science & Fitness. 2006;4(2):117–125. [Google Scholar]
- 21.Chong RK, Horak FB, Woollacott MH. Parkinson’s disease impairs the ability to change set quick. J Neurol Sci. 2000;175(1):57–70. doi: 10.1016/s0022-510x(00)00277-x. https://doi.org/10.1016/S0022-510X(00)00277-X . [DOI] [PubMed] [Google Scholar]
- 22.Visser JE, Bloem BR. Role of the basal ganglia in balance control. Neural Plast. 2005;12:161–174. doi: 10.1155/NP.2005.161. https://doi.org/10.1155/NP.2005.161 . PMid:16097484. PMCid:PMC2565457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roiz Rde M, Cacho EW, Pazinatto MM, Reis JG, Cliquet A, Jr, Barasnevicius-Quagliato EM. Gait analysis comparing Parkinson’s disease with healthy elderly subjects. Arq Neuropsiquiatr. 2010;68(1):81–6. doi: 10.1590/s0004-282x2010000100018. https://doi.org/10.1590/S0004-282X2010000100018 . PMid:20339659. [DOI] [PubMed] [Google Scholar]
- 24.Sofuwa O, Nieuwboer A, Desloovere K, Willems AM, Chavret F, Jonkers I. Quantitative gait analysis in Parkinson’s disease: comparison with a healthy control group. Arch Phys Med Rehabil. 2005;86:1007–1013. doi: 10.1016/j.apmr.2004.08.012. https://doi.org/10.1016/j.apmr.2004.08.012 . PMid:15895349. [DOI] [PubMed] [Google Scholar]
- 25.Carpinella I, Crenna P, Calabrese E, et al. Locomotor function in the early stage of Parkinson’s disease. Transac Neural Systems Rehabil Engineering. 2007;15:543–551. doi: 10.1109/TNSRE.2007.908933. https://doi.org/10.1109/TNSRE.2007.908933 . PMid:18198712. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y, Lee Y, Cheng S, Lin P, Wang R. Relationships between gait and dynamic balance in early Parkinson’s disease. Gait Posture. 2008;27:611–615. doi: 10.1016/j.gaitpost.2007.08.003. https://doi.org/10.1016/j.gaitpost.2007.08.003 . PMid:17890091. [DOI] [PubMed] [Google Scholar]
- 27.Morris ME, Iansek R, Matyas TA, Summers JJ. Stride length regulation in Parkinson’s disease. Normalization strategies and underlying mechanism. Brain. 1996;119(Pt 2):551–68. doi: 10.1093/brain/119.2.551. https://doi.org/10.1093/brain/119.2.551 . PMid:8800948. [DOI] [PubMed] [Google Scholar]
- 28.Sofuwa O, Nieuwboer A, Desloovere K, Willems AM, Chavret F, Jonkers I. Quantitative gait analysis in Parkinson’s disease: comparison with a healthy control group. Arch Phys Med Rehabil. 2005;86(5):1007–13. doi: 10.1016/j.apmr.2004.08.012. https://doi.org/10.1016/j.apmr.2004.08.012 . PMid:15895349. [DOI] [PubMed] [Google Scholar]
- 29.Svehlík M, Zwick EB, Steinwender G, Linhart WE, Schwingenschuh P, Katschnig P, Ott E, Enzinger C. Gait analysis in patients with Parkinson’s disease off dopaminergic therapy. Arch Phys Med Rehabil. 2009;90(11):1880–6. doi: 10.1016/j.apmr.2009.06.017. https://doi.org/10.1016/j.apmr.2009.06.017 . PMid:19887212. [DOI] [PubMed] [Google Scholar]
- 30.Braak H, Del Tredici K. Nervous system pathology in sporadic Parkinson disease. Neurology. 2008;70:1916–1925. doi: 10.1212/01.wnl.0000312279.49272.9f. https://doi.org/10.1212/01.wnl.0000312279.49272.9f . PMid:18474848. [DOI] [PubMed] [Google Scholar]
- 31.Ferrazzoli D, Fasano A, Maestri R, Bera R, Palamara G, Ghilardi MF, Pezzoli G, Frazzitta G. Balance Dysfunction in Parkinson’s Disease: The Role of Posturography in Developing a Rehabilitation Program. Parkinsons Dis. 2015;2015:520128. doi: 10.1155/2015/520128. https://doi.org/10.1155/2015/520128 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sidaway B, Anderson J, Danielson G, Martin L, Smith G. Effects of long-term gait training using visual cues in an individual with Parkinson disease. Phys Ther. 2006;86(2):186–94. PMid:16445332. [PubMed] [Google Scholar]
- 33.Manon H, Godau J, Kattner B, Rombach S, Grau S, Maetzler W, Berg D. Gait velocity and step length at baseline predict outcome of Nordic walking training in patients with Parkinson’s disease. Parkinsonism and Related Disorders. 2015;21:413e416. doi: 10.1016/j.parkreldis.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Vieregge P, Stolze H, Klein C, Heberlein I. Gait quantitation in Parkinson’s diseaseelocomotor disability and correlation to clinical rating scales. J Neural Transm. 1997;104:237e48. doi: 10.1007/BF01273184. [DOI] [PubMed] [Google Scholar]
- 35.Lei H, Toosizadeh N, Schwenk M, Sherman M, Sherman S, Karp S, Sternberg E, Najafi B. A Pilot Clinical Trial to Objectively Assess the Efficacy of Electroacupuncture on Gait in Patients with Parkinson’s Disease Using Body Worn Sensors. PLoS ONE. 2016;11(5):26. doi: 10.1371/journal.pone.0155613. https://doi.org/10.1371/journal.pone.0155613 . PMid:27227460. PMCid:PMC4882016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azulay JP, Mesure S, Blin O. Influence of visual cues on gait in Parkinson’s disease: contribution to attention or sensory dependence? J Neurol Sci. 2006;248(1–2):192–5. doi: 10.1016/j.jns.2006.05.008. https://doi.org/10.1016/j.jns.2006.05.008 . PMid:16765379. [DOI] [PubMed] [Google Scholar]
- 37.Bello O, Sanchez JA, Fernandez-del-Olmo M. Treadmill walking in Parkinson’s disease patients: adaptation and generalization effect. Mov Disord. 2008;23(9):1243–9. doi: 10.1002/mds.22069. https://doi.org/10.1002/mds.22069 . PMid:18464281. [DOI] [PubMed] [Google Scholar]


