Abstract
BACKGROUND:
Renal transplantation (RTx) is the treatment of choice for paediatric end-stage renal disease (ESRD). A major cause of morbidity and mortality after RTx is cardiovascular disease. Independent predictors of cardiovascular events were shown to constitute an endothelial dysfunction (ED). This study aims to evaluate Visfatin serum level in comparison to brachial artery flow-mediated dilatation (FMD) as a marker of endothelial dysfunction in paediatric RTx recipients.
METHODS:
Visfatin serum level has been evaluated in 30 patients on regular hemodialysis (HD), 36 patients post-RTx and 30 controls as a measure for ED, and has been compared to brachial artery FMD.
RESULTS:
Visfatin level in transplant recipients was significantly lower than the hemodialysis group as well as FMD was better in transplant recipients. In spite of marked improvement of FMD and marked reduction of visfatin in post-RTx no direct statistical correlation was found between serum Visfatin level and flow-mediated dilatation.
CONCLUSION:
Pediatric RTx recipients show lower serum Visfatin level and better FMD than those on regular hemodialysis, reflecting less endothelial dysfunction (ED) and less cardiovascular risk. FMD in kidney transplant recipients tends to be less than normal subjects while visfatin level of the same group is similar to controls. Pediatric RTx appears to have a positive impact on the growth development of children with ESRD.
Keywords: End stage renal disease (ESRD), Children, Endothelial dysfunction (ED), Flow- mediated dilatation (FMD), Renal transplantation (RTx), Visfatin
Introduction
The endothelium plays a central role in the control of many aspects of vascular function. It responds to changes in blood-borne signals and hemodynamic forces by releasing vasoactive substances such as nitric oxide (NO). Endothelial dysfunction may result from decreased production or availability of NO [1] endothelial dysfunction (ED). It is now generally accepted that the first step in atherosclerosis [2] ED appears to be useful in the prediction of morbidity and mortality in cardiovascular risk groups [3].
Mortality from cardiovascular events and ED has been studied in adults and children with CKD [4]. Cardiovascular disease still represents a major cause of morbidity and mortality after kidney transplantation in spite of successful renal transplantation is associated with the elimination of uremia and consequently would be expected to improve ED [5]. Visfatin, also known as a pre-B-cell colony-enhancing factor (PBEF), is a novel and ubiquitous adipokine secreted by various tissues, especially visceral and subcutaneous fat [6]. Several clinical studies have shown a positive correlation between endothelial dysfunction and enhanced visfatin plasma levels [7]. Alternative markers of endothelial damage in CKD stage 5 patients have been positively associated with increased plasma visfatin levels [8].
The aim of this work was to study plasma visfatin level in paediatric kidney transplant recipients, in comparison to those on regular HD as well as normal children and to determine the relation between plasma Visfatin level and flow- mediated dilatation and other risk factors for endothelial dysfunction in paediatric renal transplant recipients.
Subjects and Methods
This is a cross-sectional study that included 96 paediatric subjects in three groups (36 post-renal transplantations for more than 12 months, 30 on regular hemodialysis for more than 3months, 30 normal children as controls).
We exclude from the study children presenting with acute problems associated with infection or inflammation and transplanted children with moderate or severe chronic graft dysfunction (eGFR < 45 mL/min/1.73 m^2). Also, patients with DM were excluded from our study.
Patients were recruited from Center of Pediatric Nephrology and Transplantation Unit, paediatric hospital, Kasr El Aini Faculty of Medicine, Cairo University. Control subjects were healthy age and sex matched relatives of cases attending the outpatient general clinic for acute illness.
Informed consent was obtained from the legal guardians of the patients before enrollment in the study. The study protocol was approved by the Research Committee of Pediatric Department, Faculty of Medicine, and Cairo University.
All patients were subjected to the following:
1. Detailed history: focusing on; Age of diagnosis, the cause of renal failure, duration of follow-up, onset and duration of renal replacement therapy, associated problems, hypertension, medications used, complications met, family history including consanguinity and similar conditions.
2. Full clinical examination: stressing on;
a. Anthropometric measurements including Height and weight were calculated as a percent of the median for age and sex to be corrected for age-related difference. Body mass index (BMI) was calculated from the formula: BMI=weight in kg/ square the height in meter.
b. Vital signs especially heart rate and blood pressure.
Blood pressure percentiles were determined according to (Bernstein, 2011) [9], and BP was classified into i. Normal BP: <90 percentile with no antihypertensive medications; ii. Borderline hypertension: BP between 90-95th percentile without antihypertensive; iii. Controlled Hypertension: BP < 95th percentile on treatment; and iv. Uncontrolled Hypertension: BP ≥ 95th percentile with treatment.
3. For transplant patients: Review of early graft function, the occurrence of acute rejection episodes, immunosuppressive regimen and steroid doses, patient compliance as well as the presence of hypertension, anaemia, graft dysfunction or other problems.
4. Routine laboratory investigations: CBC, BUN, serum creatinine level, Serum electrolytes including potassium, sodium, calcium and phosphorus levels, fasting blood glucose and lipid profile including cholesterol level, HDL, LDL and triglycerides, Liver function tests including ALT, ALP, total bilirubin level and serum albumin.
5. Glomerular filtration rate (GFR) calculated from serum creatinine using the Schwartz formula [10] in transplanted children and assessment of delivered dialysis dose using fractional urea clearance measured by single pool KT/v [11] in dialysis patients.
6. Assessment of Brachial artery flow-mediated dilatation by Doppler.
- It was measured by the comparison between the vessel diameter at rest with that during reactive hyperemia.
- The diameter of the brachial artery was imaged above the antecubital fossa in the longitudinal plane. Arterial flow velocity was measured with a pulsed Doppler signal at a 90° angle to the vessel.
- A baseline scan was taken after the patient had an initial rest period of 15 min in the supine position. To induce increased flow, the pneumatic tourniquet was applied to the forearm and inflated to a pressure above the systolic blood pressure for 5 min, then released.
- The second scan for reactive hyperemia was taken continuously from 30 s before to 90 s after cuff deflation, with flow velocity recording for the first 30 s following cuff deflation.
- Resting and peak flow volumes (ml/min) were measured.
- Reactive hyperemia (RH) was calculated as follows: RH = (Peak flow/resting flow) ×100.
- Percent flow-mediated dilatation (FMD %) represented the difference between vessel diameter at rest (D1) and during reactive hyperemia (D2) was calculated as follows:
FMD = (D2 - D1 / D1) X 100
A percent FMD less than 5% was considered impaired.
7. Echocardiography to asses’ cardiac function in both group.
8. Measurement of plasma Visfatin level (using ELISA technique) for cases and controls: Steps: i. Serum was left to coagulate at room temperature for 10-20 minutes then centrifuged for 20 minutes at the speed of 2000-3000 round per minute; then the supernatant was removed; ii. Ten standard wells were set on the ELISA coated plates. Standard 100μl were added to the first and the second wells, then standard dilution of 50 μl was added to the first and second wells, mixed, then 100 μl were taken out of the first and second well then added to the third and fourth well separately, then standard dilution of 50 μl was added to the third and fourth wells, mixed, 50 μl were taken out from the third and fourth wells and discarded. 50 μl were added to the fifth and the sixth wells, then standard dilution 50 μl added to the fifth and the sixth wells, mixed, 50 μl taken out from the fifth and sixth wells and added to the seventh and the eighth wells, then standard dilution of 50 μl added to the seventh and eighth wells, mixed, 50 μl taken out from the seventh and eighth wells and added to the ninth and the tenth wells then standard dilution of 50 μl added to the ninth and the tenth well, mixed, 50 μl taken out from the ninth and the tenth well and discarded (a sample 50 μl was added to each well after diluting) (density 18 μg/l, 12 μg/l, 6 μg/l, 3 μg/l, 1.5 μg/l); iii. Blank wells were set separately (we didn’t add sample and HRP-conjugate reagent in blank comparison wells; other operation steps are carried out the same). We added sample dilution of 40 μl to the testing sample well, then added another testing sample of 10 μl (sample final dilution is 5-fold), trying not to touch the well as far as possible, and gently mixed; iv. Then we closed the plate with the closure plate membrane, and incubated for 30 minutes at 37°C; v. A 30 fold wash solution was diluted 30 fold with distilled water and reserved; vi. We uncovered the closure plate membrane, discarded liquid, dried by swing, added washing buffer to every well, waited for 30 s then drained, repeated 5 times, dried by Pat; vii. Then we added HRP-conjugate reagent 50 μl to each well except the blank well; viii. We repeated step iv again followed by step vi again; ix. Then we added chromogen solution A 50 μl and chromogen solution B to each well, evaded the light preservation for 15 minutes at 37°C; x. We added sulphuric acid stop solution 50 μl to each well, till the blue colour changed to yellow; xi. We considered a blank well as zero, and then read absorbance spectrophotometrically at 450 nm within 15 minutes from adding the stop solution; and xii. We took the standard density as the horizontal, the OD value for the vertical, then we plotted the standard curve on a graph paper, then we had to find out the corresponding density according to the sample OD value by the sample curve, multiplied by the dilution multiple, or we sometimes calculated the straight-line regression equation of the standard curve with the standard density and the OD value. Using with the sample OD value in the equation, we calculated the sample density, multiplied by the dilution factor to get the actual sample density.
Analysis of data
Data were tabulated and subjected to computer-assisted statistical analysis using Microsoft Excel version 2003 and the Statistical Package for Social Science (SPSS) for Windows version 16.0. Nominal data were expressed as frequency and percentage and were compared using chi- squared tests. Numerical data were expressed as a mean and standard deviation and were compared using independent samples t-tests. P Values less than 0.05 were considered significant.
Results
Ninety-six paediatric subjects were included in this study (36 post-RTx for more than 12 months, 30 on HD for more than 3 months, and 30 normal children as controls). Twenty-six (87%) of hemodialysis group were on HD for more than one year Regarding transplantation 29 paediatric patients transplanted from related living donor only 7patients 19.4% from unrelated living donor
Overall, the study groups consist of 50 males 52% and 46 females 48%. Clinical data of the study group was showed in Table 1.
Table 1.
Clinical data of transplant recipients and hemodialysis patients
| Transplant recipients (n = 36) (mean ± SD) | Hemodialysis patients (n = 30) (mean ± SD) | P value | |
|---|---|---|---|
| Age (years) | 11.5 ± 3.48 | 11.4 ± 3.34 | 0.93 |
| Weight (kg) | 32.83 ± 14.77 | 24.54 ± 9.43 | 0.008 |
| Weight (% median) | 80.3 ± 22.09 | 61.2 ± 20.8 | 0.0006 |
| Height (cm) | 123.25 ± 14.03 | 117.03 ± 13.99 | 0.08 |
| Height (%median) | 85.6 ± 5.8 | 80.4 ± 7.7 | 0.003 |
| SBP (mmHg) | 109 ± 10.5 | 113 ± 13.3 | 0.13 |
| DBP (mmHg) | 70.28 ± 8.45 | 72.63 ± 7.52 | 0.24 |
| BMI (kg/m2) | 20.76 ± 6.26 | 17.50 ± 4.11 | 0.014 |
SBP: systolic blood pressure, DBP: diastolic blood pressure, BMI: body mass index.
The most common cause of renal failure of both groups was urological abnormalities followed by undetermined cause as illustrated in Table 2.
Table 2.
Aetiology of renal disease in transplant and dialysis groups (n=66)
| All cases | D | T | ||||
|---|---|---|---|---|---|---|
| No | % | No | % | No | % | |
| CAKUT | 20 | 30.30 | 8 | 26.67 | 12 | 33.33 |
| PUJO | 1 | 1.52 | 1 | 3.33 | 0 | 0 |
| PUV | 11 | 16.67 | 5 | 16.67 | 6 | 16.67 |
| Urolithiasis | 1 | 1.515 | 0 | 0 | 1 | 2.78 |
| Reflux nephropathy | 7 | 10.61 | 2 | 6.67 | 5 | 13.89 |
| Undetermined | 17 | 25.76 | 9 | 30 | 8 | 22.22 |
| Primary glomerulopathy | 7 | 10.6 | 4 | 13.33 | 3 | 8.33 |
| NPHP | 13 | 19.69 | 5 | 16.67 | 8 | 22.22 |
| PKD | 2 | 3.03 | 0 | 0 | 2 | 5.56 |
| Oxalosis | 2 | 3.03 | 1 | 3.33 | 1 | 2.78 |
| Cystinosis | 2 | 3.03 | 1 | 3.33 | 1 | 2.78 |
| HUS | 1 | 1.52 | 1 | 3.33 | 0 | 0 |
| Chronic interstitial nephritis | 2 | 3.03 | 1 | 3.33 | 1 | 2.78 |
CAKUT (congenital anomalies of the kidney and urinary tract); PUJO (periureteric junction obstruction); PUV (posterior urethral valve), NPHP (nephronophthisis); PKD (polycystic kidney disease), HUS (hemolytic uremic syndrome).
Regarding Echocardiography of studied patients at the time of assessment, we found no significant difference between both groups regarding FS. LVEDD (cm/m2) tended to be higher in dialysis group (p=0.057).
FMD of transplanted and dialysis groups versus control group showed in Fig. 1.
Figure 1.
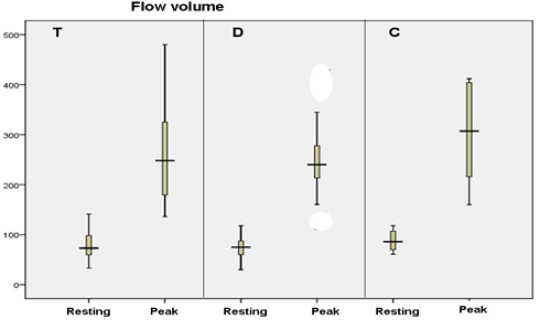
Flow- mediated dilatation (FMD) of the three studied groups (n = 96). T = transplanted; D = dialysis; C = control
As illustrated by Fig. 1, the resting and peak flow volumes were somewhat lower in the dialysis group than the transplanted group.
In comparison to the control group- the post RTx patients showed almost similar serum visfatin level (median IQR of 4 (3.5-5.8)) to the control group while in patients on dialysis the median visfatin level is almost 15 times the control group (median (IQR) 65(30-70) ng/ml, and it was statistically significant p < 0.0001 as showed in Fig. 2.
Figure 2.
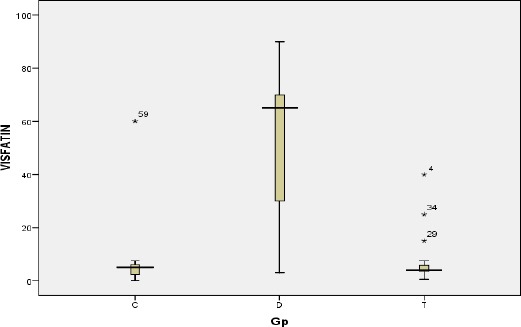
Visfatin level in the three groups ng/ml. T = transplanted; D = dialysis; C = control
Inspite marked improvement of FMD and reduction of serum visfatin level There was no significant correlation between serum visfatin and FMD; whether as an absolute value (r= -0.15, p = 0.28) or the FMD% (r = -0.10, p = 0.47).
Among clinical data obtained, significant positive correlation between duration of dialysis) and serum visfatin level (r = 0.56, p = 0.00001) and also there is negative non-significant correlation (r = -0.102, p = 0.56).
Among laboratory data obtained, significant correlations were found mainly between serum alkaline phosphatase, triglycerides level (TG) and serum visfatin level (r = 0.31, p = 0.027), (r = 0.49, p = 0.0006) respectivly.
Discussion
The burden of chronic disease throughout the world is steadily increasing. Cardiovascular disease (CVD) and chronic kidney disease (CKD) frequently coexist and represent a major challenge in today’s medicine [12]. The aim of this study was to study plasma Visfatin level in paediatric RTx recipients, in comparison to those on regular HD as well as normal children and to determine the relation between plasma Visfatin level and FMD and other risk factors for endothelial dysfunction in RTx recipients and HD patients.
The mean age of our transplant recipients was similar to that of the dialysis group. Preemptive transplantation was undertaken in 11 of 36 transplant recipients, while the others were transplanted after a mean period of 21.6 months on dialysis. Transplant recipients predictably had shorter dialysis duration than the HD group (mean 52.2 months).
Among our 36 patients who were recipients of RTx, the source was a living donor in 100% of cases, which is mainly due to the Egyptian regulations governing the transplantation procedures in Egypt.
Living donors’ allografts are favoured over those from deceased donors; Rockville 2010 found that the five-year survival for the allografts is greater in the former according to the NAPRTCS data [13].
In the aetiology of ESRD in the studied groups, the leading causes were CAKUT by 33.3% and 26.6%, of the transplanted and the dialysis groups respectively. Second, an undetermined cause which affected almost 22.2% and 30% of the transplanted and the dialysis groups respectively. These kidney pathologies resemble those in the NAPRTCS 2010 transplant and 2011 dialysis report; where CAKUT (obstructive uropathies 15.3% and 12.6%, then reflux nephropathy 5.2% and 3.5%, congenital renal aplasia/dysplasia 15.8% and 14.2% of the transplanted and the dialysis groups respectively). Cystic pathologies 5.7% and 4.9%, Focal segmental glomerulosclerosis 11.7% and 14.4% transplanted and the dialysis groups respectively were also leading causes.
Hypertension is a common complication following RTx in children and considers being a risk factor for increased cardiovascular morbidity and grafting failure [14]. In our study, there is a positive correlation between visfatin and hypertension but statistically not significant.
RTX group has significantly lower systolic and diastolic blood pressure than dialysis group (p = 0.02) at 90th centile, and a significantly lower diastolic blood pressure (p = 0.04) at the 95th centile.
Considering variables related to endothelial dysfunction the control subjects had mean FMD (%) of 16.13 The mean FMD (%) in dialysis patients was 7.6, significantly lower than normal subjects (16.1%; p < 0.001). This denotes that children with ESRD on HD have ED manifested as impaired endothelium-dependent VD.
RTx recipients in our study, they had a mean FMD of 12%; significantly better than dialysis patients (p = 0.04). They also tended to have lower FMD than normal subjects, with a difference not reaching statistical significance (p = 0.08).
Regarding serum visfatin level, the median serum visfatin in normal subjects was 5 ng/ml (IQR 2.4-6 ng/ml). HD patients had significantly higher level; with more than 10 folds of normal controls. This in agree with the study was done on 68 patients on HD patients compared with 22 healthy controls, showed a highly significant increase in serum visfatin in the CKD group on HD in comparison with the healthy control participants [15].
In RTx recipients the median serum visfatin was significantly lower than dialysis patients and near to normal subjects. This in agree with an adult study was done to measured visfatin levels in 58 living donor kidney transplant non-diabetic recipients, before transplantation and on the 30th and 90th day after transplantation [16].
The authors are aware of the relation between visfatin levels & glomerular filtration rate (GFR), possibly independently of ED; however, since patients on regular HD & RTx recipients with stable graft function each reflect essentially the same level of renal function, this is not expected to confound results significantly.
In our study, we couldn’t find a significant statistical correlation between serum visfatin and FMD; whether as an absolute value (r = -0.15, p = 0.28) or the FMD% (r = -0.10, p = 0.47). In our paediatric Rtx visfatin and FMD are near normal subjects. Therefore, the lack of statistically significant correlation can’t rule out the presence of such correlations in populations with marked endothelial dysfunction such as dialysis patients. Dyslipidemia is an established risk factor for atherosclerosis in uremic and numeric patients [17]. Among laboratory data obtained, significant correlations were found mainly between serum triglycerides and visfatin serum level (r = 0.49, p = 0.0006). The similar positive correlation was found by others [7, 15].
Whether the significant lowering in visfatin level post RTx in comparison to HD, could be a cause or a marker for the associated improved endothelial dysfunction is still to be further investigated. Longitudinal follow-up to determine patient-oriented CV outcome in correlation with FMD and serum visfatin is highly indicated. The long-term implications of vascular dysfunction should be the subject of further research.
In conclusion, paediatric RTx recipients show lower serum visfatin level and better FMD than those on regular HD, reflecting less endothelial dysfunction and less cardiovascular risk. FMD in RTx recipients tends to be less than normal subjects while visfatin level of the same group is similar to controls. Pediatric RTx appears to have a positive impact on the growth development of children with ESRD.
Acknowledgements
The authors sincerely thank the affected patients and their families for participation in our study.
Footnotes
Funding: This research did not receive any financial support.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Verma S, Anderson TJ. Fundamentals of endothelial function for the clinical cardiologist. Circulation. 2002;105:546–549. doi: 10.1161/hc0502.104540. https://doi.org/10.1161/hc0502.104540 . PMid:11827916. [DOI] [PubMed] [Google Scholar]
- 2.Pawlak K, Pawlak D, Mysliwiec M. Method of dialysis therapy and selected markers of oxidative stress and endothelial injury in patients with chronic renal failure. Pol Arch Med Wewn. 2005;113:21–26. PMid:16130597. [PubMed] [Google Scholar]
- 3.Cross J. Endothelial dysfunction in uraemia. Blood Purif. 2002;20:459–461. doi: 10.1159/000063552. https://doi.org/10.1159/000063552 . PMid:12207092. [DOI] [PubMed] [Google Scholar]
- 4.Foley RN, Murray MA, Li S, Herzog AC, McBean MA, Eggers WP, Collins AJ. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16:489–495. doi: 10.1681/ASN.2004030203. https://doi.org/10.1681/ASN.2004030203 . PMid:15590763. [DOI] [PubMed] [Google Scholar]
- 5.Kocak H, Ceken K, Yavuz A, et al. Effect of renal transplantation on endothelial function in haemodialysis patients. Nephrol Dial Transplant. 2006;21:203–207. doi: 10.1093/ndt/gfi119. https://doi.org/10.1093/ndt/gfi119 . PMid:16144848. [DOI] [PubMed] [Google Scholar]
- 6.Fukuhara A, Matsuda M, Nishizawa M. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–430. doi: 10.1126/science.1097243. https://doi.org/10.1126/science.1097243 . PMid:15604363. [DOI] [PubMed] [Google Scholar]
- 7.Mu J, Feng B, Ye Z, Yuan F, Zeng W. Visfatin is related to lipid dysregulation, endothelial dysfunction and atherosclerosis in patients with chronic kidney disease. J Nephrol. 2010;24:177–184. doi: 10.5301/jn.2010.3488. https://doi.org/10.5301/JN.2010.3488 . [DOI] [PubMed] [Google Scholar]
- 8.Kato A, Odamaki M, Ishida J, Hishida A. Relationship between serum pre-B cell colony enhancing factor/visfatin and atherosclerotic parameters in chronic hemodialysis patients. Am J Nephrol. 2009;29:31–35. doi: 10.1159/000148648. https://doi.org/10.1159/000148648 . PMid:18663287. [DOI] [PubMed] [Google Scholar]
- 9.Kliegman RM, Stanton BF, Geme JSt, Schor NF, Behrman RE, editors. Report of the Second Task Force on Blood Pressure Control in Children, Heart, Lung and Blood Institute, Bthesda. Pediatrics. 1987;79:1–25. Quoted from Bernstein D. History and physical examination. Nelson Textbook of Pediatrics; 19th edition, 2011:1534-1535. [PubMed] [Google Scholar]
- 10.Schwartz GJ, Gauthier B. A simple estimate of glomular filteration rate in adolescent boys. J Pediatr. 1985;106:522–526. doi: 10.1016/s0022-3476(85)80697-1. https://doi.org/10.1016/S0022-3476(85)80697-1 . [DOI] [PubMed] [Google Scholar]
- 11.Eknoyan G, Beck GJ, Alfred K, et al. Effect of Dialysis Dose and Membrane Flux in Maintenance Hemodialysis for the Hemodialysis (HEMO) Study Group. N Engl J Med. 2002;347:2010–2019. doi: 10.1056/NEJMoa021583. https://doi.org/10.1056/NEJMoa021583 . PMid:12490682. [DOI] [PubMed] [Google Scholar]
- 12.Lainščak. Cardiovascular Risk in Chronic Kidney Disease The journal of International Federation of Clinical Chemistry and Laboratory Medicine. 2009:72–75. [Google Scholar]
- 13.Rockville. North American Pediatric Renal Transplant Cooperative Study (NAPRTCS), 2010. Annual report. [Accessed on May 27, 2011]. Available from: http://www.naprtcs.org .
- 14.Sinha MD, Kerecuk L, Gilg J, Reid CJ. British Association for Paediatric Nephrology. Systemic arterial hypertension in children following renal transplantation: Prevalence and risk factors. Nephrol Dial Transplant. 2012;27:3359–68. doi: 10.1093/ndt/gfr804. https://doi.org/10.1093/ndt/gfr804 . PMid:22328733. [DOI] [PubMed] [Google Scholar]
- 15.Lotfy AWM, Mohammed NA, El-Tokhy HM, Attia FA. Serum visfatin in chronic renal failure patients on maintenance hemodialysis: A correlation study. Egyptian J Internal Medicine. 2013;25(4):202–208. https://doi.org/10.4103/1110-7782.124982 . [Google Scholar]
- 16.Yilmaz MI, Saglam M, Carrero JJ, et al. Normalization of endothelial dysfunction following renal transplantation is accompanied by a reduction of circulating visfatin/NAMPT. A novel marker of endothelial damage? Clinical Transplantation. 2009;23(2):241–248. doi: 10.1111/j.1399-0012.2008.00921.x. https://doi.org/10.1111/j.1399-0012.2008.00921.x . PMid:19402217. [DOI] [PubMed] [Google Scholar]
- 17.Prasad A, Tupas-Habib T, Schenke WH, Mincemoyer R, Panza JA. Acute and chronic angiotensin-1 receptor antagonism reverses endothelial dysfunction in atherosclerosis. Circulation. 2000;101(20):2349–2354. doi: 10.1161/01.cir.101.20.2349. https://doi.org/10.1161/01.CIR.101.20.2349 . PMid:10821809. [DOI] [PubMed] [Google Scholar]


