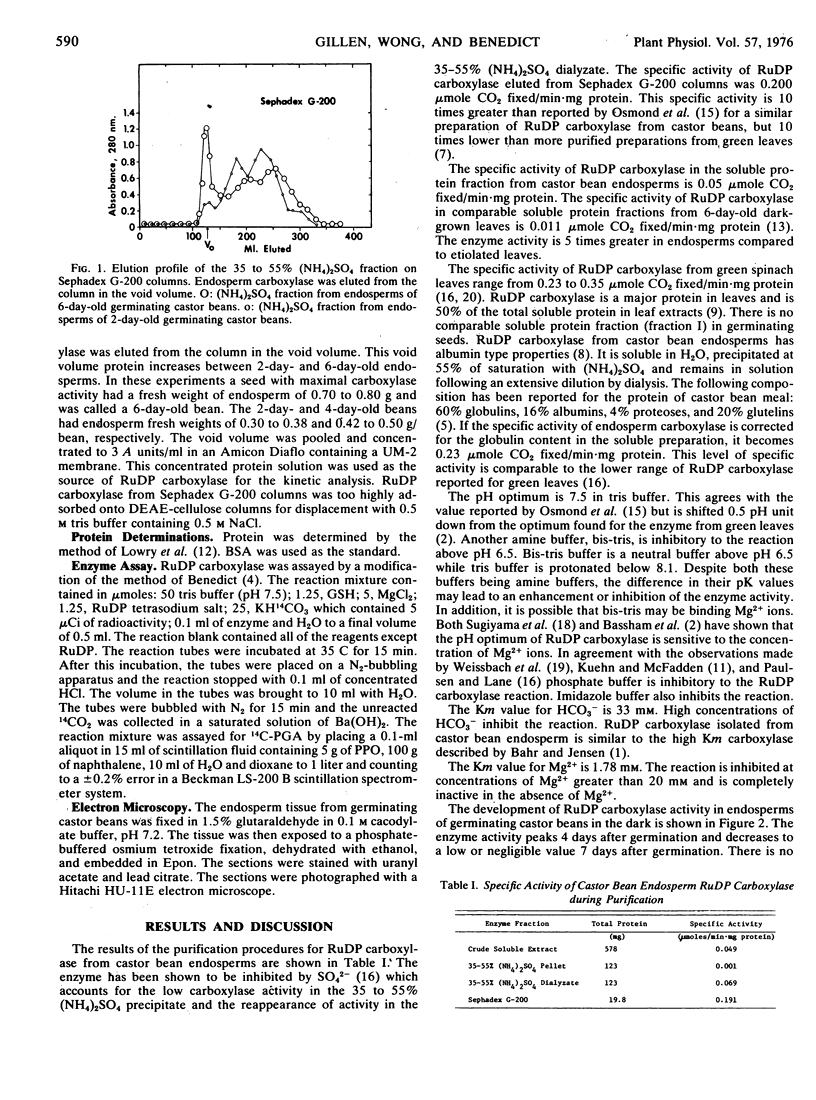
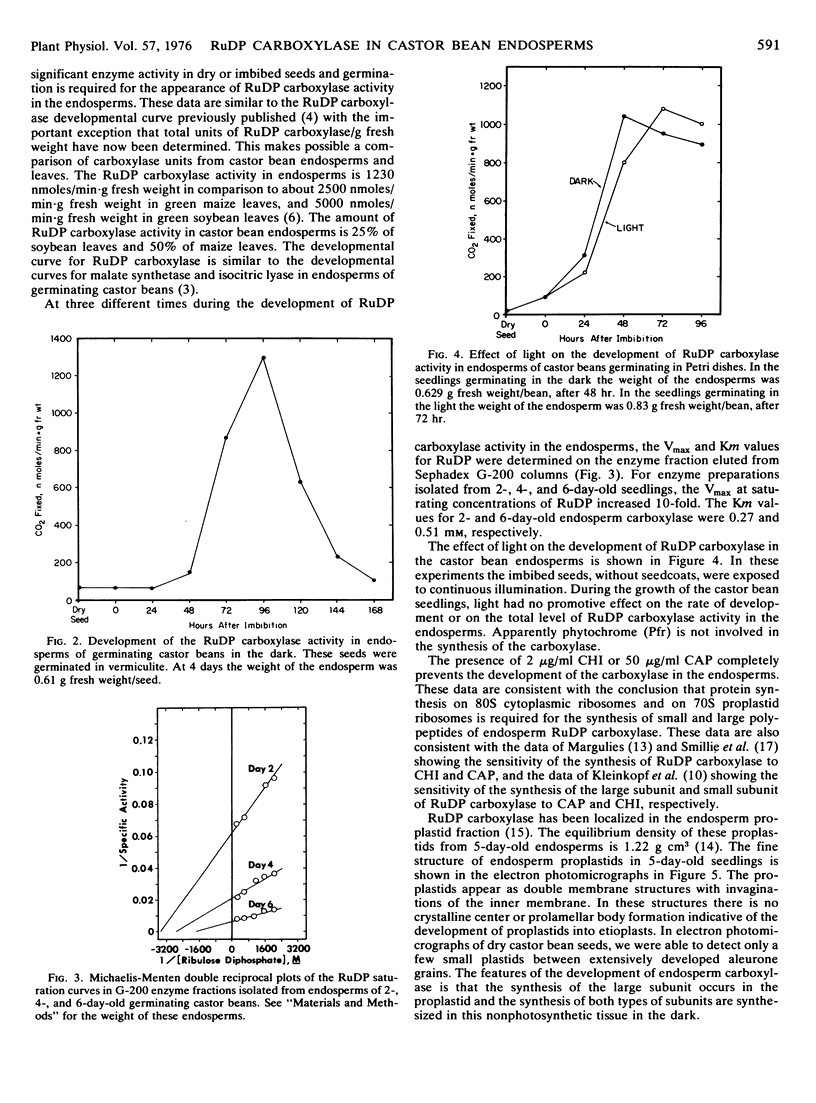
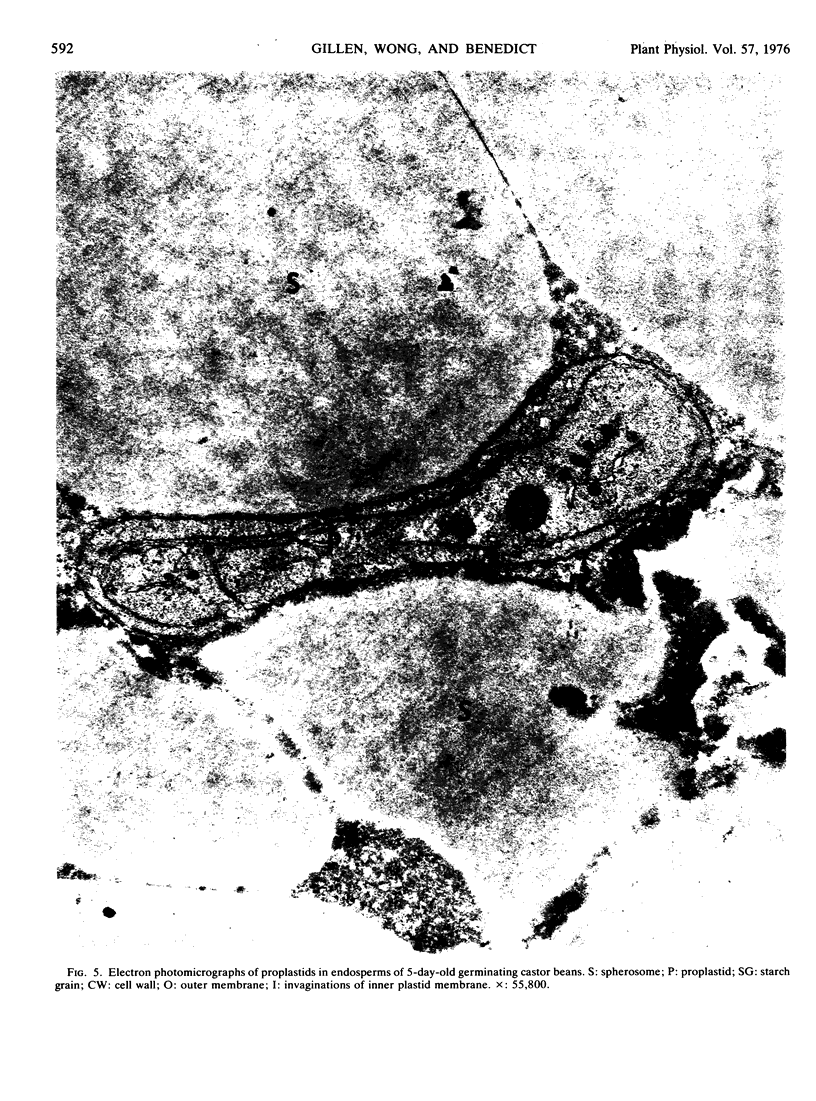
Abstract
Ribulose 1,5-diphosphate (RuDP) carboxylase has been partially purified from dark-grown nonphotosynthetic endosperms of germinating castor beans (Ricinus communis var. Hale). The Km values for RuDP, HCO3−, and Mg2+ are 0.51, 33, and 1.78 mm, respectively. The pH optimum for the carboxylation reaction is pH 7.5. Germination is required for the development of the carboxylase in the endosperms. The enzyme reaches a maximal activity in 4- to 5-day-old dark-grown seedlings (which have an endosperm weight of approximately 0.75 g fresh weight/bean) and then declines. Total endosperm carboxylase activity is 1230 nmoles/min·g fresh weight which is 25 and 50% of the total activity developed in soybean and maize leaves, respectively. Specific activity of the carboxylase in crude soluble endosperm preparations (which contain enzymic and storage protein) is 0.05 μmole/min·mg protein. This is 5 times greater than the specific activity of RuDP carboxylase in soluble preparations from etiolated leaves. During germination the Vmax of the endosperm carboxylase for RuDP increases 10-fold. Development of the enzyme is inhibited 90% by the exposure of the endosperm to 2 μg/ml cycloheximide or 50 μg/ml chloramphenicol. Light (or phytochrome Pfr) is not required for the synthesis of the enzyme. Electron photomicrographs of dark-grown endosperm cells (with peak RuDP carboxylase activity) show proplastids with several invaginations of the inner membrane but no prolamellar-like structures.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahr J. T., Jensen R. G. Ribulose Diphosphate Carboxylase from Freshly Ruptured Spinach Chloroplasts Having an in Vivo Km[CO(2)]. Plant Physiol. 1974 Jan;53(1):39–44. doi: 10.1104/pp.53.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassham J. A., Sharp P., Morris I. The effect of Mg2+ concentration on the pH optimum and Michaelis constants of the spinach chloroplast ribulosediphosphate carboxylase (carboxydismutase). Biochim Biophys Acta. 1968 May 28;153(4):898–900. doi: 10.1016/0005-2728(68)90019-4. [DOI] [PubMed] [Google Scholar]
- Beevers H. Glyoxysomes of castor bean endosperm and their relation to gluconeogenesis. Ann N Y Acad Sci. 1969 Dec 19;168(2):313–324. doi: 10.1111/j.1749-6632.1969.tb43118.x. [DOI] [PubMed] [Google Scholar]
- Kleinkopf G. E., Huffaker R. C., Matheson A. Light-induced de Novo Synthesis of Ribulose 1,5-Diphosphate Carboxylase in Greening Leaves of Barley. Plant Physiol. 1970 Sep;46(3):416–418. doi: 10.1104/pp.46.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn G. D., McFadden B. A. Ribulose 1,5-diphosphate carboxylase from Hydrogenomonas eutropha and Hydrogenomonas facilis. I. Purification, metallic ion requirements, inhibition, and kinetic constants. Biochemistry. 1969 Jun;8(6):2394–2402. doi: 10.1021/bi00834a021. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Margulies M. M. Effect of Chloramphenicol on Light-Dependent Synthesis of Proteins and Enzymes of Leaves and Chloroplasts of Phaseolus vulgaris. Plant Physiol. 1964 Jul;39(4):579–585. doi: 10.1104/pp.39.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miflin B. J., Beevers H. Isolation of intact plastids from a range of plant tissues. Plant Physiol. 1974 Jun;53(6):870–874. doi: 10.1104/pp.53.6.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond C. B., Akazawa T., Beevers H. Localization and properties of ribulose diphosphate carboxylase from castor bean endosperm. Plant Physiol. 1975 Feb;55(2):226–230. doi: 10.1104/pp.55.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen J. M., Lane M. D. Spinach ribulose diphosphate carboxylase. I. Purification and properties of the enzyme. Biochemistry. 1966 Jul;5(7):2350–2357. doi: 10.1021/bi00871a025. [DOI] [PubMed] [Google Scholar]
- Smillie R. M., Graham D., Dwyer M. R., Grieve A., Tobin N. F. Evidence for the synthesis in vivo of proteins of the Calvin cycle and of the photosynthetic electron-transfer pathway on chloroplast ribosomes. Biochem Biophys Res Commun. 1967 Aug 23;28(4):604–610. doi: 10.1016/0006-291x(67)90356-7. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Nakayama N., Akazawa T. Structure and function of chloroplast proteins. V. Homotropic effect of bicarbonate in RuDP carboxylase reaction and the mechanism of activation by magnesium ions. Arch Biochem Biophys. 1968 Sep 10;126(3):737–745. doi: 10.1016/0003-9861(68)90465-7. [DOI] [PubMed] [Google Scholar]
- WEISSBACH A., HORECKER B. L., HURWITZ J. The enzymatic formation of phosphoglyceric acid from ribulose diphosphate and carbon dioxide. J Biol Chem. 1956 Feb;218(2):795–810. [PubMed] [Google Scholar]