Abstract
We evaluated the effect of dimethyl fumarate (DMF) treatment on B‐cell memory and cytokine production in 18 patients with relapsing remitting multiple sclerosis (RRMS) using peripheral blood mononuclear cells obtained prior to and at 6 months post‐DMF initiation. We noted a decline in the absolute B‐cell number with DMF treatment, with a preferential depletion of memory B cells and a concurrent increase in naïve B cells. We noted significant reductions in GM‐CSF, TNF‐α, and IL‐6 producing B cells with DMF treatment. These effects on the B‐cell compartment may underlie the beneficial effects of DMF in RRMS.
Introduction
Dimethyl fumarate (DMF) is a commonly used disease‐modifying therapy for patients with relapsing remitting multiple sclerosis (RRMS). In pivotal phase 3 trials, this medication produced reductions in the annualized relapse rate and reduced the occurrence of new MRI disease activity.1 The immunological effects of DMF in RRMS are gradually being defined and studies have demonstrated changes in various T‐cell subsets and T‐cell cytokine production.2, 3 The importance of B cells in MS pathophysiology is now well recognized. B‐cell depletion is an effective therapy for MS and the efficacy of this approach appears to be related to its effects on the antigen presenting and cytokine‐producing functions of B cells.4 The effects of DMF on B‐cell function in RRMS patients have not been well characterized, with two small studies evaluating the longitudinal effects on B cells.5, 6
Our study utilized a prospectively collected longitudinal cohort to study the immunological effects of DMF treatment. We demonstrate the preferential depletion of memory B cells with DMF treatment and a reduction in B cells producing pro‐inflammatory cytokines including granulocyte macrophage colony‐stimulating factor (GM‐CSF), tumor necrosis factor ‐α (TNF‐α), and interleukin‐6 (IL‐6).
Methods
Participants, Standard protocol approvals, and Patient consents
We enrolled patients with RRMS, who were initiating DMF, from the Johns Hopkins MS Center. The protocol for this study was approved by the Johns Hopkins Institutional Review Board. All participants provided informed consent prior to enrollment. Participants underwent phlebotomy at baseline and at 6 months postinitiation of DMF. Additionally, demographic and disease‐related information was collected from all participants.
Isolation of Peripheral blood mononuclear cells (PBMCs)
Blood obtained at each visit was processed using a standard protocol and PBMCs were obtained by density gradient separation. These were cryopreserved until the end of the study.
B‐cell culture and flow cytometry
PBMCs were thawed and stained for viability (Live/Dead Aqua, Life Technologies) followed by surface markers. For intracellular cytokine detection, PBMCs were thawed and cultured in AIMV media (Life Technologies) overnight. The next morning, cells were stimulated with 25 ng/mL PMA, 475 ng/mL ionomycin, and BD GolgiStop (BD Biosciences) for 5 h at 37°C. This protocol is similar to a previously described strategy to identify B‐cell cytokine production.7 Following stimulation, cells were stained for viability (Viobility Green, Miltenyi), Fc blocked, stained for surface markers, fixed and permeabilized using IC fixation buffer (eBioscience), then stained for intracellular cytokines. Following staining, cells were analyzed on a MACSQuant flow cytometer (Miltenyi). Fluorescence minus one staining controls were used to determine gating. Data analysis was performed using FlowLogic (Inivai Technologies). Antibodies used included CD20, GM‐CSF, CD19, CD27, TNFα, CD38, IgD, CD45, CD24, CD16/32 (Biolegend), IL‐6, and IL‐10 (BD Biosciences). The gating strategy for various B‐cell populations is shown in Figure S1
Statistical analysis
We compared the baseline and end‐of‐study values for the various immune cell populations using a Wilcoxon signed‐rank test. We utilized a nonparametric test since some variables were not normally distributed (based on Shapiro‐Wilk test). We considered a P‐value of <0.05 as significant. We also performed sensitivity analyses excluding two participants who received intravenous steroids within 1 month of baseline visit.
Results
We enrolled 18 RRMS patients in the study. The demographic and disease characteristics are summarized in Table 1. The majority of the patients were either treatment naive or treated with an injectable disease‐modifying therapy prior to DMF initiation. Two patients received intravenous steroids within a month prior to study initiation. One participant discontinued DMF, more than 1 month prior to the end‐of‐study visit, and was excluded from our analyses.
Table 1.
Demographic and disease characteristics of participants
| Characteristic | RRMS |
|---|---|
| Number | 18 |
| Age, years (mean ± SD) | 43.9 ± 10.8 |
| Female sex, n (%) | 13 (72.2) |
| EDSS, median (IQR) | 2.0 (1.5) |
| Disease duration, years (mean ± SD) | 9.8 ± 6.2 |
| Previous treatment | |
| None | 6 |
| Glatiramer acetate | 5 |
| Interferon beta | 5 |
| Natalizumab | 2 |
DMF treatment reduced the absolute number of B cells in RRMS patients
DMF treatment resulted in a significant drop in absolute lymphocyte counts (2036 cells/mm3 vs. 1255 cells/mm3, P = 0.002), and while the proportion of lymphocytes that were B cells was not altered (P = 0.93); the absolute B‐cell counts decreased with DMF treatment (272 cells/mm3 vs. 151 cells/mm3, P = 0.005). This is in keeping with previous reports of reductions in total B‐cell numbers with DMF treatment.6
DMF treatment reduced the proportion of memory B cells and increased the proportion of naïve B cells
As demonstrated in Figure 1, DMF treatment led to a reduction in the proportion of CD27+ memory B cells (39.3%, P = 0.003) and a concomitant increase in the proportion of CD27− IgD+ naïve B cells (12.2%, P = 0.001) in RRMS patients (Table 2). This suggests that within the B‐cell compartment, DMF preferentially targets the memory population; and this was true for both CD27+ IgD− class‐switched and CD27+ IgD+ nonclass‐switched memory B cells (Table 2).
Figure 1.
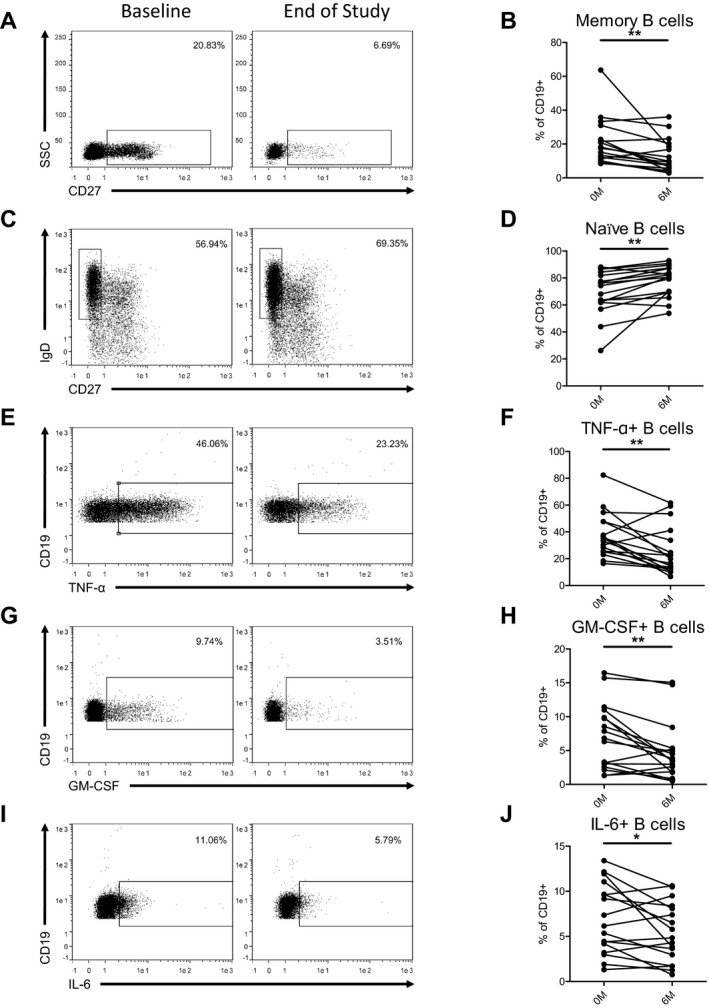
DMF treatment alters B‐cell memory subsets and reduces proinflammatory cytokine‐producing B cells. PBMCs isolated from RRMS patients (baseline and after 6 months of dimethyl fumarate treatment) were cryopreserved at the time of collection. PBMCs were thawed and then either stained for viability and surface markers, or cultured overnight then restimulated with PMA/Ionomycin/BD GolgiStop for 5 h before being stained for viability, Fc blocked, and stained for cytokine production. B cells were defined as singlet viable CD19+ lymphocytes, and this gated population of cells is shown in the representative flow cytometry plots (A, C, E, G, I). Line graphs with each line representing an individual participant depict the changes in B‐cell subpopulations (B, D, F, H, J). RRMS patients treated with dimethyl fumarate had a significant decrease in memory B cells (CD27+) (A, B) and an increase in naïve B cells (CD27− IgD+) (C, D). Dimethyl fumarate treatment also led to a significant decrease in TNF‐α (E, F), GM‐CSF (G, H), and IL‐6 (I, J) producing B cells. Wilcoxon signed‐rank tests were utilized to compare data from the two study time‐points. *P < 0.05, **P < 0.01.
Table 2.
Change in B‐cell populations in RRMS patients following DMF treatment
| Population | RRMS group | P‐value | |
|---|---|---|---|
| Absolute change mean (95% CI) [mean (95% CI)] | Relative change mean (95% CI) [mean (95% CI)] | ||
| Naïve B cells | 8.6 (3.1, 14.1) | 12.2 (4.4, 20.1) | 0.001 |
| Memory B cells | −7.9 (−13.7, −2.1) | −39.3 (−68.2, −10.4) | 0.003 |
| Class‐switched memory B cells | −4.4 (−8.6, −0.2) | −35.5 (−69.4, −1.6) | 0.019 |
| Nonclass‐switched memory B cells | −3.5 (−5.7, −1.2) | −45.5 (−74.0, −15.6) | 0.002 |
| Transitional B cells | 2.9 (−2.1, 7.8) | 34.5 (−25.0, 92.8) | 0.44 |
| B‐1 cells | −0.5 (−1.1, 0.2) | −24.1 (−56.4, 10.2) | 0.17 |
| Plasmablasts | −0.1 (−2.8, 2.6) | −2.3 (−63.2, 58.7) | 0.94 |
| GM‐CSF+ B cells | −2.4 (−3.8, −0.9) | −35.3 (−55.9, −13.2) | 0.004 |
| TNF‐α+ B cells | −11.8 (−19.4, −4.3) | −32.1 (−52.7, −11.7) | 0.007 |
| IL‐6+ B cells | −1.6 (−2.9, −0.17) | −23.9 (−43.3, −2.5) | 0.044 |
| IL‐10+ B cells | 0.01 (−0.15, 0.17) | 2.5 (−26.5, 30.0) | 0.58 |
We also examined the change in proportion of Transitional B cells (CD24high CD38high), B‐1 cells (CD43+ CD27+), and plasmablasts (CD19+ CD24− CD38high) with DMF treatment and noted no significant change in these populations (Table 2).
DMF treatment reduced the proportion of B cells producing pro‐inflammatory cytokines (GM‐CSF, TNF‐α, and IL‐6)
We then examined the production of proinflammatory cytokines by B cells using intracellular staining and flow cytometry. We noted reductions in the proportion of B cells producing proinflammatory cytokines GM‐CSF (35.3%, P = 0.004), TNF‐α (32.1%, P = 0.007), and IL‐6 (23.9%, P = 0.044) (Fig. 1, Table 2). Increased production of these cytokines by B cells has been previously noted in MS.7, 8 We observed no change in the proportion of B cells producing the anti‐inflammatory cytokine IL‐10 (P = 0.58) (Table 2). Previous studies have demonstrated that short stimulation protocols only identify a subset of B cells that produce IL‐10,9 and hence we also quantified transitional B cell and B‐1 cell populations which are thought to have regulatory properties related to IL‐10 production.10, 11 Neither of these populations changed significantly with DMF treatment (Table 2).
Exclusion of the two patients who received steroids within a month of the baseline visit from the analyses did not significantly change any of the presented results.
Discussion
This study utilized a prospectively collected cohort of RRMS patients to demonstrate that DMF treatment leads to a preferential depletion of memory B cells and reduces the proportion of GM‐CSF, IL‐6, and TNF‐α producing B cells. These changes in pathogenic B cell subsets are potentially a part of the immunological mechanism of action of DMF.
We noted a reduction in the absolute number of B cells with DMF treatment, in keeping with previous studies.6 However, within the B‐cell compartment, we noted a preferential depletion of memory B cells which is consistent with the results of a recent study.5 Memory B cells are thought to be pathogenic in MS and previous studies have noted the production of proinflammatory cytokines such as GM‐CSF and TNF‐α predominantly from memory B cells, while anti‐inflammatory cytokines such as IL‐10 are derived mainly from the naïve B‐cell population.12 Memory B cells are also more efficient antigen presenting cells and provide help to T cells involved in autoimmunity. Other disease‐modifying therapies have also been noted to selectively target the memory B‐cell population.13 Depletion of memory B cells by DMF would thus be expected to have beneficial effects on inflammatory disease activity in patients with MS.
The mechanism for this selective targeting is unknown, but may be related to the modification of reactive cysteine residues by DMF in various proteins involved in immune cell function.14 Several of these proteins identified in T cells, including NF‐kB and interferon regulatory factor 4 (IRF‐4), are also important in B‐cell maturation and could explain the preferential reduction in the B‐cell memory population.15
We also demonstrate striking effects of DMF on B‐cell cytokine production. We noted a reduction in GM‐CSF‐producing B cells with DMF treatment. This subset of B cells provides proinflammatory signals to myeloid cells and is enriched in RRMS patients.7 The efficacy of B‐cell depletion appears to be related to the depletion of this particular B‐cell subset. Additionally, other B‐cell subsets that declined with DMF treatment included IL‐6‐producing and TNF‐α‐producing B cells. Both these subsets of B cells have been implicated in MS disease pathogenesis.4 B cell IL‐6 production can lead to increased differentiation of Th17 cells which are important players in MS pathogenesis.4 Similarly, TNF‐α production from B cells also promotes inflammatory T‐cell responses.4 Thus, the reduced production of these proinflammatory cytokines from the B‐cell compartment, could lead to a reduction in myeloid and T‐cell inflammatory responses.
DMF treatment did not change the proportion of IL‐10‐producing B cells. Our protocol for assessing cytokine production may not identify a subset of B cells capable of producing IL‐10 that require more prolonged stimulation in vitro, 9 however, we also quantified other B‐cell subtypes (transitional B cells and B‐1 cells) that are associated with regulatory function and noted no significant change in these subtypes.10, 11 The lack of change in transitional B cells and B‐1 cells could be due to the short duration of this study, since another study noted changes in these B‐cell subsets primarily after 12 months of DMF therapy.6 The lack of a reduction in IL‐10‐producing B cells in combination with the decline in proinflammatory cytokine‐producing B cells, could shift the balance between these cell populations resulting in an anti‐inflammatory state. Our findings are in concordance with those from a recent study that noted an anti‐inflammatory shift in B cells in RRMS patients with DMF treatment.5
DMF treatment produced multiple changes in the B‐cell compartment that target B‐cell functions related to MS pathogenesis; these changes may underlie the beneficial effects of DMF on MS disease activity.
Author contributions
MDS performed the experiments and wrote the manuscript. KAM performed experiments and provided critical feedback on the manuscript, PAC conceived the study, obtained funding, provided supervision, designed the study and provided critical feedback on the manuscript. PB designed the study, collected samples, performed statistical analysis, and wrote the manuscript.
Conflicts of Interest
MDS reports no disclosures. KAM reports no disclosures. PAC reports grants from Biogen, during conduct of the study; grants from Teva, grants from Novartis, grants from MedImmune, personal fees from Vertex, and grants and personal fees from Biogen, outside the submitted work. PB reports no disclosures.
Supporting information
Figure S1. Flow cytometry gating strategy.
References
- 1. Fox RJ, Kita M, Cohan SL, et al. BG‐12 (dimethyl fumarate): a review of mechanism of action, efficacy, and safety. Curr Med Res Opin 2013;1–12. [DOI] [PubMed] [Google Scholar]
- 2. Spencer CM, Crabtree‐Hartman EC, Lehmann‐Horn K, et al. Reduction of CD8(+) T lymphocytes in multiple sclerosis patients treated with dimethyl fumarate. Neurol Neuroimmunol Neuroinflamm 2015;2:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Longbrake EE, Ramsbottom MJ, Cantoni C, et al. Dimethyl fumarate selectively reduces memory T cells in multiple sclerosis patients. Mult Scler 2016;22:1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li R, Rezk A, Healy LM, et al. Cytokine‐defined b cell responses as therapeutic targets in multiple sclerosis. Front Immunol 2016;6:626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li R, Rezk A, Ghadiri M, et al. Dimethyl fumarate treatment mediates an anti‐inflammatory shift in B cell subsets of patients with multiple sclerosis. J Immunol 2017;198:691–698. [DOI] [PubMed] [Google Scholar]
- 6. Lundy SK, Wu Q, Wang Q, et al. Dimethyl fumarate treatment of relapsing‐remitting multiple sclerosis influences B‐cell subsets. Neurol Neuroimmunol Neuroinflamm 2016;3:e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li R, Rezk A, Miyazaki Y, et al. Proinflammatory GM‐CSF‐producing B cells in multiple sclerosis and B cell depletion therapy. Sci Transl Med 2015;7:310ra166–310ra166. [DOI] [PubMed] [Google Scholar]
- 8. Barr TA, Shen P, Brown S, et al. B cell depletion therapy ameliorates autoimmune disease through ablation of IL‐6‐producing B cells. J Exp Med 2012;209:1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Iwata Y, Matsushita T, Horikawa M, et al. Characterization of a rare IL‐10–competent B‐cell subset in humans that parallels mouse regulatory B10 cells. Blood 2011;117:530–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blair PA, Noreña LY, Flores‐Borja F, et al. CD19+ CD24hiCD38hi B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity 2010;32:129–140. [DOI] [PubMed] [Google Scholar]
- 11. Griffin DO, Rothstein TL. Human "orchestrator&;quot; CD11b(+) B1 cells spontaneously secrete interleukin‐10 and regulate T‐cell activity. Mol Med 2012;18:1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duddy M, Niino M, Adatia F, et al. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol 2007;178:6092–6099. [DOI] [PubMed] [Google Scholar]
- 13. Rizzo F, Giacomini E, Mechelli R, et al. Interferon‐β therapy specifically reduces pathogenic memory B cells in multiple sclerosis patients by inducing a FAS‐mediated apoptosis. Immunol Cell Biol 2016;94:886–894. [DOI] [PubMed] [Google Scholar]
- 14. Blewett MM, Xie J, Zaro BW, et al. Chemical proteomic map of dimethyl fumarate‐sensitive cysteines in primary human T cells. Sci Signal 2016;9:rs10–rs10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nam S, Lim J‐S. Essential role of interferon regulatory factor 4 (IRF4) in immune cell development. Arch Pharm Res 2016;39:1548–1555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow cytometry gating strategy.


