Abstract
Objective
Amyotrophic Lateral Sclerosis (ALS) is a clinically heterogeneous neurodegenerative disorder associated with cognitive and behavioral impairment. The primary aim of this study was to identify behavioral subphenotypes in ALS using a custom designed behavioral assessment tool (Beaumont Behavioural Inventory, BBI). Secondary aims were to (1) investigate the predictive nature of cognitive assessment on behavioral change, (2) report the behavioral profile associated with the C9orf72 expansion, (3) categorize behavioral change through disease staging, and (4) to investigate the relationship between cross‐sectional behavioral classification and survival.
Methods
A cross‐sectional population‐based research design was applied to examine behavioral data from ALS patients (n = 317) and healthy controls (n = 66). Patients were screened for the C9orf72 repeat expansion. A subcohort of ALS patients completed an extensive cognitive assessment battery (n = 65), to investigate predictors of behavior change. Principal component analysis (PCA) determined factors associated with altered behavior. Survival data were extracted from the Irish ALS register.
Results
No behavioral changes were reported in 180 patients (57%); 95 patients had mild‐moderate behavioral change (30%); 42 patients met the cut‐off for Clinically Severe Behavioral Change (13%), suggestive of a bvFTD diagnosis. The most frequently endorsed behaviors in ALS were reduced concern for hygiene (36.8%), irritability (36.2%), new unusual habits (33.4%), and increased apathy (31.1%). Five independent factors were identified through factor analysis. Social cognitive performance was predictive of behavior change (P = 0.031), yielding an R 2 = 0.188. Behavioral categorization (mild/moderate/severe) at the time of assessment was not associated with survival (P = 0.198).
Interpretation
These data imply the presence of distinct subphenotypes of behavioral change in ALS, which most likely reflect subcategories of extramotor network disruption.
Introduction
Amyotrophic Lateral Sclerosis (ALS) is a clinically and genetically heterogeneous neurodegenerative disorder, of which cognitive and behavior impairment are core features.1, 2, 3, 4, 5, 6 Executive dysfunction is present in up to 50% of ALS patients, alongside behavioral features associated with frontotemporal dementia (FTD).7, 8 Approximately 13–15% of ALS patients presenting with severe behavior change meet consensus criteria for behavioral variant frontotemporal dementia (bvFTD),9 which negatively impacts on patient survival.7
Behavior change is underreported within the ALS literature,8, 9 as studies often lack a robust methodological design to infer generalizable results.10, 11, 12, 13 A recent systematic review of 21 studies found that mild to moderate behavioral changes are present in 17%–88% of ALS patients without overt dementia.4 The most commonly reported behavior changes in ALS patients are apathy, which is present in up to 60% of patients,12, 14 followed by disinhibition15 and irritability. Direct comparisons of ALS and bvFTD patients suggest that behavioral symptoms are significantly less frequent in ALS,16 though changes in behavior are qualitatively similar between the two groups.15 However, significant correlations between executive dysfunction and behavioral change have been found12, 13 and comorbidity of symptoms is reported.9, 17, 18 Behavior changes are also shown to occur independently of cognitive dysfunction.19
The anatomical degeneration of cortical regions associated with behavioral regulation has been documented in ALS studies20, 21 and have included orbitofrontal, anterior cingulate,19 and prefrontal cortical regions.22 Extensive extramotor pathology has also been demonstrated in ALS cohorts with no detectable behavioral or cognitive change, which most likely reflects presymptomatic gray matter alterations.23 Multi‐parametric imaging studies of the C9orf72 genotype demonstrate orbitofrontal, Broca's area, and cingulate pathology, as well as marked white matter degeneration in the genu of the corpus callosum.24 Correlative imaging studies of behavioral change in ALS demonstrated that apathy scores correlate with bilateral orbitofrontal cortex atrophy, decreased frontal fractional anisotropy,21 and accumbens nucleus atrophy.25 Notwithstanding, there have been few population‐based studies of sufficient magnitude to definitively subcategorize behavioral subphenotypes in ALS, and to determine whether severity of behavioral change affect clinical outcome.
The aims of this study were to examine behavioral change in a large population‐based cohort of ALS patients using a recently validated behavioral instrument26, to determine whether distinct subphenotypes of behavioral change can be discerned based on known patterns of network disruption, to consider the cognitive predictors of behavioral change, to investigate whether behavioral change correlate with clinical parameters of disease, and to examine the impact of behavioral change on survival.
Material and Methods
Participants
Recruitment of ALS patients (n = 317) and healthy controls (n = 66) was undertaken using both clinic‐based recruitment, and through the Irish ALS Register.27 Recruitment protocols have been described previously in detail.6 A subcohort of patients underwent neuropsychological assessment outlined in detail in Table 1. All patients were tested within 7.66 ± 10.54 months of diagnosis, and 22.09 ± 16.07 months of first symptom (See Table 2 for further details). Clinical stage was estimated using the King's Staging Criteria.28, 29 Matched healthy controls were recruited as part of a volunteer network (n = 66). Inclusion criterion included possible, probable or definite ALS based on the revised El Escorial Criteria30 with supported neurophysiology. Exclusion criteria included neurological conditions associated with cognitive or behavioral change, and/or high dose psychotropic medications. Patients with a diagnosis of ALS‐FTD were included; however, FTD patients without overt symptomatology of ALS were not included in this study. All but 9 of 317 patients were screened for the C9orf72 expansion.
Table 1.
Details of the neuropsychological battery
| Executive dysfunction | Impairment that is two SD below the mean for healthy controls on at least two executive tasks |
| Stroop colour‐word Test | (a) Priming trial: patients presented with a multi‐colored list of color names and asked to read as many words as they can in 2 min. (b) Inhibitory trial: a similar list is presented but color names (e.g., “blue”) are printed in an ink color not denoted by the name (e.g., red). Correct responses in two minutes are recorded |
| Nonexecutive factors (including bulbar disability) contribute equally to both trials. The difference in scores between the two trials represent the number of responses “lost” due to the delay imposed by the extra executive demands in the inhibitory trial | |
| Brixton spatial anticipation test | A rule attainment‐based task whereby the rule in operation cannot be identified by any perceptually salient aspect of the stimuli. It consists of pages showing the same basic array of 10 circles set in two rows of five, with each circle numbered from 1 to 10. The changes in position are governed by a series of simple rules, which vary without warning. |
| Backward digit span | A measure of auditory attention and working memory. This test is composed of trials where examinees are read strings of digits and are asked to repeat them back aloud. The length of the digit strings is incrementally increased over successive trials |
| Category fluency | Patients were asked to name as many animals as they could think of in 1 min (spoken only) |
| Phonemic verbal fluency | Written/spoken, number of words starting with letter “S” generated in 5 min and number of four letter words starting with letter “C” generated in 4 min. Verbal Fluency Index used to adjust for disability |
| Memory dysfunction | Impairment that is two SD below the mean for healthy controls on at least four* of the parameters highlighted using + |
| Logical memory (LM) | +LM1 (immediate recall), +LM2 (delayed recall) and +LM retention (retention) |
| Verbal paired associate (VPA) | +VPA1 (immediate recall), +VPA2 (delayed recall) and +VPA retention (retention) |
| Auditory Delayed Recognition Task | +Sum of total recognition scores on Logical Memory and Verbal Paired Associates |
| California verbal learning tests | +Total of five trials, +short delay free recall (immediate recall) +long delay free recall (delayed recall) |
| Rey‐Osterrieth complex figure test | Nonverbal memory: parameters used: +immediate and +delayed recall trials |
| Language dysfunction | Impairment that is two SD below the mean for healthy controls on this task |
| Boston naming test | |
| Visuospatial dysfunction | Impairment that is two SD below the mean for healthy controls on copy trial of this task |
| Rey‐Osterrieth Complex Figure Test |
Table 2.
Baseline demographics of participants Mean ± SD
| Demographic variable | Healthy controls (N = 66) | ALS (N = 317) | P |
|---|---|---|---|
| Age at assessment | 61.39 ± 13.67 | 63.00 ± 11.13 | 0.333 |
| Males % | 45% | 51% | 0.023 |
| Years of Education | 13.51 ± 3.76 | 12.91 ± 3.77 | 0.252 |
| Disease onset (%) | |||
| Spinal Onset | – | 56.7 | – |
| Bulbar Onset | – | 23.2 | – |
| Respiratory | – | 2.9 | – |
| Time since diagnosis (months) | – | 7.66 ± 10.54 | – |
| Disease duration (months) | – | 22.09 ± 16.07 | – |
| Total ALSFRS‐R (±SD) | – | 35.17 ± 7.88 | |
| Bulbar total | – | 9.42 ± 2.96 | – |
| Spinal total | – | 15.23 ± 5.88 | – |
| Respiratory total | – | 10.51 ± 2.40 | – |
| Disease staging (as per King's criteria) | |||
| Stage 1 | – | 21% | – |
| Stage 2 | – | 18% | – |
| Stage 3 | – | 14% | – |
| Stage 4 | – | 47% | – |
All participants provided written consent as per the approved protocol. The work was approved by the Beaumont Hospital Ethics Committee and meets the standards and requirements of the Declaration of Helsinki.
Cognitive, behavioral, and functional assessment
A 41‐item proxy‐report behavioral questionnaire was administered to each participant in this study (The Beaumont Behavioural Inventory: BBI), which was custom designed and validated for use with ALS patients.26 The BBI has high internal consistency (Cronbach's = 0.89), and high sensitivity and specificity (87.9% and 78.8%, respectively) for identifying mild behavior change, and using a cut‐off score of 22.5 can identify ALS‐FTD (90% sensitivity; 96% specificity). The presence of symptoms is graded on a scale of 1 to 3 (mild; moderate; severe). The results are then categorized based on the total score (No Behavioral Dysfunction ≤6; Mild Behavioral Change 7‐22; Severe Behavioral Dysfunction ≥23).
Detailed neuropsychological assessment data were available for a subcohort of participants (n = 65), who were assessed using an extensive battery of standardized neuropsychological measures (Table 1) sensitive to cognitive changes in ALS, as part of a parallel study.6 This cohort underwent cognitive testing as part of a larger study investigating cognitive dysfunction in a population‐based cohort of ALS patients. These patients were recruited using the outlined protocol, and were incident‐based ALS patients.
The revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS‐R) is a clinically validated tool used to document disease progression over time and was utilized in this study to compare disease severity within patient groups.31
Statistical analysis
Considering the large cohort of ALS patients recruited to this study, Cronbach's alpha was determined for the BBI as a means of validation. Cronbach's alpha was previously reported for the BBI at 0.89 as reported above. Group comparisons were undertaken using independent sample t‐test and ANOVA. Multiple comparisons were considered for all variables and analyses where relevant. Logistic regression was used to investigate the value of cognitive scores in predicting behavioral impairment. The predictive value of the model was estimated using the C‐statistic. A linear regression was also employed to investigate the predictive value of cognitive outcome data, and measures of mood. All tests were two‐tailed and alpha was set at 0.05. IBM Statistical Package for the Social Sciences (SPSS) 21.0 was used for analyses.
Factor extraction
With regard to statistical power, n = 100 is the recommended minimum number required to undertake a factor analysis.32 However, five respondents per item has also been suggested.33 Considering these data, a Kaiser‐Meyer‐Olkin (KMO) test yielded P = 0.824, suggesting that this study meets these three criteria for sufficient sample size.
Principal Components Analysis (PCA) has been previously used within the literature on neurodegenerative diseases, that is, Huntington's disease (HD), to examine behavioral clustering and identify factors/principal components of measurement tools.34 PCA examines the interrelationship of variables, and an orthogonal varimax rotation was employed.35 The method of PCA and varimax rotation was adopted for this study, due to the congruency between observed cognitive and behavioral changes within both HD and ALS,36 as the varimax rotation aims to investigate and address the total variance accounted for by each item, rather than the degree to which each factor is correlated. An oblique rotation method, such as direct oblimin (delta=0), differs in that it investigates the correlations between factors. For completeness, an oblique rotation has been performed and is reported within the supplementary material which will identify the degree to which the factor structures correlate. To decide upon the number of emerging factors, Kaiser's criterion for factors with an eigenvalue of >1 was employed, in line with previously published studies investigating behavioral phenotypes in neurodegenerative diseases.37, 38, 39, 40 The generated scree plot associated with Kaiser's criterion is available as supplementary information (Figure S1).
Results
Prevalence of behavioral change
Of the total ALS cohort (N = 317), 180 patients (57%) had no behavioral dysfunction (BBI ≤6), 95 patients (30%) had mild‐moderate behavioral impairment (BBI = 7–22), and 42 patients (13%) had severe behavioral dysfunction (BBI ≥23) as illustrated in Figure 1. Cronbach's alpha was employed to investigate the reliability of the BBI total score. Our findings are consistent with the validation of the BBI,26 with Cronbach's alpha = 0.906.
Figure 1.
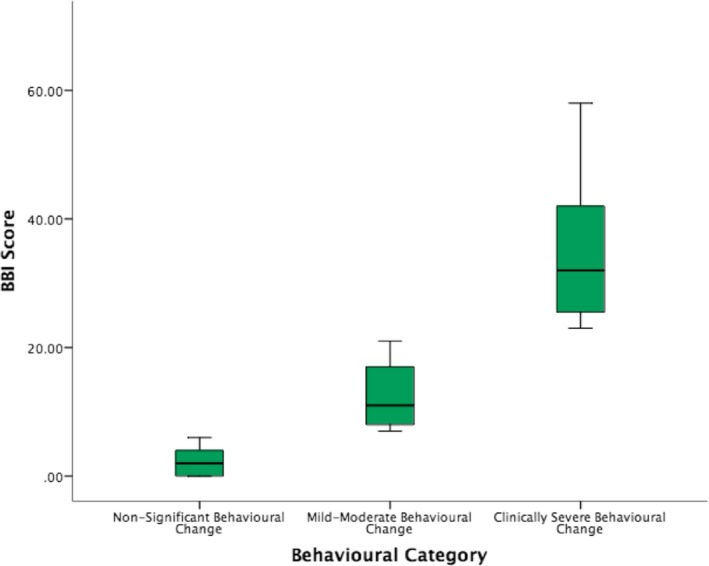
Box‐plots representing the behavioral data stratified by severity as nonsignificant behavioral change (n = 180), mild‐moderate behavioral change (n = 95), and clinically severe behavior change (n = 42).
Bulbar‐onset disease had no effect on overall behavioral impairment (P = 0.643) but was associated with higher rates of emotional lability (56.5% vs. 40.8%, P = 0.002). No significant association between behavioral impairment and gender, site of onset, or disease duration, was observed.
The most frequently described new‐onset behavior in ALS was emotional lability (44%; P < 0.001), followed by reduced concern for hygiene (36.8%; P < 0.001), irritability (36.2%; P = 0.002), new “unusual habits” (33.4%; P < 0.001), and increased apathy (31.1%; P < 0.001). The classification of “unusual habits” was determined by caregivers, who outlined behaviors that were previously uncharacteristic of the person. The remaining behavioral changes can be seen in Table 3, alongside the caregiver endorsement of mild, moderate, and severe change.
Table 3.
Total ALS cohort's behavioral change on BBI (n = 317)
| BBI symptom | No change (%) | Mild (%) | Moderate (%) | Severe (%) |
|---|---|---|---|---|
| Increased irritability | 63.8 | 20.4 | 13.2 | 2.6 |
| Less aware of physical senses | 84.1 | 6.3 | 8.3 | 1.3 |
| Difficulties with conversation | 76.2 | 14.5 | 6.6 | 2.6 |
| Not aware of mistakes | 75 | 14.1 | 7.9 | 3.0 |
| Less reactive to difficulties | 68.9 | 12.9 | 13.6 | 4.6 |
| Acts without thinking | 73 | 10.9 | 8.6 | 7.6 |
| Emotional lability | 56 | 21.5 | 15.6 | 6.8 |
| Lack of appropriate embarrassment | 77.5 | 13.2 | 6.6 | 2.6 |
| Less Concerned about others | 80.3 | 10.5 | 4.3 | 4.9 |
| More selfish behavior | 87.1 | 7.9 | 3.0 | 2.0 |
| New unusual habits | 66.6 | 20.5 | 9.3 | 3.6 |
| More withdrawn | 82.3 | 10.5 | 5.2 | 2.0 |
| Perseverative speech | 81.4 | 13.1 | 4.6 | 1.0 |
| Less concerned about hygiene | 63.2 | 22.4 | 7.9 | 6.6 |
| More aggressive | 83.6 | 9.2 | 5.2 | 2.0 |
| Obsessive Counting | 96.4 | 2.6 | 0.3 | 0.7 |
| Excessively storing food in mouth | 92.8 | 4.6 | 2.3 | 0.3 |
| Eats much more | 89.5 | 6.5 | 3.6 | 0.3 |
| Hyperorality | 97.7 | 1 | 1 | 0.3 |
| Overly sensitive to stimuli | 71.9 | 15.7 | 10.1 | 2.3 |
| New food preferences | 81.8 | 13 | 3.9 | 1.3 |
| Less picky about food | 86.6 | 9.5 | 2.3 | 1.6 |
| Difficulty with everyday language | 92.6 | 2.7 | 3.1 | 1.6 |
| Smokes more | 92.6 | 2.7 | 3.1 | 1.6 |
| Change in sexual interest | 85.9 | 5.2 | 3.3 | 5.6 |
| Sees/Hears Things/People | 97.8 | 0.7 | 0.7 | 0.7 |
| New bizarre beliefs | 95.6 | 1.5 | 2.9 | 0 |
| Repetitious behavior | 82.6 | 9.8 | 4.6 | 3.0 |
| Repeats phrases again and again | 88.8 | 5.3 | 4.0 | 2.0 |
| Dislikes change in routine | 82.5 | 11.4 | 4.2 | 1.9 |
| Seeks social contact | 89.5 | 5.2 | 4.2 | 1 |
| Constantly aligns things | 88.8 | 6.6 | 3.3 | 1.3 |
| Overly concerned with neatness | 95.7 | 2.3 | 2.0 | 0 |
| Constantly checking clocks etc. | 93.1 | 4.6 | 1.6 | 0.7 |
| Shows less emotion | 84.3 | 8.5 | 4.6 | 2.6 |
| Acts inappropriately in public | 94.4 | 2.6 | 2.6 | 0.3 |
| Developed unusual rituals | 99.7 | 0.7 | 0 | 0 |
| Hoards, hides, or collects things | 93.1 | 3.9 | 2.6 | 0.3 |
| Handles things for no reason | 97.1 | 1.6 | 1 | 0.3 |
| More distractible | 77.5 | 13.4 | 4.6 | 4.6 |
| Mimics/repeats words/phrases | 93.1 | 4.0 | 1.3 | 1.7 |
Bold text implies significantly different to controls, at a group level, where P < 0.05. These data were transposed to percentages for ease of viewing after analyses were conducted for significance.
Within the patient cohort, ALS patients without behavioral impairment (n = 180), with mild behavioral impairment (n = 95), and severe behavioral impairment (n = 42) were stratified to investigate the most common types of behavior change. Patients exhibiting mild behavior change were also reported to have emotional lability (57%), apathy (48%), and an altered sensory perception to external stimuli (41.1%), according to their primary caregiver. This cohort further exhibited new‐onset unusual habits (44.7%) and less concern for hygiene (52.1%). Patients with severe behavioral impairment were reported to be more apathetic (87.5%), not as aware of mistakes as before (82.5%), more impulsive (82.9%), and less responsive to others' needs (71.4%). Through the itemization of the scale in Table 4, it can be seen that many of the same new‐onset behaviors are endorsed across all groups, with higher frequency in the severely impaired group.
Table 4.
ALS cohort stratified by behavioral category, displaying % of endorsed items per group
| BBI symptom | No behavior change (n = 180) | Mild‐moderate behavioral change (n = 95) | Clinically severe behavioral change (n = 42) |
|---|---|---|---|
| Increased irritability | 35.7 | 32.6 | 46.3 |
| Less aware of physical senses | 3.6 | 22.3 | 53.8 |
| Difficulties with conversation | 7.7 | 36.8 | 60 |
| Not aware of mistakes | 4.7 | 36.8 | 82.5 |
| Less reactive to difficulties | 8.3 | 48.4 | 87.5 |
| Acts without thinking | 7.7 | 36.8 | 82.9 |
| Emotional lability | 28.7 | 57.4 | 76.2 |
| Lack of appropriate embarrassment | 6.5 | 33 | 65 |
| Less concerned about others | 0.6 | 30.5 | 71.4 |
| More selfish behavior | 1.8 | 19.1 | 43.9 |
| New unusual habits | 19.8 | 44.7 | 66.4 |
| More withdrawn | 3.6 | 21.1 | 68.3 |
| Perseverative speech | 6.5 | 26.3 | 51.2 |
| Less concerned about hygiene | 17.2 | 52.1 | 82.9 |
| More aggressive | 2.4 | 21.1 | 61.9 |
| Obsessive counting | 0 | 3.2 | 20 |
| Excessively storing food in mouth | 0 | 9.5 | 31 |
| Eats much more | 5.9 | 13.7 | 22 |
| Hyperorality | 0.6 | 0 | 14.6 |
| Overly sensitive to stimuli (Noise) | 15.3 | 41.1 | 51.2 |
| New food preferences | 5.3 | 33.7 | 35.7 |
| Less picky about food | 1.8 | 26.3 | 31.7 |
| Difficulty with everyday language | 8.4 | 27.7 | 65.9 |
| Smokes more | 4.9 | 9.4 | 14.3 |
| Change in sexual interest | 7.9 | 17.1 | 33.3 |
| Sees/Hears Things/People | 0 | 3 | 11.8 |
| New bizarre beliefs | 0 | 9.1 | 17.6 |
| Repetitious behavior | 4.1 | 25.5 | 53.7 |
| Repeats phrases again and again | 0 | 12.8 | 53.7 |
| Dislikes change in routine | 4.1 | 25.6 | 54.8 |
| Seeks social contact | 1.2 | 12.6 | 43.9 |
| Constantly aligns things | 0.6 | 13.4 | 48.8 |
| Overly concerned with neatness | 0.6 | 4.3 | 20 |
| Constantly checking clocks etc. | 0.6 | 8.5 | 29.3 |
| Shows less emotion | 1.2 | 22.3 | 61 |
| Acts inappropriately in public | 0 | 4.2 | 31.7 |
| Developed unusual rituals | 0 | 1.1 | 2.6 |
| Hoards, hides, or collects things | 0.6 | 6.3 | 35 |
| Handles things for no reason | 0 | 0 | 22 |
| More distractible | 4.7 | 35.8 | 65.9 |
| Mimics/Repeats Words/Phrases | 0 | 5.3 | 39 |
ALS patients were stratified by King's Staging Criteria. Behavioral reports were significantly different between King's stages (P = 0.016). Post hoc analysis showed, on a group level, that caregivers of patients in Stage 4 reported significantly greater behavioral change in patients, compared to caregivers of patients in Stage 1. The distribution of scores on a group level, stratified by severity of behavioral change can be seen in Figure 2.
Figure 2.
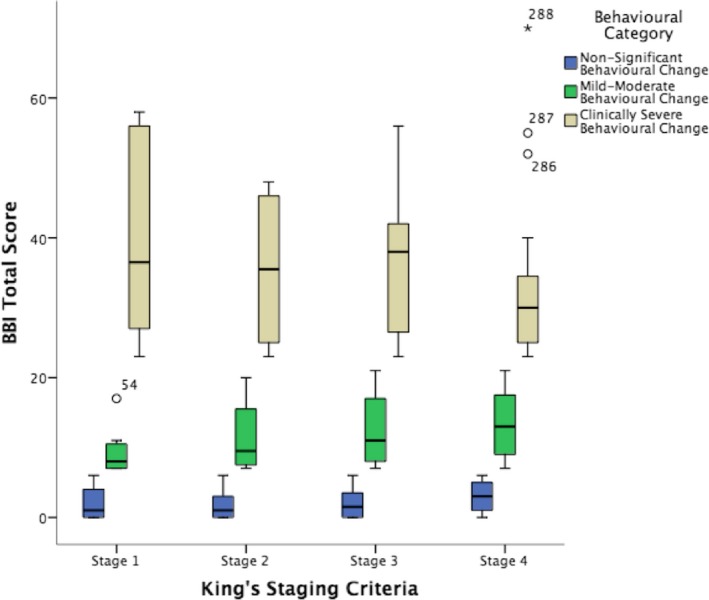
ALS patient behavioral data stratified by King's stage, and trichotomized by behavioral severity. ALS patients were classified by the King's staging criteria as follows: Stage 1, 21%; Stage 2, 18%; Stage 3 14%; Stage 4, 47%. In Stage 1, 77% had no behavioral change (1.84 ± 2.0), 13% were in the mild‐moderate change category (9.37 ± 3.46), and 10% were in the clinically severe range (39.50 ± 14.57); Stage 2, 67% of patients had no behavioral change (1.63 ± 1.93), 24% had mild‐moderate behavioral change (11.41 ± 4.92), and 8% were within the clinically severe range (35.50 ± 12.34); Stage 3 patients recorded mostly no behavioral change (50%: 2.1 ± 2.22), with 32% in the mild‐moderate range (12.38 ± 4.83), and 18% (36.28 ± 11.78) in the clinically severe range; Stage 4, 46% of the cohort has no behavioral change (2.93 ± 2.09), 40% exhibited mild‐moderate behavioral change (13.34 ± 4.48), with 14% within the clinically severe range (33.57 ± 12.64).
Cognitive function as a predictor of behavior change
A subcohort of ALS patients underwent detailed cognitive evaluation (n = 65) as part of a parallel study investigating ALS patients' cognitive function. All patients enrolled within the cognitive study, which was population‐based, who also completed the BBI were considered for this study. Twenty‐four patients had evidence of single‐ or multi‐domain cognitive impairment (37%). Of those who were cognitively intact (n = 41), 30% had evidence of mild‐moderate behavioral change (n = 12), with 7% reported to have severe behavioral impairment (n = 3). Executive dysfunction was more likely to be an evidence in those with behavioral impairment (P = 0.001). Spearman correlation analyses revealed that patients exhibiting severe behavioral impairment performed poorly on the Stroop Colour‐Word Interference Task (r = −0.549, P < 0.0001), VFI (r = 0.504, P < 0.0001) and, to a lesser extent, the Brixton Spatial Anticipation task (r = −0.300, P = 0.036). The Brixton Spatial Anticipation task did not meet the more stringent cut‐off when controlling for multiple comparisons within the cognitive data. Table S1 reports cognitive outcome data for this subcohort in greater detail, inclusive of significant and nonsignificant findings in relation to behavioral change.
Severe behavioral impairment was significantly correlated with poor scores on The Reading the Mind in the Eye Test (RMET), which is a task of social cognition (r = −0.453, P = 0.002). A binary logistic regression model defined a predictive value of abnormal performance on measures of executive function, that is, the Stroop task, verbal fluency index, and RMET with the BBI as the outcome variable (P = 0.011, C‐statistic = 0.794). A significant predictor of behavioral dysfunction in this model was impaired performance on the RMET (P = 0.031, odds ratio = 8.8, 95% CI: 1.2–63.7). A linear regression model was then created to include cognitive outcome data, and the Hospital Anxiety and Depression Scale (HADS: Table S1). The only significant predictor within the battery of tests was the RMET (P = 0.031), yielding an individual R2 = 0.188, with the total battery yielding R 2 = 0.616, when the BBI total sum score was the specific outcome variable. Variables reported within Table S1 were included in the linear regression model.
Factor analysis of the BBI
As shown in Table 5, utilizing patient data, five independent factors were identified using varimax rotation. An oblique methodology was also employed using a direct oblimin rotation (delta = 0) which identified identical principle components within the factor structures, though the rank order of the factors differed (see Table S2). Although the BBI focuses on dysexecutive behavior, Factor 1 identified in the model accounts for the greatest amount of variance, which describes a broad range of disinhibited items of the scale (i.e., acts without thinking; acts inappropriately; excessively stores food in mouth; and hyperorality), Factor 2 reflects a clustering of items related to an irregularity of reward and impulse control (i.e., eats much more, is less picky about food, smokes more, is more distractible, etc.). Factor 3 reports dysexecutive behaviors (i.e., aggressive behavior, irritability, less concern about hygiene, and less concern for others). Factor 4 details a group of behaviors focusing around cognitive rigidity (i.e., overly concerned with neatness, aligning things a certain way, checking clocks/switches). Factor 5 is reflective of behavioral changes likely to be seen within ALS‐FTD, and are characterized by their neuropsychiatric nature (i.e., visual and auditory hallucinations, new bizarre beliefs, hoards/hides/collects things, and handles things for no apparent reason). Many of the behaviors apparent in Factor 5 were represented within the C9orf72‐positive group. Table S3 shows the breakdown between ALS patients and healthy controls based on the itemized loading onto factors within the BBI, and the comparative result. See Figure S2 for a behavioral schema of these factors.
Table 5.
Item loading and relevant cross‐loading into five identified factors (varimax rotation)
| Beaumont behaviour inventory questions | Identified BBI components | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| 1 | Repeats words/sentences said by others | 0.745 | ||||
| Not aware of mistakes | 0.647 | |||||
| Excessively stores food in mouth | 0.632 | |||||
| More withdrawn | 0.607 | 0.442 | ||||
| Lack of embarrassment | 0.594 | |||||
| Acts without thinking | 0.580 | |||||
| Difficulties with conversation | 0.564 | |||||
| Difficulty with everyday language | 0.550 | 0.471 | ||||
| Less able to react to difficulties | 0.549 | |||||
| Hyperorality | 0.541 | |||||
| Repeats phrases again and again | 0.520 | 0.482 | ||||
| Acts inappropriately in public | 0.481 | |||||
| Obsessive countinga | 0.306 | |||||
| Seeks social contact | 0.672 | |||||
| Shows less emotion | 0.623 | |||||
| More distractible/unable to concentrate | 0.539 | |||||
| 2 | New food preferences | 0.502 | ||||
| Dislikes change in routine | 0.448 | |||||
| Smokes more | 0.415 | |||||
| Eats much morea | 0.270 | |||||
| Less picky about food preferencesa | 0.245 | |||||
| New unusual habits | 0.621 | |||||
| More aggressive | 0.617 | |||||
| Less concerned about others | 0.496 | 0.543 | ||||
| Increased irritability | 0.497 | |||||
| Perseverative speech | 0.478 | |||||
| 3 | More selfish | 0.474 | ||||
| Less concerned about hygiene | 0.460 | 0.464 | ||||
| Emotional lability | 0.444 | |||||
| Repetitious behaviora | 0.383 | |||||
| Overly sensitive to sensations (noise)a | 0.371 | |||||
| Less aware of physical sensationa | 0.326 | |||||
| Developed unusual ritualsa | 0.297 | |||||
| Overly concerned with neatness | 0.751 | |||||
| 4 | Constantly aligns things in a certain way | 0.720 | ||||
| Constantly checking clocks/switches | 0.477 | 0.483 | ||||
| Change in sexual interest | 0.441 | |||||
| Sees/Hears Things/People | 0.839 | |||||
| 5 | Handles things for no apparent reason | 0.736 | ||||
| New bizarre beliefs | 0.674 | |||||
| Hoards, hides, or collects things | 0.586 | |||||
| Variance explained | 24.4 | 6.2 | 5.7 | 4.6 | 4.1 | |
Extraction Method: Principal Component Analysis. Rotation Method: Varimax with Kaiser Normalization. Bold figures display which factor the items loaded best with. BBI, Beaumont Behavioural Inventory.
Items which are considered weak, that is, <0.4, which do not load to an individual factor.
The C9orf72 expansion and behavior change in ALS
C9orf72 hexanucleotide repeat screening was undertaken in 308/317 ALS patients (97.16%). Of the patients who had not undergone genetic testing, seven had no significant behavior change, and two fell within the mild‐moderate range. Forty of the ALS patients tested (12.98%) were positive for the C9orf72 hexanucleotide repeat expansion. These patients were significantly younger at time of testing compared to patients without the genetic expansion (59.8 ± 9.0 vs. 63.7 ± 11.26, P = 0.037).
The presence of the positive C9orf72 gene expansion was associated with greater caregiver endorsement on the BBI, although 40% of these patients had no significant behavior change (n = 16); 40% were reported to have mild‐moderate behavioral change; the remaining 20% (n = 8) were within the Clinically Severe Behavioral Change group. Four patients with the C9orf72 gene expansion had evidence of cognitive impairment, two within the mild‐moderate behavior change group, and two within the Clinically Severe Behavioral Change group.
C9orf72‐positive patients were less aware of mistakes (45% vs. 22.7%; P = 0.007); were more aggressive (25% vs. 15.5%; P = 0.026); reported seeing/hearing things/people (7.7% vs. 1.7%; P = 0.031); demonstrated new bizarre beliefs (14.3% vs. 3.5%; P = 0.024); and showed evidence of hoarding, hiding, or collecting objects (15% vs. 5.8%; P = 0.045).
ALS patient survival and behavior change
Of the total cohort of 317 patients tested, 175 are deceased. Of these, 85 had no behavioral change; 65 had mild‐moderate behavioral change, and 25 patients were within the clinically severe range. As seen in Figure 3, there was no significant difference between the groups at the beginning of the survival curve (Breslow P = 0.105), middle (Tarone‐Ware P = 0.139), or end (Log Rank P = 0.198), implying that behavior change, as assessed early in the disease, was not a predictor of survival.
Figure 3.
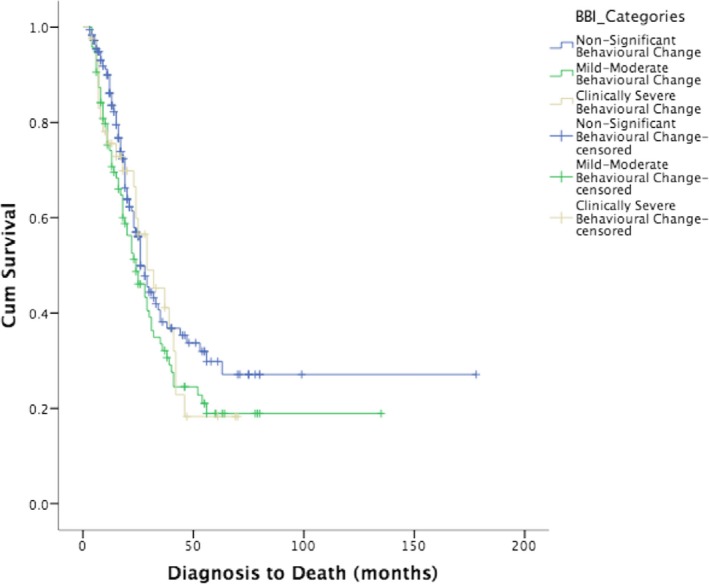
Survival data of ALS patients (n = 175) stratified by the severity of behavioral change.
Discussion
The aim of this study was to examine behavioral change in a large population‐based cohort of ALS patients using a validated disease‐specific behavioral scale. Our data confirm those of previous studies that emotional lability is a frequent finding in ALS (44%). Other common behavioral changes included a reduced concern for hygiene (36.8%), increased irritability (36.2%), new unusual habits (33.4%), and increased apathy (31.1%). As noted previously, 37% of patients with behavioral change were cognitively normal.
Emotional lability, or “pseudobulbar affect” is recognized as a manifestation of extensive upper motor neuron pathology, and is generally considered separately from other types of behavioral change. In our cohort, increased rates of emotional lability were associated with bulbar‐onset disease, which is congruent with other studies. Emotional lability is considered to be a disconnection between mechanisms which mediate emotions and associated motor responses, and primarily presents secondary to lesions, atrophy, or injury to the cortico‐limbic‐subcortico‐thalamic‐ponto‐cerebellar network.41
The majority of patients exhibiting mild behavior change had evidence of emotional lability (57.4%). Apathy was also commonly reported (48.4%). This cohort further exhibited new‐onset unusual habits (44.7%) and less concern for hygiene (52.1%). Patients with severe behavioral impairment exhibited apathy (87.5%); were not as aware of mistakes as before (82.5%), more impulsive (82.9%), and less responsive to others' needs (71.4%). Clinically relevant behavioral changes were also noted, that is, increased sensitivity to sensory stimuli (>28%), likely resulting from pathophysiological changes in the afferent system.42
Our findings concur with previous findings which suggest that behavioral changes in ALS are qualitatively similar regardless of whether a patient presents with mild or more severe behavior change.8, 15 These qualitative similarities likely represent shared disrupted neurologic pathways, with greater atrophy resulting in increased behavioral severity as seen with other neurodegenerative conditions. Notwithstanding, some behaviors, that is, hallucinations, were almost exclusively present in the Clinically Severe Behavioral Change group (See Tables 3 and 4).
Our findings extend those of previous studies both by the large size of the study population, and by our data‐driven observations indicating that specific behavioral subphenotypes can be discerned in ALS to a greater extent than previously reported.9, 43 By mapping, from a neuropsychological perspective, these altered behaviors onto known anatomic pathways, the behavioral categories broadly reflect differential impairments within the corticostriatal circuits known to be compromised in ALS. For example, the neuropsychological categorization of behaviors within Factor 1 are reflective of disruptions in pathways involving medial prefrontal and orbitofrontal cortices, as well as the anterior insula.44, 45 Behaviors characteristics within Factor 2 implicate orbitofrontal and anterior cingulate pathways46, 47; and those within Factor 3 are suggestive of disruptions in right prefrontal regions.48, 49, 50, 51 The clusters of behavioral change within Factor 4 are associated with alterations in the dorsolateral prefrontal cortex and temporolimbic networks.52, 53 Behaviors described by Factor 5 are not predictive of specific neuroanatomical pathways but are closely associated with the C9orf72 expansion which is known to implicate extensive cortical and subcortical frontotemporal regions.24 See Figure S1 for a graphical illustration of the behavioral schema.
Further analysis of behavioral impairment in ALS demonstrated that changes occurred in a mutually exclusive manner, which adds validity to the findings (see Table 5). This is evident through the statistical convergence, that is, Factor 1: “Lack of Embarrassment”, and “Acts Inappropriately in Public” occurred together. Moreover, the statistical divergence of the scale items illustrates the exclusivity of these behavioral changes between different factor, that is, Factor 1: “More Withdrawn”, and Factor 2: “Seeks Social Contact”. Employing an oblique rotation method yielded identical factor structures (Table S2), however, the rank order differed. Using the oblimin rotation, the factor which represented C9orf72 features was more closely correlated with the primary factor, that is, disinhibition. Clinically, this finding reflects the widely reported association between these observed behaviors, and the C9orf72 expansion in ALS patients.2, 3, 4, 9, 10, 12, 13, 15, 16
The cumulative variance accounted for by our principal component analysis are consistent with the methodology employed, whereby one principal factor is identified (Factor 1: See Table 5), with subcomponents identified which carry less variance. These factors met the stringent statistical cut‐off of behaviors which cluster together in ALS. Considering these data, the BBI should be considered as a reliable and valid tool for identifying behavioral change in ALS when the total score is adopted, as previously reported (Cronbach's alpha = 0.906). However, considering these data, specific subdomains of behavioral clusters have been outlined which may represent specific behavioral phenotypes in ALS, and specific subscales of the BBI.
One of the mostly widely accepted nondysexecutive behavioral subphenotype in ALS2, 54 relates to Factor 5 which, from a neuropsychological perspective, is predictive of more extensive network disruption. In our study cohort, this constellation of behavioral change was associated primarily with the presence of the C9orf72 expansion.54 In our cohort, 20% (n = 8) of the C9orf72‐positive patients met the cut‐off score for clinically severe change, which according to the BBI, is suggestive of bvFTD.26
Extensive cognitive assessments were conducted with a subcohort of ALS patients (n = 65). Although 37% of patients with behavioral change were cognitively normal, there were significant correlations between the measures of executive function (Stroop Colour‐Word Interference Task, VFI and the Brixton Spatial Anticipation task) and severe behavioral change. Notably, these tasks are dependent on the integrity of multiple frontostriatal circuits, requiring both inhibition of unwanted responses and close error monitoring.55 More severe behavioral impairment also correlated with lower scores on the RMET, which was a reliable predictor of behavioral change in ALS.
Overall, these data confirm the relationship between executive function and severe behavioral change and infer that behavior changes in ALS are associated with frontal pathology. Indeed, the strongest predictor of behavioral change in our neuropsychological model was a measure of social cognition, which anatomically correlates with core circuitry involving the dorsolateral prefrontal cortical regions and the anterior cingulate.56
Although primarily cross‐sectional in nature, the large population‐based sample permitted an assessment of the relationship between behavioral change and disease stage as defined by the King's Staging System. Our findings suggest that while physical disability may contribute to milder degrees of behavioral impairment, it is unlikely to be a major determinant of overall behavioral impairment. We found no significant differences on survival between the groups with and without behavioral impairment, in contrast to our previous findings that executive impairment is a significant negative prognostic indicator.57
This study has limitations. The data are drawn from a cross‐sectional analysis, albeit with survival data, and longitudinal analyses are required to confirm our findings. Although this study incorporated a relatively large cohort of C9orf72‐positive patients, larger samples with differing behavioral profiles are required. Notwithstanding, this study, which to the best of our knowledge is the largest analysis of behavioral change in ALS to date and facilitates for the first time a factor analysis of behavior, has identified a characteristic behavioral phenotype associated with the C9orf72 repeat expansion, and several further behavioral subphenotypes. These factor loadings represent the psychometric and statistical clustering of the items on the scale, and further external validation studies are needed, as well as neuroimaging data or postmortem analyses to confirm the behavioral mapping to neuropathological regions, known to be implicated in ALS.
Our neuropsychology‐based analyses support the hypothesis that behavioral subphenotypes in ALS reflects differential disruption of extramotor networks involving frontostriatal, orbitofrontal, and anterior cingulate pathways and indicate that the neuropsychological profile of ALS patients that manifest as behavioral change is heterogeneous, reflecting differential disruption of neuroanatomic circuits. Further longitudinal studies are currently underway to assess the interaction between behavioral change and clinical staging, and between neuropsychological changes and those observed by neuroimaging.
Author Contribution
TB contributed to the process of data collection, analyzed the data and wrote the manuscript; PB, AV, RLM, KL, MOS, and MPG contributed to the process of data collection, edited, and revised the intellectual content of the manuscript; NP supervised the neuropsychological and statistical aspects of the study and revised the manuscript from a neurocognitive and behavioral perspective; and OH supervised all clinical aspects of the study in all stages of development and revised the manuscript from a clinical perspective.
Conflict of Interest
Dr Tom Burke, Ms Marta Pinto‐Grau, Dr Peter Bede, Ms. Katie Lonergan, Dr Alice Vajda, Dr Russell McLaughlin, Mr Mark Heverin, and Dr Niall Pender have nothing to disclose. Prof. Orla Hardiman has received fees for consultation work from Biogen Idec, Cytokinetics, and Novartis. She serves as Editor‐in‐Chief of Amyotrophic Lateral Sclerosis. The authors report no conflict of interests.
Supporting information
Figure S1. Scree plot derived from the factor analysis of BBI data.
Figure S2. A schema of the identified behavioral factors and their neuroanatomical correlates based on the literature. mPFC: medial prefrontal cortex; OFC: orbitofrontal cortex; aI: anterior Insula; ACC: anterior cingulate; dlPFC: dorsolateral prefrontal regions TL temporolimbic area. Factor 1: mPFC‐OFC‐aI (black); Factor 2: OFC‐ACC (blue); Factor 3: OFC‐ mPFC‐dlPFC (red); Factor 4: dlPFC‐OFC‐TL (orange). See Bede et al., 2013 for characteristics of cortical and subcortical changes in ALS with the C9orf72 expansion.
Table S1. Additional data on the subcohort completing the cognitive assessment.
Table S2. Item loading and cross‐loading on five identified factors (oblique method, oblimin rotation).
Table S3. Frequency of all reported behaviors in the ALS cohort versus healthy controls.
Acknowledgments
The authors thank the patients and caregivers who took part in this study. This research has received funding from the Health Seventh Framework Programme (FP7/2007‐2013) under grant agreement #259867, ALSA (the ALS Association), HRB (the Health Research Board, grant H01300), Joint Programme in Neurodegeneration (JPND), The Irish Institute of Clinical Neuroscience (grant #12549.201616) and Research Motor Neuron (previously named Motor Neuron Disease Research Foundation).
References
- 1. Bak TH, Chandran S. What wires together dies together: verbs, actions and neurodegeneration in motor neuron disease. Cortex 2012;48:936–944. [DOI] [PubMed] [Google Scholar]
- 2. Byrne S, Elamin M, Bede P, et al. Cognitive and clinical characteristics of patients with amyotrophic lateral sclerosis carrying a C9orf72 repeat expansion: a population‐based cohort study. Lancet Neurol 2012;11:232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goldstein LH, Abrahams S. Changes in cognition and behaviour in amyotrophic lateral sclerosis: nature of impairment and implications for assessment. Lancet Neurol 2013;12:368–380. [DOI] [PubMed] [Google Scholar]
- 4. Raaphorst J, Beeldman E, De Visser M, et al. A systematic review of behavioural changes in motor neuron disease. Amyotroph Lateral Scler 2012;13:493–501. [DOI] [PubMed] [Google Scholar]
- 5. Consonni M, Catricalà E, Dalla Bella E, et al. Beyond the consensus criteria: multiple cognitive profiles in amyotrophic lateral sclerosis? Cortex 2016;31:162–167. [DOI] [PubMed] [Google Scholar]
- 6. Phukan J, Elamin M, Bede P, et al. The syndrome of cognitive impairment in amyotrophic lateral sclerosis: a population‐based study. J Neurol Neurosurg Psychiatry 2012;83:102–108. [DOI] [PubMed] [Google Scholar]
- 7. Elamin M, Bede P, Byrne S, et al. Cognitive changes predict functional decline in ALS a population‐based longitudinal study. Neurology 2013;80:1590–1597. [DOI] [PubMed] [Google Scholar]
- 8. Beeldman E, Raaphorst J, Twennaar MK, et al. The cognitive profile of ALS: a systematic review and meta‐analysis update. J Neurol Neurosurg Psychiatry 2016;87:611–619. [DOI] [PubMed] [Google Scholar]
- 9. Strong MJ, Grace GM, Freedman M, et al. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2009;10:131–146. [DOI] [PubMed] [Google Scholar]
- 10. Gibbons ZC, Richardson A, Neary D, et al. Behaviour in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2008;9:67–74. [DOI] [PubMed] [Google Scholar]
- 11. Girardi A, MacPherson SE, Abrahams S. Deficits in emotional and social cognition in amyotrophic lateral sclerosis. Neuropsychology. 2011;25:53. [DOI] [PubMed] [Google Scholar]
- 12. Grossman AB, Woolley‐Levine S, Bradley WG, et al. Detecting neurobehavioral changes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2007;8:56–61. [DOI] [PubMed] [Google Scholar]
- 13. Witgert M, Salamone AR, Strutt AM, et al. Frontal‐lobe mediated behavioral dysfunction in amyotrophic lateral sclerosis. Eur J Neurol 2010;17:103–110. [DOI] [PubMed] [Google Scholar]
- 14. Burke T, Elamin M, Galvin M, et al. Caregiver burden in amyotrophic lateral sclerosis: a cross‐sectional investigation of predictors. J Neurol 2015;262:1526–1532. [DOI] [PubMed] [Google Scholar]
- 15. Lillo P, Mioshi E, Zoing MC, et al. How common are behavioural changes in amyotrophic lateral sclerosis? Amyotroph Lateral Scler 2011;12:45–51. [DOI] [PubMed] [Google Scholar]
- 16. De Silva D, Hsieh S, Caga J, et al. Motor function and behaviour across the ALS‐FTD spectrum. Acta Neurol Scand 2016;133(5):367–372. [DOI] [PubMed] [Google Scholar]
- 17. Christodoulou G, Gennings C, Hupf J, et al. Telephone based cognitive‐behavioral screening for frontotemporal changes in patients with amyotrophic lateral sclerosis (ALS). Amyotroph Lateral Scler Frontotemporal Degener 2016;27:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mioshi E, Caga J, Lillo P, et al. Neuropsychiatric changes precede classic motor symptoms in ALS and do not affect survival. Neurology 2014;82:149–155. [DOI] [PubMed] [Google Scholar]
- 19. Woolley SC, Zhang Y, Schuff N, et al. Neuroanatomical correlates of apathy in ALS using 4 Tesla diffusion tensor MRI. Amyotroph Lateral Scler 2011;12:52–58. [DOI] [PubMed] [Google Scholar]
- 20. Mioshi E, Lillo P, Yew B, et al. Cortical atrophy in ALS is critically associated with neuropsychiatric and cognitive changes. Neurology 2013;80:1117–1123. [DOI] [PubMed] [Google Scholar]
- 21. Tsujimoto M, Senda J, Ishihara T, et al. Behavioral changes in early ALS correlate with voxel‐based morphometry and diffusion tensor imaging. J Neurol Sci 2011;307:34–40. [DOI] [PubMed] [Google Scholar]
- 22. Brettschneider J, Del Tredici K, Toledo JB, et al. Stages of pTDP‐43 pathology in amyotrophic lateral sclerosis. Ann Neurol 2013;74:20–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bede P, Bokde A, Elamin M, et al. Grey matter correlates of clinical variables in amyotrophic lateral sclerosis (ALS): a neuroimaging study of ALS motor phenotype heterogeneity and cortical focality. J Neurol Neurosurg Psychiatry 2013;84:766–773. [DOI] [PubMed] [Google Scholar]
- 24. Bede P, Bokde AL, Byrne S, et al. Multi‐parametric MRI study of ALS stratified for the C9orf72 genotype. Neurology 2013;81:361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Machts J, Loewe K, Kaufmann J, et al. Basal ganglia pathology in ALS is associated with neuropsychological deficits. Neurology 2015;85:1301–1309. [DOI] [PubMed] [Google Scholar]
- 26. Elamin M, Pinto‐Grau M, Burke T, et al. Identifying behavioural changes in ALS: validation of the Beaumont Behavioural Inventory (BBI). Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration 2016;18:68–73. [DOI] [PubMed] [Google Scholar]
- 27. O'Toole O, Traynor BJ, Brennan P, et al. Epidemiology and clinical features of amyotrophic lateral sclerosis in Ireland between 1995 and 2004. J Neurol Neurosurg Psychiatry 2008;79:30–32. [DOI] [PubMed] [Google Scholar]
- 28. Balendra R, Jones A, Jivraj N, et al. Estimating clinical stage of amyotrophic lateral sclerosis from the ALS Functional Rating Scale. Amyotroph Lateral Scler Frontotemporal Degener 2014;15:279–284. [DOI] [PubMed] [Google Scholar]
- 29. Roche JC, Rojas‐Garcia R, Scott KM, et al. A proposed staging system for amyotrophic lateral sclerosis. Brain 2012;135:847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brooks BR, Miller RG, Swash M, et al. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000;1:293–299. [DOI] [PubMed] [Google Scholar]
- 31. Cedarbaum JM, Stambler N, Malta E, et al. 1A complete listing of the BDNF Study Group. The ALSFRS‐R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci 1999;169:13–21. [DOI] [PubMed] [Google Scholar]
- 32. Ferguson E, Cox T. Exploratory factor analysis: a users' guide. Int J Select Assess 1993;1:84–94. [Google Scholar]
- 33. Bryman A, Cramer D. Quantitative data analysis with SPSS for Windows. London: Routledge, 1997. [Google Scholar]
- 34. Rickards H, De Souza J, Van Walsem M, et al. Factor analysis of behavioural symptoms in Huntington's disease. J Neurol Neurosurg Psychiatry 2011;82:411–412. [DOI] [PubMed] [Google Scholar]
- 35. Rattray J, Jones MC. Essential elements of questionnaire design and development. J Clin Nurs 2007;16:234–243. [DOI] [PubMed] [Google Scholar]
- 36. Burke T, Doherty CP, Koroshetz W, Pender N. Huntington's Disease In: Editors: Hardiman O., Doherty C.P., Elamin M. Bede P. (Eds.). neurodegenerative Disorders. Switzerland : Springer International Publishing; 2016. p. 167–179. [Google Scholar]
- 37. Kingma EM, van Duijn E, Timman R, et al. Behavioural problems in Huntington's disease using the Problem Behaviours Assessment. Gen Hosp Psychiatry 2008;30:155–161. [DOI] [PubMed] [Google Scholar]
- 38. Starkstein SE, Jorge R, Mizrahi R, Robinson RG. A diagnostic formulation for anosognosia in Alzheimer's disease. J Neurol Neurosurg Psychiatry 2006;77:719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ott BR, Tate CA, Gordon NM, Heindel WC. Gender differences in the behavioral manifestations of Alzheimer's disease. J Am Geriatr Soc 1996;44:583–587. [DOI] [PubMed] [Google Scholar]
- 40. Spalletta G, Baldinetti F, Buccione I, et al. Cognition and behaviour are independent and heterogeneous dimensions in Alzheimer's disease. J Neurol 2004;251:688–695. [DOI] [PubMed] [Google Scholar]
- 41. Parvizi J, Coburn KL, Shillcutt SD, et al. Neuroanatomy of pathological laughing and crying: a report of the American Neuropsychiatric Association Committee on Research. J Neuropsy Clin Neurosci 2009;21:75–87. [DOI] [PubMed] [Google Scholar]
- 42. Palmieri A, Abrahams S, Sorarù G, et al. Emotional Lability in MND: relationship to cognition and psychopathology and impact on caregivers. J Neurol Sci 2009;278:16–20. [DOI] [PubMed] [Google Scholar]
- 43. Gothelf D, Farber N, Raveh E, et al. Hyperacusis in Williams syndrome Characteristics and associated neuroaudiologic abnormalities. Neurology 2006;66:390–395. [DOI] [PubMed] [Google Scholar]
- 44. Josephs KA, Whitwell JL, Eggers SD, et al. Gray matter correlates of behavioral severity in progressive supranuclear palsy. Mov Disord 2011;26:493–498. [DOI] [PubMed] [Google Scholar]
- 45. Abrahams S, Newton J, Niven E, et al. Screening for cognition and behaviour changes in ALS. Amyotroph Lateral Scler Frontotemporal Degener 2014;15:9–14. [DOI] [PubMed] [Google Scholar]
- 46. Seeley WW. Anterior insula degeneration in frontotemporal dementia. Brain Struct Funct 2010;214:465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Perry RJ, Graham A, Williams G, et al. Patterns of frontal lobe atrophy in frontotemporal dementia: a volumetric MRI study. Dement Geriatr Cogn Disord 2006;22:278–287. [DOI] [PubMed] [Google Scholar]
- 48. Perry DC, Sturm VE, Seeley WW, et al. Anatomical correlates of reward‐seeking behaviours in behavioural variant frontotemporal dementia. Brain 2014;137:1621–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 2010;35:4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Meier SL, Charleston AJ, Tippett LJ. Cognitive and behavioural deficits associated with the orbitomedial prefrontal cortex in amyotrophic lateral sclerosis. Brain 2010;133:3444–3447. [DOI] [PubMed] [Google Scholar]
- 51. Modirrousta M, Price BH, Dickerson BC. Neuropsychiatric symptoms in primary progressive aphasia: phenomenology, pathophysiology, and approach to assessment and treatment. Neurodegener Dis Manag 2013;3:133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rohrer JD, Warren JD. Phenomenology and anatomy of abnormal behaviours in primary progressive aphasia. J Neurol Sci 2010;293:35–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Piras F, Piras F, Chiapponi C, et al. Widespread structural brain changes in OCD: a systematic review of voxel‐based morphometry studies. Cortex 2015;31:89–108. [DOI] [PubMed] [Google Scholar]
- 54. Majounie E, Renton AE, Mok K, et al. Frequency of the C9orf72 hexanucleotide repeat expansion in patients with amyotrophic lateral sclerosis and frontotemporal dementia: a cross‐sectional study. Lancet Neurol 2012;11:323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hoerold D, Pender NP, Robertson IH. Metacognitive and online error awareness deficits after prefrontal cortex lesions. Neuropsychologia 2013;51:385–391. [DOI] [PubMed] [Google Scholar]
- 56. Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci 2005;6:691–702. [DOI] [PubMed] [Google Scholar]
- 57. Elamin M, Phukan J, Bede P, et al. Executive dysfunction is a negative prognostic indicator in patients with ALS without dementia. Neurology 2011;76:1263–1269. doi:10.1212/WNL.0b013e318214359f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Scree plot derived from the factor analysis of BBI data.
Figure S2. A schema of the identified behavioral factors and their neuroanatomical correlates based on the literature. mPFC: medial prefrontal cortex; OFC: orbitofrontal cortex; aI: anterior Insula; ACC: anterior cingulate; dlPFC: dorsolateral prefrontal regions TL temporolimbic area. Factor 1: mPFC‐OFC‐aI (black); Factor 2: OFC‐ACC (blue); Factor 3: OFC‐ mPFC‐dlPFC (red); Factor 4: dlPFC‐OFC‐TL (orange). See Bede et al., 2013 for characteristics of cortical and subcortical changes in ALS with the C9orf72 expansion.
Table S1. Additional data on the subcohort completing the cognitive assessment.
Table S2. Item loading and cross‐loading on five identified factors (oblique method, oblimin rotation).
Table S3. Frequency of all reported behaviors in the ALS cohort versus healthy controls.


