Abstract
Background: Exosomes, cell-derived vesicles encompassing lipids, DNA, proteins coding genes and noncoding RNAs (ncRNAs) are present in diverse body fluids. They offer novel biomarker and drug therapy potential for diverse diseases, including cancer. Materials and Methods: Using gene ontology, exosomal genes database and GeneCards metadata analysis tools, a database of cancer-associated protein coding genes and ncRNAs (n=2,777) was established. Variant analysis, expression profiling and pathway mapping were used to identify putative pancreatic cancer exosomal gene candidates. Results: Five hundred and seventy-five protein-coding genes, 26 RNA genes and one pseudogene directly associated with pancreatic cancer were identified in the study. Nine open reading frames (ORFs) encompassing enzymes, apoptosis and transcriptional regulators, and secreted factors and five cDNAs (enzymes) emerged from the analysis. Among the ncRNA class, 26 microRNAs (miRs), one pseudogene, one long noncoding RNA (LNC) and one antisense gene were identified. Furthermore, 22 exosome-associated protein-coding targets (a cytokine, enzymes, membrane glycoproteins, receptors, and a transporter) emerged as putative leads for pancreatic cancer therapy. Seven of these protein-coding targets are FDA-approved and the drugs-based on these could provide repurposing opportunities for pancreatic cancer. Conclusion: The database of exosomal genes established in this study provides a framework for developing novel biomarkers and drug therapy targets for pancreatic cancer.
Keywords: Carcinoma, drug targets, gene ontology, open reading frame, non-coding RNAs, secretome
Pancreatic ductal adenocarcinoma is predicted to overtake breast cancer and become the third leading cause of cancer-related deaths in the United States (1). Pancreatic cancer has a very poor prognosis in most patients and seriously lacks effective therapies. Early diagnosis continues to be a challenge in clinics due to advanced-stage presentation. Metastasis and resistance to current chemotherapeutics further contributes to the poor prognosis (2). Most therapies for pancreatic cancer still revolve around the use of ineffective cytotoxic drugs. Novel drug targets and early-stage biomarkers are urgently needed for this untreatable cancer.
Exosomes are 40-150 nm extracellular vesicles (EVs) released by all cell types (3-4). Exosomes are highly heterogeneous (5) and likely reflect the phenotype of the cell that generate them. Similar to cells, exosomes are composed of a lipid bi-layer and contain all known molecular constituents of a cell, including proteins, microRNAs (miRs), mRNA, and DNA (6-8). This lipid bi-layer offers high stability in body fluids, making the exosomes highly attractive targets for diagnostic markers (9). Exosomal secretion involves either a constitutive release via the trans-Golgi network or by an inducible release mechanism (10). While the precise function of the exosomes is unclear, it is suggested they may be involved in removal of cellular waste (11)).
The levels of exosomes in cancer patients are higher than the levels found in normal patients (12-14). This phenomenon may be due to altered physiology in diseased organs. Tumor-derived exosomes have ant- tumorigenic properties including induction of apoptosis and enhancement of anti-tumor immunity as well as pro-tumorigenic properties such as tumor growth, angiogenesis and progression (14-16). The exosomal proteins, nucleic acids and noncoding RNAs detectable in diverse body fluids offer novel biomarker potential for cancer (17-20). For early detection of pancreatic cancer, secreted biomarkers could make a significant improvement over the current options (6,21).
As an initial step towards developing a comprehensive knowledgebase of pancreatic cancer exosomes, a working database of cancer-associated exosomal genes was generated using bioinformatics approaches. From this database of cancer exosomal genes, pancreatic cancer targets encompassing protein-coding genes, open reading frames (ORFs) and non-coding RNAs, (ncRNAs) including antisense, pseudogenes, long noncoding RNAs (LNC) and microRNAs (miRs) were identified. Enrichment analysis involving somatic mutations, mRNA expression, genome-wide association, protein expression and pancreatic cancer pathways enabled the identification of pancreatic cancer lead candidate genes. Druggable classes of proteins including enzymes, membrane glycoproteins, receptors, and transporters were identified. Seven protein-coding genes from these leads are current target for FDA approved drugs. The targets identified in the study offer a strong rationale for novel biomarkers discovery for the diagnosis and therapy of pancreatic cancer.
Materials and Methods
Knowledge databases. Disease and knowledge-oriented databases MalaCards (22), DisGeNET (23), UCSC genome browser (24) and European Bioinformatics Institute (http://www.ebi.ac.uk/) were used to obtain general information. The Gene Ontology tool, QuickGO (25), was used to classify exosome-associated genes. Exosome encyclopedia, Exocarta (26), was used to develop an initial working database of exosomal protein-coding genes, ncRNAs, secreted products in diverse body fluids and individual cancer-related genes. Comprehensive bioinformatics analysis of the database of exosomal genes was performed using the GeneALaCart tool from the LifeMap GeneCards Suite (27). The GeneAnalytics Gene Analysis tool (27) was used to categorize results into lists of matched tissues, cells, diseases, pathways, compounds and gene ontology (GO) terms to enhance gene set interpretation. Associations between genes and diseases/phenotypes based on shared pathways, interaction networks and paralogy relations were inferred using the VarElect NGS Phenotyper of the GeneCards Suite (27). VarElect utilizes the Deep LifeMap Knowledgebase to infer direct, as well as indirect, links between genes and phenotypes. Indirect association between genes and disease are based on shared pathways and interaction networks analysis.
Other bioinformatics analyses. Protein expression of the candidate genes was investigated using the Human Proteome Map (28) and the Proteomics DB (29). Genome-wide association was verified using the NCBI Phenome- Genome Integrator (PheGenI) (30) and the Genome-Wide Association Studies Catalogue, GWAS (31). Clinical variations were verified using the ClinVar database (32). Protein motifs and domains were verified using the Prosite-Expasy (33). The protein-related information was obtained from the UniProtKB (34). Cancer-related databases COSMIC (35), cBIOPortal (36), International Cancer Genome Consortium (ICGC) (37) and Oncomine (38) were used to verify cancer relevance of hits. The Human Genome Nomenclature Committee was used to verify the gene symbols used in the database (39).
Uncharacterized ORF proteins were compared against the dark matter ORF database previously described (40). Various ncRNA databases MIRMine (http://guanlab.ccmb.med.umich.edu/mirmine/), MIR Cancer (41), mir2Disease (42), MIRDB (43), MIRTarBase (44), miRFocus (http://mirfocus.org), LNC2Cancer (45), lncRNA and Disease (46), lncRNA DB (47) and StarBase (48) were used to verify long noncoding RNAs (Lnc RNAs), long intergenic RNAs (Linc RNAs), micro RNAs (miRs), pseudogenes and antisense RNAs. Pancreatic cancer expression was verified using the Array express from European Bioinformatics Institute (49), pancreatic cancer database (http://www.pancreaticcancerdatabase.org) and Pancreatic expression database (50). Other bioinformatics tools used in the study have been described earlier (51,52).
MicroRNA nomenclature. A capitalized "miR-" refers to the mature form of the miRNA, while the uncapitalized "mir-" refers to the pre-miRNA and the pri-miRNA, and "MIR" refers to the gene that encodes them (53).
Results
Identification of exosomal genes by gene ontology. Gene ontology provides a valuable approach to identify a broad class of genes based on functional query. An initial database of exosome-associated genes was developed using the QuickGo gene ontology annotation tool encompassing known protein-coding genes and uncharacterized Open Reading Frames (ORF). Classes of exosomal proteins (cytoplasmic, nuclear, extracellular, secreted, assembly, biogenesis and regulators) were categorized (Figure 1). In addition to 2,777 known protein-coding genes, uncharacterized cDNAs (n=6), ORFS (n=9) were identified. A majority of the exosomal proteins were extracellular (2748/2777). This initial database of exosomal proteins provided the framework for cancer target discovery.
Figure 1. Exosomal landscape. The Gene Ontology tool QuickGo (EBI) was used to enrich exosomal proteins using various filters. The number of genes for each of the classes of exosomes is shown.
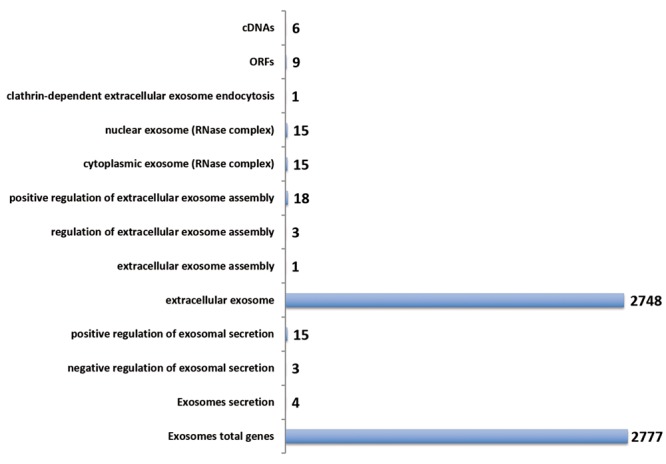
Datamining the ExoCarta database. ExoCarta, a vesicle encyclopedia (Compendium on extracellular vesicles) is a powerful consolidated exosome database encompassing datasets and links to other bioinformatics resources for protein coding genes (mRNAs and proteins), ncRNAs and lipid particles. The entire database (V5, July 2015) was downloaded and filtering options were used to classify output of genes into classes of coding and noncoding genes (Figure 2). In addition to the protein-coding genes (1,028) and mRNAs (1,767) the noncoding miRs (1,308) were the largest class of genes obtained from the ExoCarta database (Figure 2). This database was cross- verified with the QuickGo-based working database of exosomal proteins and a consolidated database was generated. The consolidated database provided the starting point for target(s) discovery by additional datamining.
Figure 2. Exosomal proteins and non-coding genes. The ExoCarta database was classified into indicated class of coding and noncoding genes. The numbers indicate the number of genes for each class of genes.
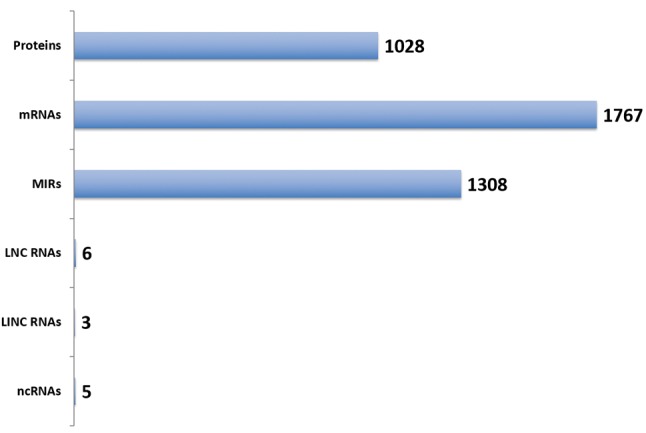
Exosomal secretome genes. Secreted proteins and noncoding genes (ncRNAs) present in diverse body fluids offer a biomarker potential for early diagnosis and pharmacogenomics. Hence it was of interest to sub-classify the database of exosomal genes into expression profile, based on detection in the body fluids (Figure 3). Exosomal gene expression was seen in amniotic fluids, ascites, milk, serum, saliva, semen etc. Urinary secretome encompassed the largest number of exosomal genes (n=1,505). These secreted exosomal proteins and ncRNAs would provide the basis for biomarker discoveries for diverse cancers.
Figure 3. Exosomal secretome. The ExoCarta database was data mined and classified into gene expression pattern for body fluid types. The numbers represent the hits for each body fluid type.
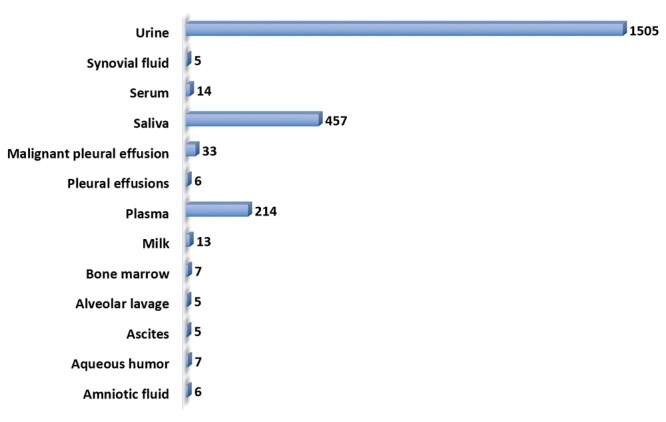
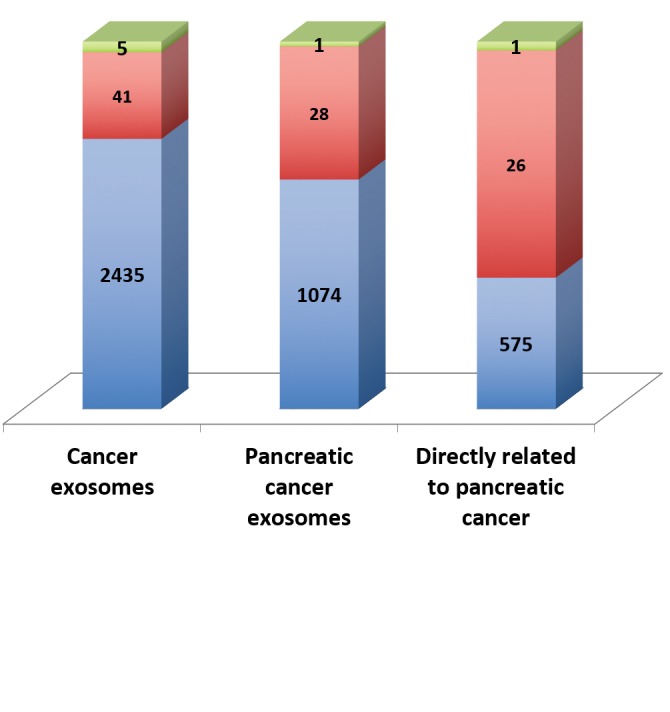
Cancer profile of the exosomal genes. Identification of exosomal genes present in individual cancer types is an attractive starting point for cancer-specific target discovery. Individual cancer-related exosomal genes were identified using the Exocarta tool and the cancer profile of the exosomal genes was established (Figure 4). Exosomal genes were present in diverse cancer types including sarcomas, carcinomas and leukemia. The largest number of hits was seen for colon (n=1,615), hepatocellular (n=1,138), neuroblastoma (n=1,279), ovarian (n=2,032), prostate (n=1,048) and squamous carcinoma (n=904). Rat pancreatic cancer genes identified in the study were used to identify the human homologues (n=165). The cancer type- related set of exosomal genes provide an opportunity for cancer-specific target discovery.
Figure 4. Cancer exosomal genes. The ExoCarta database was mined and indicated cancer type-associated proteins and ncRNAs. The numbers indicate the number of hits for each of the cancer types. Data for non-human species (basophilic leukemia, mammary adenocarcinoma and pancreatic cancer) are included. The human homologues of the rat pancreatic cancer genes were inferred from the functional annotation tool, DAVID.
Pancreatic cancer exosomal targets. The GeneCards Meta analysis tool was used to develop pancreatic cancer exosomal genes. The output data was filtered for protein coding genes, RNA genes and pseudogenes (Figure 5). The pancreatic cancer genes were further subjected to variant analysis using the VarElect tool and genes predicted to be directly related were identified. The list of genes included protein coding genes (n=575), ncRNAs (n=26) and pseudogene (n=1). The coding genes included druggable classes of proteins (adhesion molecules, enzymes, receptors, transporters, channel proteins), growth factors, cytokines and chemokines, oncogenes and tumor suppressor genes.
Figure 5. Identification of pancreatic cancer exosomal genes. The GeneCards database was mined for all cancers and pancreatic cancerassociated exosomal genes. The VarElect tool was used to identify directly associated genes with pancreatic cancer. The number of hits for the indicated class of genes is shown.
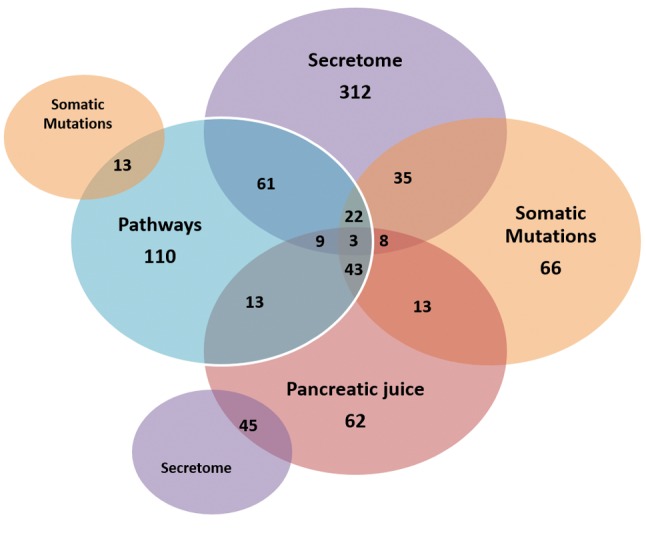
Enrichment of pancreatic cancer-associated exosomal protein coding genes. The pancreatic cancer associated list of exosomal genes shown in Figure 5 was subjected to additional enrichment analysis using the VarElect and GeneAnalytic tool of the GeneCards Suite. Exosomal protein-coding genes related to pancreatic cancer pathways, tissue specific expression, secretome nature and somatic mutations were identified (Figure 6). Twenty-two protein-coding targets (cytokines, enzymes, membrane glycoproteins, receptors, transporter) were shared across pancreatic cancer pathways, expression, somatic mutations and were in the secretome. These 22 exosomal proteins offer lead potential for drug discovery.
Figure 6. Profiling of pancreatic cancer exosomal protein coding genes. Using the VarElect and GeneAnalytics tool of the GeneCards Suite, pancreatic cancer genes were enriched for mutations, expression, pathways and secretome. The number of genes for each classifier is shown.
Exosomal ncRNAs in pancreatic cancer. The pancreatic cancer-related ncRNAs identified in Figure 5 were further subjected to variant analysis using the VarElect tool. Twenty-four miRs, one pseudogene, one LNC RNA and one antisense gene were identified as directly related to pancreatic cancer. Two miRs (mir206 and mir30A) were indirectly related to pancreatic cancer (Table I). These lncRNAs and miRs were subjected to comprehensive datamining using diverse ncRNA and miR databases as indicated in the Materials and Methods Section. Fifteen of these miRs target 19 pancreatic cancer exosomal protein-coding genes identified in the study. The lncRNAs, H19 and HOTAIR, were up-regulated in pancreatic cancers (lnc2cancer and lncRNA database). In addition, fifteen MIRs (MIR100, MIR10b, MIR125B1, MIR145, MIR155, MIR196A1, MIR200A, MIR200C, MIR203A, MIR21, MIR210, MIR212, MIR221, MIR222 and MIR23B) were up-regulated and four MIRs (MIR126, MIR141, MIR373 and MIR375) were down-regulated in pancreatic cancer. Among the miRs identified, four of the MIRs (MIR21, MIR34a, MIR155, and MIR221) were also causally linked to pancreatic cancer as shown by multiple databases (miR2sdisease, pancreatic cancer expression database, and miR cancer). Furthermore, eight of the ncRNAs (H19, MIR126, MIR141, MIR200a, MIR200c, MIR203a, MIR210, MIR34a, MIR373 and MIR375) were amplified or deleted in over 10% of the pancreatic cancer patients analyzed, n=109 (cBioPortal).
Table I. Pancreatic cancer exosomal ncRNAs.
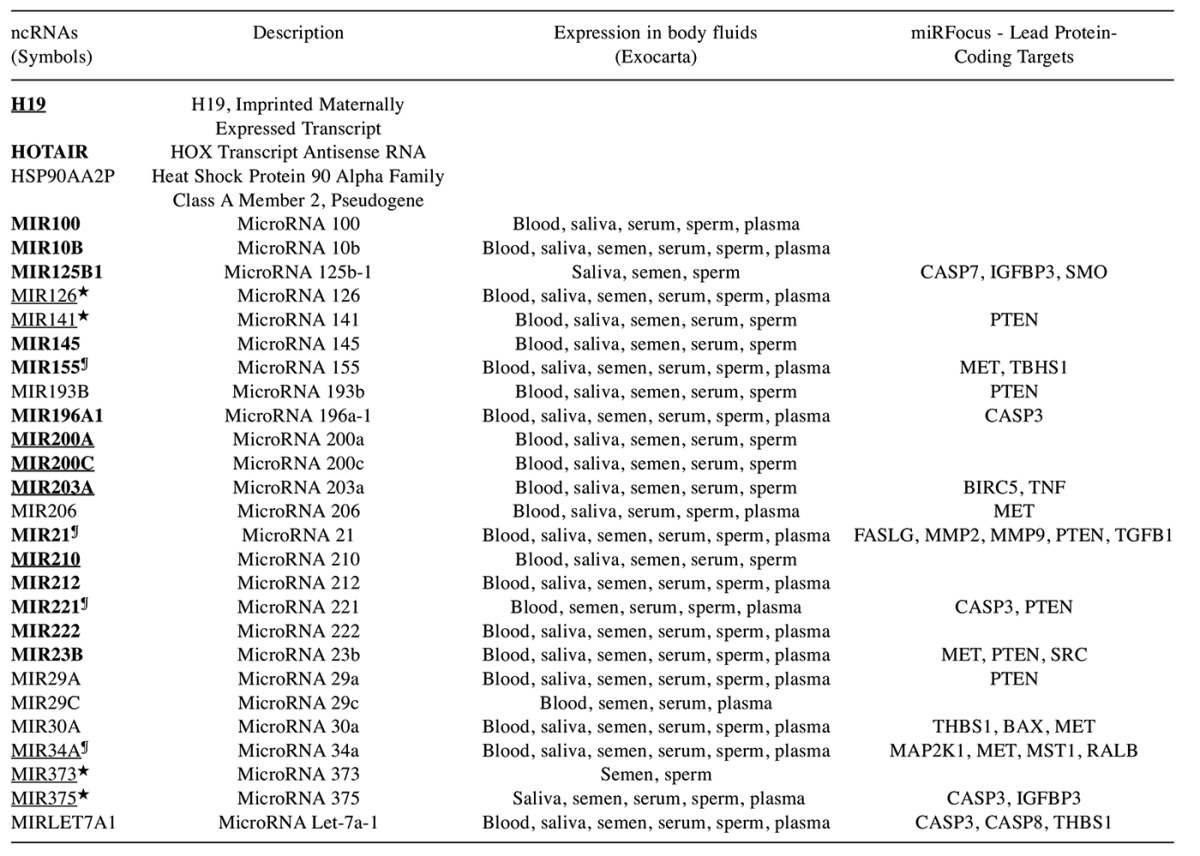
The exosomal ncRNAs identified in the study were analyzed using pancreatic cancer expression, mutations and ncRNA databases. Up-regulated ncRNAs in pancreatic cancer are shown bolded. Over 10% of the patients showing gene amplifications or deletions are underlined. The miR targets for the 22 protein coding genes were identified by miRFocus tool. ★Down-regulated in pancreatic cancers, ¶Causally linked.
The MIRs (MIR200a, MIR200c, MIR203a, MIR210, and MIR34a) showed a correlation of gene amplification (as measured by cBioportal) with an up-regulation of gene expression in the pancreatic cancers (pancreatic cancer expression database). Expression of the miRs was detected in blood, saliva, semen, serum, sperm, and plasma. These exosomal and secreted LncRNAs and the miRs offer the potential to develop a panel of biomarkers for pancreatic cancer diagnosis.
Novel pancreatic cancer ORF protein targets. QuickGo gene ontology analysis identified (see Figure 1) six uncharacterized cDNAs, two of which shared the same symbol and was therefore considered a single gene, and nine ORFS. Reasoning that these uncharacterized genes may offer a potential for novel target discovery, these genes were subjected to a comprehensive bioinformatics analysis (Table II). The novel protein targets were classified into enzymes, secretome, transcriptional regulator and transmembrane proteins. Expression in diverse body fluids was detected for the ORFs. Genetic association studies revealed an association with asthma, cancer, diabetes, inflammation, obesity, osteoporosis, bone density, metabolic syndrome X, chronic kidney disease and substance-related disorders. Nine of the uncharacterized proteins (C11orf54, C16orf69, C17orf80, C19orf18, C1orf123, C5orf46, C6orf120, EXOSC2 and MARK3) were up-regulated and three of the RNA-processing enzymes (EXOSC8, EXOSC9 and EXOSC10) were down-regulated in pancreatic cancer. Furthermore, eight of these uncharacterized proteins (C17orf80, C19orf18, C1orf123, C2orf16, C6orf120, EXOSC2, EXOSC10 and MARK3) were found to be altered in more than 5% of the pancreatic cancer patients (ICGC dataset). Transcriptome and proteome analysis revealed that the ORFs (C19orf18, C16orf89, C2orf16, C5orf46 and C6orf120) had expression in limited tissues and were absent in the pancreas.
Table II. Pancreatic cancer exosomal uncharacterized proteins.
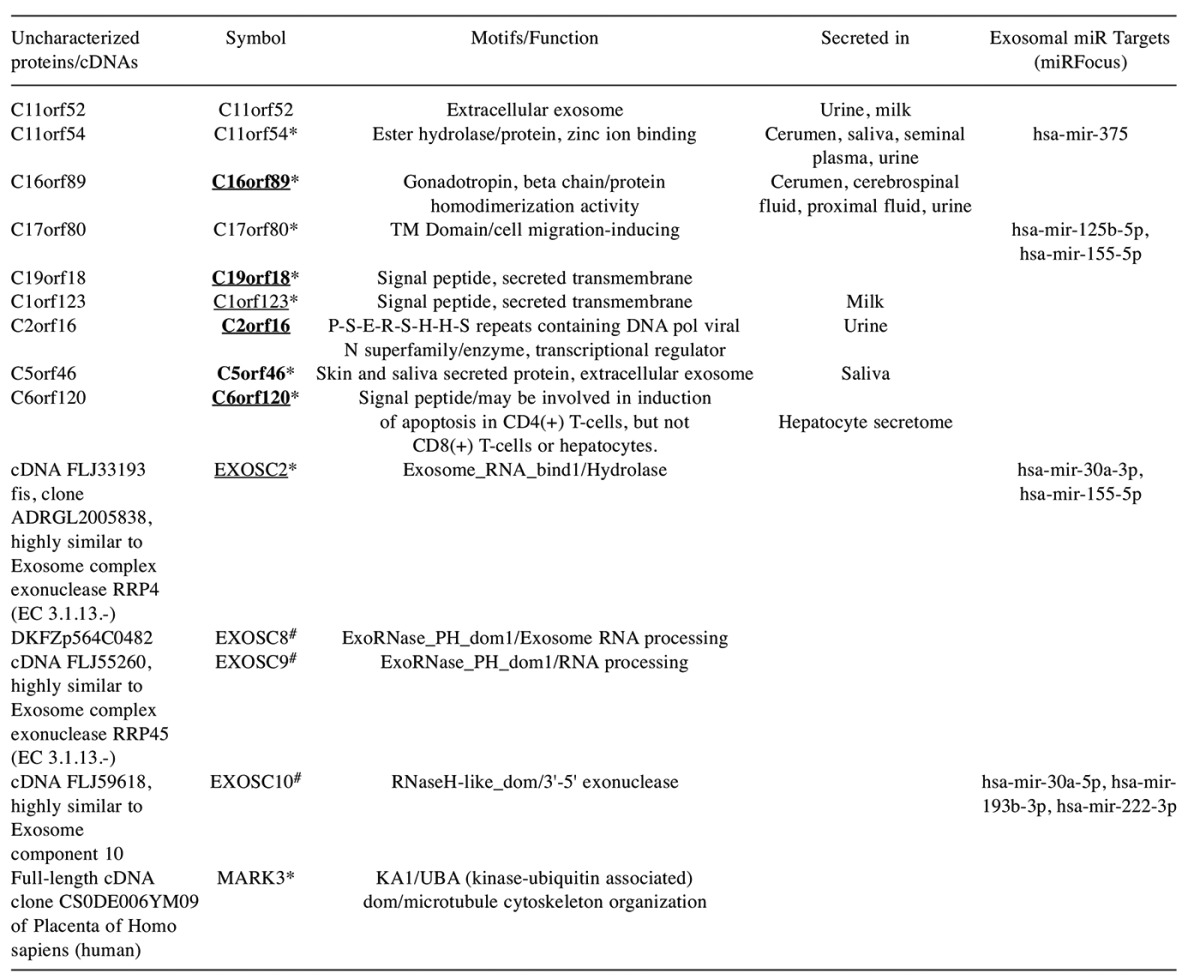
The uncharacterized ORF proteins were characterized using the Dark matter ORF database. The cDNAs were analyzed using the UniProtKB database. Approved symbols, protein motifs and function are shown. Disease association was inferred from DisGeNet, MalaCards, GWAS catalogue and the NCBI PheGenI databases. *Up-regulated in pancreatic cancer, #down-regulated in pancreatic cancer; over 5% of the patients showing gene amplifications or deletions are underlined. Genes with restricted expression and lack of expression in normal pancreas are in bold.
Putative pancreatic cancer exosomal lead genes. The 575 exosomal genes directly related to pancreatic cancer identified by variant analysis (Figure 5), were further enriched using filters encompassing somatic mutations, pathways, secretome nature and expression in the pancreatic juice. Twenty-two putative exosomal lead proteins emerged from these studies. These proteins encompassed druggable classes (enzymes, transporters and receptors), a cytokine, tumor suppressor genes and proteins involved in apoptosis, cell proliferation and differentiation, angiogenesis and genes involved in nucleotide and protein binding (Table III).
Table III. Pancreatic cancer exosomal genes.
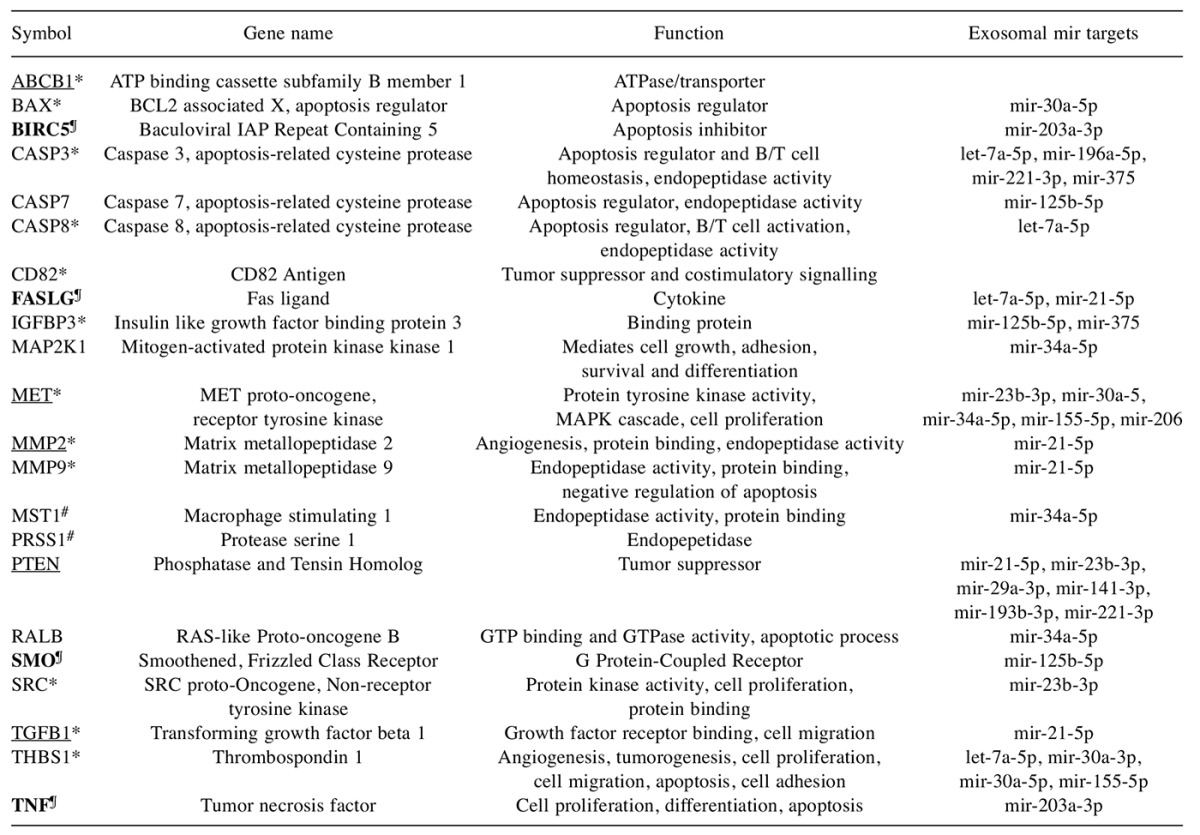
The lead known protein-coding genes are shown. Gene symbols, HGNC approved names and functional class is shown. *Up-regulated in pancretic cancer, #down-regulated in pancretic cancer (Pancreatic Expression database), ¶genetic association with neoplasms (DisGeNet). Genes with restricted expression and lack of expression in normal pancreas are bolded. Targets showing mutations >10% of the patients analyzed by ICGC are underlined. Target exosomal miRs were identified using the miR Focus.
Fifteen proteins ATP binding cassette subfamily B member 1 (ABCB1), BCL2 associated X, apoptosis regulator (BAX1), baculoviral IAP repeat containing 5 (BIRC5), caspase 3, apoptosis-related cysteine protease (CASP3), caspase 8, apoptosis-related cysteine protease (CASP8), CD82 antigen (CD82), Fas ligand (FASLG), Insulin like growth factor binding protein 3 (IGFBP3), MET proto-oncogene, receptor tyrosine kinase (MET), matrix metallopeptidase 2 (MMP2), matrix metallopeptidase 9 (MMP9), smoothened, frizzled class receptor (SMO), SRC proto-oncogene, non-receptor tyrosine kinase (SRC), transforming growth factor beta 1 (TGFB1) and tumor necrosis factor (TNF) were up-regulated in pancreatic cancer. Some of the up-regulated proteins (BIRC5, FASLG, SMO and TNF) were also found to be down-regulated in distinct patient samples suggesting patient-specific variations. Genetic association studies revealed that the proteins BIRC5, FASLG, SMO and TNF had association with multiple cancers including pancreatic neoplasm. These four proteins also showed restricted expression in multiple tissues and were absent in the normal pancreas. The ICGC dataset analysis showed that the proteins ABCB1, MET, MMP2, Phosphatase and Tensin Homolog (PTEN) and TGFB1 harbored mutations in more than 10% of the pancreatic cancer patients. Interestingly, nineteen of the twenty-two pancreatic cancer exosomal proteins were also targets for the exosomal miRs identified in the study.
Comparison of these 22 protein targets against the DrugBank list of targets identified seven Federal Drug Administration (FDA) approved targets for diverse cancers. These targets include Mitogen-activated protein kinase kinase 1.
MAPK1 (metastatic melanoma, unresectable melanoma), MET (Advanced renal cell carcinoma, progressive metastatic medullary thyroid cancer), MMP2 (angiogenesis and metastasis inhibitor), MMP9 (angiogenesis and metastasis inhibitor), SMO (Locally advanced basal cell carcinoma, metastatic basal cell carcinoma), SRC (Chronic myelogenous leukemia) and TNF (multiple myeloma).
Discussion
Extracellular vesicular exosomes are becoming attractive targets for diagnosis and therapy and may provide a basis for drug delivery. Secretion of proteins and miRs in the exosomes provides a framework for biomarkers development. The field of exosomes research is continuing to evolve rapidly. In the last twenty years, the number of peer-reviewed publications on exosomes has increased exponentially. The precise function of the exosomes in normal cell physiology and in pathogenesis of diseased phenotype is unclear. Accumulating knowledgebases of exosomes in cancer (54), neurological (55), metabolic and cardiac disease (56), immune function (57) and virus infections (58) is beginning to provide a strong rationale for intervention and therapy for diverse diseases.
Increasing results demonstrate the relevance of exosomal proteins and miRs in the progression of pancreatic cancer (59-61). In pancreatic ductal adenocarcinoma (PDAC) patients, serum-derived exosomes possess significantly higher glypican-1 (GPC1) in 100% cases when compared to the exosomes from healthy donors (12). MET proto-oncogene, receptor tyrosine kinase (MET) is implicated in chemoresistance in PDAC and offers a therapeutic target (62). The PRSS1 gene intron mutations may be a common perturbation in chronic pancreatitis of pancreatic cancer (63).
In PDAC, the involvement of different microRNAs including miR-10a, miR-21, miR-34a miR-150, miR-155, miR-203, miR-210, miR-212-3p let-7, miR-744, miR-1246 and others are documented in several studies (64). Exosomal microRNA such as miR-212-3p, miR-203, miR-21 have been shown to enhance invasion, modulate immune response and drug resistance (65,66).
In addition to miRs, long non-coding RNAs (lncRNA) were also identified in the study. One of the lncRNAs, HOX Transcript Antisense RNA (HOTAIR) has been shown to be strongly up-regulated in the saliva of pancreatic cancer patients (67). The H19, imprinted maternally expressed transcript (H19) is involved in promotion of pancreatic cancer metastasis perhaps by involving epithelial-mesenchymal transition (68). Polymorphisms in the H19 gene is associated with risk for bladder cancer development (69). The pseudogene, heat shock protein 90 alpha family class A member 2, pseudogene (HSP90AA2P) is significantly associated with perineural invasion and may provide a biomarker (70).
The 22 lead candidate protein-coding genes identified in this study include several druggable classes such as enzymes (protease, metallopeptidases, kinases, GTPase and caspases); receptor (Frizzled Class) and transporters (ATP binding cassette). In addition, cytokines, growth and angiogenic factors were also identified.
Seven of these targets (MAPK1, MET, MMP2, MMP9, SMO, SRC and TNF) are FDA approved drug targets and the drugs are already used in the clinic for diverse cancer therapy. These drugs can be readily repurposed for pancreatic cancer treatment. The other fifteen proteins identified in the study offer new opportunities for cancer-related drug discovery efforts.
Nine exosomal ORF proteins and five cDNAs were identified in the study. These novel uncharacterized genes included enzymes (RNA processing exosomal enzymes), transcriptional regulators and secreted molecules. The cancer association of the C16orf19 (Lung cancer, renal cell carcinoma), C5orf46 (Renal cell carcinoma), EXOSC2 (Liver neoplasms) and MARK3 (brain stem astrocytic neoplasm) provides additional tumor markers potential. The C5orf46 skin and saliva secreted extracellular exosomal protein may provide a noninvasive diagnostic potential (71).
The 22 exosomal protein-coding genes identified in the study in turn were regulated by the exosomal miRs (Tables I and III). This suggests a network of gene regulation for these pancreatic cancer exosomal targets. Further investigations with these genes might offer a potential for development of multiple markers for diagnosis.
Pancreatic cancer diagnosis and therapy needs urgent molecular targets. The poor prognosis of pancreatic cancer due to advanced-stage presentation in most patients necessitates the discovery of novel, reliable, and non-invasive or minimally invasive biomarkers for early detection. The database of pancreatic cancer-related secreted exosomal genes and ncRNAs identified in this study could offer clinical relevance in early detection, improved prognosis and development of targeted therapies. The database of genes developed in the study will be made available freely to investigators as supplemental table upon request.
In summary, the results demonstrate the usefulness of bioinformatics approaches to pancreatic cancer target discovery. The database of pancreatic cancer exosomal proteins and miRs established in the study provides a starting point for discovery of novel diagnostics and therapeutics. A panel of pancreatic cancer related biomarkers encompassing the protein coding genes and ncRNAs can be tested for clinical validation.
Conflicts of Interest
The Authors declare no conflicts of interest.
Data Availability
Complete data is available upon request.
Acknowledgements
This work was supported in part by the Genomics of Cancer Fund, Florida Atlantic University Foundation. The Authors thank Jeanine Narayanan for editorial assistance.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, Wu CC, LeBleu VS, Kalluri R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527(7579):525–530. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahlert C, Kalluri R. Exosomes in tumor microenvironment influence cancer progression and metastasis. J Mol Med (Berl) 2013;91(4):431–437. doi: 10.1007/s00109-013-1020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Thery C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA. 2016;113(8):E968–977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahlert C, Melo SA, Protopopov A, Tang J, Seth S, Koch M, Zhang J, Weitz J, Chin L, Futreal A, Kalluri R. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem. 2014;289(7):3869–3875. doi: 10.1074/jbc.C113.532267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 8.Balaj L, Lessard R, Dai L, Cho YJ, Pomeroy SL, Breakefield XO, Skog J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun. 2011;2:180. doi: 10.1038/ncomms1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keller S, Ridinger J, Rupp AK, Janssen JW, Altevogt P. Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med. 2011;9:86. doi: 10.1186/1479-5876-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Record M, Subra C, Silvente-Poirot S, Poirot M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol. 2011;81(10):1171–1182. doi: 10.1016/j.bcp.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 12.Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, Reissfelder C, Pilarsky C, Fraga MF, Piwnica-Worms D, Kalluri R. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523(7559):177–182. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou B, Xu JW, Cheng YG, Gao JY, Hu SY, Wang L, Zhan HX. Early detection of pancreatic cancer: Where are we now and where are we going? Int J Cancer. 2017 doi: 10.1002/ijc.30670. doi:10.1002/ijc.30670. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126(4):1208–1215. doi: 10.1172/JCI81135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Yuan X, Shi H, Wu L, Qian H, Xu W. Exosomes in cancer: small particle, big player. Journal of Hematology & Oncology. 2015;8(1):83. doi: 10.1186/s13045-015-0181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karagiannis GS, Pavlou MP, Diamandis EP. Cancer secretomics reveal pathophysiological pathways in cancer molecular oncology. Mol Oncol. 2010;4(6):496–510. doi: 10.1016/j.molonc.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.San Lucas FA, Allenson K, Bernard V, Castillo J, Kim DU, Ellis K, Ehli EA, Davies GE, Petersen JL, Li D, Wolff R, Katz M, Varadhachary G, Wistuba I, Maitra A, Alvarez H. Minimally invasive genomic and transcriptomic profiling of visceral cancers by next-generation sequencing of circulating exosomes. Ann Oncol. 2016;27(4):635–641. doi: 10.1093/annonc/mdv604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadota T, Yoshioka Y, Fujita Y, Kuwano K, Ochiya T. Extracellular vesicles in lung cancer-From bench to bedside. Semin Cell Dev Biol. 2017;S1084-9521(17):30140–30144. doi: 10.1016/j.semcdb.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Rabinowits G, Gercel-Taylor C, Day JM, Taylor DD, Kloecker GH. Exosomal microRNA: a diagnostic marker for lung cancer. Clin Lung Cancer. 2009;10(1):42–46. doi: 10.3816/CLC.2009.n.006. [DOI] [PubMed] [Google Scholar]
- 20.Majem B, Rigau M, Reventos J, Wong DT. Non-coding RNAs in saliva: emerging biomarkers for molecular diagnostics. Int J Mol Sci. 2015;16(4):8676–8698. doi: 10.3390/ijms16048676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel GK, Patton MC, Singh S, Khushman M, Singh AP. Pancreatic Cancer Exosomes: Shedding Off for a Meaningful Journey. Pancreat Disord Ther. 2016;6(2):e148. doi: 10.4172/2165-7092.1000e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rappaport N, Twik M, Nativ N, Stelzer G, Bahir I, Stein TI, Safran M, Lancet D. MalaCards: A Comprehensive Automatically-Mined Database of Human Diseases. Curr Protoc Bioinformatics. 2014;47(1.24):1–19. doi: 10.1002/0471250953.bi0124s47. [DOI] [PubMed] [Google Scholar]
- 23.Pinero J, Queralt-Rosinach N, Bravo A, Deu-Pons J, Bauer-Mehren A, Baron M, Sanz F, Furlong LI. DisGeNET: a discovery platform for the dynamical exploration of human diseases and their genes. Database. 2015;2015:bav028. doi: 10.1093/database/bav028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karolchik D, Barber GP, Casper J, Clawson H, Cline MS, Diekhans M, Dreszer TR, Fujita PA, Guruvadoo L, Haeussler M, Harte RA, Heitner S, Hinrichs AS, Learned K, Lee BT, Li CH, Raney BJ, Rhead B, Rosenbloom KR, Sloan CA, Speir ML, Zweig AS, Haussler D, Kuhn RM, Kent WJ. The UCSC Genome Browser database: 2014 update. Nucleic Acids Res. 2014;42:D764–770. doi: 10.1093/nar/gkt1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huntley RP, Sawford T, Mutowo-Meullenet P, Shypitsyna A, Bonilla C, Martin MJ, O'Donovan C. The GOA database: gene Ontology annotation updates for 2015. Nucleic Acids Res. 2015;43:D1057–1063. doi: 10.1093/nar/gku1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, Samuel M, Pathan M, Jois M, Chilamkurti N, Gangoda L, Mathivanan S. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J Mol Biol. 2016;428(4):688–692. doi: 10.1016/j.jmb.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Safran M, Dalah I, Alexander J, Rosen N, Iny Stein T, Shmoish M, Nativ N, Bahir I, Doniger T, Krug H, Sirota-Madi A, Olender T, Golan Y, Stelzer G, Harel A, Lancet D. GeneCards Version 3: the human gene integrator. Database. 2010;2010:baq020. doi: 10.1093/database/baq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim MS, Pinto SM, Getnet D, Nirujogi RS, Manda SS, Chaerkady R, Madugundu AK, Kelkar DS, Isserlin R, Jain S, Thomas JK, Muthusamy B, Leal-Rojas P, Kumar P, Sahasrabuddhe NA, Balakrishnan L, Advani J, George B, Renuse S, Selvan LD, Patil AH, Nanjappa V, Radhakrishnan A, Prasad S, Subbannayya T, Raju R, Kumar M, Sreenivasamurthy SK, Marimuthu A, Sathe GJ, Chavan S, Datta KK, Subbannayya Y, Sahu A, Yelamanchi SD, Jayaram S, Rajagopalan P, Sharma J, Murthy KR, Syed N, Goel R, Khan AA, Ahmad S, Dey G, Mudgal K, Chatterjee A, Huang TC, Zhong J, Wu X, Shaw PG, Freed D, Zahari MS, Mukherjee KK, Shankar S, Mahadevan A, Lam H, Mitchell CJ, Shankar SK, Satishchandra P, Schroeder JT, Sirdeshmukh R, Maitra A, Leach SD, Drake CG, Halushka MK, Prasad TS, Hruban RH, Kerr CL, Bader GD, Iacobuzio-Donahue CA, Gowda H, Pandey A. A draft map of the human proteome. Nature. 2014;509(7502):575–581. doi: 10.1038/nature13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilhelm M, Schlegl J, Hahne H, Moghaddas Gholami A, Lieberenz M, Savitski MM, Ziegler E, Butzmann L, Gessulat S, Marx H, Mathieson T, Lemeer S, Schnatbaum K, Reimer U, Wenschuh H, Mollenhauer M, Slotta-Huspenina J, Boese JH, Bantscheff M, Gerstmair A, Faerber F, Kuster B. Mass-spectrometry-based draft of the human proteome. Nature. 2014;509(7502):582–587. doi: 10.1038/nature13319. [DOI] [PubMed] [Google Scholar]
- 30.Ramos EM, Hoffman D, Junkins HA, Maglott D, Phan L, Sherry ST, Feolo M, Hindorff LA. Phenotype-Genotype Integrator (PheGenI): synthesizing genome-wide association study (GWAS) data with existing genomic resources. Eur J Hum Genet. 2014;22(1):144–147. doi: 10.1038/ejhg.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welter D, MacArthur J, Morales J, Burdett T, Hall P, Junkins H, Klemm A, Flicek P, Manolio T, Hindorff L, Parkinson H. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 2014;42(Database issue):D1001–1006. doi: 10.1093/nar/gkt1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landrum MJ, Lee JM, Riley GR, Jang W, Rubinstein WS, Church DM, Maglott DR. ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 2014;42(Database issue):D980–985. doi: 10.1093/nar/gkt1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Artimo P, Jonnalagedda M, Arnold K, Baratin D, Csardi G, de Castro E, Duvaud S, Flegel V, Fortier A, Gasteiger E, Grosdidier A, Hernandez C, Ioannidis V, Kuznetsov D, Liechti R, Moretti S, Mostaguir K, Redaschi N, Rossier G, Xenarios I, Stockinger H. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012;40(Web Server issue):W597–603. doi: 10.1093/nar/gks400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.UniProt C. Update on activities at the Universal Protein Resource (UniProt) in 2013. Nucleic Acids Res. 2013;41(Database issue):D43–47. doi: 10.1093/nar/gks1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forbes SA, Bindal N, Bamford S, Cole C, Kok CY, Beare D, Jia M, Shepherd R, Leung K, Menzies A, Teague JW, Campbell PJ, Stratton MR, Futreal PA. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2011;39(Database issue):D945–950. doi: 10.1093/nar/gkq929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.International Cancer Genome C, Hudson TJ, Anderson W, Artez A, Barker AD, Bell C, Bernabe RR, Bhan MK, Calvo F, Eerola I, Gerhard DS, Guttmacher A, Guyer M, Hemsley FM, Jennings JL, Kerr D, Klatt P, Kolar P, Kusada J, Lane DP, Laplace F, Youyong L, Nettekoven G, Ozenberger B, Peterson J, Rao TS, Remacle J, Schafer AJ, Shibata T, Stratton MR, Vockley JG, Watanabe K, Yang H, Yuen MM, Knoppers BM, Bobrow M, Cambon-Thomsen A, Dressler LG, Dyke SO, Joly Y, Kato K, Kennedy KL, Nicolas P, Parker MJ, Rial-Sebbag E, Romeo-Casabona CM, Shaw KM, Wallace S, Wiesner GL, Zeps N, Lichter P, Biankin AV, Chabannon C, Chin L, Clement B, de Alava E, Degos F, Ferguson ML, Geary P, Hayes DN, Hudson TJ, Johns AL, Kasprzyk A, Nakagawa H, Penny R, Piris MA, Sarin R, Scarpa A, Shibata T, van de Vijver M, Futreal PA, Aburatani H, Bayes M, Botwell DD, Campbell PJ, Estivill X, Gerhard DS, Grimmond SM, Gut I, Hirst M, Lopez-Otin C, Majumder P, Marra M, McPherson JD, Nakagawa H, Ning Z, Puente XS, Ruan Y, Shibata T, Stratton MR, Stunnenberg HG, Swerdlow H, Velculescu VE, Wilson RK, Xue HH, Yang L, Spellman PT, Bader GD, Boutros PC, Campbell PJ, Flicek P, Getz G, Guigo R, Guo G, Haussler D, Heath S, Hubbard TJ, Jiang T, Jones SM, Li Q, Lopez-Bigas N, Luo R, Muthuswamy L, Ouellette BF, Pearson JV, Puente XS, Quesada V, Raphael BJ, Sander C, Shibata T, Speed TP, Stein LD, Stuart JM, Teague JW, Totoki Y, Tsunoda T, Valencia A, Wheeler DA, Wu H, Zhao S, Zhou G, Stein LD, Guigo R, Hubbard TJ, Joly Y, Jones SM, Kasprzyk A, Lathrop M, Lopez-Bigas N, Ouellette BF, Spellman PT, Teague JW, Thomas G, Valencia A, Yoshida T, Kennedy KL, Axton M, Dyke SO, Futreal PA, Gerhard DS, Gunter C, Guyer M, Hudson TJ, McPherson JD, Miller LJ, Ozenberger B, Shaw KM, Kasprzyk A, Stein LD, Zhang J, Haider SA, Wang J, Yung CK, Cros A, Liang Y, Gnaneshan S, Guberman J, Hsu J, Bobrow M, Chalmers DR, Hasel KW, Joly Y, Kaan TS, Kennedy KL, Knoppers BM, Lowrance WW, Masui T, Nicolas P, Rial-Sebbag E, Rodriguez LL, Vergely C, Yoshida T, Grimmond SM, Biankin AV, Bowtell DD, Cloonan N, deFazio A, Eshleman JR, Etemadmoghadam D, Gardiner BB, Kench JG, Scarpa A, Sutherland RL, Tempero MA, Waddell NJ, Wilson PJ, McPherson JD, Gallinger S, Tsao MS, Shaw PA, Petersen GM, Mukhopadhyay D, Chin L, DePinho RA, Thayer S, Muthuswamy L, Shazand K, Beck T, Sam M, Timms L, Ballin V, Lu Y, Ji J, Zhang X, Chen F, Hu X, Zhou G, Yang Q, Tian G, Zhang L, Xing X, Li X, Zhu Z, Yu Y, Yu J, Yang H, Lathrop M, Tost J, Brennan P, Holcatova I, Zaridze D, Brazma A, Egevard L, Prokhortchouk E, Banks RE, Uhlen M, Cambon-Thomsen A, Viksna J, Ponten F, Skryabin K, Stratton MR, Futreal PA, Birney E, Borg A, Borresen-Dale AL, Caldas C, Foekens JA, Martin S, Reis-Filho JS, Richardson AL, Sotiriou C, Stunnenberg HG, Thoms G, van de Vijver M, van't Veer L, Calvo F, Birnbaum D, Blanche H, Boucher P, Boyault S, Chabannon C, Gut I, Masson-Jacquemier JD, Lathrop M, Pauporte I, Pivot X, Vincent-Salomon A, Tabone E, Theillet C, Thomas G, Tost J, Treilleux I, Calvo F, Bioulac-Sage P, Clement B, Decaens T, Degos F, Franco D, Gut I, Gut M, Heath S, Lathrop M, Samuel D, Thomas G, Zucman-Rossi J, Lichter P, Eils R, Brors B, Korbel JO, Korshunov A, Landgraf P, Lehrach H, Pfister S, Radlwimmer B, Reifenberger G, Taylor MD, von Kalle C, Majumder PP, Sarin R, Rao TS, Bhan MK, Scarpa A, Pederzoli P, Lawlor RA, Delledonne M, Bardelli A, Biankin AV, Grimmond SM, Gress T, Klimstra D, Zamboni G, Shibata T, Nakamura Y, Nakagawa H, Kusada J, Tsunoda T, Miyano S, Aburatani H, Kato K, Fujimoto A, Yoshida T, Campo E, Lopez-Otin C, Estivill X, Guigo R, de Sanjose S, Piris MA, Montserrat E, Gonzalez-Diaz M, Puente XS, Jares P, Valencia A, Himmelbauer H, Quesada V, Bea S, Stratton MR, Futreal PA, Campbell PJ, Vincent-Salomon A, Richardson AL, Reis-Filho JS, van de Vijver M, Thomas G, Masson-Jacquemier JD, Aparicio S, Borg A, Borresen-Dale AL, Caldas C, Foekens JA, Stunnenberg HG, van't Veer L, Easton DF, Spellman PT, Martin S, Barker AD, Chin L, Collins FS, Compton CC, Ferguson ML, Gerhard DS, Getz G, Gunter C, Guttmacher A, Guyer M, Hayes DN, Lander ES, Ozenberger B, Penny R, Peterson J, Sander C, Shaw KM, Speed TP, Spellman PT, Vockley JG, Wheeler DA, Wilson RK, Hudson TJ, Chin L, Knoppers BM, Lander ES, Lichter P, Stein LD, Stratton MR, Anderson W, Barker AD, Bell C, Bobrow M, Burke W, Collins FS, Compton CC, DePinho RA, Easton DF, Futreal PA, Gerhard DS, Green AR, Guyer M, Hamilton SR, Hubbard TJ, Kallioniemi OP, Kennedy KL, Ley TJ, Liu ET, Lu Y, Majumder P, Marra M, Ozenberger B, Peterson J, Schafer AJ, Spellman PT, Stunnenberg HG, Wainwright BJ, Wilson RK, Yang H. International network of cancer genome projects. Nature. 2010;464(7291):993–998. doi: 10.1038/nature08987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rhodes DR, Kalyana-Sundaram S, Mahavisno V, Varambally R, Yu J, Briggs BB, Barrette TR, Anstet MJ, Kincead-Beal C, Kulkarni P, Varambally S, Ghosh D, Chinnaiyan AM. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 2007;9(2):166–180. doi: 10.1593/neo.07112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gray KA, Yates B, Seal RL, Wright MW, Bruford EA. genenames.org: the HGNC resources in 2015. Nucleic Acids Res. 2015;43(Database issue):D1079–85. doi: 10.1093/nar/gku1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delgado A, Chapado MJ, Brandao P, Hamid S, Narayanan R. Atlas of the Open reading Frames in human diseases: Dark matter of the human genome. MOJ Proteomics Bioinform. 2015;2(1):00036. [PubMed] [Google Scholar]
- 41.Xie B, Ding Q, Han H, Wu D. miRCancer: a microRNA-cancer association database constructed by text mining on literature. Bioinformatics. 2013;29(5):638–644. doi: 10.1093/bioinformatics/btt014. [DOI] [PubMed] [Google Scholar]
- 42.Jiang Q, Wang Y, Hao Y, Juan L, Teng M, Zhang X, Li M, Wang G, Liu Y. miR2Disease: a manually curated database for microRNA deregulation in human disease. Nucleic Acids Res. 2009;37(Database issue):D98–104. doi: 10.1093/nar/gkn714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X. miRDB: a microRNA target prediction and functional annotation database with a wiki interface. RNA. 2008;14(6):1012–1017. doi: 10.1261/rna.965408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chou CH, Chang NW, Shrestha S, Hsu SD, Lin YL, Lee WH, Yang CD, Hong HC, Wei TY, Tu SJ, Tsai TR, Ho SY, Jian TY, Wu HY, Chen PR, Lin NC, Huang HT, Yang TL, Pai CY, Tai CS, Chen WL, Huang CY, Liu CC, Weng SL, Liao KW, Hsu WL, Huang HD. miRTarBase 2016: updates to the experimentally validated miRNA-target interactions database. Nucleic Acids Res. 2016;44(D1):D239–247. doi: 10.1093/nar/gkv1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ning S, Zhang J, Wang P, Zhi H, Wang J, Liu Y, Gao Y, Guo M, Yue M, Wang L, Li X. Lnc2Cancer: a manually curated database of experimentally supported lncRNAs associated with various human cancers. Nucleic Acids Res. 2016;44(D1):D980–985. doi: 10.1093/nar/gkv1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen G, Wang Z, Wang D, Qiu C, Liu M, Chen X, Zhang Q, Yan G, Cui Q. LncRNADisease: a database for long-non-coding RNA-associated diseases. Nucleic Acids Res. 2013;41(Database issue):D983–986. doi: 10.1093/nar/gks1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amaral PP, Clark MB, Gascoigne DK, Dinger ME, Mattick JS. lncRNAdb: a reference database for long noncoding RNAs. Nucleic Acids Res. 2011;39(Database issue):D146–151. doi: 10.1093/nar/gkq1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang JH, Li JH, Shao P, Zhou H, Chen YQ, Qu LH. starBase: a database for exploring microRNA-mRNA interaction maps from Argonaute CLIP-Seq and Degradome-Seq data. Nucleic Acids Res. 2011;39(Database issue):D202–209. doi: 10.1093/nar/gkq1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parkinson H, Sarkans U, Shojatalab M, Abeygunawardena N, Contrino S, Coulson R, Farne A, Lara GG, Holloway E, Kapushesky M, Lilja P, Mukherjee G, Oezcimen A, Rayner T, Rocca-Serra P, Sharma A, Sansone S, Brazma A. ArrayExpress – a public repository for microarray gene expression data at the EBI. Nucleic Acids Res. 2005;33(Database issue):D553–555. doi: 10.1093/nar/gki056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dayem Ullah AZ, Cutts RJ, Ghetia M, Gadaleta E, Hahn SA, Crnogorac-Jurcevic T, Lemoine NR, Chelala C. The pancreatic expression database: recent extensions and updates. Nucleic Acids Res. 2014;42(Database issue):D944–949. doi: 10.1093/nar/gkt959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Narayanan R. Phenome-Genome Association Studies of Pancreatic Cancer: New Targets for Therapy and Diagnosis. Cancer Genomics Proteomics. 2015;12(1):9–19. [PubMed] [Google Scholar]
- 52.Narayanan R. Druggable Cancer Secretome: Neoplasm-associated Traits. Cancer Genomics Proteomics. 2015;12(3):119–131. [PubMed] [Google Scholar]
- 53.Wright MW, Bruford EA. Naming 'junk': human non-protein coding RNA (ncRNA) gene nomenclature. Hum Genomics. 2011;5(2):90–98. doi: 10.1186/1479-7364-5-2-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whiteside TL. Tumor-Derived Exosomes and Their Role in Cancer Progression. Adv Clin Chem. 2016;74:103–141. doi: 10.1016/bs.acc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Howitt J, Hill AF. Exosomes in the Pathology of Neurodegenerative Diseases. J Biol Chem. 2016;291(52):26589–26597. doi: 10.1074/jbc.R116.757955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iisalo E, Kanto J, Aaltonen L, Makela J. Flunitrazepam as an induction agent in children. A clinical and pharmacokinetic study. Br J Anaesth. 1984;56(8):899–902. doi: 10.1093/bja/56.8.899. [DOI] [PubMed] [Google Scholar]
- 57.Greening DW, Gopal SK, Xu R, Simpson RJ, Chen W. Exosomes and their roles in immune regulation and cancer. Semin Cell Dev Biol. 2015;40:72–81. doi: 10.1016/j.semcdb.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 58.Madison MN, Okeoma CM. Exosomes: Implications in HIV-1 Pathogenesis. Viruses. 2015;7(7):4093–4118. doi: 10.3390/v7072810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu L, Risch HA. Exosomes: potential for early detection in pancreatic cancer. Future Oncol. 2016;12(8):1081–1090. doi: 10.2217/fon-2015-0005. [DOI] [PubMed] [Google Scholar]
- 60.Takikawa T, Masamune A, Yoshida N, Hamada S, Kogure T, Shimosegawa T. Exosomes Derived From Pancreatic Stellate Cells: MicroRNA Signature and Effects on Pancreatic Cancer Cells. Pancreas. 2017;46(1):19–27. doi: 10.1097/MPA.0000000000000722. [DOI] [PubMed] [Google Scholar]
- 61.Tang YT, Xu XH, Yang XD, Hao J, Cao H, Zhu W, Zhang SY, Cao JP. Role of non-coding RNAs in pancreatic cancer: the bane of the microworld. World J Gastroenterol. 2014;20(28):9405–9417. doi: 10.3748/wjg.v20.i28.9405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Delitto D, Vertes-George E, Hughes SJ, Behrns KE, Trevino JG. c-Met signaling in the development of tumorigenesis and chemoresistance: potential applications in pancreatic cancer. World J Gastroenterol. 2014;20(26):8458–8470. doi: 10.3748/wjg.v20.i26.8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gao F, Liu QC, Zhang S, Zhuang ZH, Lin CZ, Lin XH. PRSS1 intron mutations in patients with pancreatic cancer and chronic pancreatitis. Mol Med Rep. 2012;5(2):449–451. doi: 10.3892/mmr.2011.684. [DOI] [PubMed] [Google Scholar]
- 64.Srivastava SK, Arora S, Singh S, Bhardwaj A, Averett C, Singh AP. MicroRNAs in pancreatic malignancy: progress and promises. Cancer Lett. 2014;347(2):167–174. doi: 10.1016/j.canlet.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ding G, Zhou L, Qian Y, Fu M, Chen J, Chen J, Xiang J, Wu Z, Jiang G, Cao L. Pancreatic cancer-derived exosomes transfer miRNAs to dendritic cells and inhibit RFXAP expression via miR-212-3p. Oncotarget. 2015;6(30):29877–29888. doi: 10.18632/oncotarget.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou M, Chen J, Zhou L, Chen W, Ding G, Cao L. Pancreatic cancer derived exosomes regulate the expression of TLR4 in dendritic cells via miR-203. Cell Immunol. 2014;292(1-2):65–69. doi: 10.1016/j.cellimm.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 67.Xie Z, Chen X, Li J, Guo Y, Li H, Pan X, Jiang J, Liu H, Wu B. Salivary HOTAIR and PVT1 as novel biomarkers for early pancreatic cancer. Oncotarget. 2016;7(18):25408–25419. doi: 10.18632/oncotarget.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma C, Nong K, Zhu H, Wang W, Huang X, Yuan Z, Ai K. H19 promotes pancreatic cancer metastasis by derepressing let-7's suppression on its target HMGA2-mediated EMT. Tumour Biol. 2014;35(9):9163–9169. doi: 10.1007/s13277-014-2185-5. [DOI] [PubMed] [Google Scholar]
- 69.Verhaegh GW, Verkleij L, Vermeulen SH, den Heijer M, Witjes JA, Kiemeney LA. Polymorphisms in the H19 gene and the risk of bladder cancer. Eur Urol. 2008;54(5):1118–1126. doi: 10.1016/j.eururo.2008.01.060. [DOI] [PubMed] [Google Scholar]
- 70.Jiang H, Duan B, He C, Geng S, Shen X, Zhu H, Sheng H, Yang C, Gao H. Cytoplasmic HSP90alpha expression is associated with perineural invasion in pancreatic cancer. Int J Clin Exp Pathol. 2014;7(6):3305–3311. [PMC free article] [PubMed] [Google Scholar]
- 71.Clark HF, Gurney AL, Abaya E, Baker K, Baldwin D, Brush J, Chen J, Chow B, Chui C, Crowley C, Currell B, Deuel B, Dowd P, Eaton D, Foster J, Grimaldi C, Gu Q, Hass PE, Heldens S, Huang A, Kim HS, Klimowski L, Jin Y, Johnson S, Lee J, Lewis L, Liao D, Mark M, Robbie E, Sanchez C, Schoenfeld J, Seshagiri S, Simmons L, Singh J, Smith V, Stinson J, Vagts A, Vandlen R, Watanabe C, Wieand D, Woods K, Xie MH, Yansura D, Yi S, Yu G, Yuan J, Zhang M, Zhang Z, Goddard A, Wood WI, Godowski P, Gray A. The secreted protein discovery initiative (SPDI), a large-scale effort to identify novel human secreted and transmembrane proteins: a bioinformatics assessment. Genome Res. 2003;13(10):2265–2270. doi: 10.1101/gr.1293003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Complete data is available upon request.


