Abstract
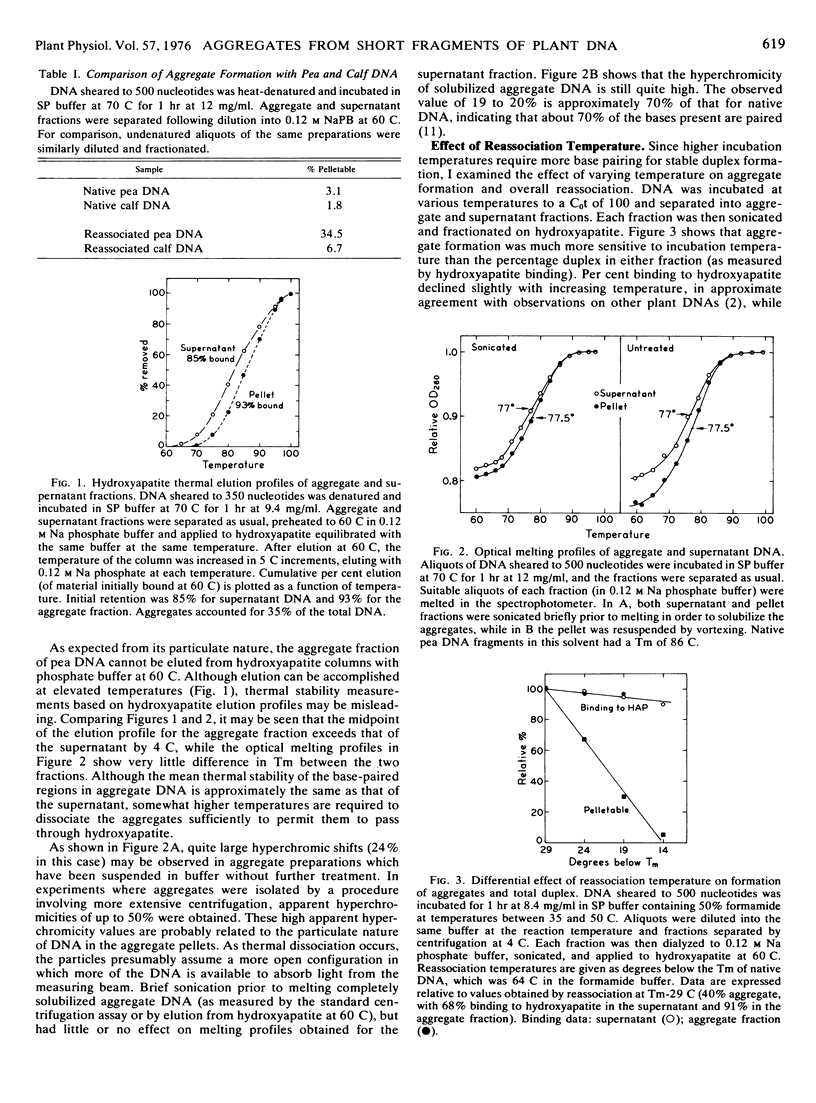
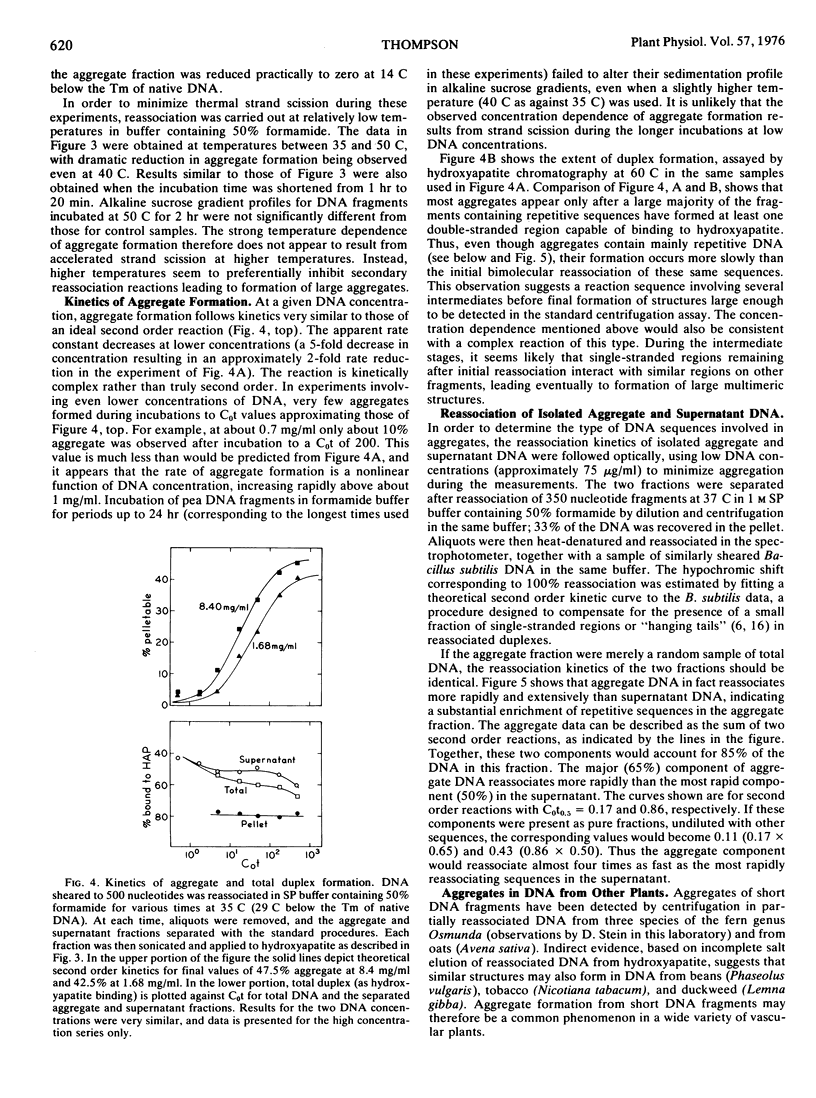
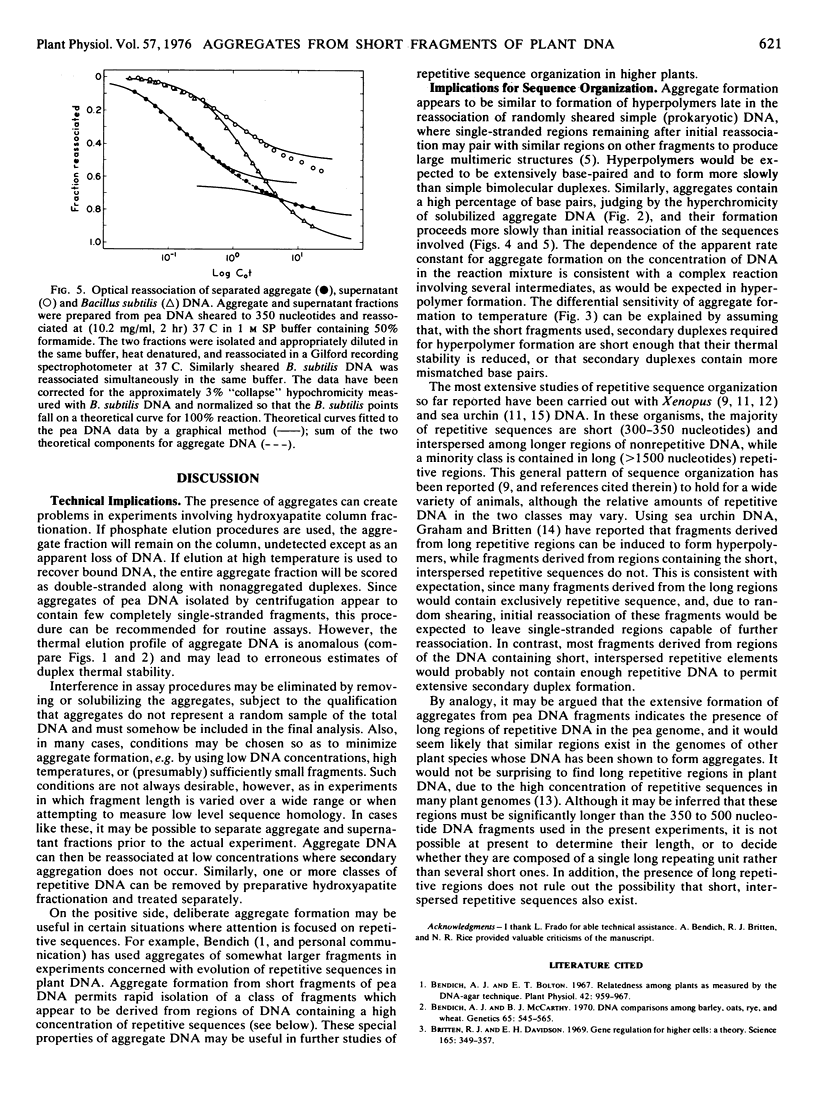
Large aggregates have been observed after partial reassociation of pea (Pisum sativum L.) DNA preparations sheared to mean single strand fragment lengths as short as 350 nucleotides. At high DNA concentrations and conditions of salt and temperature which require only moderate precision of base pairing, aggregates pelletable by brief centrifugation account for 30 to 40% of the total DNA from peas, while calf thymus DNA reassociated under similar conditions forms less than 10% pelletable structures. In contrast to networks formed during the reassociation of long DNA fragments containing interspersed repetitive sequences, these aggregates contain a high percentage of double-stranded DNA and are enriched in repetitive sequences.
Aggregates detectable by centrifugation do not begin to appear until after extensive repetitive sequence reassociation has already occurred. The results are consistent with a model involving secondary reassociation between single-stranded regions (“hanging tails”) remaining after initial duplex formation. This process would lead to formation of large multimers of the original fragments, analogous to the large hyperpolymers which have been observed in extensively reassociated prokaryotic DNA. Randomly sheared fragments containing short (about 300 base pairs) repetitive sequences interspersed with single copy DNA would not be expected to hyperpolymerize significantly under these conditions. I suggest, as a working hypothesis, that much of the repetitive sequence DNA in peas is contained in regions considerably longer than 300 base pairs.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendich A. J., Bolton E. T. Relatedness Among Plants as Measured by the DNA-Agar Technique. Plant Physiol. 1967 Jul;42(7):959–967. doi: 10.1104/pp.42.7.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendich A. J., McCarthy B. J. DNA Comparisons among Barley, Oats, Rye, and Wheat. Genetics. 1970 Aug;65(4):545–565. doi: 10.1093/genetics/65.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Davidson E. H. Gene regulation for higher cells: a theory. Science. 1969 Jul 25;165(3891):349–357. doi: 10.1126/science.165.3891.349. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Davidson E. H. Repetitive and non-repetitive DNA sequences and a speculation on the origins of evolutionary novelty. Q Rev Biol. 1971 Jun;46(2):111–138. doi: 10.1086/406830. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Chamberlin M. E., Britten R. J., Davidson E. H. Sequence organization in Xenopus DNA studied by the electron microscope. J Mol Biol. 1975 Aug 5;96(2):317–333. doi: 10.1016/0022-2836(75)90351-4. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Britten R. J. Organization, transcription, and regulation in the animal genome. Q Rev Biol. 1973 Dec;48(4):565–613. doi: 10.1086/407817. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Graham D. E., Neufeld B. R., Chamberlin M. E., Amenson C. S., Hough B. R., Britten R. J. Arrangement and characterization of repetitive sequence elements in animal DNAs. Cold Spring Harb Symp Quant Biol. 1974;38:295–301. doi: 10.1101/sqb.1974.038.01.033. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Hough B. R., Amenson C. S., Britten R. J. General interspersion of repetitive with non-repetitive sequence elements in the DNA of Xenopus. J Mol Biol. 1973 Jun 15;77(1):1–23. doi: 10.1016/0022-2836(73)90359-8. [DOI] [PubMed] [Google Scholar]
- Flavell R. B., Bennett M. D., Smith J. B., Smith D. B. Genome size and the proportion of repeated nucleotide sequence DNA in plants. Biochem Genet. 1974 Oct;12(4):257–269. doi: 10.1007/BF00485947. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Thermal renaturation of deoxyribonucleic acids. J Mol Biol. 1961 Oct;3:585–594. doi: 10.1016/s0022-2836(61)80023-5. [DOI] [PubMed] [Google Scholar]
- Thompson W. F., Cleland R. Auxin and ribonucleic Acid synthesis in pea stem tissue as studied by deoxyribonucleic Acid-ribonucleic Acid hybridization. Plant Physiol. 1971 Dec;48(6):663–670. doi: 10.1104/pp.48.6.663. [DOI] [PMC free article] [PubMed] [Google Scholar]


