Abstract
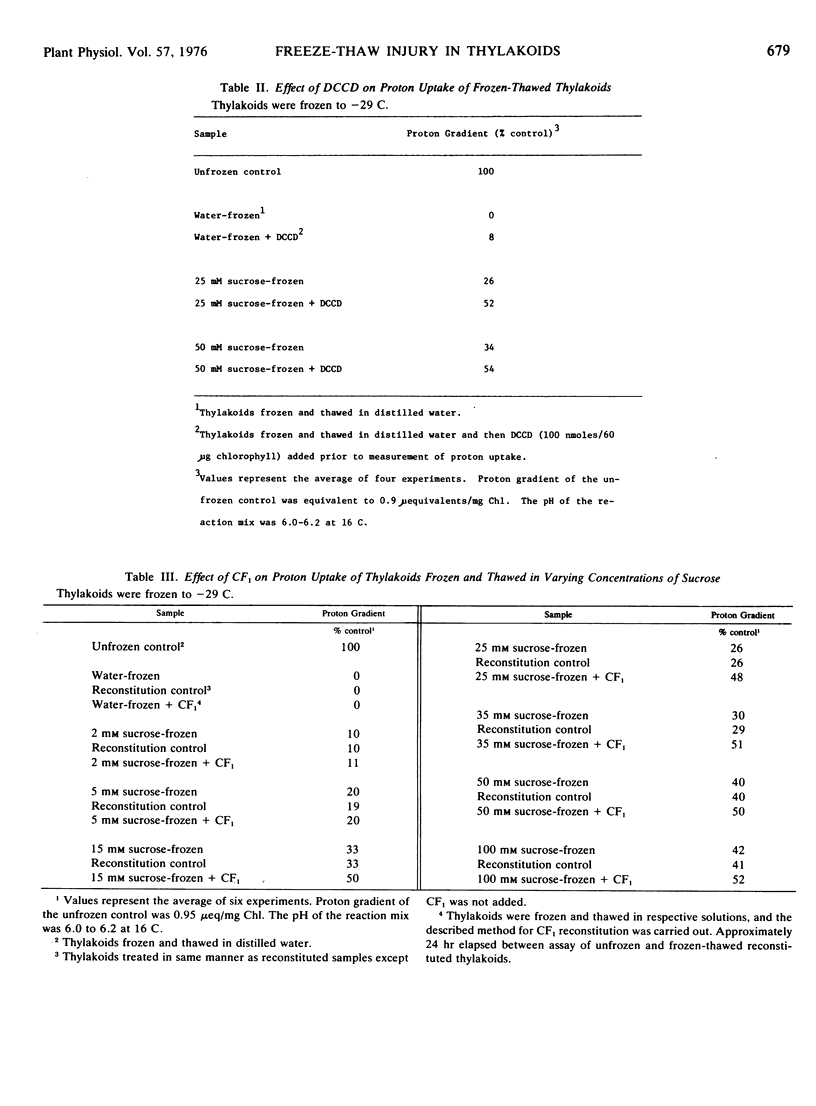
Plastocyanin and chloroplast coupling factor 1 (CF1) are released from spinach (Spinacia oleracea L.) thylakoids during a slow freezethaw cycle. CF1 addition increases the proton uptake of thylakoids previously frozen in sucrose concentrations of 15 mm to 100 mm. Addition of CF1 and plastocyanin restores the proton uptake of thylakoids frozen in 100 mm sucrose. Plastocyanin and CF1 release is a manifestation, not the cause, of freeze-thaw damage.
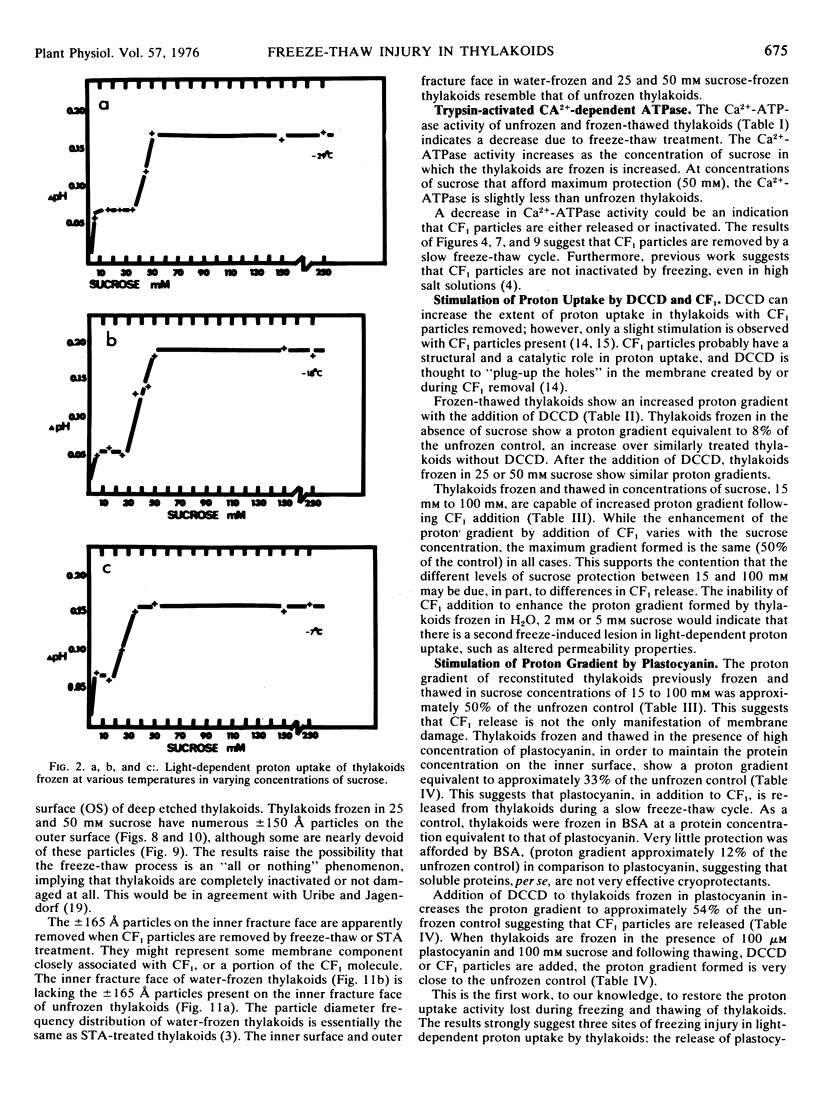
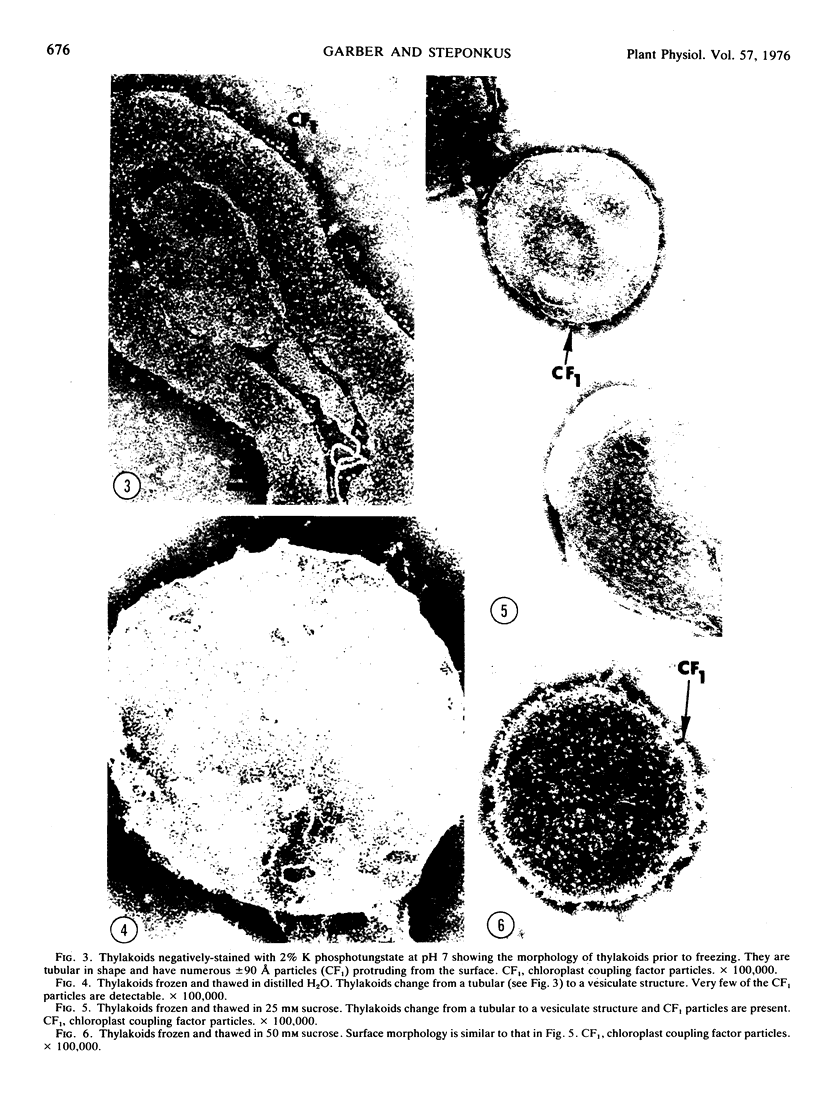
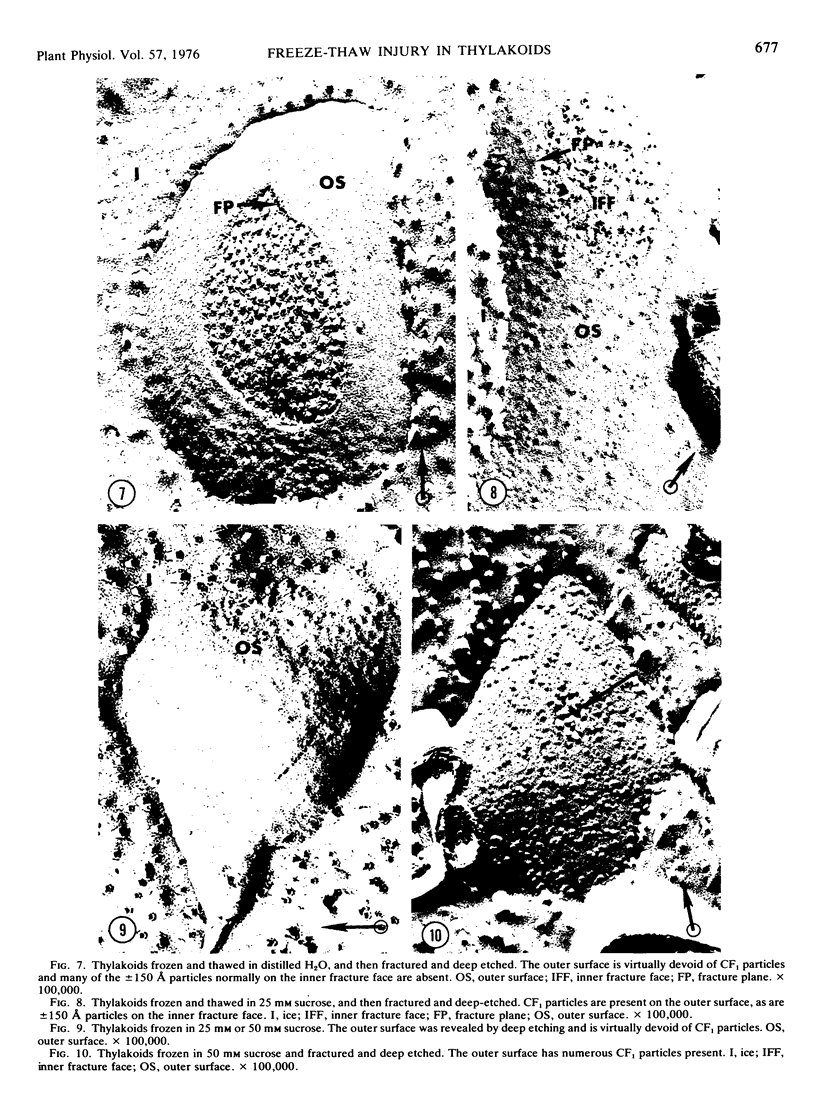
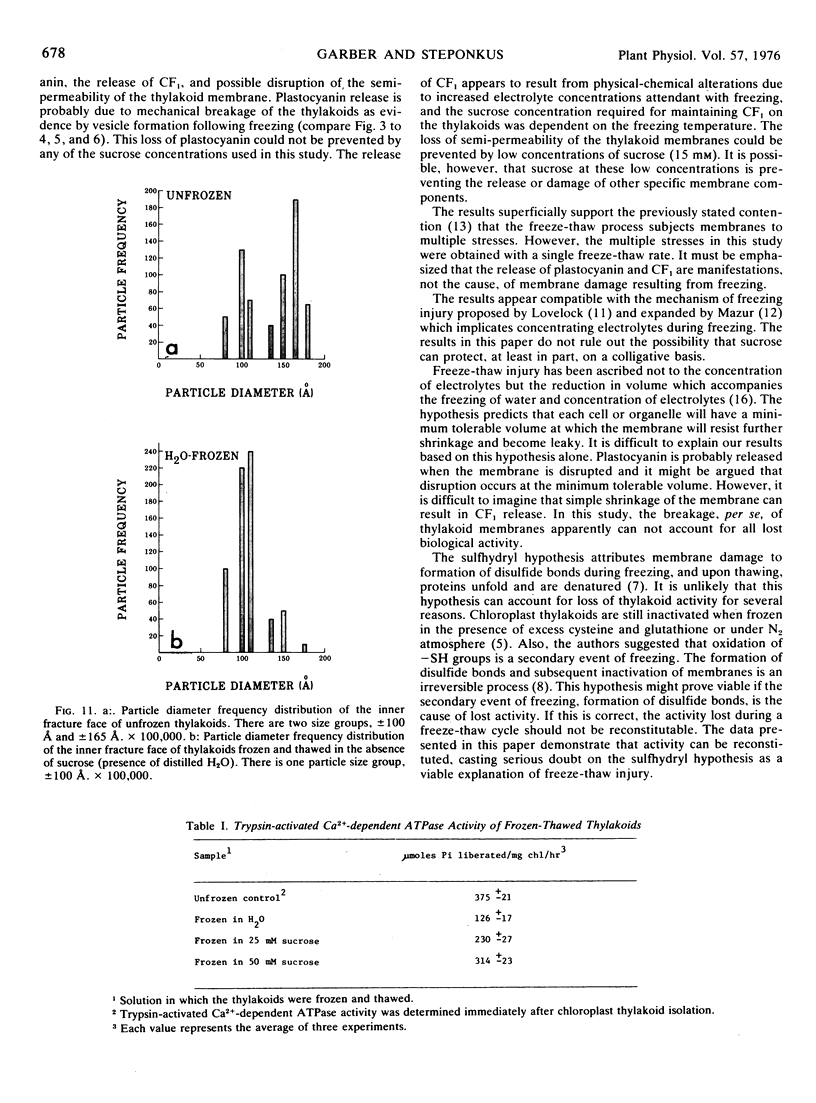
Frozen-thawed thylakoids appear to exhibit two levels of response to sucrose as measured by light-dependent proton uptake. Different levels of protection afforded by sucrose may be due, in part, to quantitative differences in CF1 release. The results suggest at least three freeze-induced lesions in light-dependent proton uptake by thylakoids: plastocyanin release, CF1 release, and disruption of the semi-permeability of thylakoids.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. M., McCarty R. E. The effects of plastocyanin on photophosphorylation. Biochim Biophys Acta. 1969 Oct 21;189(2):193–206. doi: 10.1016/0005-2728(69)90047-4. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
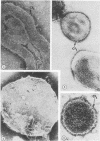
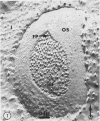
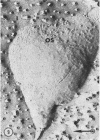
- Garber M. P., Steponkus P. L. Identification of chloroplast coupling factor by freeze-etching and negative-staining techniques. J Cell Biol. 1974 Oct;63(1):24–34. doi: 10.1083/jcb.63.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U. W., Santarius K. A. Loss of Adenosine Triphosphate Synthesis Caused by Freezing and Its Relationship to Frost Hardiness Problems. Plant Physiol. 1964 Sep;39(5):712–719. doi: 10.1104/pp.39.5.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U. Freezing injury and uncoupling of phosphorylation from electron transport in chloroplasts. Plant Physiol. 1967 Oct;42(10):1343–1350. doi: 10.1104/pp.42.10.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U., Tyankova L., Santarius K. A. Effects of freezing on biological membranes in vivo and in vitro. Biochim Biophys Acta. 1973 Jan 2;291(1):23–37. doi: 10.1016/0005-2736(73)90057-6. [DOI] [PubMed] [Google Scholar]
- LOVELOCK J. E. The haemolysis of human red blood-cells by freezing and thawing. Biochim Biophys Acta. 1953 Mar;10(3):414–426. doi: 10.1016/0006-3002(53)90273-x. [DOI] [PubMed] [Google Scholar]
- Lien S., Racker E. Partial resolution of the enzymes catalyzing photophosphorylation. 8. Properties of silicotungstate-treated subchloroplast particles. J Biol Chem. 1971 Jul 10;246(13):4298–4307. [PubMed] [Google Scholar]
- Mazur P. Cryobiology: the freezing of biological systems. Science. 1970 May 22;168(3934):939–949. doi: 10.1126/science.168.3934.939. [DOI] [PubMed] [Google Scholar]
- Mazur P. The role of cell membranes in the freezing of yeast and other single cells. Ann N Y Acad Sci. 1965 Oct 13;125(2):658–676. doi: 10.1111/j.1749-6632.1965.tb45420.x. [DOI] [PubMed] [Google Scholar]
- McCarty R. E., Racker E. Effect of a coupling factor and its antiserum on photophosphorylation and hydrogen ion transport. Brookhaven Symp Biol. 1966;19:202–214. [PubMed] [Google Scholar]
- Meryman H. T. Modified model for the mechanism of freezing injury in erythrocytes. Nature. 1968 Apr 27;218(5139):333–336. doi: 10.1038/218333a0. [DOI] [PubMed] [Google Scholar]
- NEUMANN J., JAGENDORF A. T. LIGHT-INDUCED PH CHANGES RELATED PHOSPHORYLATION BY CHLOROPLASTS. Arch Biochem Biophys. 1964 Jul;107:109–119. doi: 10.1016/0003-9861(64)90276-0. [DOI] [PubMed] [Google Scholar]
- Uribe E. G., Jagendorf A. T. Membrane permeability and internal volume as factors in ATP synthesis by spinach chloroplasts. Arch Biochem Biophys. 1968 Nov;128(2):351–359. doi: 10.1016/0003-9861(68)90041-6. [DOI] [PubMed] [Google Scholar]
- Williams R. J., Meryman H. T. Freezing injury and resistance in spinach chloroplast grana. Plant Physiol. 1970 Jun;45(6):752–755. doi: 10.1104/pp.45.6.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Sakai A. Phospholipid degradation in frozen plant cells associated with freezing injury. Plant Physiol. 1974 Mar;53(3):509–511. doi: 10.1104/pp.53.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]