Abstract
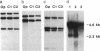
Osteopetrotic (op/op) mutant mice suffer from congenital osteopetrosis due to a severe deficiency of osteoclasts. Furthermore, the total number of mononuclear phagocytes is extremely low in affected mice. Serum, 11 tissues, and different cell and organ conditioned media from op/op mice were shown to be devoid of biologically active colony-stimulating factor 1 (CSF-1), whereas all of these preparations from littermate control +/+ and +/op mice contained the growth factor. The deficiency was specific for CSF-1 in that serum or conditioned media from op/op mice possessed elevated levels of at least three other macrophage growth factors. Partial correction of the op/op defect was observed following intraperitoneal implantation of diffusion chambers containing L929 cells, which in culture produce CSF-1 as their sole macrophage growth factor. No rearrangement of the CSF-1 gene in op/op mice was detected by Southern analysis. However, in contrast to control lung fibroblasts, which contained 4.6- and 2.3-kilobase CSF-1 mRNAs, only the 4.6-kilobase species was detected in op/op cells. An alteration in the CSF-1 gene is strongly implicated as the primary defect in op/op mice because they do not contain detectable CSF-1, their defect is correctable by administration of CSF-1, the op locus and the CSF-1 gene map within the same region of mouse chromosome 3, their CSF-1 mRNA biosynthesis is altered, and the op/op phenotype is consistent with the phenotype expected in a CSF-1 deficient mouse.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arceci R. J., Shanahan F., Stanley E. R., Pollard J. W. Temporal expression and location of colony-stimulating factor 1 (CSF-1) and its receptor in the female reproductive tract are consistent with CSF-1-regulated placental development. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8818–8822. doi: 10.1073/pnas.86.22.8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartocci A., Mastrogiannis D. S., Migliorati G., Stockert R. J., Wolkoff A. W., Stanley E. R. Macrophages specifically regulate the concentration of their own growth factor in the circulation. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6179–6183. doi: 10.1073/pnas.84.17.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartocci A., Pollard J. W., Stanley E. R. Regulation of colony-stimulating factor 1 during pregnancy. J Exp Med. 1986 Sep 1;164(3):956–961. doi: 10.1084/jem.164.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley T. R., Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966 Jun;44(3):287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Garland J., Scott D., Scolnick E., Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980 Oct 1;152(4):1036–1047. doi: 10.1084/jem.152.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisselbrecht S., Sola B., Fichelson S., Bordereaux D., Tambourin P., Mattei M. G., Simon D., Guenet J. L. The murine M-CSF gene is localized on chromosome 3. Blood. 1989 May 1;73(6):1742–1745. [PubMed] [Google Scholar]
- Hattersley G., Dorey E., Horton M. A., Chambers T. J. Human macrophage colony-stimulating factor inhibits bone resorption by osteoclasts disaggregated from rat bone. J Cell Physiol. 1988 Oct;137(1):199–203. doi: 10.1002/jcp.1041370125. [DOI] [PubMed] [Google Scholar]
- Hume D. A., Pavli P., Donahue R. E., Fidler I. J. The effect of human recombinant macrophage colony-stimulating factor (CSF-1) on the murine mononuclear phagocyte system in vivo. J Immunol. 1988 Nov 15;141(10):3405–3409. [PubMed] [Google Scholar]
- Kelso A., Owens T. Production of two hemopoietic growth factors is differentially regulated in single T lymphocytes activated with an anti-T cell receptor antibody. J Immunol. 1988 Feb 15;140(4):1159–1167. [PubMed] [Google Scholar]
- Ladner M. B., Martin G. A., Noble J. A., Wittman V. P., Warren M. K., McGrogan M., Stanley E. R. cDNA cloning and expression of murine macrophage colony-stimulating factor from L929 cells. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6706–6710. doi: 10.1073/pnas.85.18.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald B. R., Mundy G. R., Clark S., Wang E. A., Kuehl T. J., Stanley E. R., Roodman G. D. Effects of human recombinant CSF-GM and highly purified CSF-1 on the formation of multinucleated cells with osteoclast characteristics in long-term bone marrow cultures. J Bone Miner Res. 1986 Apr;1(2):227–233. doi: 10.1002/jbmr.5650010210. [DOI] [PubMed] [Google Scholar]
- Marks S. C., Jr, Lane P. W. Osteopetrosis, a new recessive skeletal mutation on chromosome 12 of the mouse. J Hered. 1976 Jan-Feb;67(1):11–18. doi: 10.1093/oxfordjournals.jhered.a108657. [DOI] [PubMed] [Google Scholar]
- Minkin C. Bone acid phosphatase: tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcif Tissue Int. 1982 May;34(3):285–290. doi: 10.1007/BF02411252. [DOI] [PubMed] [Google Scholar]
- Moore M. A. G-CSF: its relationship to leukemia differentiation-inducing activity and other hemopoietic regulators. J Cell Physiol Suppl. 1982;1:53–64. doi: 10.1002/jcp.1041130411. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Fong T. A. Specific assays for cytokine production by T cells. J Immunol Methods. 1989 Jan 17;116(2):151–158. doi: 10.1016/0022-1759(89)90198-1. [DOI] [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D., Matsumoto M., Johnson G. R. Purification of a factor inducing differentiation in murine myelomonocytic leukemia cells. Identification as granulocyte colony-stimulating factor. J Biol Chem. 1983 Jul 25;258(14):9017–9023. [PubMed] [Google Scholar]
- Pojda Z., Szczylik C., Wiktor-Jedrzejczak W. Multiple lineage colony growth from human marrow in plasma clot diffusion chambers. Exp Hematol. 1987 Oct;15(9):922–927. [PubMed] [Google Scholar]
- Pollard J. W., Bartocci A., Arceci R., Orlofsky A., Ladner M. B., Stanley E. R. Apparent role of the macrophage growth factor, CSF-1, in placental development. Nature. 1987 Dec 3;330(6147):484–486. doi: 10.1038/330484a0. [DOI] [PubMed] [Google Scholar]
- Stanley E. R. Colony-stimulating factor (CSF) radioimmunoassay: detection of a CSF subclass stimulating macrophage production. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2969–2973. doi: 10.1073/pnas.76.6.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. R. The macrophage colony-stimulating factor, CSF-1. Methods Enzymol. 1985;116:564–587. doi: 10.1016/s0076-6879(85)16044-1. [DOI] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W. W., Ahmed A., Szczylik C., Skelly R. R. Hematological characterization of congenital osteopetrosis in op/op mouse. Possible mechanism for abnormal macrophage differentiation. J Exp Med. 1982 Nov 1;156(5):1516–1527. doi: 10.1084/jem.156.5.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wijngaert F. P., Tas M. C., van der Meer J. W., Burger E. H. Growth of osteoclast precursor-like cells from whole mouse bone marrow: inhibitory effect of CSF-1. Bone Miner. 1987 Nov;3(2):97–110. [PubMed] [Google Scholar]