Abstract
IMPORTANCE
During hospitalization for hematopoietic stem cell transplantation (HCT), patients receive high-dose chemotherapy before transplantation and experience significant physical and psychological symptoms and poor quality of life (QOL).
OBJECTIVE
To assess the effect of inpatient palliative care on patient- and caregiver-reported outcomes during hospitalization for HCT and 3 months after transplantation.
DESIGN, SETTING, AND PARTICIPANTS
Nonblinded randomized clinical trial among 160 adults with hematologic malignancies undergoing autologous/allogeneic HCT and their caregivers (n = 94). The study was conducted from August 2014 to January 2016 in a Boston hospital; follow-up was completed in May 2016.
INTERVENTIONS
Patients assigned to the intervention (n=81) were seen by palliative care clinicians at least twice a week during HCT hospitalization; the palliative intervention was focused on management of physical and psychological symptoms. Patients assigned to standard transplant care (n=79) could be seen by palliative care clinicians on request.
MAIN OUTCOMES AND MEASURES
Primary: change in patient QOL from baseline to week 2; secondary: patient-assessed mood, fatigue, and symptom burden scores at baseline, 2 weeks, and 3 months after HCT and caregiver-assessed QOL and mood at baseline and 2 weeks after HCT.
RESULTS
Among 160 patients (mean age, 60 [SD, 13.3] years;91 women [56.9%]; median hospital stay, 21 days) and 94 caregivers, 157 (98.1%) and 89 (94.7%), respectively, completed 2-weekfollow-up, and 149 patients (93.1%) completed 3-monthfollow-up. Intervention patients reported a smaller decrease in QOL from baseline to week 2 vs controls. Intervention patients had less increase in depression, lower anxiety, no difference in fatigue, and less increase in symptom burden. At 3 months, intervention patients had higher QOL and less depression but no significant differences in anxiety, fatigue, or symptom burden. From baseline to week 2 after HCT, caregivers of intervention patients vs controls reported no significant differences in QOL or anxiety but had a smaller increase in depression (mean, 0.25 vs 1.80; mean difference, 1.55; 95%CI, 0.14–2.96; P = .03).
| Patient Outcomes | Mean Score at Week 2
|
Mean Difference Between Groups (95% CI) | P Value | |
|---|---|---|---|---|
| Standard Care | Palliative Care | |||
| Quality of life (change from baseline) | −21.54 | −14.72 | −6.82 (−13.48 to −0.16) | .045 |
| Fatigue | −13.65 | −10.30 | −3.34 (−7.25 to 0.56) | .09 |
| Symptom burden | 23.14 | 17.35 | 5.80 (0.49 to 11.10) | .03 |
| Depression | 3.92 | 2.43 | 1.49 (0.20 to 2.78) | .02 |
| Anxiety | 1.12 | −0.80 | 1.92 (0.83 to 3.01) | <.001 |
CONCLUSIONS AND RELEVANCE
Among adults at a single institution undergoing HCT for hematologic malignancy, the use of inpatient palliative care compared with standard transplant care resulted in a smaller decrease in QOL 2 weeks after transplantation. Further research is needed for replication and to assess longer-term outcomes and cost implications.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT02207322
Patients with hematologic malignancies hospitalized for hematopoietic stem cell transplantation (HCT) experience physical symptoms due to chemotherapy-induced toxic effects and early post transplantation complications.1–4 These symptoms, along with the physical isolation patients experience during the 3- to 4-week hospitalization, can contribute to a decline in their quality of life (QOL) and mood throughout their hospital stay.4–6 Furthermore, the distress patients experience during transplantation often persists and has long-term QOL and psychological consequences, further exacerbating the morbidity of HCT.2,5,7 Despite the physical and psychological burden experienced by patients undergoing HCT, studies of interventions to improve their QOL and reduce their distress during HCT are limited.1,4,8–10 Clinicians often perceive patients’ physical and psychological symptoms during transplantation to be expected and unmodifiable.4,11 Although palliative care clinicians are increasingly asked to care for patients with solid tumors, they are infrequently consulted for patients with hematologic malignancies.12–14
This randomized clinical trial assessed the effect of inpatient palliative care integrated with standard transplant care on patient-reported QOL, mood, and symptom burden during hospitalization for HCT and at 3 months after HCT. The study also explored the effect of the intervention on caregivers’ QOL and mood during patients’ HCT hospitalization. The study hypotheses were that patients receiving the intervention would have a smaller decrease in their QOL and mood, lower symptom burden during their transplant hospitalization, and lower psychological distress at 3 months after HCT compared with patients receiving standard transplant care.
Methods
Study Procedures
This study was approved by the Dana Farber Harvard Cancer Center Institutional Review Board and willing participants provided written informed consent (see trial protocol in Supplement 1). From August 12, 2014, to January 26, 2016, adult patients with hematologic malignancies admitted for autologous and allogeneic HCT at Massachusetts General Hospital and their caregivers were enrolled in a nonblinded randomized clinical trial of early palliative care integrated with standard transplant care vs standard transplant care alone. Consecutively eligible patients with planned autologous or allogeneic HCT were identified during the weekly transplant team meetings. Research staff obtained permission by email from the treating oncologist to approach eligible patients and their caregivers within 72 hours of their transplant admission (HCT hospitalization). After providing informed consent, participants completed baseline study questionnaires. Patients were then randomized to the palliative care intervention or standard transplant care using a computer-generated 1:1 randomization stratified by type of HCT (autologous, myeloablative allogeneic, or reduced-intensity allogeneic HCT).
Participants
Patients aged 18 years or older who could speak English or complete questionnaires with minimal assistance from an interpreter were eligible to participate. Patients with a history of HCT or those with psychiatric or comorbid disease that the oncologist believed would interfere with adherence to study procedures were excluded. Enrolled patients were asked to identify a caregiver (a relative or a friend who either lived with the patient or had in-person contact with him/her at least twice per week) who could be invited to participate in the caregiver portion of this study. Patients without a caregiver were still eligible to participate.
Palliative Care Intervention
Intervention patients met with the inpatient palliative care physician or advanced practice nurse within 72 hours of randomization. The palliative care clinician followed patients up longitudinally during their hospitalization, seeing them at least twice per week. Patients, caregivers, and the palliative care clinicians were permitted to initiate additional visits as needed.
Given the focus on the transplant hospitalization period, the palliative care intervention primarily focused on managing patients’ physical and psychological symptoms during hospitalization for HCT and did not include additional components of palliative care such as advance care planning, goals-of-care and code status discussions, or end-of-life decision making.
The palliative care intervention was developed based on a review of the literature, findings of prior preliminary work examining the experience of patients hospitalized for HCT,4 and input from 3 palliative care clinicians. Study investigators created an intervention manual (eAppendix in Supplement 2) that provided guidelines for addressing nausea, pain and mucositis, fatigue, insomnia, bowel problems, and psychological distress. Both pharmacological recommendations and behavioral interventions were included in the manual. The study manual did not mandate the timing of addressing each symptom because patients may experience symptoms at different points during their HCT. Palliative care clinicians documented the symptoms and topics addressed during each visit using Research Electronic Data Capture (REDCap). After each visit, the palliative care clinicians communicated their recommendations in person to the transplant team and documented their recommendations in the medical record. The palliative care visits were billed as part of the inpatient hospitalization bill to the patient’s insurance company.
Standard Transplant Care
Control patients received standard transplant care with the supportive care measures instituted by the transplant team. Patients, caregivers, and transplant clinicians were permitted to request consultation with palliative care clinicians.
Study Measures
Participants completed study questionnaires prior to randomization and during the second week of hospitalization for HCT (at patients’ blood count nadir; ie, the period during HCT hospitalization when patients experience the lowest blood cell counts and highest toxicity and symptom burden: day 5 after stem cell infusion for autologous and day 8 after stem cell infusion for allogeneic HCT, with a 2-day window) and at 3 and 6 months after HCT.
Patient-Reported Measures
To describe participants’ characteristics, patients self-reported their race, sex, relationship status, education, and income using fixed categories. The 47-itemFunctional Assessment of Cancer Therapy–Bone Marrow Transplant (FACT-BMT; range, 0–164; higher scores represent better QOL), which includes subscales assessing physical, functional, emotional, social well-being, and bone marrow transplant–specific concerns during the past week, was used to assess patients’ QOL.15 A 5-point change in the FACT-BMT score is considered clinically significant.4,15 Fatigue was measured using the 13-item FACT Fatigue subscale (range, 0–52).16 Higher scores on both the FACT-BMT and the FACT Fatigue subscale indicate better QOL and lower fatigue.
Patients’ anxiety and depression were measured with the 14-itemHospital Anxiety and Depression Scale (HADS), which consists of subscales assessing depression (HADS-D) and anxiety (HADS-A) symptoms in the past week, with scores ranging from 0 (no distress) to 21 (maximum distress) and cutoff scores greater than 7 indicating clinically significant symptoms.17 Patients also completed the Patient Health Questionnaire 9 (PHQ-9; range, 0–27), a 9-item measure that detects symptoms of major depressive disorder according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) and can be evaluated continuously, with higher scores indicating worse depression.18
The revised Edmonton Symptom Assessment Scale (ESAS) measures 10 symptoms on a 0- to 10-point scale, with higher scores indicating greatersymptomburden.19 Because of a clerical error, the first 38 study patients did not complete the nausea item, which was therefore omitted from the composite ESAS score analyses (range, 0–90).
Patients’ posttraumatic stress disorder (PTSD) symptoms were measured at baseline and 3 months after HCT using the PTSD Checklist–Civilian Version (range, 17–85). The PTSD Checklist is a 17-itemself-reportedmeasure that evaluates the severity of PTSD symptoms with higher scores indicating worse PTSD symptoms.
The study team reviewed patients’ electronic health records to obtain their cancer diagnosis, comorbidities, and date of transplantation. For each patient, the HCT Comorbidity Index20 was calculated at the time of their transplant consultation.
Caregiver-Reported Measures
To describe caregiver characteristics, caregivers self-reported their age, sex, race, ethnicity, religion, education, and relationship to the patient using fixed categories. The Care Giver Oncology QOL questionnaire (range, 0–116), a 29-item instrument that measures 10 QOL domains and can be analyzed by domain or using a composite score, was used tomeasureQOL.21 Caregivers also completed the HADS andPHQ-9. Caregiver outcomes were obtained only at baseline and week 2.
Study Outcomes
Primary End Point
The primary study end point was the comparison between study groups of the change in patient QOL, as measured by the FACT-BMT, from baseline to week 2 of hospitalization for HCT.
Secondary End Points
Of 26 prespecified secondary outcomes, 13 were analyzed for this study. Secondary study end points reported herein include changes in patient mood (depression and anxiety, as measured with the HADS and PHQ-9), fatigue (FACT Fatigue subscale), symptom burden (ESAS), and caregiver QOL and mood (HADS and PHQ-9) from baseline to week 2 of HCT hospitalization, as well as a comparison of patient-reported QOL, mood, fatigue, symptom burden, and PTSD between the 2 study groups 3 months after HCT. Additional secondary end points included in the protocol that will be reported separately include comparison of patient distress using the National Comprehensive Cancer Network Distress Thermometer Checklist, patient-reported outcomes (QOL, mood, fatigue, symptom burden, and PTSD) 6 months after HCT, incidence of acute and chronic graft-vs-host disease, and nonrelapse mortality and overall survival.
Statistical Analysis
Participants’ baseline characteristics between randomized groups were summarized using frequency and percentage for categorical variables and means and standard deviations for continuous variables. The primary study end point was the comparison of the change in FACT-BMT score during hospitalization from baseline to week 2 between study groups using a 2-sample t test. The second week of hospitalization was chosen for the primary end point of the study because this is the most symptomatic phase of the transplant hospitalization.4 The secondary outcomes, including changes inpatients’ depression, anxiety, fatigue, and symptom burden, were also compared from baseline to week 2 between study groups using a 2-sample t test. The same statistical approach was used to examine changes in caregiver-reported outcomes. The HADS subscale scores were dichotomized as described above to compare frequencies of depression and anxiety symptoms between the 2 study groups at week 2 and 3 months after HCT using the Fisher exact test.
Separate exploratory post hoc analyses of covariance were conducted to compare changes in all patient-reported outcomes from baseline to week 2 and 3 months after HCT, adjusting for baseline outcome scores between the 2 study groups. In addition to complete case analyses, multiple imputations were used for missing observations as prespecified in the study protocol. The multiple imputation approach used baseline characteristics (age, sex, education, transplant type, HCT comorbidity index, and performance status) to build a regression model to impute missing outcomes data with 100 imputations. Mixed linear-effects models adjusting for baseline scores were also used on the imputed data set to assess the intervention effect on patient- and caregiver-reported outcomes at week 2 and 3 months after HCT. Transplant type (autologous vs allogeneic) was assessed as a potential moderator of the effect of the intervention using interaction terms that were added to the models.
A sample size of 160 patients (80 patients in each group) was estimated to be sufficient with 80% power to detect a 6-point change in QOL (FACT-BMT) from baseline to week 2 using a 2-sample t test with an α=.05 statistical significance level and a rate of attrition of 15%. All reported P values are 2-sidedwithP < .05 considered statistically significant. Analyses did not adjust for multiple comparisons; thus, all secondary end point analyses are exploratory. All data analyses were conducted using Stata version 9.3.
Results
Patient Participants
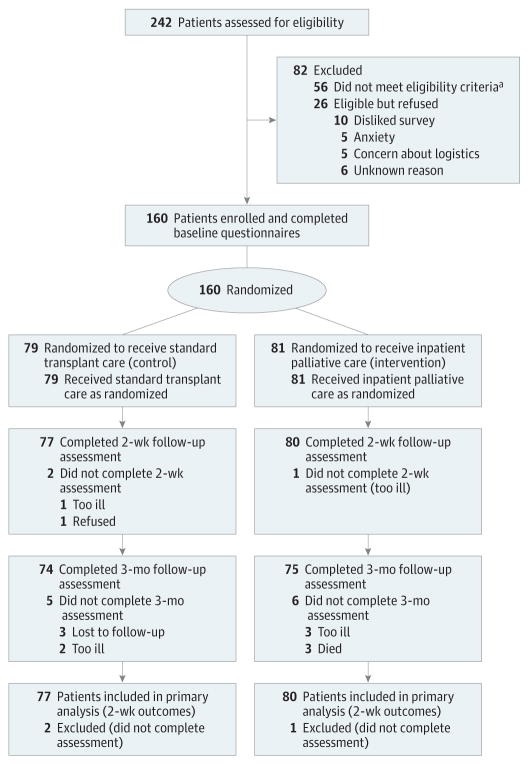
A total of 242 patients were screened for eligibility (Figure 1). One hundred eighty-six eligible patients were approached and 160 (86%) were enrolled in the study. Enrolled patients were mostly white (n = 139 [86.9%]), with a mean age of 57.1 (SD, 13.3) years; 56.9%(n = 91) were female and 50%(n = 80)were undergoing allogeneic HCT (Table 1). There were no meaningful differences in clinical characteristics between study groups, including baseline measures on the FACT-BMT, HADS, PHQ-9, FACT Fatigue, ESAS, and PTSD Checklist. Three patients (1.9%) and 11 patients (6.9%) had missing data at 2 weeks and 3 months after HCT, respectively.
Figure 1. Participant Flow in a Randomized Clinical Trial of Inpatient Palliative Care Compared With Standard Transplant Care Among Patients Hospitalized for Hematopoietic Stem Cell Transplantation (HCT).
aReasons for ineligibility included language barrier (n = 10), benign disease (n = 6), previous HCT (n=15), clinician refusal (n = 2), enrollment in another supportive care trial (n = 8), transplantation aborted within 24 hours of admission (n = 6), combined solid organ transplantation and HCT (n = 3), and primarily outpatient transplantation (n = 6).
Table 1.
Patient Baseline Characteristicsa
| Characteristics | Standard Care (n = 79) | Palliative Care (n = 81) |
|---|---|---|
| Age, mean (SD), y | 56.9 (14.1) | 57.2 (12.7) |
| Female, No. (%) | 43 (54.4) | 48 (59.3) |
| Diagnosis, No. (%) | ||
| Acute lymphoblastic leukemia | 7 (9.0) | 4 (5.0) |
| Acute myeloid leukemia/myelodysplastic syndrome | 23 (29.5) | 24 (30.0) |
| Myelofibrosis/chronic myeloid leukemia | 7 (9.0) | 8 (10.0) |
| Lymphoma | 26 (33.3) | 18 (22.5) |
| Multiple myeloma | 15 (19.2) | 26 (32.5) |
| Race, No. (%) | ||
| White | 70 (88.6) | 69 (85.2) |
| Other | 9 (11.4) | 12 (14.8) |
| Relationship status, No. (%) | ||
| Married | 55 (69.6) | 63 (77.8) |
| Divorced | 9 (11.4) | 5 (6.2) |
| Single | 10 (12.7) | 10 (12.4) |
| Widowed | 5 (6.3) | 3 (3.7) |
| Education, No. (%) | ||
| High school | 24 (30.4) | 23 (28.4) |
| College | 42 (53.2) | 35 (43.2) |
| Postgraduate | 13 (16.5) | 23 (28.4) |
| Annual income, No. (%), $ | ||
| ≤50 999 | 29 (40.3) | 20 (27.4) |
| 51 000–100 000 | 19 (26.4) | 29 (39.7) |
| >100 000 | 24 (33.3) | 24 (32.9) |
| Missing | 7 (8.9) | 8 (9.9) |
| HCT Comorbidity Index, median (IQR) | 3 (2) | 3 (3) |
| ECOG Performance Status score, No. (%) | ||
| 0 | 28 (35.4) | 27 (33.3) |
| 1 | 49 (62.0) | 54 (66.7) |
| 2 | 2 (2.5) | 0 |
| Transplant type, No. (%) | ||
| Autologous HCT | 39 (49.4) | 41 (50.6) |
| Myeloablative allogeneic HCT | 14 (17.7) | 16 (19.8) |
| Reduced-intensity allogeneic HCT | 26 (32.9) | 24 (29.6) |
| Donor type (allogeneic), No. (%) | ||
| Matched related donor | 11 (27.5) | 11 (27.5) |
| Matched unrelated donor | 23 (57.5) | 22 (55) |
| Haploidentical donor | 4 (10.0) | 7 (17.5) |
| Cord | 2 (5.0) | 0 |
| HCT hospital length of stay, mean (SD), d | 21.7 (5.4) | 21.9 (11.2) |
| FACT-BMT score, mean (SD)a | 107.3 (20.7) | 110.3 (19.1) |
| FACT Fatigue score, mean (SD)b | 36.9 (10.8) | 38.1 (10.3) |
| PHQ-9 score, mean (SD)c | 5.4 (4.7) | 4.8 (4.4) |
| HADS Depression score, mean (SD)d | 4.9 (4.1) | 4.0 (3.2) |
| HADS Anxiety score, mean (SD)d | 5.4 (3.8) | 4.6 (3.6) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; HCT, hematopoietic stem cell transplantation; IQR, interquartile range.
The range for the Functional Assessment of Cancer Therapy–Bone Marrow Transplant (FACT-BMT) is 0 to 164.
The range for the Functional Assessment of Cancer Therapy Fatigue subscale (FACT Fatigue) is 0 to 52.
The range for the Patient Health Questionnaire 9 (PHQ-9) is 0 to 27.
The range for the Hospital Anxiety and Depression Scale (HADS) Anxiety and Depression subscales is 0 to 21.
Palliative Care Visits
The median durations of HCT hospitalization for the intervention and control groups were 20 (range, 12–102) days and 21 (range, 13–40) days, respectively. All intervention patients had at least 2 palliative care visits during the first 2 weeks of their hospitalization (median number of visits, 4; range, 2–7). Intervention participants had at least 4 palliative care visits during their entire hospitalization (median number of visits, 8; range, 4–40). Two control patients received a palliative care consultation. Table 2 depicts the most commonly addressed symptoms and topics during the palliative care visits. A total of 41.8%(146/349) of palliative care visits occurred while a family member was present. The mean duration of the initial palliative care consultation visit was 59.2 (SD, 8.8) minutes, and clinicians reported spending a mean time of 60.2 (SD, 33.3) minutes per week on subsequent visits.
Table 2.
Visit Content and Symptoms Addressed During Palliative Care Visits
| Visit Content and Symptoms Addressed | No. (%) | |
|---|---|---|
| Initial Palliative Care Consultation Visit (n = 81 Visits) | Subsequent Palliative Care Visits (n = 268 Visits) | |
| Visit content | ||
| Rapport building | 80 (98.8) | 182 (67.9) |
| Symptoms | 72 (88.9) | 237 (88.4) |
| Coping | 69 (85.2) | 170 (63.4) |
| Illness understanding | 10 (12.3) | 22 (8.2) |
| Treatment decision making | 2 (2.5) | 4 (1.5) |
| Advance care planning | 2 (2.5) | 8 (3.0) |
| Symptoms addressed | ||
| Nausea | 55 (67.9) | 187 (69.8) |
| Pain | 53 (65.4) | 142 (53.0) |
| Diarrhea | 43 (53.1) | 102 (38.1) |
| Constipation | 45 (55.6) | 34 (12.7) |
| Fatigue | 31 (38.3) | 55 (20.5) |
| Insomnia | 27 (33.3) | 36 (13.4) |
| Anxiety | 27 (33.3) | 25 (9.3) |
| Depression | 9 (11.1) | 7 (2.6) |
Primary Outcome
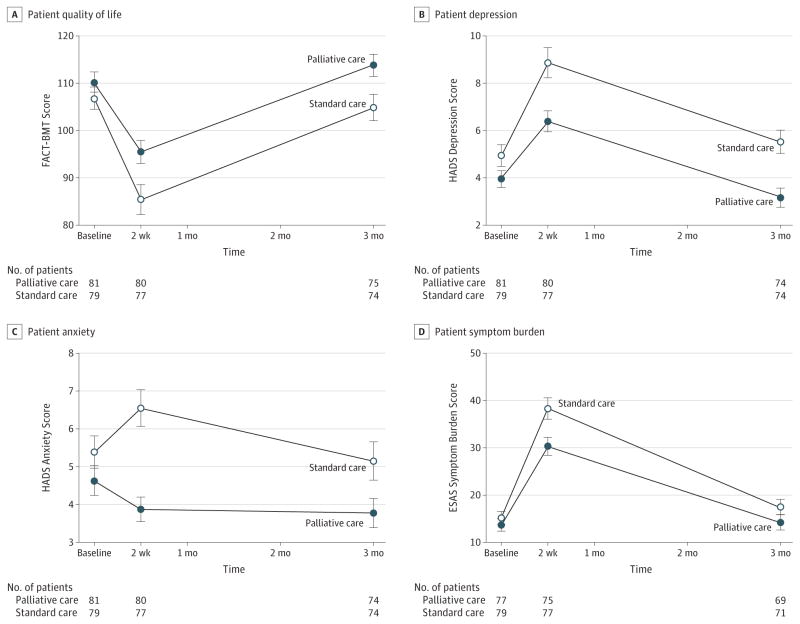
Figure 2A depicts changes in patient QOL across all time points. Intervention patients reported a lower decrease in QOL from baseline to week 2 vs control patients (intervention: mean baseline FACT-BMT score, 110.26; week 2 score, 95.46, mean change, −14.72; control: mean baseline score, 106.83; week 2 score, 85.42; mean change, −21.54; difference between groups, −6.82;95% CI, −13.48 to −0.16; P = .045).
Figure 2. Patient-Reported Quality of Life, Depression, Anxiety, and Symptom Burden Outcomes Across All Time Points by Treatment Group.
Error bars indicate 95% CIs. FACT-BMT indicates Functional Assessment of Cancer Therapy–Bone Marrow Transplant; HADS, Hospital Anxiety and Depression Scale; ESAS, Edmonton Symptom Assessment Scale.
Secondary Outcomes
As shown in Figure 2B, patients in the intervention group, compared with patients in the control group, had lower mean depression scores at 2 weeks on the HADS-D (intervention: baseline score, 3.95; week 2 score, 6.39; mean change, 2.43; control: baseline score, 4.94; week 2 score, 8.86; mean change, 3.92; difference between groups, 1.49; 95% CI, 0.20–2.78; P = .02) as well as at 3 months.
As shown in Figure 2C, patients in the intervention group reported a decrease in anxiety symptoms, whereas control patients reported an increase in anxiety symptoms from baseline to week 2 on the HADS-A (intervention: mean baseline score, 4.64; mean week 2 score, 3.87; mean change, −0.80; control: mean baseline score, 5.39; mean week 2 score, 6.55; mean change, 1.12; difference between groups, 1.92; 95% CI, 0.83–3.01; P < .001) but no significant difference at 3 months. During week 2, intervention patients were less likely to have clinically significant depression symptoms on the HADS-D (30.0% vs 59.7% among controls; P < .001) and anxiety symptoms on the HADS-A (10.0% vs 41.6% among controls; P < .001), but there were no significant differences in PHQ-9 scores (intervention: mean baseline score, 4.75; mean week 2 score, 8.03; mean change, 3.24; control: mean baseline score, 5.40; mean week 2 score, 9.75; mean change, 4.35; difference between groups, 1.11; 95% CI, −0.47 to 2.70; P = .17).
There were no significant differences between the groups in reported fatigue at 2 weeks (intervention: mean baseline score, 38.14; mean week 2 score, 27.93; mean change, −10.30; control: mean baseline score, 36.89; mean week 2 score, 23.35; mean change, −13.65; difference between groups, −3.35; 95% CI, −7.25 to 0.56; P = .09) or at 3 months.
Patients in the intervention group, compared with patients in the control group, reported lower increases in symptom burden (intervention: mean baseline score, 13.68; mean week 2 score, 30.31; mean change, 17.35; control: mean baseline score, 15.15; mean week 2 score, 38.29; mean change, 23.14; difference between groups, 5.80; 95% CI, 0.49–11.10; P = .03) from baseline to week 2 (Figure 2D). There was no significant difference at 3 months.
In models adjusting for baseline scores, intervention assignment was significantly associated with patient-reported QOL, depression, anxiety, fatigue, and symptom burden at week 2 after HCT (Table 3). Moreover, after adjusting for baseline scores, intervention assignment was significantly associated with patient-reported QOL, depression, and PTSD but was not significantly associated with anxiety, fatigue, or symptom burden at 3 months after HCT (Table 3). At 3 months after HCT, intervention patients were less likely to have clinically significant depression symptoms (9.5%vs 28.4%; P = .006) but had no difference in anxiety. Similar results were obtained with mixed linear-effects models adjusting for baseline scores with multiple imputations to examine the intervention effect at both time points (eTable 1 in Supplement 2). The effect of the intervention did not differ by transplant type (no significant interaction involving intervention and transplant type).
Table 3.
Adjusted Analyses of Patient-Reported Outcomes 2 Weeks and 3 Months After Hematopoietic Stem Cell Transplantationa
| Outcomes | Sample Size, No. | Adjusted Mean Score (95% CI) | Adjusted Mean Difference Between Groups (95% CI) | P Value |
|---|---|---|---|---|
| At 2 weeks | ||||
| FACT-BMT score (primary outcome) | ||||
| Standard care | 77 | 86.60 (82.00–91.20) | 7.73 (1.27 to 14.19) | .02 |
| Palliative care | 80 | 94.33 (89.81–98.84) | ||
| FACT Fatigue score | ||||
| Standard care | 77 | 23.71 (21.11–26.31) | 3.88 (0.21 to 7.54) | .04 |
| Palliative care | 79 | 27.59 (25.01–30.16) | ||
| ESAS symptom burden score | ||||
| Standard care | 77 | 37.74 (34.09–41.40) | −6.26 (−11.46 to −1.05) | .02 |
| Palliative care | 75 | 31.49 (27.79–35.19) | ||
| HADS Depression subscale score | ||||
| Standard care | 77 | 8.49 (7.59–9.39) | −1.74 (−3.01 to −0.47) | .008 |
| Palliative care | 80 | 6.74 (5.86–7.63) | ||
| HADS Anxiety subscale score | ||||
| Standard care | 77 | 6.33 (5.64–7.02) | −2.26 (−3.22 to −1.29) | <.001 |
| Palliative care | 80 | 4.08 (3.40–4.75) | ||
| PHQ-9 depression symptom score | ||||
| Standard care | 77 | 9.52 (8.42–10.63) | −1.28 (−2.82 to 0.27) | .10 |
| Palliative care | 80 | 8.25 (7.16–9.33) | ||
| At 3 months | ||||
| FACT-BMT score | ||||
| Standard care | 74 | 106.66 (102.91–110.41) | 5.34 (0.04 to 10.65) | .048 |
| Palliative care | 75 | 112.00 (108.28–115.73) | ||
| FACT Fatigue score | ||||
| Standard care | 74 | 35.60 (33.43–37.77) | 2.00 (−1.08 to 5.09) | .20 |
| Palliative care | 73 | 37.60 (35.42–39.79) | ||
| ESAS symptom burden score | ||||
| Standard care | 71 | 17.06 (14.40–19.73) | −2.44 (−6.29 to 1.41) | .21 |
| Palliative care | 69 | 14.62 (11.84–17.40) | ||
| HADS Depression subscale score | ||||
| Standard care | 74 | 5.19 (4.45–5.93) | −1.70 (−2.75 to −0.65) | .002 |
| Palliative care | 74 | 3.49 (2.75–4.23) | ||
| HADS Anxiety subscale score | ||||
| Standard care | 74 | 4.84 (4.15–5.53) | −0.76 (−1.73 to 0.23) | .13 |
| Palliative care | 74 | 4.08 (3.39–4.78) | ||
| PHQ-9 depression symptom score | ||||
| Standard care | 74 | 5.94 (5.02–6.86) | −2.12 (−3.42 to −0.814) | .002 |
| Palliative care | 75 | 3.82 (2.91–4.74) | ||
| PTSD Checklist score | ||||
| Standard care | 67 | 25.79 (23.79–27.79) | −4.35 (−7.12 to −1.58) | .002 |
| Palliative care | 72 | 21.44 (19.53–23.35) | ||
Abbreviations: ESAS, Edmonton Symptom Assessment Scale; FACT-BMT, Functional Assessment of Cancer Therapy–Bone Marrow Transplant; FACT Fatigue, Functional Assessment of Cancer Therapy Fatigue subscale; HADS, Hospital Anxiety and Depression Scale; PHQ-9, Patient Health Questionnaire 9; PTSD, posttraumatic stress disorder.
Analysis of covariance adjusting for baseline scores.
Caregiver Outcomes
Among the 160 enrolled patients, 94 had caregivers who were randomized as part of a patient-caregiver dyad to the intervention group (n = 49) or control group (n = 45), 18 patients did not identify a caregiver whom they were willing to have research staff approach for study participation, 37 caregivers were unable to be reached, and 11 refused to participate. Baseline caregiver characteristics were well balanced between the study groups (Table 4). The majority of caregivers were the patient’s spouse (n = 76 [80.9%]). Only 5 caregivers (5.3%) had missing data at week 2.
Table 4.
Caregiver Baseline Characteristicsa
| Characteristics | Standard Care (n = 45) | Palliative Care (n = 49) |
|---|---|---|
| Age, mean (SD), y | 54.3 (13.7) | 54.4 (14.6) |
| Female | 33 (73.3) | 33 (66.7) |
| Race | ||
| White | 43 (95.6) | 42 (85.7) |
| Other | 2 (4.4) | 7 (14.3) |
| Hispanic ethnicity | 1 (2.2) | 1 (2.0) |
| Religion | ||
| Catholic | 19 (42.2) | 17 (34.7) |
| Protestant | 9 (20.0) | 14 (28.6) |
| Jewish | 2 (4.4) | 2 (4.1) |
| Muslim | 3 (6.7) | 1 (2.0) |
| None | 9 (20.0) | 9 (18.4) |
| Other | 3 (6.7) | 6 (12.2) |
| Relationship to patient | ||
| Married/partner | 38 (84.4) | 38 (77.6) |
| Other | 7 (15.6) | 11 (22.4) |
| Education | ||
| High school or some college | 16 (35.6) | 22 (44.9) |
| College graduate or higher | 28 (62.2) | 26 (53.1) |
| Missing data | 1 (2.2) | 1 (2.0) |
| Caregiver lives with patient | ||
| Yes | 40 (88.9) | 41 (83.7) |
| No | 5 (11.1) | 8 (16.3) |
Data are expressed as No. (%) of caregivers unless otherwise indicated.
From baseline to week 2, there were no significant differences between caregivers of patients assigned to the intervention group and caregivers of patients assigned to the control group in overall QOL (intervention: mean baseline score, 118.81; mean week 2 score, 118.72; mean change, −0.58; control: mean baseline score, 116.85; mean week 2 score, 113.32; mean change, −3.56; difference between groups, −2.98; 95%CI, −7.96 to 1.99; P = .24). Caregivers of intervention patients vs those of controls reported less increase in depression symptoms from baseline to week 2 on the HADS-D (intervention: mean baseline score, 3.98; mean week 2 score, 4.05; mean change, 0.25; control: mean baseline score, 4.30; mean week 2 score,6.00; mean change, 1.80; difference between groups, 1.55; 95% CI, 0.14–2.96; P = .03).
There were no significant differences in caregivers’ anxiety from baseline to week 2 between the study groups on the HADS-A(intervention: mean baseline score, 6.90; mean week 2 score, 6.24; mean change, −0.67; control: mean baseline score, 7.24; mean week 2 score, 6.55; mean change, −0.62; difference between groups, 0.05; 95%CI, −1.38 to 0.10; P = .95). There were also no significant differences in depression as measured by the PHQ-9 from baseline to week 2 between the study groups (intervention: mean baseline score, 3.84; mean week 2 score, 4.51; mean change,0.84; control:mean baseline score, 4.22; mean week 2 score, 4.96; mean change, 0.84; difference between groups, 0.01; 95% CI, −1.44 to 1.45; P = .99). Similar results were obtained for caregiver outcomes at week 2 using multiple imputations (eTable 2 in Supplement 2).
However, caregivers of patients in the intervention group, vs caregivers of patients in the control group, reported improvement in coping (intervention: mean baseline score, 12.28; mean week 2 score, 12.70; mean change, 0.23; control: mean baseline score, 12.18; mean week 2 score, 11.59; mean change, −0.74; difference between groups, −0.97; 95% CI, −1.79 to −0.15; P = .02) and improvement in administrative and financial domains of QOL(intervention: mean baseline score, 13.02; mean week 2 score, 13.22; mean change, 0.24; control: mean baseline score, 13.23; mean week 2 score, 12.71; mean change, −0.46; difference between groups, −0.70; 95% CI, −1.31 to −0.09; P = .02) from baseline to week 2.
Discussion
In this study of patients with hematologic malignancies undergoing HCT, involvement of palliative care compared with standard transplant care led to a lower decrease in QOL at week 2 of hospitalization for HCT, the primary outcome. Because a 5-point change in the FACT-BMT score is considered clinically important,4,15 the intervention led to a clinically meaningful difference in QOL compared with standard transplant care. In addition, exploratory secondary outcomes also showed that patients in the palliative care group benefited, with less increase in their depression symptoms, lower anxiety symptoms, and less increase in symptom burden compared with those receiving standard transplant care. Thus, palliative care may help to lessen the decline in QOL experienced by patients during hospitalization for HCT, which has long been perceived as a natural aspect of the transplantation process.1,4,11
To our knowledge, this is the first study to evaluate the effect of a palliative care intervention for patients receiving potentially curative therapy, specifically those with hematologic malignancies hospitalized for HCT. Multiple randomized trials have demonstrated that concurrent palliative and oncology care leads to improvement in QOL and symptom burden in patients with advanced cancer.22–26 However, these findings have not affected the care of patients with hematologic malignancies, in part because of the lack of evidence supporting the benefits of palliative care in this population.27 In the present study, only 2 patients randomized to receive standard transplant care received a palliative care consultation, illustrating the infrequent use of palliative care in this population. This study also suggests that the benefits of palliative care may extend beyond patients with solid tumors. Hematopoietic stem cell transplantation is a highly specialized procedure only offered at large academic hospitals and comprehensive cancer centers, which often have inpatient palliative care services. Thus, access to inpatient palliative care services should be available for the majority of patients undergoing HCT.
This is also the first trial to our knowledge of a palliative care intervention that demonstrates reduction in patient anxiety at week 2 following HCT.22,23,25,26 Palliative care clinicians frequently focused on coping and managing expectations, which likely explains the improvement in psychological outcomes, including anxiety. The effects of the intervention were sustained, with better QOL and lower depression and PTSD symptoms at 3 months after HCT, although the improvements in fatigue, symptom burden, and anxiety observed at 2 weeks were no longer significant at 3 months. Prior work has suggested that the extent of decline in QOL and increase in depression symptoms during patients’ hospitalization for HCT are associated with their QOL and PTSD symptoms up to 6 months after transplantation.7 Future work should examine the effect of integrating palliative care on longer-term patient outcomes, as well as health care utilization and end-of-life outcomes, given the high risk of morbidity and mortality in this population.1,28,29
This work also demonstrates that a palliative care intervention targeted to the needs of patients can affect their caregivers’ outcomes. Although this study was underpowered to examine caregiver outcomes, the palliative care intervention led to improvements in caregivers’ coping and depression symptoms at 2 weeks but no significant improvement in caregivers’ QOL or anxiety symptoms. These findings suggest that modifying patients’ experience during HCT may have some positive effects on some aspects of caregivers’ well-being. Future studies should be adequately powered to better assess the effect of palliative care involvement on caregiver outcomes, given the critical role that caregivers play in providing care and support for this population.
This study has several important limitations. First, the investigation was performed at a single tertiary care site with a specialized group of transplant and palliative care clinicians, and the patient population lacked racial and ethnic diversity, potentially limiting the generalizability of the results to other care settings or transplant centers with different practices. Second, patients and clinicians could not be blinded to the intervention, which may have introduced bias. The lack of blinding is particularly relevant because all outcome measures were participant reported. Involvement of palliative care in the care of patients randomized to the intervention on the same transplant floor may have altered clinicians’ behaviors in the control group, which may have diluted the study findings.
Third, numerous secondary outcomes were assessed without adjustment for multiple comparisons and therefore should be considered exploratory findings. Fourth, the intervention only entailed visits by a palliative care physician or advanced nurse practitioner and did not include other members of the palliative care team such as social workers, psychologists, or chaplains.
Fifth, data on the cost of the palliative care intervention were not collected. Future work should include an evaluation of the cost-effectiveness of the palliative care intervention in this population. Sixth, an attention-control placebo group was not used in this study and, thus, it is unclear to what extent participants’ outcomes were are affected by the amount of time spent with a supportive individual as opposed to a clinician with palliative care expertise. Given the potential limitations in generalizability and uncertainty about cost-effectiveness, additional research (including replication) is needed before recommendation about dissemination can be made.
Conclusions
Among adults at a single institution undergoing HCT for hematologic malignancies, the use of inpatient palliative care compared with standard transplant care resulted in a smaller decrease in QOL after week 2 of the transplant hospitalization. Further research is needed to replicate these findings and assess the long-term outcomes and cost implications.
Supplementary Material
Key Points.
Question
What is the effect of an inpatient palliative care intervention on the quality of life of patients with hematologic malignancies during hospitalization for hematopoietic stem cell transplantation (HCT)?
Findings
In this randomized clinical trial of 160 adults, patients assigned to an inpatient palliative care intervention reported a 14.72-point decrease in their quality of life from the time of admission for HCT to week 2 of hospitalization compared with a 21.54-point decrease in quality of life for patients assigned to transplant care alone, a statistically significant difference.
Meaning
Among patients with hematologic malignancies undergoing HCT, involvement of palliative care, compared with transplant care alone, led to a smaller decrease in quality of life at 2 weeks after transplantation.
Acknowledgments
Funding/Support: This work was supported by funds from the National Palliative Care Research Foundation (to Dr El-Jawahri) and grant K24 CA 181253 (to Dr Temel) from the National Cancer Institute.
Glossary
- ESAS
Edmonton Symptom Assessment Scale
- FACT-BMT
Functional Assessment of Cancer Therapy–Bone Marrow Transplant
- HADS
Hospital Anxiety and Depression Scale
- HCT
hematopoietic stem cell transplantation
- PHQ-9
Patient Health Questionnaire 9
- PTSD
posttraumatic stress disorder
- QOL
quality of life
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Chen reports receipt of personal fees for consulting or advisory board membership from Bayer, Millennium, Incyte, Seattle Genetics, and Insys. No other disclosures were reported.
Role of the Funder/Sponsor: The study funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Author Contributions: Dr El-Jawahri had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: El-Jawahri, Greer, Jackson, McAfee, Chen, Temel.
Acquisition, analysis, or interpretation of data: El-Jawahri, LeBlanc, VanDusen, Traeger, Greer, Pirl, Jackson, Telles, Rhodes, Spitzer, Chen, Lee, Temel.
Drafting of the manuscript: El-Jawahri, Pirl, Jackson, Chen.
Critical revision of the manuscript for important intellectual content: El-Jawahri, LeBlanc, VanDusen, Traeger, Greer, Jackson, Telles, Rhodes, Spitzer, McAfee, Chen, Lee, Temel.
Statistical analysis: El-Jawahri, Traeger, Greer.
Administrative, technical, or material support: El-Jawahri, VanDusen, Pirl, Jackson, Telles.
References
- 1.Pidala J, Anasetti C, Jim H. Health-related quality of life following haematopoietic cell transplantation: patient education, evaluation and intervention. Br J Haematol. 2010;148(3):373–385. doi: 10.1111/j.1365-2141.2009.07992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pidala J, Anasetti C, Jim H. Quality of life after allogeneic hematopoietic cell transplantation. Blood. 2009;114(1):7–19. doi: 10.1182/blood-2008-10-182592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McQuellon RP, Russell GB, Rambo TD, et al. Quality of life and psychological distress of bone marrow transplant recipients: the “time trajectory” to recovery over the first year. Bone Marrow Transplant. 1998;21(5):477–486. doi: 10.1038/sj.bmt.1701115. [DOI] [PubMed] [Google Scholar]
- 4.El-Jawahri AR, Traeger LN, Kuzmuk K, et al. Quality of life and mood of patients and family caregivers during hospitalization for hematopoietic stem cell transplantation. Cancer. 2015;121(6):951– 959. doi: 10.1002/cncr.29149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prieto JM, Atala J, Blanch J, et al. Patient-rated emotional and physical functioning among hematologic cancer patients during hospitalization for stem-cell transplantation. Bone Marrow Transplant. 2005;35(3):307–314. doi: 10.1038/sj.bmt.1704788. [DOI] [PubMed] [Google Scholar]
- 6.Fife BL, Huster GA, Cornetta KG, Kennedy VN, Akard LP, Broun ER. Longitudinal study of adaptation to the stress of bone marrow transplantation. J Clin Oncol. 2000;18(7):1539–1549. doi: 10.1200/JCO.2000.18.7.1539. [DOI] [PubMed] [Google Scholar]
- 7.El-Jawahri AR, Vandusen HB, Traeger LN, et al. Quality of life and mood predict posttraumatic stress disorder after hematopoietic stem cell transplantation. Cancer. 2016;122(5):806–812. doi: 10.1002/cncr.29818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeFor TE, Burns LJ, Gold EM, Weisdorf DJ. A randomized trial of the effect of a walking regimen on the functional status of 100 adult allogeneic donor hematopoietic cell transplant patients. Biol Blood Marrow Transplant. 2007;13(8):948–955. doi: 10.1016/j.bbmt.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Syrjala KL, Donaldson GW, Davis MW, Kippes ME, Carr JE. Relaxation and imagery and cognitive-behavioral training reduce pain during cancer treatment: a controlled clinical trial. Pain. 1995;63(2):189–198. doi: 10.1016/0304-3959(95)00039-U. [DOI] [PubMed] [Google Scholar]
- 10.Syrjala KL, Cummings C, Donaldson GW. Hypnosis or cognitive behavioral training for the reduction of pain and nausea during cancer treatment: a controlled clinical trial. Pain. 1992;48(2):137–146. doi: 10.1016/0304-3959(92)90049-H. [DOI] [PubMed] [Google Scholar]
- 11.Lee SJ, Joffe S, Kim HT, et al. Physicians’ attitudes about quality-of-life issues in hematopoietic stem cell transplantation. Blood. 2004;104(7):2194–2200. doi: 10.1182/blood-2003-07-2430. [DOI] [PubMed] [Google Scholar]
- 12.Howell DA, Shellens R, Roman E, Garry AC, Patmore R, Howard MR. Haematological malignancy: are patients appropriately referred for specialist palliative and hospice care? a systematic review and meta-analysis of published data. Palliat Med. 2011;25(6):630–641. doi: 10.1177/0269216310391692. [DOI] [PubMed] [Google Scholar]
- 13.Manitta VJ, Philip JA, Cole-Sinclair MF. Palliative care and the hemato-oncological patient: can we live together? a review of the literature. J Palliat Med. 2010;13(8):1021–1025. doi: 10.1089/jpm.2009.0267. [DOI] [PubMed] [Google Scholar]
- 14.Epstein AS, Goldberg GR, Meier DE. Palliative care and hematologic oncology: the promise of collaboration. Blood Rev. 2012;26(6):233–239. doi: 10.1016/j.blre.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 15.McQuellon RP, Russell GB, Cella DF, et al. Quality of life measurement in bone marrow transplantation: development of the Functional Assessment of Cancer Therapy–Bone Marrow Transplant (FACT-BMT) scale. Bone Marrow Transplant. 1997;19(4):357–368. doi: 10.1038/sj.bmt.1700672. [DOI] [PubMed] [Google Scholar]
- 16.Santana MJ, Au HJ, Dharma-Wardene M, et al. Health-related quality of life measures in routine clinical care: can FACT-Fatigue help to assess the management of fatigue in cancer patients? Int J Technol Assess Health Care. 2009;25(1):90–96. doi: 10.1017/S0266462309090126. [DOI] [PubMed] [Google Scholar]
- 17.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 18.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanabe SM, Nekolaichuk CL, Beaumont C. The Edmonton Symptom Assessment System, a proposed tool for distress screening in cancer patients: development and refinement. Psychooncology. 2012;21(9):977–985. doi: 10.1002/pon.1996. [DOI] [PubMed] [Google Scholar]
- 20.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)–specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912– 2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minaya P, Baumstarck K, Berbis J, et al. The CareGiver Oncology Quality of Life questionnaire (CarGOQoL): development and validation of an instrument to measure the quality of life of the caregivers of patients with cancer. Eur J Cancer. 2012;48(6):904–911. doi: 10.1016/j.ejca.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 23.Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741–749. doi: 10.1001/jama.2009.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmermann C, Riechelmann R, Krzyzanowska M, Rodin G, Tannock I. Effectiveness of specialized palliative care: a systematic review. JAMA. 2008;299(14):1698–1709. doi: 10.1001/jama.299.14.1698. [DOI] [PubMed] [Google Scholar]
- 25.Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383(9930):1721–1730. doi: 10.1016/S0140-6736(13)62416-2. [DOI] [PubMed] [Google Scholar]
- 26.Bakitas MA, Tosteson TD, Li Z, et al. Early vs delayed initiation of concurrent palliative oncology care: patient outcomes in the ENABLE III randomized controlled trial. J Clin Oncol. 2015;33(13):1438–1445. doi: 10.1200/JCO.2014.58.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LeBlanc TW, El-Jawahri A. When and why should patients with hematologic malignancies see a palliative care specialist? Hematology Am Soc Hematol Educ Program. 2015;2015(1):471–478. doi: 10.1182/asheducation-2015.1.471. [DOI] [PubMed] [Google Scholar]
- 28.Syrjala KL, Chapko MK, Vitaliano PP, Cummings C, Sullivan KM. Recovery after allogeneic marrow transplantation: prospective study of predictors of long-term physical and psychosocial functioning. Bone Marrow Transplant. 1993;11(4):319–327. [PubMed] [Google Scholar]
- 29.Fraser CJ, Bhatia S, Ness K, et al. Impact of chronic graft-vs-host disease on the health status of hematopoietic cell transplantation survivors: a report from the Bone Marrow Transplant Survivor Study. Blood. 2006;108(8):2867–2873. doi: 10.1182/blood-2006-02-003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.