Abstract
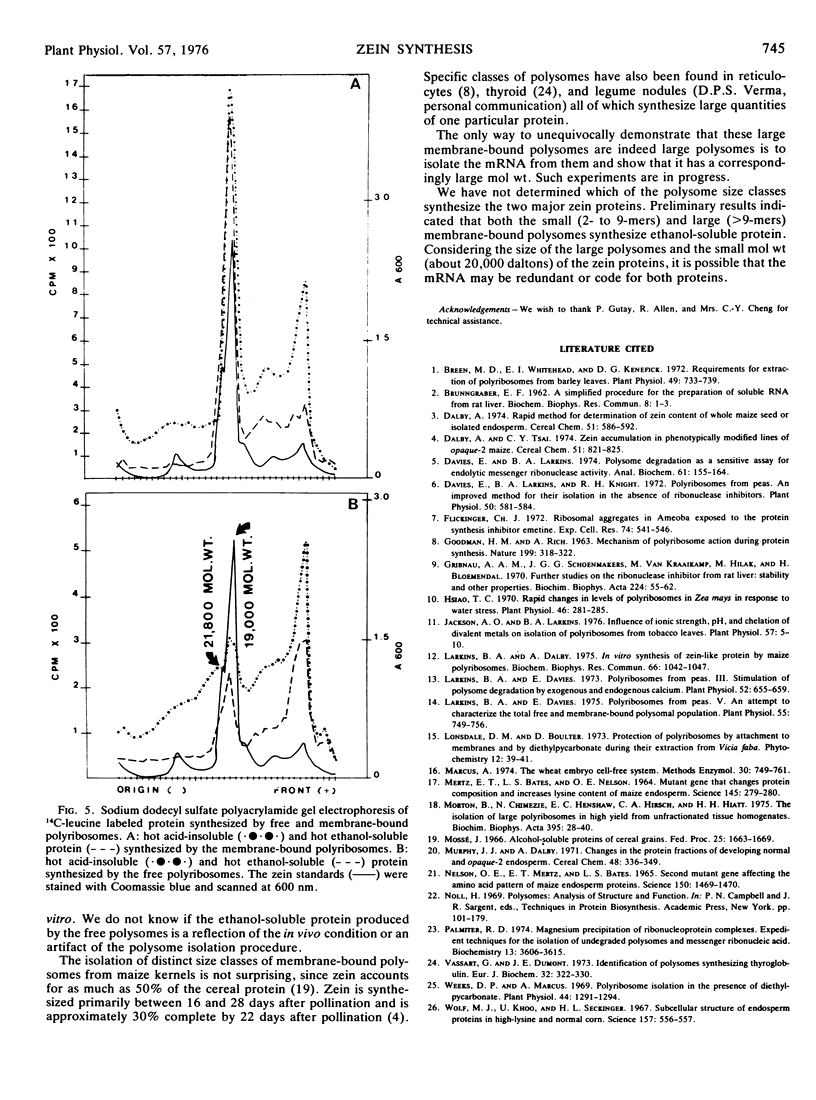
Undegraded free and membrane-bound polysomes were isolated from developing kernels of Zea mays L. frozen in liquid nitrogen. Freezing in liquid nitrogen was a prerequisite for preserving polysome structure in stored kernels. Membrane-bound polysomes from 22-day post-pollination kernels ground in high pH buffers containing 50 mm Mg2+ contained unique classes of large polysomes. These large polysomes were sensitive to ribonuclease, and electron micrographs verified that they were not formed by aggregation. The membrane-bound polysomes were the principal site of zein synthesis, since the major protein synthesized in vitro was similar to purified zein in its ethanol solubility and mobility on sodium dodecyl sulfate polyacrylamide gels.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRUNNGRABER E. F. A simplified procedure for the preparation of "soluble" RNA from rat liver. Biochem Biophys Res Commun. 1962 Jun 19;8:1–3. doi: 10.1016/0006-291x(62)90223-1. [DOI] [PubMed] [Google Scholar]
- Breen M. D., Whitehead E. I., Kenefick D. G. Requirement for extraction of polyribosomes from barley tissue. Plant Physiol. 1972 May;49(5):733–739. doi: 10.1104/pp.49.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies E., Larkins B. A., Knight R. H. Polyribosomes from peas: an improved method for their isolation in the absence of ribonuclease inhibitors. Plant Physiol. 1972 Nov;50(5):581–584. doi: 10.1104/pp.50.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies E., Larkins B. A. Polyribosome degradation as a sensitive assay for endolytic messenger-ribonuclease activity. Anal Biochem. 1974 Sep;61(1):155–164. doi: 10.1016/0003-2697(74)90342-x. [DOI] [PubMed] [Google Scholar]
- Divakaran P., Kumar S. Formation of acids shorter than palmitic by rat liver cytosol. Biochem Biophys Res Commun. 1975 Oct 6;66(3):1042–1047. doi: 10.1016/0006-291x(75)90745-7. [DOI] [PubMed] [Google Scholar]
- GOODMAN H. M., RICH A. MECHANISM OF POLYRIBOSOME ACTION DURING PROTEIN SYNTHESIS. Nature. 1963 Jul 27;199:318–322. doi: 10.1038/199318a0. [DOI] [PubMed] [Google Scholar]
- Gribnau A. A., Schoenmakers J. G., van Kraaikamp M., Hilak M., Bloemendal H. Further studies on the ribonuclease inhibitor from rat liver: stability and other properties. Biochim Biophys Acta. 1970 Nov 12;224(1):55–62. doi: 10.1016/0005-2787(70)90619-2. [DOI] [PubMed] [Google Scholar]
- Hsiao T. C. Rapid Changes in Levels of Polyribosomes in Zea mays in Response to Water Stress. Plant Physiol. 1970 Aug;46(2):281–285. doi: 10.1104/pp.46.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkins B. A., Davies E. Polyribosomes from Peas: III. Stimulation of Polysome Degradation by Exogenous and Endogenous Calcium. Plant Physiol. 1973 Dec;52(6):655–659. doi: 10.1104/pp.52.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkins B. A., Davies E. Polyribosomes from Peas: V. An Attempt to Characterize the Total Free and Membrane-bound Polysomal Population. Plant Physiol. 1975 Apr;55(4):749–756. doi: 10.1104/pp.55.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERTZ E. T., BATES L. S., NELSON O. E. MUTANT GENE THAT CHANGES PROTEIN COMPOSITION AND INCREASES LYSINE CONTENT OF MAIZE ENDOSPERM. Science. 1964 Jul 17;145(3629):279–280. doi: 10.1126/science.145.3629.279. [DOI] [PubMed] [Google Scholar]
- Marcus A., Efron D., Weeks D. P. The wheat embryo cell-free system. Methods Enzymol. 1974;30:749–754. doi: 10.1016/0076-6879(74)30073-0. [DOI] [PubMed] [Google Scholar]
- Morton B., Nwizu C., Henshaw E. C., Hirsch C. A., Hiatt H. H. The isolation of large polysomes in high yield from unfractionated tissue homogenates. Biochim Biophys Acta. 1975 Jun 2;395(1):28–40. doi: 10.1016/0005-2787(75)90230-0. [DOI] [PubMed] [Google Scholar]
- Mossé J. Alcohol-soluble proteins of cereal grains. Fed Proc. 1966 Nov-Dec;25(6):1663–1669. [PubMed] [Google Scholar]
- Nelson O. E., Mertz E. T., Bates L. S. Second Mutant Gene Affecting the Amino Acid Pattern of Maize Endosperm Proteins. Science. 1965 Dec 10;150(3702):1469–1470. doi: 10.1126/science.150.3702.1469. [DOI] [PubMed] [Google Scholar]
- Vassart G., Dumont J. E. Identification of polysomes synthesizing thyroglobulin. Eur J Biochem. 1973 Jan 15;32(2):322–330. doi: 10.1111/j.1432-1033.1973.tb02613.x. [DOI] [PubMed] [Google Scholar]
- Weeks D. P., Marcus A. Polyribosome isolation in the presence of diethyl pyrocarbonate. Plant Physiol. 1969 Sep;44(9):1291–1294. doi: 10.1104/pp.44.9.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M. J., Khoo U., Seckinger H. L. Subcellular structure of endosperm protein in high-lysine and normal corn. Science. 1967 Aug 4;157(3788):556–557. doi: 10.1126/science.157.3788.556. [DOI] [PubMed] [Google Scholar]



