Abstract
AIM
To evaluate the role of oral curcumin in inducing clinical remission in patients with mild to moderate ulcerative colitis (UC).
METHODS
A prospective randomized double-blind placebo-controlled trial comparing the remission inducing effect of oral curcumin and mesalamine 2.4 g with placebo and mesalamine 2.4 g in patients of ulcerative colitis with mild to moderate severity was conducted from January 2003 to March 2005. The included patients received 1 capsule thrice a day of placebo or curcumin (150 mg) for 8 wk. Patients were evaluated clinically and endoscopically at 0, 4 and 8 wk. The primary outcome was clinical remission at 8 wk and secondary outcomes were clinical response, mucosal healing and treatment failure at 8 wk. The primary analysis was intention to treat worst case scenario (ITT-WCS).
RESULTS
Of 300 patients with UC, 62 patients (curcumin: 29, placebo: 33) fulfilled the inclusion criteria and were randomized at baseline. Of these, 21 patients did not complete the trial, 41 patients (curcumin: 16, placebo: 25) finally completed 8 wk. There was no significant difference in rates of clinical remission (31.3% vs 27.3%, P = 0.75), clinical response (20.7% vs 36.4%, P = 0.18), mucosal healing (34.5% vs 30.3%, P = 0.72), and treatment failure (25% vs 18.5%, P = 0.59) between curcumin and placebo at 8 wk.
CONCLUSION
Low dose oral curcumin at a dose of 450 mg/d was ineffective in inducing remission in mild to moderate cases of UC.
Keywords: Curcumin, Mesalamine, Ulcerative colitis, Ulcerative colitis disease activity index, Mucosal healing
Core tip: Not all patients with mild to moderate ulcerative colitis (UC) respond to available treatment options. Curcumin, an active ingredient of turmeric has anti-inflammatory properties and has been shown to play a protective role in chemically induced mouse models of inflammatory bowel disease and to reduce relapse rates in human UC. However, optimum dose ranging studies for curcumin in ulcerative colitis have not been performed. The present study shows that low dose curcumin (450 mg/d) is ineffective in inducing remission in mild to moderate ulcerative colitis. Therefore, higher doses with effective modes of delivery are required for optimal efficacy of curcumin.
INTRODUCTION
Ulcerative colitis (UC) is a chronic relapsing and remitting inflammatory condition of the intestinal tract without a known etiology[1,2]. The interaction between environmental factors, genetic susceptibility, and immune dysregulation is implicated in the pathogenesis of UC, although their precise contributions remain incompletely understood[3-6].
Oral 5-aminosalicylates (5-ASA) compounds are the first line therapy used for inducing clinical remission in mild to moderate UC. Treatment options for patients not responding to oral 5-ASA include oral corticosteroids, immunomodulators such as 6-mercaptopurine and azathioprine, topical agents like 5-ASA and steroid enemas and biologicals. However, steroids are associated with significant side effects, immunomodulators are slow to act, topical agents would only be effective in left-sided colitis and biologicals are costly and not every patient can afford them, especially in resource constraint countries like India. Surgery is an option but every patient does not want it, and one likes to defer surgery in mild to moderate cases. Therefore, there is a need for an agent which is safe, efficacious and cheap and can be added with 5-ASA to increase the remission rates, especially in developing countries like India, where the incidence of IBD is on the rise[7].
Pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, IL-12, and interferon (IFN)-γ are upregulated in patients with UC[8]. Nuclear factor (NF)κB is the main up-regulator of expression of these cytokines and is strongly activated in UC and Crohn’s disease suggesting the important role in pathogenesis. Curcumin is the major constituent of turmeric powder extracted from the rhizomes of the plant Curcuma longa Linn. Turmeric is used as a spice to give the specific flavor and yellow color to curry. Curcumin has been identified as the most active constituent of turmeric and has been described as an anti-inflammatory, antioxidant, pro-apoptotic, and anti-proliferative compound[8,9]. As a traditional medicine, turmeric has also been widely used for centuries to treat inflammatory disorders in India[9]. The pleiotropic effects of curcumin owe to inhibition of transcriptional factor nuclear NF-κB. Curcumin blocks a signal upstream of NF-κB-inducing kinase and IκB kinase in intestinal epithelial cells[10]. The effects of curcumin on the immune response (both innate and adaptive) have been a subject of much attention in the past decade[11-15]. Curcumin has been shown to play a protective role in chemically induced mouse models of IBD[16-19] and to reduce the relapse rate in human UC[20-22].
Hence this study was carried out to determine the efficacy and safety of oral curcumin therapy in inducing remission in mild to moderate cases of UC.
MATERIALS AND METHODS
Study design
This study was a single center prospective randomized double-blind placebo-controlled trial comparing the remission inducing effect of oral curcumin and mesalamine (2.4 g/d in three divided doses) with placebo and mesalamine (2.4 g/d in three divided doses) in patients with UC with mild to moderate severity. The study was carried out from January 2003 to March 2005. The study was approved by the institutional ethics committee.
Participants
All patients were recruited from the Inflammatory Bowel Disease clinic at All India Institute of Medical Sciences, New Delhi, India. Adult patients (≥ 18 years) who had mild-to-moderately active UC [Ulcerative Colitis Disease Activity Index (UCDAI) score[23], 3-9]; with a minimum sigmoidoscopic score of 2 with at least one previously documented attack of active disease were included in this study. Patients were excluded if they had evidence of severe disease (UCDAI, > 10), concurrent enteric infection, use of oral steroids within the past 4 wk, use of antibiotics within the past 2 wk, change in dose of oral mesalamine within the past 4 wk, and initiation of azathioprine less than 6 mo before initiation of the study. Patients requiring hospitalization and imminent need for surgery, lactating and pregnant women, and those who received any investigational medicines within 3 mo were excluded. Patients with significant hepatic, renal, endocrine, respiratory, neurologic, or cardiovascular diseases also were excluded. Demographic information was recorded on a structured proforma.
Randomization
Sequence generation: The random numbers were generated by computerized random number (The RAND corp. Inc). The randomization list and numbered packing of the intervention were prepared by a person not involved in the study. Randomization was performed using permuted blocks of 6.
Randomization-allocation concealment: Allocation concealment was ensured by the use of sequentially numbered boxes coded using alphabet containing identical curcumin or placebo capsules, according to the allocation sequence.
Randomization implementation: All the study personals were blinded to the treatment assignment (placebo or curcumin) for the duration of the study. Placebo and curcumin capsules were similar in appearance and in their method of administration. The codes were reserved with a no-interest party and were revealed only after the recruitment and data collection had been completed.
Blinding
The individual sealed box method was used to maintain blinding of the investigators and study participants.
Study intervention
Included patients were randomized to receive 1 capsule thrice a day of either placebo or curcumin for a duration of 8 wk. Each curcumin capsule contained 150 mg of purified curcumin. The placebo was supplied as an indistinguishable capsule containing starch with a yellow edible dye-caramel yellow. The curcumin and placebo capsules were supplied by Himalaya Drug Company (Bangalore, India). The patients in both the groups received mesalamine at a dose of 2.4 g/d. Other supportive treatment and standard care were provided to both the groups.
Follow-up
Patients were evaluated at the study center at weeks 0, 4, 8 (or as required) after recruitment. Clinical Assessment was done on the basis of UCDAI score. A sigmoidoscopic evaluation with endoscopic scoring was also done according to Baron score[24] at each visit. Biochemical parameters like hemoglobin, erythrocyte sedimentation rate, leukocyte count, urea, creatinine, bilirubin, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and albumin were performed at each follow-up visit.
Compliance was measured by obtaining a detailed study history during a personal interview as well as compliance was judged at the 4 and 8 wk follow-up visits by a blister count of the remaining capsules. Non-compliance was defined as failure to take ≥ 80% of the medication.
Outcomes
The primary outcome measure was clinical remission (UCDAI ≤ 2) at 8 wk. Secondary outcomes were clinical response (defined as a reduction from baseline in the UCDAI of ≥ 3, sigmoidoscopic remission (Baron endoscopic score of 0/1), and treatment failure defined by an increase in UCDAI scores by ≥ 3 points or treatment intolerance by the patient.
Activity of UC
The activity of UC was assessed using the UCDAI[23]. The UCDAI was calculated by the investigator by adding the individual scores of the 4 parameters: Bowel frequency, rectal bleeding, endoscopic score, and physician’s rating of severity. (Rectal bleeding and stool frequency score was assessed by asking the patient about his/her symptoms over the past 3 d. The score for these parameters was calculated individually by taking the average of the scores for the last available 3 d before the study visit. The composite score ranges from 0 (inactive disease) to 12 (severe disease activity). The Baron score[24], represents an endoscopic classification, ranges from 0 to 3, with 0 denoting normal mucosa, (1) granularity of mucosa with loss of vascular pattern; (2) bleeding to touch; and (3) spontaneous bleeding. Sigmoidoscopic remission was defined by a Baron score of 0 or 1 (normal looking mucosa or mucosal edema alone as indicated by loss of normal vascular pattern).
Sample size estimation and statistical analysis
This study was conducted in 2003 and the efficacy of curcumin in induction of remission in UC had not been studied previously in any human trial. Hence, this was an exploratory pilot trial where consecutive patients of UC fulfilling the inclusion criteria were enrolled over a period of January 2003 to March 2005. The primary analysis was intention to treat worst-case scenario.
The data was entered in a Microsoft excel spreadsheet (MS Office version 2003). Descriptive statistics including measures of central tendency and dispersion were calculated for all variables. Continuous variables were compared using t-test for independent samples and categorical variables were compared using χ2 test. Measures of risk were computed along with 95%CI. Changes in symptom scores and clinical sign scores from baseline to the final follow-up visit were calculated and compared between the placebo and curcumin groups. A P-value of < 0.05 was considered statistically significant. All calculations were done with SPSS soft- ware (v. 16).
RESULTS
A total of 300 patients presenting at the Inflammatory Bowel Disease clinic of the Department of Gastroenterology, at All India Institute of Medical Sciences (AIIMS), New Delhi, from January 2003 to March 2005 were assessed for eligibility. Of them, 62 patients fulfilled the inclusion criteria and agreed to participate (Figure 1). Thirty three patients were randomized to the placebo group and 29 to curcumin group. A total of 21 patients did not complete the trial (8 patients in the placebo group, and 13 patients in curcumin group). Thus, a total of 41 participants, 25 in the placebo group and 16 in curcumin group, completed the trial and were analyzed. The participant flow through the trial is given in Figure 1.
Figure 1.
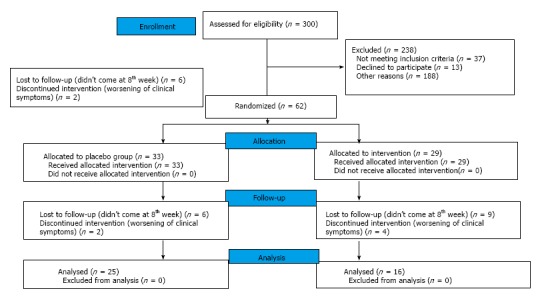
Flowchart demonstrating patient recruitment in curcumin and placebo group.
Demographic and clinical characteristics
Baseline characteristics were comparable between the 2 groups (Table 1). The mean baseline UCDAI score was also comparable between the two groups (5.2 ± 2 vs 5.5 ± 1.9, P = 0.63) (Table 2). All subsequent analyses are presented according to ITT-WCS.
Table 1.
Baseline clinical and biochemical parameters of the randomized patients n (%)
| Curcumin group (n = 29) | Placebo group (n = 33) | |
| Age (yr) | 36 ± 12 | 34 ± 7 |
| Sex (females) | 13 (44.83) | 8 (24.24) |
| Weight (kg) | 55.1 ± 10.0 | 55.7 ± 11.7 |
| BMI (kg/m2) | 20.8 ± 3.1 | 20.5 ± 3.3 |
| Disease duration (yr) | 3.83 ± 4.00 | 3.64 ± 2.59 |
| Disease extent | ||
| E3 (pancolitis) | 7 (25.9) | 6 (20.7) |
| E2 (left sided colitis) | 17 (58.6) | 21 (63.6) |
| E1 (proctitis) | 3 (11.1) | 2 (6.90) |
| Current smoking | 5 (17.24) | 4 (14.81) |
| Current alcohol use | 3 (10.34) | 4 (14.81) |
| Hemoglobin (g/dL) | 12.12 ± 2.76 | 13.35 ± 1.72 |
| Total leukocyte count (per cubic millimeter) | 8586 ± 2306 | 8221 ± 2104 |
| Platelet count(× 1000/mm3) | 256.25 ± 131.98 | 257.53 ± 113.01 |
| ESR (mm/1st h) | 3 ± 2 | 4 ± 3 |
| Urea (mg/dL) | 22.0 ± 5.2 | 21.5 ± 5.2 |
| Creatinine (mg/dL) | 0.84 ± 0.26 | 1.19 ± 1.69 |
| Potassium (meq/L) | 4.41 ± 0.33 | 4.26 ± 0.78 |
| Bilirubin (mg/dL) | 0.7 ± 0.2 | 0.6 ± 0.1 |
| Aspartate aminotransferase (U/L) | 28 ± 6 | 30 ± 8 |
| Alanine aminotransferase(U/L) | 26 ± 9 | 28 ± 19 |
| Total protein (g/L) | 8.0 ± 0.2 | 7.9 ± 0.8 |
| Albumin (g/L) | 4.4 ± 0.5 | 4.5 ± 0.3 |
| Current treatment | ||
| 5-ASA | 29 (100) | 33 (100) |
| Steroids | 0 | 0 |
| Azathioprine | 2 (6.9) | 2 (6.2) |
| Rectal steroids | 0 | 0 |
| Mesalamine enema | 0 | 0 |
Data are given as mean ± SD. 5-ASA: 5-aminosalicylates; BMI: Body mass index; ESR: Erythrocyte sedimentation rate.
Table 2.
Comparison of the Ulcerative Colitis Disease Activity Index between the two randomized groups at baseline, 4, and 8 wk
|
Curcumin group |
Placebo group |
Mean difference (95%CI) | Significance | |||
| n | UCDAI | n | UCDAI | |||
| Week 0 | 29 | 5.2 ± 2.0 | 33 | 5.5 ± 1.9 | -0.244 (-1.256 to 0.77) | 0.632 |
| Week 4 | 16 | 3.6 ± 2.4 | 23 | 4.4 ± 3.2 | -0.823 (-2.678 to 1.020) | 0.37 |
| Week 8 | 16 | 3.4 ± 3.1 | 25 | 3.8 ± 2.8 | -0.362 (-2.343 to 1.168) | 0.711 |
UCDAI is expressed as mean ± SD. UCDAI: Ulcerative Colitis Disease Activity Index.
Primary and Secondary outcome measures
Induction of clinical remission (Table 3): Clinical remission was achieved in 31.03% of patients (9 out of 29) in curcumin group and 27.27% (9 out of 33) in the placebo group at 8 wk, the difference being statistically insignificant (OR = 1.20, 95%CI: 0.40-3.60; P = 0.745).
Table 3.
Comparison of clinical remission, improvement in Ulcerative Colitis Disease Activity Index and Baron’s score, and mucosal healing at 8th week between two randomized groups
| Curcumin group | Placebo group | OR (95%CI) | P value | |
| Clinical remission | ||||
| PP1 | 9/16 (56.25%) | 9/25 (36%) | 2.28 (0.634-8.264) | 0.202 |
| ITT | 9/29 (31.03%) | 9/33 (27.27%) | 1.20 (0.40-3.60) | 0.745 |
| UCDAI improvement by ≥ 3 | ||||
| PP | 6/16 (37.5%) | 12/25 (48%) | 0.65 (0.18-2.34) | 0.509 |
| ITT | 6/29 (20.69%) | 12/33 (36.36%) | 0.46 (0.14-1.43) | 0.175 |
| Mucosal healing1 | ||||
| PP | 10/16 (62.5%) | 10/25 (40%) | 2.50 (0.69-9.09) | 0.16 |
| ITT | 10/29 (34.5%) | 10/33 (30.3%) | 1.21 (0.4 – 3.5) | 0.72 |
Healing defined by either endoscopically normal mucosa or only mucosal granularity. Any ulceration or friability was taken as non-healed mucosa. PP: Per-protocol; ITT: Intention to treat; UCDAI: Ulcerative Colitis Disease Activity Index.
Improvement in UCDAI (Table 3): The UCDAI was similar between the two randomized groups at baseline (Table 2). There was no difference in the UCDAI among the randomized patients at 4 and 8 wk. Six out of 29 (20.69%) patients in curcumin group reported an improvement in DAI score by 3 or more as compared to 12 out of 33 (36.36%) in the placebo group but the difference was not statistically significant (OR = 0.46, 95%CI: 0.14-1.43; P = 0.175).
Mucosal healing (Table 3): Mucosal healing was achieved in 34.48% of patients (10 out of 29) in curcumin group and 30.30% (10 out of 33) in the placebo group at 8 wk, the difference being statistically insignificant (OR = 1.21, 95%CI: 0.42-3.52; P = 0.725).
Treatment failure: Amongst the patients who completed the study, 1 out of 16 in curcumin group and 3 out of 25 in the placebo group were found to be the cases of treatment failure defined as increase in UCDAI score by 3 or more. The difference between the two groups was not statistically significant (OR = 0.489, 95%CI: 0.046-5.155; P = 0.545).
Moreover, 4 out of 13 dropouts in curcumin group and 2 out of 8 dropouts in placebo group cited worsening of clinical symptoms as reasons for dropout were also categorized as treatment failure as per protocol. Hence, the total treatment failure rate in curcumin and placebo groups were 25% (5 out of 20) and 18.52% (5 out of 27) respectively. The difference between the treatment failure rates in the two groups was not statistically significant (OR = 1.47, 95%CI: 0.361-5.952; P = 0.591).
Comparison of laboratory parameters between the two randomized groups
No significant improvement in hemoglobin or albumin was reported within either group at 8th week. On comparing the two groups, no significant difference was found between any laboratory parameter at either 4 or 8 wk (Table 4).
Table 4.
Comparison of the biochemical parameters between the two randomized groups at 4 and 8 wk
|
Week 4 |
Week 8 |
|||||
| Curcumin group | Placebo group | P value | Curcumin group | Placebo group | P value | |
| Hemoglobin (g/dL) | 12.0 ± 2.3 | 13.3 ± 2.2 | 0.235 | 12.1 ± 2.7 | 13.2 ± 2.6 | 0.404 |
| Total leukocyte count (per cubic millimeter) | 7900 ± 2449 | 8085 ± 2494 | 0.87 | 8957 ± 1705 | 7086 ± 1969 | 0.082 |
| ESR (mm/1st h) | 21.5 ± 13.4 | 22.2 ± 15.2 | 0.91 | 23.0 ± 17.1 | 25.9 ± 9.4 | 0.707 |
| Urea (mg/dL) | 23.1 ± 8.5 | 22.8 ± 7.0 | 0.922 | 24.7 ± 6.0 | 24.3 ± 6.7 | 0.89 |
| Alanine aminotransferase (U/L) | 26.5 ± 8.4 | 35.5 ± 20.6 | 0.178 | 29.5 ± 15.4 | 25.3 ± 5.4 | 0.516 |
| Total protein (g/L) | 7.9 ± 0.4 | 8.1 ± 0.8 | 0.666 | 8.3 ± 0.3 | 8.3 ± 0.9 | 0.963 |
| Albumin (g/L) | 4.7 ± 0.2 | 4.6 ± 0.3 | 0.869 | 4.8 ± 0.2 | 4.6 ± 0.2 | 0.169 |
Compliance
In the placebo group, 8 out of 33 patients did not complete the study. Two of them cited worsening of clinical symptoms, categorized as treatment failure, to be the cause of dropout. Others were lost to follow-up. In curcumin group, 13 out of 29 patients did not complete the study. Four of them cited worsening clinical symptoms, categorized as treatment failure, to be the cause of dropout. Patient drop out due to worsening of symptoms is the main reason for reporting the ITT-WCS analysis. In patients continuing in the trial, the compliance was more than 80% in all patients in both the treatment arms.
Safety and adverse drug reactions
No adverse clinical or biochemical effects were observed in either group. One patient complained of self-limited arthralgia in the placebo group.
DISCUSSION
This was the first randomized controlled trial of oral curcumin in the induction of remission in UC. This study showed that oral curcumin at a dose of 450 mg a day was ineffective in inducing remission or attaining clinical response. Curcumin has been shown to play a protective role in chemically induced mouse models of IBD[16-19]. Mechanisms by which curcumin exerts its pharmacological effects are thought to involve antioxidation[4], inhibition of kinases, interference with the activity of transcription factors such as NF-κB and AP-1[5]. Cyclooxygenase-2 (COX-2) and lipoxygenase (LOX) are inhibited by curcumin through NF-κB dependent or independent pathway[6,7]. NF-κB has been shown to activate, via transcription, the genes encoding pro-inflammatory cytokines (TNF-α, IL-1β and IL-12), cell adhesion molecules (vascular cell adhesion molecule (VCAM)-1 and intercellular cell adhesion molecule (ICAM)-1, inducible nitric oxide synthase (iNOS) and COX-2[25-27].
We recently published a randomized controlled trial using curcumin enemas in patients with mild to moderate distal colitis[28]. Per protocol analysis revealed significantly better outcomes in curcumin enema group, in terms of clinical response (92.9% vs 50%, P = 0.01), clinical remission (71.4% vs 31.3%, P = 0.03), and improvement on endoscopy (85.7% vs 50%, P = 0.04). However, in the present study, oral administration of curcumin did not induce remission after 8 wk of therapy. In a recent randomized controlled trial from Israel which enrolled 50 patients with mild to moderate UC, oral curcumin was found to be effective in inducing remission[29]. The dose of curcumin used was 3 g/d. In the intention-to-treat analysis, 14 patients (53.8%) receiving curcumin achieved clinical remission at week 4, compared with none of the patients receiving placebo (P = 0.01). Clinical response (reduction of ≥ 3 points in SCCAI) was achieved by 17 patients (65.3%) in the curcumin group vs 3 patients (12.5%) in the placebo group (P < 0.001).
We did not find a significant effect of using curcumin on the response, remission, or mucosal healing at 4 and 8 wk as compared with placebo. The following factors were likely responsible for observed non-response. The first reason could be the use of an inadequate dose of curcumin. A daily total of 450 mg curcumin per day in three divided doses was used in this study. In another study where curcumin was used for inducing remission, a 3 g/d dose was used[29], which is much higher than the dose used in our study. In a second study, curcumin in combination with 5-ASA was shown to be effective in maintaining remission in UC patients as compared to placebo[20,22]. Again the dose of curcumin used in that study was 2 g/d, much higher than the present study. However, at the same time, none of these studies had incorporated a dose finding study design. Hence, the present study clearly adds to the knowledge that low dose oral curcumin is not effective in inducing remission in UC. The second reason could be poor bioavailability. A phase I clinical trial conducted on 25 patients with various precancerous conditions indicated that curcumin is poorly absorbed and may have limited systemic bioavailability. Because of curcumin’s rapid plasma clearance and conjugation, its therapeutic usefulness has been somewhat limited, leading researchers to investigate the benefits of complexing curcumin with other substances to increase systemic bioavailability. Other studies have also demonstrated the safety of curcumin, including a phase-1 trial in which doses of up to 8000 mg of curcumin per day were administered without toxicity[30]. We have not used any such complex formulations, which could have produced a difference. We have shown the efficacy of topical curcumin enemas in combination with oral 5-ASA in inducing remission in a similar group of patients. The dose of curcumin used in this study was just 140 mg which was lower than the dose used in this study, indicating that curcumin indeed would be effective but with proper dosage and route of administration. There have been multiple studies on this aspect that have investigated various formulations of curcumin, some of which increase systemic bioavailability of curcumin and some have lead to increased colonic delivery[31,32]. In a mice study, Curcumin-Zn(II) complex was prepared by stirring curcumin with anhydrous zinc chloride at a molar ratio of 1:1. Kinetic stability studies showed a good stability of the metallo-complex with zinc and in vivo study revealed a significant reduction in severity and extent of colonic damage with this preparation[31]. Another study assessed the role of pH-triggered Eudragit-coated chitosan microspheres of curcumin in managing UC. In vivo organ bio-distribution study showed a negligible amount of curcumin in the stomach and small intestine and there was a significant reduction in extent and severity of colonic damage with these microspheres[32]. A trial investigating these newer formulations of curcumin could better define the role of curcumin in UC. Another limitation of this study is the small sample size as it was an exploratory pilot study. In absence of any data on the use of curcumin in UC, when this study was conducted, no sample size calculations could be done. However, the study by Lang et al which had a small sample size of 50 UC patients showed the efficacy of curcumin in inducing remission as compared to placebo[29]. Future studies are needed to prove (or disprove) the hypothesis that oral curcumin is effective in induction of remission in mild to moderate UC[33]. The dose can range from 1 to 4 g considering that doses up to 8 g are safe[30]. The sample sizes for these studies would have to be approximately 100 or 170 in each arm to detect an absolute difference of 20% and 15% respectively, assuming the baseline remission rate of 27% (from the placebo group).
In conclusion, low dose oral curcumin for 8 wk is not effective in inducing clinical remission or response in patients with mild to moderate UC. A multicenter collaborative trial using newer formulations of curcumin with higher bioavailability and a dose defining study design is required to conclusively answer this research question.
COMMENTS
Background
There is a therapeutic gap in inducing clinical remission in patients with ulcerative colitis (UC) with the available treatment options. Curcumin, an active ingredient of turmeric powder, because of it anti-inflammatory properties, can decrease the mucosal inflammation in patients with active UC. The present study was designed to evaluate the role of curcumin in inducing clinical remission in patients with mild to moderate UC.
Research frontiers
Oral 5-aminosalicylates (5-ASA) compounds are the first line therapy used for inducing clinical remission in mild to moderate UC. Treatment options for patients not responding to oral 5-ASA include oral corticosteroids, immunomodulators such as 6-mercaptopurine and azathioprine, topical agents like 5-ASA and steroid enemas, and biologicals. However, each of these agents is associated with its own side-effects and is not effective in every patient. Therefore, there is a need for an agent which is safe, efficacious and cheap and can be added with 5-ASA to increase the remission rates, especially in developing countries like India, where the incidence of inflammatory bowel disease is on the rise.
Innovations and breakthroughs
This was the first dose ranging study to evaluate the efficacy of oral curcumin in patients with mild to moderate UC. They compared oral curcumin (450 mg/d) with mesalamine (2.4 g/d) vs placebo with mesalamine in inducing remission in patients with mild to moderate UC and found that oral curcumin at a dose of 450 mg/d was ineffective in inducing remission in mild to moderate UC.
Applications
The results of this study indicate that low dose curcumin is ineffective in mild to moderate cases of UC. Therefore further studies with higher doses of curcumin or with better drug delivery systems are required to evaluate the efficacy of curcumin in UC.
Terminology
Low dose oral curcumin is ineffective in patients with mild to moderate UC.
Peer-review
This is a good study to point out Low dose oral curcumin at a dose of 450 mg/d, which was ineffective in inducing remission in mild to moderate cases of UC.
Footnotes
Institutional review board statement: The study was reviewed and approved institute ethics committee at All India Institute of Medical Sciences, New Delhi.
Informed consent statement: All study participants provided informed written consent prior to study enrollment.
Clinical trial registration statement: This trial was done from 2003 till 2005. Since there was no trial registry in India at that time, this trial did not have a registration number.
Conflict-of-interest statement: No conflict of interest for all authors.
Data sharing statement: No additional data are available.
Manuscript source: Invited manuscript
Specialty Type: Gastroenterology and hepatology
Country of Origin: India
Peer-Review Report Classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: October 19, 2016
First decision: December 27, 2016
Article in press: March 14, 2017
P- Reviewer: Freeman HJ, Guo J, Guo N, Tambuwala MM S- Editor: Gong ZM L- Editor: A E- Editor: Lu YJ
References
- 1.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 2.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 3.Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 4.Danese S, Sans M, Fiocchi C. Inflammatory bowel disease: the role of environmental factors. Autoimmun Rev. 2004;3:394–400. doi: 10.1016/j.autrev.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Lawrance IC, Fiocchi C, Chakravarti S. Ulcerative colitis and Crohn’s disease: distinctive gene expression profiles and novel susceptibility candidate genes. Hum Mol Genet. 2001;10:445–456. doi: 10.1093/hmg/10.5.445. [DOI] [PubMed] [Google Scholar]
- 6.Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut. 1998;42:477–484. doi: 10.1136/gut.42.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahuja V, Tandon RK. Inflammatory bowel disease in the Asia-Pacific area: a comparison with developed countries and regional differences. J Dig Dis. 2010;11:134–147. doi: 10.1111/j.1751-2980.2010.00429.x. [DOI] [PubMed] [Google Scholar]
- 8.Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991;57:1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- 9.Jain SK. Ethnobotany and research on medicinal plants in India. Ciba Found Symp. 1994;185:153–164; discussion 164-168. [PubMed] [Google Scholar]
- 10.Jobin C, Bradham CA, Russo MP, Juma B, Narula AS, Brenner DA, Sartor RB. Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. J Immunol. 1999;163:3474–3483. [PubMed] [Google Scholar]
- 11.Jagetia GC, Aggarwal BB. “Spicing up” of the immune system by curcumin. J Clin Immunol. 2007;27:19–35. doi: 10.1007/s10875-006-9066-7. [DOI] [PubMed] [Google Scholar]
- 12.Yadav VS, Mishra KP, Singh DP, Mehrotra S, Singh VK. Immunomodulatory effects of curcumin. Immunopharmacol Immunotoxicol. 2005;27:485–497. doi: 10.1080/08923970500242244. [DOI] [PubMed] [Google Scholar]
- 13.Kim GY, Kim KH, Lee SH, Yoon MS, Lee HJ, Moon DO, Lee CM, Ahn SC, Park YC, Park YM. Curcumin inhibits immunostimulatory function of dendritic cells: MAPKs and translocation of NF-kappa B as potential targets. J Immunol. 2005;174:8116–8124. doi: 10.4049/jimmunol.174.12.8116. [DOI] [PubMed] [Google Scholar]
- 14.Bhaumik S, Jyothi MD, Khar A. Differential modulation of nitric oxide production by curcumin in host macrophages and NK cells. FEBS Lett. 2000;483:78–82. doi: 10.1016/s0014-5793(00)02089-5. [DOI] [PubMed] [Google Scholar]
- 15.Joe B, Vijaykumar M, Lokesh BR. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit Rev Food Sci Nutr. 2004;44:97–111. doi: 10.1080/10408690490424702. [DOI] [PubMed] [Google Scholar]
- 16.Jian YT, Mai GF, Wang JD, Zhang YL, Luo RC, Fang YX. Preventive and therapeutic effects of NF-kappaB inhibitor curcumin in rats colitis induced by trinitrobenzene sulfonic acid. World J Gastroenterol. 2005;11:1747–1752. doi: 10.3748/wjg.v11.i12.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salh B, Assi K, Templeman V, Parhar K, Owen D, Gómez-Muñoz A, Jacobson K. Curcumin attenuates DNB-induced murine colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G235–G243. doi: 10.1152/ajpgi.00449.2002. [DOI] [PubMed] [Google Scholar]
- 18.Sugimoto K, Hanai H, Tozawa K, Aoshi T, Uchijima M, Nagata T, Koide Y. Curcumin prevents and ameliorates trinitrobenzene sulfonic acid-induced colitis in mice. Gastroenterology. 2002;123:1912–1922. doi: 10.1053/gast.2002.37050. [DOI] [PubMed] [Google Scholar]
- 19.Ukil A, Maity S, Karmakar S, Datta N, Vedasiromoni JR, Das PK. Curcumin, the major component of food flavour turmeric, reduces mucosal injury in trinitrobenzene sulphonic acid-induced colitis. Br J Pharmacol. 2003;139:209–218. doi: 10.1038/sj.bjp.0705241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanai H, Iida T, Takeuchi K, Watanabe F, Maruyama Y, Andoh A, Tsujikawa T, Fujiyama Y, Mitsuyama K, Sata M, et al. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2006;4:1502–1506. doi: 10.1016/j.cgh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–1629. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- 22.Kumar S, Ahuja V, Sankar MJ, Kumar A, Moss AC. Curcumin for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012;10:CD008424. doi: 10.1002/14651858.CD008424.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutherland LR, Martin F, Greer S, Robinson M, Greenberger N, Saibil F, Martin T, Sparr J, Prokipchuk E, Borgen L. 5-Aminosalicylic acid enema in the treatment of distal ulcerative colitis, proctosigmoiditis, and proctitis. Gastroenterology. 1987;92:1894–1898. doi: 10.1016/0016-5085(87)90621-4. [DOI] [PubMed] [Google Scholar]
- 24.Baron JH, Connell AM, Lennard-Jones JE. Variation between observers in describing mucosal appearances in proctocolitis. Br Med J. 1964;1:89–92. doi: 10.1136/bmj.1.5375.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaltschmidt B, Baeuerle PA, Kaltschmidt C. Potential involvement of the transcription factor NF-kappa B in neurological disorders. Mol Aspects Med. 1993;14:171–190. doi: 10.1016/0098-2997(93)90004-w. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, Yang L, Lee TJ. Oroxylin A inhibition of lipopolysaccharide-induced iNOS and COX-2 gene expression via suppression of nuclear factor-kappaB activation. Biochem Pharmacol. 2000;59:1445–1457. doi: 10.1016/s0006-2952(00)00255-0. [DOI] [PubMed] [Google Scholar]
- 27.Pahan K, Sheikh FG, Namboodiri AM, Singh I. Lovastatin and phenylacetate inhibit the induction of nitric oxide synthase and cytokines in rat primary astrocytes, microglia, and macrophages. J Clin Invest. 1997;100:2671–2679. doi: 10.1172/JCI119812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singla V, Pratap Mouli V, Garg SK, Rai T, Choudhury BN, Verma P, Deb R, Tiwari V, Rohatgi S, Dhingra R, et al. Induction with NCB-02 (curcumin) enema for mild-to-moderate distal ulcerative colitis - a randomized, placebo-controlled, pilot study. J Crohns Colitis. 2014;8:208–214. doi: 10.1016/j.crohns.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Lang A, Salomon N, Wu JC, Kopylov U, Lahat A, Har-Noy O, Ching JY, Cheong PK, Avidan B, Gamus D, et al. Curcumin in Combination With Mesalamine Induces Remission in Patients With Mild-to-Moderate Ulcerative Colitis in a Randomized Controlled Trial. Clin Gastroenterol Hepatol. 2015;13:1444–9.e1. doi: 10.1016/j.cgh.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 30.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 31.Sareen R, Jain N, Dhar KL. Curcumin-Zn(II) complex for enhanced solubility and stability: an approach for improved delivery and pharmacodynamic effects. Pharm Dev Technol. 2016;21:630–635. doi: 10.3109/10837450.2015.1041042. [DOI] [PubMed] [Google Scholar]
- 32.Sareen R, Jain N, Rajkumari A, Dhar KL. pH triggered delivery of curcumin from Eudragit-coated chitosan microspheres for inflammatory bowel disease: characterization and pharmacodynamic evaluation. Drug Deliv. 2016;23:55–62. doi: 10.3109/10717544.2014.903534. [DOI] [PubMed] [Google Scholar]
- 33.Hanai H, Sugimoto K. Curcumin has bright prospects for the treatment of inflammatory bowel disease. Curr Pharm Des. 2009;15:2087–2094. doi: 10.2174/138161209788489177. [DOI] [PubMed] [Google Scholar]


