Abstract
Flavones are a class of flavonoids that are a subject of increasing interest because of their biological activities in vitro and in vivo. This article reviews the major sources of flavones and their concentrations in food and beverages, which vary widely between studies. It also covers the roles of flavones in plants, the influence of growing conditions on their concentrations, and their stability during food processing. The absorption and metabolism of flavones are also reviewed, in particular the intestinal absorption of both O- and C-glycosides. Pharmacokinetic studies in both animals and humans are described, comparing differences between species and the effects of glycosylation on bioavailability. Biological activity in animal models and human dietary intervention studies is also reviewed. A better understanding of flavone sources and bioavailability is needed to understand mechanisms of action and nutritional intervention.
Keywords: flavonoids, absorption, metabolism, apigenin, luteolin, diosmetin, pharmacokinetics
Introduction
Flavonoids are widespread throughout the plant kingdom, and several reviews have examined their food sources and the bioavailability, metabolism, and biological activity of these compounds in humans (1–4). Although extensive, these reviews omitted or only briefly mentioned flavones, likely because of the limited information available in comparison with other flavonoids. Since the publication of these reviews, flavones have been the subject of numerous promising in vitro studies, and many publications have further expanded our knowledge of their pharmacokinetics and activity in humans and in animal models.
This review explores the most common flavones in the diet, their biological role in plants, and the most abundant food sources containing them. It will also delve into the bioavailability of flavones from foods and their metabolism in humans. Finally, the biological activity of flavones in human clinical trials will be discussed.
Current Status of Knowledge
Flavone functions and variability with growing conditions
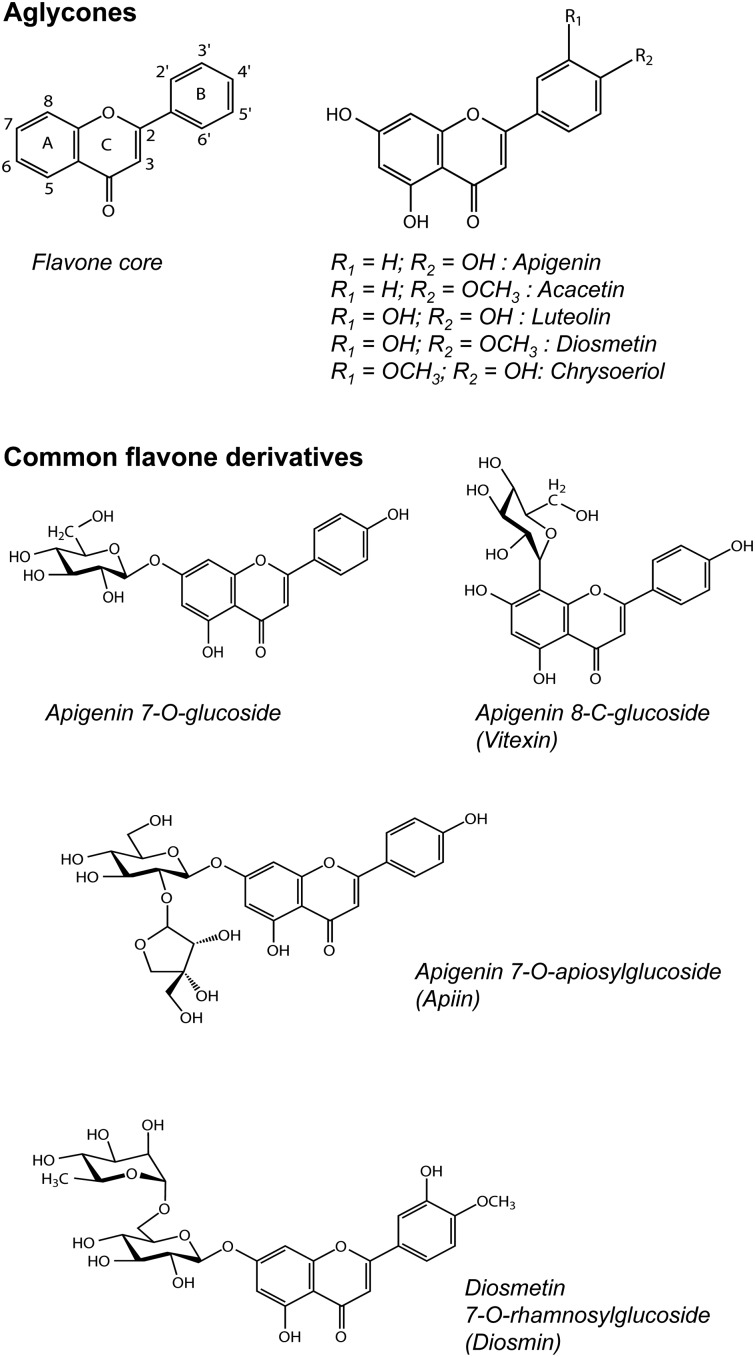
Flavones differ from other flavonoids in that they have a double bond between C2 and C3 in the flavonoid skeleton, there is no substitution at the C3 position (5), and they are oxidized at the C4 position (Figure 1). These compounds play a variety of roles in plants. Along with flavonols, they are the primary pigments in white- and cream-colored flowers (6) and act as copigments with anthocyanins in blue flowers (7). Flavones and other flavonoids can also act as UVB protectants in plants, because they absorb in the 280- to 315-nm range (7). Their synthesis can be upregulated by UV light in parsley cells (8), resulting in apigenin and luteolin concentrations in celery leaves >20 times higher than those in stalks (9) and field-grown plants with higher concentrations than greenhouse grown plants (10, 11). Flavones can also act as natural pesticides in plants, providing protection against insects (6) and fungal diseases (12, 13). Plants grown under organic conditions (with presumably more pest pressure), however, have in most cases shown no higher concentrations of flavones than their conventional counterparts (14–17). Flavones also act as signaling molecules for plants, promoting colonization of roots by nitrogen-fixing bacteria (18) and mycorrhizal fungi (19).
FIGURE 1.
Structures of flavone aglycones and common flavone derivatives.
Food sources of flavones
Flavones from plants are typically conjugated as 7-O-glycosides (Figure 1) (5), and may also have acetyl or malonyl moieties. Flavone C-glycosides are most commonly detected as 6-C- and 8-C-glucosides. Whereas flavone O-glycosides can be hydrolyzed with enzymes or acid before analysis, flavone C-glycosides are resistant to both processes and must be analyzed in their native forms (20). Both glycosides and aglycones are commonly used for quantitation in the literature. Reports on the daily intake of flavones vary, ranging from 0.7 to 9.0 mg/d in adults in Europe (21–23), 1.1 to 1.6 mg/d in women in the United States (24, 25), and 1.9 to 4.2 mg/d in female adolescents in China (26).
Concentrations of flavones in dried tea leaves and herbs are listed in Table 1. Chamomile and parsley have the highest flavone concentrations, with as much as 5320 mg apigenin O-glycosides/100 g dried chamomile flowers or 1350 mg/100 g dried parsley leaf (28, 29). The apigenin conjugates in chamomile have been identified as acetyl, malonyl, and caffeoyl derivatives of apigenin 7-O-glucoside (39), whereas those in parsley are predominantly apigenin 7-O-malonyl apiosylglucoside (malonylapiin) and apigenin 7-O-apiosylglucoside (apiin) (29). Luteolin 8-C-glucoside (orientin) and luteolin 6-C-glucoside (isoorientin) are most abundant in rooibos tea (32). Green, black, and oolong teas contain orientin and isoorientin, as well as a variety of apigenin mono- and di-C-glycosides (20). Flavone O-glycosides are also reported in many plants from the mint family (Lamiaceae), but their reported concentrations vary widely between sources. For example, luteolin glycosides in peppermint ranged from 42 to 3070 mg/100 g dry leaf, varying with the source and the analytic methods used (35, 36).
TABLE 1.
Concentrations of flavones in teas and dry herbs (2 g dry material/serving)
| Botanical name (family) | Common name | Flavone | Flavone, mg/100 g dry weight | Flavone, mg/serving | Reference |
| Chamaemelum nobile (Asteraceae) | Roman chamomile flowers | Apigenin O-glycosides | 2531 | 51 | 27 |
| Apigenin | 395 | 8 | |||
| Luteolin O-glycosides | 81 | 1 | |||
| Luteolin | 56 | 1 | |||
| Matricaria chamomilla (Asteraceae) | Chamomile flowers | Apigenin O-glycosides | 5010–5320 | 100–106 | 28 |
| Petroselinum crispum (Apiaceae) | Parsley | Apigenin/apigenin O-glycosides | 1200–1350 | 24–27 | 29, 30 |
| Luteolin | 19.8 | <1 | |||
| Tanacetum vulgare (Asteraceae) | Tansy leaf | Apigenin | 165 | 3 | 31 |
| Luteolin | 848 | 17 | |||
| Aspalathus linearis (Fabaceae) | Rooibos/red bush tea | Apigenin C-glycosides | 46–116 | 1–2 | 32–34 |
| Luteolin C-glycosides | 172–591 | 3–12 | |||
| Trigonella foenum-graecum (Fabaceae) | Fenugreek seed | Apigenin | 731 | 15 | 31 |
| Luteolin | 512 | 10 | |||
| Mentha x piperita (Lamiaceae) | Peppermint | Apigenin O-glycosides | <5.6–27 | <1 | 35, 36 |
| Luteolin O-glycosides | 42–3070 | 1–61 | |||
| Origanum vulgare (Lamiaceae) | Oregano | Apigenin | 15.6–19.4 | <1 | 30 |
| Luteolin | 901–1137 | 18–23 | |||
| Perilla frutescens (Lamiaceae) | Shiso | Apigenin O-glycosides | 280–920 | 6–18 | 37 |
| Luteolin O-glycosides | 30–790 | 1–16 | |||
| Rosmarinus officinalis (Lamiaceae) | Rosemary | Apigenin | 43.8 | 1 | 31, 38 |
| Luteolin/luteolin O-glycosides | 10–661 | 0–13 | |||
| Diosmetin O-glycosides | ND1–250 | <1–5 | |||
| Salvia officinalis (Lamiaceae) | Sage | Luteolin/luteolin O-glycosides | 49.6–1110 | 1–22 | 31, 36 |
| Camellia sinensis (Theaceae) | Green tea | Apigenin C-glycosides | 47.8–246.8 | 1–5 | 20 |
| Luteolin C-glycosides | 2.5–21.9 | <1 | |||
| Camellia sinensis (Theaceae) | Black tea | Apigenin C-glycosides | 41.4–175.7 | 1–4 | 20 |
| Luteolin C-glycosides | 5.9–14.0 | <1 | |||
| Camellia sinensis (Theaceae) | Oolong tea | Apigenin C-glycosides | 198.6 | 4 | 20 |
| Luteolin C-glycosides | 14.8 | <1 |
ND, not detected/limit of detection not specified (38).
A survey of flavones reported in juice and wine is shown in Table 2. All of the juices with appreciable flavone concentrations are in the citrus family and contain methylated flavones, e.g., acacetin, diosmetin, and chrysoeriol glycosides. Bergamot juice contains the highest concentrations of total flavone glycosides, including both flavone O- and C-glycosides (40, 41). Apigenin and diosmetin 6,8-di-C-glucosides were found in bergamot, mandarin orange, orange, and citron juices (40). Although kumquats (Fortunella crassifolia) contain >21 mg apigenin/100 g fresh weight (50), kumquat juice (F. japonica) has <1 mg/100 g (45). This may be due to differences in species or to the amount of peel included in the sample. In grapefruit, peels contained >100 times the concentration of naringin as the juice (51).
TABLE 2.
Concentrations of flavones in juices and wines
| Botanical name (family) | Common name (serving size, mL) | Flavone | Flavone, mg/100 g fresh weight | Flavone, mg/serving | Reference |
| Citrus bergamia (Rutaceae) | Bergamot juice (250) | Apigenin C-glycosides | 3.8–7.2 | 10–18 | 40, 41 |
| Apigenin O-glycosides | 6.1–7.7 | 15–19 | |||
| Luteolin C-glycosides | 0.6–0.8 | 2 | |||
| Chrysoeriol C-glycosides | 1.3–1.5 | 3–4 | |||
| Chrysoeriol O-glycosides | 5.4–6.9 | 14–17 | |||
| Diosmetin C-glycosides | 2.9–5.9 | 7–15 | |||
| Diosmetin O-glycosides | 1.9–2.7 | 5–7 | |||
| Citrus deliciosa (Rutaceae) | Mandarin orange juice (250) | Apigenin C-glycosides | 2.3–2.7 | 6–7 | 40 |
| Diosmetin C-glycosides | 0.6–0.8 | 2 | |||
| Citrus medica (Rutaceae) | Citron juice (250) | Apigenin C-glycosides | 0.6–0.8 | 2 | 40 |
| Diosmetin C-glycosides | 6.1–6.8 | 15–17 | |||
| Citrus sinensis (Rutaceae) | Orange juice (250) | Apigenin C-glycosides | 1.0–8.0 | 3–20 | 40, 42–44 |
| Apigenin O-glycosides | 0.2–0.7 | 1–2 | |||
| Luteolin C-glycosides | 0.2–0.5 | 1 | |||
| Chrysoeriol C-glycosides | 0.2 | 1 | |||
| Diosmetin C-glycosides | 0.2–0.5 | 1 | |||
| Fortunella japonica (Rutaceae) | Kumquat juice (250) | Acacetin O-glycosides | 0.1 | <1 | 45 |
| Acacetin C-glycosides | 0.1 | <1 | |||
| Apigenin C-glycosides | <0.1 | <1 | |||
| Vitis vinifera (Vitaceae) | Red and white wine (100) | Apigenin | <0.11–0.6 | <1 | 30, 46–49 |
| Luteolin | <0.11–0.4 | <1 |
The concentrations of flavones in fruits, vegetables, olive oil, and honey are listed in Table 3. Most of the studies reported values for aglycones after acid hydrolysis, although some gave concentrations of flavone O-glycosides. Fresh parsley had the highest flavone concentrations of the fresh foods, with ≤1484 mg apigenin/100 g (60). Chicory had ≤333 mg luteolin/100 g in one study (14), but luteolin was not detected in another (62). Wide variations in flavone concentrations between studies were also observed for many other fresh foods. Vegetables with the highest concentrations were in the sunflower family (Asteraceae) and carrot family (Apiaceae).
TABLE 3.
Concentrations of flavones in fruits, vegetables, olive oil, and honey
| Botanical name (family) | Common name (serving size, g) | Flavone | Flavone, mg/100 g fresh weight | Flavone, mg/serving | Reference |
| Actinidia deliciosa (Actinidiaceae) | Kiwi fruit (100) | Luteolin/luteolin glycosides | <1.21–2.2 | <1–2 | 30, 50, 52, 53 |
| Spinacia oleracea (Amaranthaceae) | Spinach (200) | Apigenin | <0.12 | <1 | 30, 54–58 |
| Luteolin | <0.13–6.6 | <1–13 | |||
| Petroselinum crispum (Apiaceae) | Parsley (5) | Apigenin | <2.44–1484 | <1–74 | 9, 30, 50, 57–60 |
| Luteolin | <0.44–22 | <1–1 | |||
| Apium graveolens var. dulce (Apiaceae) | Celery stalks (200) | Apigenin | 1.3–10.8 | 3–22 | 9, 30, 52, 61 |
| Luteolin | <1.2–2.2 | <2–4 | |||
| Celery hearts (200) | Apigenin | 19.1 | 38 | 30 | |
| Luteolin | 3.5 | 7 | |||
| Apium graveolens var. secalinum (Apiaceae) | Chinese celery (200) | Apigenin | 24.0 | 48 | 30 |
| Luteolin | 34.9 | 70 | |||
| Lactuca sativa (Asteraceae) | Lettuce (100) | Apigenin | <0.75–2.7 | <1–3 | 15, 30, 52, 54–58, 62–65 |
| Luteolin | <1.26–8.8 | <1–9 | |||
| Cynara scolymus (Asteraceae) | Artichoke heads (200) | Apigenin/apigenin glycosides | 3.9–18.9 | 8–38 | 30, 66–70 |
| Luteolin/luteolin glycosides | 2.3–7.5 | 5–15 | |||
| Chicorium intybus (Asteraceae) | Chicory leaves (100) | Apigenin/apigenin O-glycosides | <0.1–68 | <1–68 | 14, 30, 62, 71 |
| Luteolin/luteolin O-glycosides | <0.1–333 | <1–333 | |||
| Brassica napus var. napobrassica (Brassicaceae) | Rutabaga (200) | Apigenin | <0.1–15.4 | <1–31 | 30, 56 |
| Luteolin | <0.1 | <1 | |||
| Brassica oleracea (Brassicaceae) | Broccoli (200) | Luteolin/luteolin glycosides | <0.44–7.5 | <1–15 | 30, 50, 55, 58, 72 |
| Brassica oleracea var. acephala (Brassicaceae) | Kohlrabi (200) | Luteolin | ND7–1.3 | <1–3 | 30, 58 |
| Brassica rapa var. chinensis (Brassicaceae) | Chinese cabbage (200) | Apigenin | <0.1–4.5 | <1–9 | 15, 30, 54, 55, 58, 65 |
| Luteolin | <0.1–1.2 | <1–2 | |||
| Citrullus lanatus (Cucurbitaceae) | Watermelon (200) | Luteolin | <1.28–1.8 | <2–4 | 30, 52, 53, 57, 73 |
| Cucumis melo (Cucurbitaceae) | Muskmelon (200) | Luteolin | <1.28–2.6 | <2–5 | 30, 52, 53 |
| Cucirbota sp. (Cucurbitaceae) | Pumpkin (200) | Luteolin | <0.1–1.6 | <1–3 | 30, 53, 72 |
| Vaccinium sp. (Ericaceae) | Blueberry (100) | Luteolin | <1.2–1.8 | <1–2 | 9, 30, 52, 55 |
| Pisum sativum (Fabaceae) | Peas (100) | Apigenin | <0.12–17.6 | <1–18 | 30, 55, 72 |
| Luteolin | <0.1–0.4 | <1 | |||
| Olea europaea (Oleaceae) | Black olives (50) | Apigenin | 6.5 | 3 | 30, 74 |
| Luteolin | 3.2–17.5 | 2–9 | |||
| Olea europaea (Oleaceae) | Green olives (50) | Luteolin | 0.2–1.2 | <1–1 | 30 |
| Olea europaea (Oleaceae) | Olive oil (15) | Apigenin | <0.1–0.2 | <1 | 30, 75 |
| Luteolin | <0.1–0.7 | <1 | |||
| Citrus paradisi (Rutaceae) | Grapefruit (200) | Apigenin/apigenin glycosides | <0.35–4.9 | <1–10 | 30, 50, 52, 53, 55 |
| Luteolin | <1.2–1.4 | <1–3 | |||
| Citrus sinensis (Rutaceae) | Orange (200) | Luteolin | <0.1–1.5 | <1–3 | 30, 53, 55, 76 |
| Fortunella crassifolia (Rutaceae) | Kumquat (100) | Apigenin glycosides | 21.9 | 22 | 30, 50 |
| Capsicum annuum (Solanaceae) | Bell peppers (100) | Luteolin/luteolin glycosides | 0.1–12.9 | <1–13 | 9, 30, 50, 58, 62 |
| Vitis sp. (Vitaceae) | Grapes (200) | Luteolin | <0.1–2.6 | <1–5 | 9, 30, 53, 55, 56 |
| Honey (20) | Apigenin | <0.1–29.3 | <1–6 | 30, 77–82 | |
| Luteolin | <0.49–3.2 | <1–1 |
The concentrations of flavones in cereals and legumes are listed in Table 4. Millet and sorghum provided the highest concentrations of flavones of all cereals and legumes, providing 15 mg apigenin and 35 mg luteolin/100 g millet and ≤29 mg apigenin and 18 mg luteolin/100 g sorghum. Although these levels are lower than those seen in other foods, such as parsley, celery and chicory, it is important to consider the flavone concentrations in these foods. Some areas of the world, including Africa and some Asian countries, consume very high amounts of these grains; thus, millet and sorghum can contribute substantially to flavone intake (91). However, reported amounts were variable, with no flavones detected in some cases.
TABLE 4.
Concentration of flavones in cereals and legumes (28 g dry grain or legume/serving)
| Botanical name (family) | Common name | Flavone | Flavone, mg/100 g dry weight | Flavone, mg/serving | Reference |
| Pisum sativum (Fabaceae) | Field pea | Apigenin C-glycosides | <0.31–4.6 | <1–1 | 83 |
| Luteolin C-glycosides | <0.11–1.1 | <1 | |||
| Luteolin O-glycosides | <1.61–4.6 | <1–1 | |||
| Cicer arietinum (Fabaceae) | Chickpea | Luteolin C-glycosides | <0.11–0.1 | <1 | 83 |
| Vicia faba (Fabaceae) | Fava bean | Apigenin C-glycosides | <0.31–0.5 | <1 | 30, 83 |
| Apigenin O-neohesperidoside | <0.31–0.3 | <1 | |||
| Luteolin C-glycosides | <0.11–0.9 | <1 | |||
| Digitaria exilis (Poaceae) | Millet, fonio | Apigenin | 15.0 | 4 | 84 |
| Luteolin | 35.0 | 10 | |||
| Sorghum bicolor (Poaceae) | Lemon-yellow sorghum | Apigenin | 0.1–28.7 | <1–8 | 85 |
| Luteolin | 0.3–7.5 | <1–2 | |||
| Sorghum bicolor (Poaceae) | Red sorghum | Apigenin | <0.2–20.4 | <1–6 | 30, 85, 86 |
| Luteolin | <0.2–18.2 | <1–5 | |||
| Triticum monococcum (Poaceae) | Wheat grain | Apigenin C-glycosides | 2.1 | <1 | 87 |
| Triticum urartu (Poaceae) | Wheat grain | Apigenin C-glycosides | 3.8 | 1 | |
| Triticum dicoccum (Poaceae) | Wheat grain | Apigenin C-glycosides | 9.5 | 3 | |
| Triticum carthlicum (Poaceae) | Wheat grain | Apigenin C-glycosides | 10.5 | 3 | |
| Triticum polonicum (Poaceae) | Wheat grain | Apigenin C-glycosides | 7.4 | 2 | |
| Triticum turgidum (Poaceae) | Wheat grain | Apigenin C-glycosides | 12.7 | 4 | |
| Triticum durum (Poaceae) | Wheat grain | Apigenin C-glycosides | 7.8 | 2 | |
| Triticum spelta (Poaceae) | Wheat grain | Apigenin C-glycosides | 17.9 | 5 | |
| Triticum aestivum(Poaceae) | Wheat grain | Apigenin C-glycosides | 8.0 | 2 | |
| Oryza sativa (Poaceae) | Black rice | Apigenin C-glycosides | 6.3 | 2 | 88 |
| Luteolin C-glycosides | 1.4 | <1 | |||
| Red rice | Apigenin C-glycosides | 0.7 | <1 | ||
| Luteolin C-glycosides | 0.7 | <1 | |||
| Brown rice | Apigenin C-glycosides | 1.8 | <1 | ||
| Luteolin C-glycosides | 0.3 | <1 | |||
| White rice | Apigenin C-glycosides | 1.6 | <1 | ||
| Luteolin C-glycosides | 0.8 | <1 | |||
| Fagopyrum esculentum (Polygonaceae) | Dehulled, roasted buckwheat | Apigenin C-glycosides | 0.2–0.7 | <1 | 30, 89 |
| Whole buckwheat | Apigenin C-glycosides | 0.3–3.3 | <1 | 90 | |
| Luteolin C-glycosides | 0.2–3.4 | <1–1 |
Limit of detection not specified but estimated with the use of the lowest reported value (83).
Effects of processing on flavone glycosides
As with other flavonoids, flavones are typically present in plants as glycosides. Flavone O-glycosides are composed of the aglycone moiety, with ≥1 sugar attached with a β linkage (Figure 1). These compounds may be modified slightly by endogenous enzymes, such as malonyl esterases (e.g., conversion from malonylapiin to apiin) (92), but remain as glycosides after processing such as shredding (63), juicing (69, 92), and heating (43). Flavone C-glycosides are reportedly stable to fermentation (33), as well as juicing, concentration, spray drying, and pasteurization (43, 93). Substantial degradation of flavone C-glycosides, however, was found after processing for 15 min at 121°C or 4 min at 135°C (93). Compared with most other flavonoid glycosides, flavones are very stable. For example, flavonol O-glycosides can be enzymatically hydrolyzed during shredding, heat processing, or fermentation (63, 94), and anthocyanins and isoflavones can be degraded during thermal processing (95, 96). Flavanone O-glycosides, however, are fairly stable during heat processing (43).
Absorption and metabolism of flavone O-glycosides
Given that dietary flavones are ingested predominantly as glycosides, their fate is determined by how and where they are absorbed, metabolized, transported, and excreted. The absorption of orally delivered flavone O-glycosides and aglycones has been the subject of many animal studies, particularly in rats. Most of these rat studies show that apigenin, luteolin, and their simple glucosides are absorbed quickly. The time of maximum plasma concentration (Tmax)3 was generally ≤1 h, with a maximum plasma concentration (Cmax) of 1–100 μmol/L (97–100), depending on the dose and the type of food coconsumed (food matrix). The shortest Tmax values were seen with pure luteolin dissolved in DMSO/polyethyleneglycol, propyleneglycol, or ethanol (97–99), suggesting that the absence of a food matrix speeds absorption. A longer Tmax was seen with luteolin and apigenin from Verbena officinalis extract, and, although the flavone form was not specified (12), the native forms are flavone mono- and diglucuronides (101), which would need to be hydrolyzed to aglycones before absorption. The longest Tmax and greatest Cmax was seen in rats fed radiolabeled apigenin, in which the values included all metabolites of apigenin detected as radioactivity (102), and not just the forms typically measured by HPLC. Limited studies have evaluated the absorption of flavones in mice (103, 104), but showed very low plasma flavone concentrations (<1 μmol/L) relative to rats after feeding.
In contrast to pharmacokinetic studies of flavones in rats, those in humans show plasma concentrations of <1 μmol/L after consumption (Table 5). Many human studies used celery leaves or parsley as their flavone source (105–108), which contain primarily flavone apiosylglucosides (29, 114). Although an older study that used HPLC-UV did not find flavones in serum (107), 2 newer reports that used HPLC–electrochemical detection found concentrations averaging <0.2 μmol/L with Tmax values of >7 h (105, 108). The latter suggests that the flavone apiosylglucosides are absorbed in the colon, which may contribute to their low bioavailability. The colon has less surface area for absorption and is also a site for extensive metabolism and degradation by gut microbiota, as will be discussed later. Subjects who consumed comparable amounts of flavones as simple glucosides from artichoke leaf extract had Tmax values of <1 h and maximal plasma concentrations of 0.2–0.5 μmol/L (111). Whereas <1% of flavones from parsley were found in urine (105, 106), after the consumption of aglycone-rich hydrolyzed Chrysanthemum morifolium extract, urinary excretion was 2% of luteolin and 6% of apigenin intake (110).
TABLE 5.
Bioavailability of flavone O-glycosides in humans1
| Source | Subjects, n | Dose | Intervention duration | Plasma Tmax, h | Plasma concentration, μmol/L | AUC | Urinary excretion, % of intake | Elimination half-life, h | Reference |
| Parsley | 6 men and 5 women | 2 g parsley/kg body weight (0.24 mg apigenin/kg body weight) | Single dose | 7.2 | 0.127 | 61.98 min · μmol− · L− | 0.22 | — | 105 |
| Parsley | 7 men and 7 women | 20 g parsley/d (4.5 mg apigenin/d) | 7 d | — | — | — | 0.58 | — | 106 |
| Parsley | 9 men and 9 women | 4.9 g dried parsley/d (84 mg apigenin/d) | 7 d | — | <1.1 | — | — | — | 107 |
| Celery leaf | 10 men and 10 women | 2 g celery leaf/kg body weight (apigenin dose unknown) | Single dose | 7.70 | 0.190 | 89.02 min · μmol− · L− | — | — | 108 |
| 40 mL chamomile extract (ethanol/water) | 1 woman | 90.2 mg apigenin 7-glucoside, 4.2 mg luteolin 7-glucoside, 6.1 mg apigenin, 2.4 mg luteolin | Single dose | — | <0.74 | — | ND | — | 109 |
| Extract of C. morifolium tablets | 4 men and 4 women | 85.56 mg luteolin | 12 h | — | — | — | 2.30 luteolin | — | 110 |
| 65.04 mg apigenin | 6.09 apigenin (12 h) | ||||||||
| Cooked artichoke heads (61.7 g) | 2 men and 3 women | 4.9 mg luteolin glycoside | Single dose | — | ND | — | — | 67 | |
| 6.0 mg apigenin glycoside | |||||||||
| Artichoke leaf extract | 7 men and 7 women | 14.4 or 35.2 mg luteolin eq. | Single dose | 0.36 or 0.46 | 0.206 or 0.546 | 168.6 or 499.6 ng · h− · mL− | 1.72 or 1.99 | 2.50 or 2.45 | 111 |
| Diosmin (diosmetin 7-O-rutinoside) | 1 man | 1000 mg (493 mg diosmetin eq.) | Single dose | 3.00 | 0.170 | 142.84 ng · h− · mL− | 0.003 | 1.10 | 112 |
| Diosmin | 2 men and 3 women | 10 mg diosmin/kg body weight (4.93 mg diosmetin eq./kg body weight) | Single dose | 1 | 1.388 | 5617 ng · h− · mL− | ND | 31.5 | 113 |
eq., equivalent; ND, not detected/limit of detection not specified; Tmax, time of maximum plasma concentration.
Although numerous studies have shown that flavones are absorbed systemically (Table 5), they are typically found in plasma or urine as glucuronide or sulfate metabolites, rather than the original flavone O-glycosides (111, 113, 115). As with many other flavonoid glycosides in foods, flavones must first be hydrolyzed to aglycones for absorption (116), and are then metabolized to glucuronidated or sulfated forms before reaching systemic circulation (117). A brief summary of the sites of metabolism and enzymes involved is presented in Table 6.
TABLE 6.
Most common metabolic conversions of flavone O-glycosides1
| Site of metabolism | Enzyme | Conversion | Reference |
| Small intestine | β-glucosidase (LPH) | Flavone 7-O-glucoside to aglycone | 118 |
| Small intestine, liver | UGT1A1, UGT1A8, and UGT1A9 | Aglycone to flavone glucuronide | 119 |
| Liver | SULT1A1 and SULT1E1 | Aglycone to flavone sulfate | 103, 120, 121 |
| Large intestine | Intestinal microbiota | Flavone 7-O-glucoside to aglycone, 3-(4-hydroxyphenyl) propionic acid | 100 |
LPH, lactase-phlorizin hydrolase; SULT1, sulfotransferase 1; UGT1A, UDP glucuronosyltransferase family 1 member A.
It is not clear what role the stomach plays in the absorption of flavones in humans. Although few compounds are absorbed in the human stomach because of its relatively small surface area (122), ex vivo models show that flavonoid glycosides can be absorbed through the human stomach and cleaved by stomach-specific β-glucosidase (123). Rapid absorption, rapid elimination, and poor absorption efficiency are considered to be consistent with absorption in the stomach, and anthocyanins are among the flavonoids that fit this description (3). Human clinical trials with flavones, although limited, suggest that they might behave in a similar manner. Whereas studies that collected plasma samples starting 4 h after dosing found Tmax values of >7 h for apigenin (105, 108), Tmax values of <0.5 h were obtained when blood collection was begun 0.25 h after dosing (111). It is possible that rats have a greater ability to absorb flavones through the stomach lumen, and there is evidence that flavone glycosides can be absorbed there (124), which would provide an explanation for the higher levels of flavone absorption seen in rats.
The small intestine, which is longer than the stomach and contains villi and microvilli, is much more effective at absorbing compounds (122). There is also evidence that the small intestine is a major site of hydrolysis and metabolism of flavone O-glycosides. Cleavage of flavonoid glycosides by β-glucosidase in the small intestine was tested in vitro with a Caco-2 model and cultured small intestinal cells from 10 people. The human small intestinal cells showed ≤87-fold interindividual variation in β-glucosidase activity, with variation also depending on the flavonoid conjugate being hydrolyzed (116). Two β-glucosidases in small intestinal cells can cleave flavonoid glycosides, with the greatest effect on flavonol, flavone, flavanone, and isoflavone O-glucosides (116, 118). The most likely to act on flavone glycosides in the small intestine is lactase-phlorizin hydrolase, a membrane-bound enzyme. Of the various flavonoids tested, it cleaved glucose but did not hydrolyze rhamnose, xylose, arabinose, and galactose to yield aglycones (116). Lactase-phlorizin hydrolase also hydrolyzes lactose, and is therefore not produced in lactose-intolerant individuals; this is a possible source of interindividual variation (116).
The second glucosidase found in intestinal cells is cytosolic β-glucosidase (CBG), which also resides in the cytosol of human liver, spleen, and kidney cells. It has been shown to preferentially hydrolyze β-d-fucose, β-d-glucose, α-l-arabinose, and β-d-galactose in vitro, but it can also hydrolyze β-l-arabinose and β-d-xylose linkages (118). In order for CBG to cleave flavonoid glycosides, the molecules must first be actively or passively transported into the cytosol. Although it has been proposed that flavonoids such as quercetin are transported into cells by passive diffusion (125), this would favor the transport of less polar (e.g., aglycone) molecules through the lipid bilayer membrane. And although the sodium-dependent glucose transporter 1 is involved in the uptake of some flavonoid glucosides such as quercetin 3-glucoside and 4′-glucoside (126), it does not appear to transport flavones and, in fact, may be inhibited by luteolin (127). Therefore, flavone O-glycosides must be cleaved to aglycones before they are absorbed into the small intestinal epithelial cells, and CBG does not play a role in their deglycosylation.
Flavones may be metabolized in the small intestine, and if flavones and their metabolites are transported to the basolateral side of the small intestine, they are carried to the liver via the hepatic portal vein (122). As with other flavonoids, flavones are metabolized by phase II enzymes in both the small intestine and liver. They are extensively metabolized by intestinal cells to glucuronidated and sulfated forms and then effluxed back to both the intestinal lumen and the bloodstream. The efflux of apigenin sulfate is mediated by both multidrug resistance–related proteins and organic anion transporters, whereas the efflux of apigenin glucuronide is mediated by multidrug resistance–related proteins. This efflux of flavone metabolites is likely a rate-limiting step in transport across the intestinal membrane (117). In vitro studies with human liver and intestinal microsomes found that luteolin was glucuronidated primarily at the 7 position in liver cells and at the 3′ and 4′ positions in intestinal cells. This differed markedly from rats, in which liver microsomes glucuronidate luteolin primarily at the 3′ position. Intestinal microsomes conjugated nearly 3 times as much luteolin as liver microsomes. When individual enzymes were tested, some glucuronidated luteolin much more efficiently than others, with human UDP glucuronosyltransferase family 1 member A (UGT1A) 1, UGT1A8, and UGT1A9 being especially effective (119). When apigenin was incubated with microsomes and cytosol isolated from human liver and intestinal tissue, monoglucuronides were produced by microsomes and monosulfates were produced in the cytosol. Apigenin was more rapidly glucuronidated than sulfonated (103). Another study that used HEPG2 human liver cells found that luteolin absorbed into the cells was predominantly methylated, indicating catechol-O-methyltransferase activity, which was not observed with apigenin (120). Evidence of catechol-O-methyltransferase activity for luteolin but not apigenin has also been observed in rats (128, 129). Based on these observations, future analytic methods will need to include methylated metabolites of luteolin, such as chrysoeriol and diosmetin, because they are not typically converted to luteolin during sample preparation.
Flavonoids that reach the colon can be further hydrolyzed and metabolized by bacteria. Rats were either kept germ free or associated with human intestinal microbiota. After giving the rats apigenin via an intragastric 7-glucoside dose, different levels of apigenin, apigenin conjugates, and other metabolites were excreted in the urine and feces (100). Rats with human intestinal microbiota excreted primarily conjugated apigenin and free 3-(4-hydroxyphenyl)propionic acid in the urine and free apigenin in feces compared with conjugated apigenin in the urine and free and conjugated apigenin in the feces of germ-free rats. Rats with human intestinal microbiota excreted more of the following metabolites in their urine: free and conjugated naringenin, free phloretin, conjugated luteolin, free 3,4-dihydroxyphenylpropionic acid, free and conjugated 3-(4-hydroxyphenyl)propionic acid and 4-hydroxycinnamic acid, and free 3-hydroxyphenylpropionic acid (100). It is interesting to note that luteolin and naringenin were also found in germ-free rats, indicating that they may be products of metabolism by the rats themselves. The metabolism of apigenin to luteolin has previously been reported in rats, mediated by the phase I enzyme cytochrome P450 (130), and this conversion has also been demonstrated in human liver microsomes (131). To our knowledge, no other studies have reported naringenin as a metabolite of apigenin, but it can be detected in samples if unpurified β-glucuronidase from Helix pomatia is used in the sample preparation (132).
In vitro experiments with Eubacterium ramulus, an obligate anaerobe found in the human colon, showed that eriodictyol and 3-(3,4-dihydroxyphenyl)propionic acid were metabolites of luteolin (133). Studies with Clostridium orbiscindens, which is found in numbers similar to those for E. ramulus in the intestinal tract, also showed that eriodictyol was a product of luteolin metabolism and that naringenin was a product of apigenin. Phloretin, 3-(4-dihydroxyphenyl)propionic acid, and phloroglucinol were end products of both apigenin and luteolin degradation (134).
Absorption and metabolism of flavone C-glycosides
As with for flavone O-glycosides, rats are the most common animal model used for flavone C-glycoside absorption. The Tmax for vitexin (apigenin 8-C-glycoside) derivatives was <1 h, and the Cmax was 1–29 μmol/L, varying with the dose (135–137). Urinary excretion of <1% confirms that flavone C-glycosides are poorly absorbed (136), and 10–88% recovery from feces indicates that they may be resistant to degradation by gut bacteria in rats (136, 138). As with flavone O-glycosides, the C-glycosides are less bioavailable in humans than in rats (Table 7). Although metabolites of apigenin or luteolin C-glycosides were found in plasma after human subjects drank rooibos tea, the concentrations were <1 nmol/L (34). Gastrointestinal and feces extracts showed 3 primary metabolites by intestinal bacteria: phloroglucinol, hydrocaffeic acid, and phloretic acid (138). When luteolin 6-C-glucoside (isoorientin) was incubated with human intestinal bacteria, only a small amount of luteolin aglycone was produced. The most abundant metabolites were 3,4-dihydroxyphenylpropionic acid and eriodictyol, with small amounts of 6-C-glucosyleriodictyol and phloroglucinol (140).
TABLE 7.
Bioavailability of flavone C-glycosides in humans1
| Source | Subjects, n | Dose | Plasma Tmax, h | Plasma concentration, μmol/L | Urinary excretion, % of intake | Reference |
| Rooibos teas (fermented and unfermented leaves) | 5 men and 5 women | 0.1–0.2 mg luteolin, 12.2–12.6 mg luteolin C-glycosides, and 2.0–2.2 mg apigenin C-glycosides | — | ND | ND | 139 |
| Rooibos tea (unfermented leaves) | 10 men | 43 mg luteolin C-glycosides, 5.9 mg apigenin C-glycosides, and 0.9 mg luteolin O-glycosides | 1.5–3 | <0.001 | — | 34 |
ND, not detected/limit of detection not specified; Tmax, time of maximum plasma concentration.
Biological activity of flavones in humans
Several intervention studies have been carried out with flavones and flavone-containing foods in humans. A trial that used fresh and cooked parsley as a flavone source found that the treatment group had higher blood antioxidant enzyme activities, including superoxide dismutase, glutathione peroxidase, glutathione reductase, and catalase (106). Other interventions with flavone-rich foods showed that they differed in their effects on biomarkers for heart disease and stroke. In a crossover study, subjects showed no differences in platelet aggregation after consuming a dried parsley supplement for 7 d. Although apigenin aglycone appeared effective at doses as low as 2.5 μmol/L in vitro, it did not show an effect at 250 nmol/L (107), which is closer to the concentrations expected in plasma. The metabolism of apigenin aglycone to conjugates might also explain the lack of efficacy. In another study, an 84-d trial that used artichoke leaf extract as an apigenin source found that the treatment group had lower total cholesterol than those taking placebo, but there were no significant differences in HDL cholesterol, LDL cholesterol, or TGs (141). Supplementation with vitexin-rich hawthorn extract lowered total and LDL cholesterol after 6 mo, but also had no effect on HDL cholesterol or TGs (142). Diosmetin rutinoside supplements have been successfully used in Europe to reduce bleeding and improve wound healing (143, 144). The proposed mechanisms of action include increasing venous tone and countering inflammatory mediators (144), congruent with the anti-inflammatory activity of flavones already demonstrated in animal studies.
The efficacy of flavones in human clinical trials cannot always be predicted by in vitro, animal, or bioavailability studies. One reason is that although flavones are found in circulation as glucuronide or sulfate conjugates, most in vitro experiments are done with aglycones or glucoside conjugates. It is possible for neutrophils to deconjugate luteolin glucuronide to aglycone in circulation, releasing the biologically active aglycone (145), but the extent of this process in vivo is unknown. Flavones may also be active in the intestinal lumen rather than in circulation, providing an explanation for the biological activity of the poorly available flavone C-glycosides. For example, apigenin and luteolin inhibit TNF-induced proinflammatory gene expression in murine intestinal epithelial cells (146). Similar effects were demonstrated with luteolin in human epithelial cells, indicating potential activity against inflammatory bowel disease (147).
Conclusions
Flavones occur in a wide variety of fruits, vegetables, and beverages. In their native forms, they are found as both O- and C-glycosides, with O-glycosides being more common and usually better absorbed. Relative to other polyphenols, flavones are not well absorbed, with plasma concentrations typically <1 μmol/L in humans. However, flavones have demonstrated many potentially beneficial activities in animal studies and human trials, and these should be further explored. Future clinical trials should not only take into consideration the dose but also the flavone form, because some native glycosides are poorly available and could be improved through processing.
Acknowledgments
We thank Andrea Doseff and Erich Grotewold for reviewing the manuscript. GLH wrote the original paper and had primary responsibility for the final content; RAR contributed substantially to revisions; and SJS provided advice and consultation. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: CBG, cytosolic β-glucosidase; Cmax, maximum plasma concentration; Tmax, time of maximum plasma concentration; UGT1A, UDP glucuronosyltransferase family 1 member A.
References
- 1.Hollman PCH. Absorption, bioavailability, and metabolism of flavonoids. Pharm Biol 2004;42:74–83. [Google Scholar]
- 2.Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr 2004;79:727–47. [DOI] [PubMed] [Google Scholar]
- 3.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr 2005;81:230S–242S. [DOI] [PubMed] [Google Scholar]
- 4.Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am J Clin Nutr 2005;81:243S–255S. [DOI] [PubMed] [Google Scholar]
- 5.Martens S, Mithöfer A. Flavones and flavone synthases. Phytochemistry 2005;66:2399–407. [DOI] [PubMed] [Google Scholar]
- 6.Harborne JB, Grayer RJ. Flavonoids and insects. In: Harborne JB, editor. The flavonoids: advances in research since 1986. 1st ed. London: Chapman & Hall; 1994. p. 589–618. [Google Scholar]
- 7.Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry 2000;55:481–504. [DOI] [PubMed] [Google Scholar]
- 8.Bruns B, Hahlbrock K, Schäfer E. Fluence dependence of the ultraviolet-light-induced accumulation of chalcone synthase mRNA and effects of blue and far-red light in cultured parsley cells. Planta 1986;169:393–8. [DOI] [PubMed] [Google Scholar]
- 9.Justesen U, Knuthsen P, Leth T. Quantitative analysis of flavonols, flavones, and flavanones in fruits, vegetables and beverages by high-performance liquid chromatography with photo-diode array and mass spectrometric detection. J Chromatogr A 1998;799:101–10. [DOI] [PubMed] [Google Scholar]
- 10.Pedneault K, Léonhart S, Gosselin A, Papadopoulos AP, Angers P, Dorais M. Variations in concentration of active compounds in four hydroponically-and field-grown medicinal plant species. Acta Hortic 2002;580:255–62. [Google Scholar]
- 11.Wildanger W, Herrmann K. Flavonols and flavones of vegetables. 1. Flavonols of Brassicae. Z Lebensm Unters Forsch 1973;152:134–7. [Google Scholar]
- 12.McNally DJ, Wurms KV, Labbé C, Quideau S, Bélanger RR. Complex C-glycosyl flavonoid phytoalexins from Cucumis sativus. J Nat Prod 2003;66:1280–3. [DOI] [PubMed] [Google Scholar]
- 13.Lattanzio V, De Cicco V, Di Venere D, Lima G, Salerno M. Antifungal activity of phenolics against fungi commonly encountered during storage. Ital J Food Sci 1994;6:23–30. [Google Scholar]
- 14.Heimler D, Isolani L, Vignolini P, Romani A. Polyphenol content and antiradical activity of Cichorium intybus L. from biodynamic and conventional farming. Food Chem 2009;114:765–70. [Google Scholar]
- 15.Young JE, Zhao X, Carey EE, Welti R, Yang S-S, Wang W. Phytochemical phenolics in organically grown vegetables. Mol Nutr Food Res 2005;49:1136–42. [DOI] [PubMed] [Google Scholar]
- 16.Chassy AW, Bui L, Renaud ENC, Van Horn M, Mitchell AE. Three-year comparison of the content of antioxidant microconstituents and several quality characteristics in organic and conventionally managed tomatoes and bell peppers. J Agric Food Chem 2006;54:8244–52. [DOI] [PubMed] [Google Scholar]
- 17.Zhao X, Carey EE, Young JE, Wang W, Iwamoto T. Influences of organic fertilization, high tunnel environment, and postharvest storage on phenolic compounds in lettuce. HortScience 2007;42:71–76. [Google Scholar]
- 18.Rolfe BG. Flavones and isoflavones as inducing substances of legume nodulation. Biofactors 1988;1:3–10. [PubMed] [Google Scholar]
- 19.Siqueira JO, Safir GR, Nair MG. Stimulation of vesicular-arbuscular mycorrhiza formation and growth of white clover by flavonoid compounds. New Phytol 1991;118:87–93. [Google Scholar]
- 20.Engelhardt UH, Finger A, Kuhr S. Determination of flavone C-glycosides in tea. Z Lebensm Unters Forsch 1993;197:239–44. [DOI] [PubMed] [Google Scholar]
- 21.Zamora-Ros R, Knaze V, Luján-Barroso L, Slimani N, Romieu I, Fedirko V, de Magistris MS, Ericson U, Amiano P, Trichopoulou A, et al. . Estimated dietary intakes of flavonols, flavanones and flavones in the European Prospective Investigation into Cancer and Nutrition (EPIC) 24 hour dietary recall cohort. Br J Nutr 2011;106:1915–25. [DOI] [PubMed] [Google Scholar]
- 22.Pounis G, Di Castelnuovo A, Bonaccio M, Costanzo S, Persichillo M, Krogh V, Donati MB, de Gaetano G, Iacoviello L. Flavonoid and lignan intake in a Mediterranean population: proposal for a holistic approach in polyphenol dietary analysis, the Moli-sani Study. Eur J Clin Nutr 2016;70:338–45. [DOI] [PubMed] [Google Scholar]
- 23.Jennings A, Welch AA, Spector T, Macgregor A, Cassidy A. Intakes of anthocyanins and flavones are associated with biomarkers of insulin resistance and inflammation in women. J Nutr 2014;144:202–8. [DOI] [PubMed] [Google Scholar]
- 24.Cassidy A, Huang T, Rice MS, Rimm EB, Tworoger SS. Intake of dietary flavonoids and risk of epithelial ovarian cancer. Am J Clin Nutr 2014;100:1344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Gapstur SM, Gaudet MM, Peterson JJ, Dwyer JT, McCullough ML. Evidence for an association of dietary flavonoid intake with breast cancer risk by estrogen receptor status is limited. J Nutr 2014;144:1603–11. [DOI] [PubMed] [Google Scholar]
- 26.Sun C, Wang H, Wang D, Chen Y, Zhao Y, Xia W. Using an FFQ to assess intakes of dietary flavonols and flavones among female adolescents in the Suihua area of northern China. Public Health Nutr 2015;18:632–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carnat A, Carnat AP, Fraisse D, Ricoux L, Lamaison JL. The aromatic and polyphenolic composition of Roman chamomile tea. Fitoterapia 2004;75:32–8. [DOI] [PubMed] [Google Scholar]
- 28.Svehlíková V, Repcák M. Apigenin chemotypes of Matricaria chamomilla L. Biochem Syst Ecol 2006;34:654–7. [Google Scholar]
- 29.Lechtenberg M, Zumdick S, Gerhards C, Schmidt TJ, Hensel A. Evaluation of analytical markers characterising different drying methods of parsley leaves (Petroselinum crispum L.). Pharmazie 2007;62:949–54. [PubMed] [Google Scholar]
- 30.Bhagwat S, Haytowitz DB, Holden JM. USDA database for the flavonoid content of selected foods, release 3.1 [Internet]. 2014. [cited 2016 Oct 19]. Available from: https://www.ars.usda.gov/ARSUserFiles/80400525/Data/Flav/Flav_R03–1.pdf.
- 31.Wojdylo A, Oszmianski J, Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem 2007;105:940–9. [Google Scholar]
- 32.Bramati L, Aquilano F, Pietta P. Unfermented rooibos tea: quantitative characterization of flavonoids by HPLC-UV and determination of the total antioxidant activity. J Agric Food Chem 2003;51:7472–4. [DOI] [PubMed] [Google Scholar]
- 33.Pengilly M, Joubert E, van Zyl WH, Botha A, Bloom M. Enhancement of rooibos (Aspalathus linearis) aqueous extract and antioxidant yield with fungal enzymes. J Agric Food Chem 2008;56:4047–53. [DOI] [PubMed] [Google Scholar]
- 34.Breiter T, Laue C, Kressel G, Gröll S, Engelhardt UH, Hahn A. Bioavailability and antioxidant potential of rooibos flavonoids in humans following the consumption of different rooibos formulations. Food Chem 2011;128:338–47. [DOI] [PubMed] [Google Scholar]
- 35.Areias FM, Valentão P, Andrade PB, Ferreres F, Seabra RM. Phenolic fingerprint of peppermint leaves. Food Chem 2001;73:307–11. [Google Scholar]
- 36.Fecka I, Turek S. Determination of water-soluble polyphenolic compounds in commercial herbal teas from Lamiaceae: peppermint, melissa, and sage. J Agric Food Chem 2007;55:10908–17. [DOI] [PubMed] [Google Scholar]
- 37.Meng L, Lozano Y, Bombarda I, Gaydou EM, Li B. Polyphenol extraction from eight Perilla frutescens cultivars. C R Chim 2009;12:602–11. [Google Scholar]
- 38.del Baño MJ, Lorente J, Castillo J, Benavente-García O, Marín MP, Del Río JA, Ortuño A, Ibarra I. Flavonoid distribution during the development of leaves, flowers, stems, and roots of Rosmarinus officinalis. Postulation of a biosynthetic pathway. J Agric Food Chem 2004;52:4987–92. [DOI] [PubMed] [Google Scholar]
- 39.Svehliková V, Bennett RN, Mellon FA, Needs PW, Piacente S, Kroon PA, Bao Y. Isolation, identification and stability of acylated derivatives of apigenin 7-O-glucoside from chamomile (Chamomilla recutita [L.] Rauschert). Phytochemistry 2004;65:2323–32. [DOI] [PubMed] [Google Scholar]
- 40.Caristi C, Bellocco E, Gargiulli C, Toscano G, Leuzzi U. Flavone-di-C-glycosides in citrus juices from Southern Italy. Food Chem 2006;95:431–7. [Google Scholar]
- 41.Gattuso G, Caristi C, Gargiulli C, Bellocco E, Toscano G, Leuzzi U. Flavonoid glycosides in bergamot juice (Citrus bergamia Risso). J Agric Food Chem 2006;54:3929–35. [DOI] [PubMed] [Google Scholar]
- 42.Fernandez de Simon B, Perez-Ilzarbe J, Hernandez T, Gomez-Cordoves C, Estrella I. Importance of phenolic compounds for the characterization of fruit juices. J Agric Food Chem 1992;40:1531–5. [Google Scholar]
- 43.Gil-Izquierdo A, Gil MI, Ferreres F. Effect of processing techniques at industrial scale on orange juice antioxidant and beneficial health compounds. J Agric Food Chem 2002;50:5107–14. [DOI] [PubMed] [Google Scholar]
- 44.Mullen W, Marks SC, Crozier A. Evaluation of phenolic compounds in commercial fruit juices and fruit drinks. J Agric Food Chem 2007;55:3148–57. [DOI] [PubMed] [Google Scholar]
- 45.Barreca D, Bellocco E, Caristi C, Leuzzi U, Gattuso G. Kumquat (Fortunella japonica Swingle) juice: flavonoid distribution and antioxidant properties. Food Res Int 2011;44:2190–7. [Google Scholar]
- 46.Gambelli L, Santaroni GP. Polyphenols content in some Italian red wines of different geographical origins. J Food Compos Anal 2004;17:613–8. [Google Scholar]
- 47.Sun Y, Fang N, Chen DDY, Donkor KK. Determination of potentially anti-carcinogenic flavonoids in wines by micellar electrokinetic chromatography. Food Chem 2008;106:415–20. [Google Scholar]
- 48.Bevilacqua L, Buiarelli F, Coccioli F, Jasionowska R. Identification of compounds in wine by HPLC-tandem mass spectrometry. Ann Chim 2004;94:679–89. [DOI] [PubMed] [Google Scholar]
- 49.Fang F, Li J-M, Zhang P, Tang K, Wang W, Pan Q-H, Huang W-D. Effects of grape variety, harvest date, fermentation vessel and wine ageing on flavonoid concentration in red wines. Food Res Int 2008;41:53–60. [Google Scholar]
- 50.Sakakibara H, Honda Y, Nakagawa S, Ashida H, Kanazawa K. Simultaneous determination of all polyphenols in vegetables, fruits, and teas. J Agric Food Chem 2003;51:571–81. [DOI] [PubMed] [Google Scholar]
- 51.Wu T, Guan Y, Ye J. Determination of flavonoids and ascorbic acid in grapefruit peel and juice by capillary electrophoresis with electrochemical detection. Food Chem 2007;100:1573–9. [Google Scholar]
- 52.Harnly JM, Doherty RF, Beecher GR, Holden JM, Haytowitz DB, Bhagwat S, Gebhardt S. Flavonoid content of U.S. fruits, vegetables, and nuts. J Agric Food Chem 2006;54:9966–77. [DOI] [PubMed] [Google Scholar]
- 53.Lugasi A, Takács M. Flavonoid aglycons in foods of plant origin II. Fresh and dried fruits. Acta Aliment Hung 2002;31:63–71. [Google Scholar]
- 54.Chu Y-H, Chang C-L, Hsu H-F. Flavonoid content of several vegetables and their antioxidant activity. J Sci Food Agric 2000;80:561–6. [Google Scholar]
- 55.Franke AA, Custer LJ, Arakaki C, Murphy SP. Vitamin C and flavonoid levels of fruits and vegetables consumed in Hawaii. J Food Compos Anal 2004;17:1–35. [Google Scholar]
- 56.Hertog MGL, Hollman PCH, Katan MB. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J Agric Food Chem 1992;40:2379–83. [Google Scholar]
- 57.Arai Y, Watanabe S, Kimira M, Shimoi K, Mochizuki R, Kinae N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J Nutr 2000;130:2243–50. [DOI] [PubMed] [Google Scholar]
- 58.Lugasi A, Hóvári J. Flavonoid aglycones in foods of plant origin I. Vegetables. Acta Aliment Hung 2000;29:345–52. [Google Scholar]
- 59.Justesen U, Knuthsen P. Composition of flavonoids in fresh herbs and calculation of flavonoid intake by use of herbs in traditional Danish dishes. Food Chem 2001;73:245–50. [Google Scholar]
- 60.Mattila P, Astola J, Kumpulainen J. Determination of flavonoids in plant material by HPLC with diode-array and electro-array detections. J Agric Food Chem 2000;48:5834–41. [DOI] [PubMed] [Google Scholar]
- 61.Hertog MGL, Hollman PCH, Venema DP. Optimization of a quantitative HPLC determination of potentially anticarcinogenic flavonoids in vegetables and fruits. J Agric Food Chem 1992;40:1591–8. [Google Scholar]
- 62.Arabbi PR, Genovese MI, Lajolo FM. Flavonoids in vegetable foods commonly consumed in Brazil and estimated ingestion by the Brazilian population. J Agric Food Chem 2004;52:1124–31. [DOI] [PubMed] [Google Scholar]
- 63.DuPont MS, Mondin Z, Williamson G, Price KR. Effect of variety, processing, and storage on the flavonoid glycoside content and composition of lettuce and endive. J Agric Food Chem 2000;48:3957–64. [DOI] [PubMed] [Google Scholar]
- 64.Huber LS, Hoffmann-Ribani R, Rodriguez-Amaya DB. Quantitative variation in Brazilian vegetable sources of flavonols and flavones. Food Chem 2009;113:1278–82. [Google Scholar]
- 65.Bahorun T, Luximon-Ramma A, Crozier A, Aruoma OI. Total phenol, flavonoid, proanthocyanidin and vitamin C levels and antioxidant activities of Mauritian vegetables. J Sci Food Agric 2004;84:1553–61. [Google Scholar]
- 66.Ferracane R, Pellegrini N, Visconti A, Graziani G, Chiavaro E, Miglio C, Fogliano V. Effects of different cooking methods on antioxidant profile, antioxidant capacity, and physical characteristics of artichoke. J Agric Food Chem 2008;56:8601–8. [DOI] [PubMed] [Google Scholar]
- 67.Azzini E, Bugianesi R, Romano F, Di Venere D, Miccadei S, Durazzo A, Foddai MS, Catasta G, Linsalata V, Maiani G. Absorption and metabolism of bioactive molecules after oral consumption of cooked edible heads of Cynara scolymus L. (cultivar Violetto di Provenza) in human subjects: a pilot study. Br J Nutr 2007;97:963–9. [DOI] [PubMed] [Google Scholar]
- 68.Wang M, Simon JE, Aviles IF, He K, Zheng Q-Y, Tadmor Y. Analysis of antioxidative phenolic compounds in artichoke (Cynara scolymus L.). J Agric Food Chem 2003;51:601–8. [DOI] [PubMed] [Google Scholar]
- 69.Schütz K, Kammerer D, Carle R, Schieber A. Identification and quantification of caffeoylquinic acids and flavonoids from artichoke (Cynara scolymus L.) heads, juice, and pomace by HPLC-DAD-ESI/MS(n). J Agric Food Chem 2004;52:4090–6. [DOI] [PubMed] [Google Scholar]
- 70.Lattanzio V, van Sumere CF. Changes in phenolic compounds during the development and cold storage of artichoke (Cynara scolymus L.) heads. Food Chem 1987;24:37–50. [Google Scholar]
- 71.Innocenti M, Gallori S, Giaccherini C, Ieri F, Vincieri FF, Mulinacci N. Evaluation of the phenolic content in the aerial parts of different varieties of Cichorium intybus L. J Agric Food Chem 2005;53:6497–502. [DOI] [PubMed] [Google Scholar]
- 72.Miean KH, Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J Agric Food Chem 2001;49:3106–12. [DOI] [PubMed] [Google Scholar]
- 73.Sampson L, Rimm E, Hollman PCH, de Vries JHM, Katan MB. Flavonol and flavone intakes in US health professionals. J Am Diet Assoc 2002;102:1414–20. [DOI] [PubMed] [Google Scholar]
- 74.Owen RW, Haubner R, Mier W, Giacosa A, Hull WE, Spiegelhalder B, Bartsch H. Isolation, structure elucidation and antioxidant potential of the major phenolic and flavonoid compounds in brined olive drupes. Food Chem Toxicol 2003;41:703–17. [DOI] [PubMed] [Google Scholar]
- 75.Ouni Y, Taamalli A, Gómez-Caravaca AM, Segura-Carretero A, Fernández-Gutiérrez A, Zarrouk M. Characterisation and quantification of phenolic compounds of extra-virgin olive oils according to their geographical origin by a rapid and resolutive LC–ESI-TOF MS method. Food Chem 2011;127:1263–7. [DOI] [PubMed] [Google Scholar]
- 76.Hoffmann-Ribani R, Huber LS, Rodriguez-Amaya DB. Flavonols in fresh and processed Brazilian fruits. J Food Compos Anal 2009;22:263–8. [Google Scholar]
- 77.Iurlina MO, Saiz AI, Fritz R, Manrique GD. Major flavonoids of Argentinean honeys. Optimisation of the extraction method and analysis of their content in relationship to the geographical source of honeys. Food Chem 2009;115:1141–9. [Google Scholar]
- 78.Karakaya S, Nehir ELS. Quercetin, luteolin, apigenin and kaempferol contents of some foods. Food Chem 1999;66:289–92. [Google Scholar]
- 79.Yao L, Jiang Y, D’Arcy B, Singanusong R, Datta N, Caffin N, Raymont K. Quantitative high-performance liquid chromatography analyses of flavonoids in Australian Eucalyptus honeys. J Agric Food Chem 2004;52:210–4. [DOI] [PubMed] [Google Scholar]
- 80.Gil MI, Ferreres F, Ortiz A, Subra E, Tomas-Barberan FA. Plant phenolic metabolites and floral origin of rosemary honey. J Agric Food Chem 1995;43:2833–8. [Google Scholar]
- 81.Kenjerić D, Mandić ML, Primorac L, Čačić F. Flavonoid pattern of sage (Salvia officinalis L.) unifloral honey. Food Chem 2008;110:187–92. [DOI] [PubMed] [Google Scholar]
- 82.Yao L, Jiang Y, Singanusong R, D’Arcy B, Datta N, Caffin N, Raymont K. Flavonoids in Australian Melaleuca, Guioa, Lophostemon, Banksia and Helianthus honeys and their potential for floral authentication. Food Res Int 2004;37:166–74. [Google Scholar]
- 83.Magalhães SCQ, Taveira M, Cabrita ARJ, Fonseca AJM, Valentão P, Andrade PB. European marketable grain legume seeds: further insight into phenolic compounds profiles. Food Chem 2017;215:177–84. [DOI] [PubMed] [Google Scholar]
- 84.Sartelet H, Serghat S, Lobstein A, Ingenbleek Y, Anton R, Petitfrère E, Aguie-Aguie G, Martiny L, Haye B. Flavonoids extracted from fonio millet (Digitaria exilis) reveal potent antithyroid properties. Nutrition 1996;12:100–6. [DOI] [PubMed] [Google Scholar]
- 85.Dykes L, Peterson GC, Rooney WL, Rooney LW. Flavonoid composition of lemon-yellow sorghum genotypes. Food Chem 2011;128:173–9. [DOI] [PubMed] [Google Scholar]
- 86.Wu G, Johnson SK, Bornman JF, Bennett SJ, Clarke MW, Singh V, Fang Z. Growth temperature and genotype both play important roles in sorghum grain phenolic composition. Sci Rep 2016;6:21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wijaya GY, Mares DJ. Apigenin di-C-glycosides (ACG) content and composition in grains of bread wheat (Triticum aestivum) and related species. J Cereal Sci 2012;56:260–7. [Google Scholar]
- 88.Pereira-Caro G, Cros G, Yokota T, Crozier A. Phytochemical profiles of black, red, brown, and white rice from the Camargue region of France. J Agric Food Chem 2013;61:7976–86. [DOI] [PubMed] [Google Scholar]
- 89.Dietrych-Szostak D, Oleszek W. Effect of processing on the flavonoid content in buckwheat (Fagopyrum esculentum Möench) grain. J Agric Food Chem 1999;47:4384–7. [DOI] [PubMed] [Google Scholar]
- 90.Kiprovski B, Mikulic-Petkovsek M, Slatnar A, Veberic R, Stampar F, Malencic D, Latkovic D. Comparison of phenolic profiles and antioxidant properties of European Fagopyrum esculentum cultivars. Food Chem 2015;185:41–7. [DOI] [PubMed] [Google Scholar]
- 91.Production and utilization. Sorghum and millets in human nutrition [Internet]. 1995 [cited 2016 Oct 26]. Rome (Italy): FAO of the UN. Available from: http://www.fao.org/docrep/T0818E/T0818E00.htm#Contents.
- 92.Hostetler GL, Riedl KM, Schwartz SJ. Endogenous enzymes, heat, and pH affect flavone profiles in parsley (Petroselinum crispum var. neapolitanum) and celery (Apium graveolens) during juice processing. J Agric Food Chem 2012;60:202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Joubert E, Viljoen M, De Beer D, Manley M. Effect of heat on aspalathin, iso-orientin, and orientin contents and color of fermented rooibos (Aspalathus linearis) iced tea. J Agric Food Chem 2009;57:4204–11. [DOI] [PubMed] [Google Scholar]
- 94.Aherne SA, O’Brien NM. Dietary flavonols: chemistry, food content, and metabolism. Nutrition 2002;18:75–81. [DOI] [PubMed] [Google Scholar]
- 95.Patras A, Brunton NP, O’Donnell C, Tiwari BK. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci Technol 2010;21:3–11. [Google Scholar]
- 96.Jackson C-JC, Dini JP, Lavandier C, Rupasinghe HPV, Faulkner H, Poysa V, Buzzell D, DeGrandis S. Effects of processing on the content and composition of isoflavones during manufacturing of soy beverage and tofu. Process Biochem 2002;37:1117–23. [Google Scholar]
- 97.Shimoi K, Okada H, Furugori M, Goda T, Takase S, Suzuki M, Hara Y, Yamamoto H, Kinae N. Intestinal absorption of luteolin and luteolin 7-O-beta-glucoside in rats and humans. FEBS Lett 1998;438:220–4. [DOI] [PubMed] [Google Scholar]
- 98.Sarawek S, Derendorf H, Butterweck V. Pharmacokinetics of luteolin and metabolites in rats. Nat Prod Commun 2008;3:2029–36. [Google Scholar]
- 99.Zhou P, Li L-P, Luo S-Q, Jiang H-D, Zeng S. Intestinal absorption of luteolin from peanut hull extract is more efficient than that from individual pure luteolin. J Agric Food Chem 2008;56:296–300. [DOI] [PubMed] [Google Scholar]
- 100.Hanske L, Loh G, Sczesny S, Blaut M, Braune A. The bioavailability of apigenin-7-glucoside is influenced by human intestinal microbiota in rats. J Nutr 2009;139:1095–102. [DOI] [PubMed] [Google Scholar]
- 101.Bilia AR, Giomi M, Innocenti M, Gallori S, Vincieri FF. HPLC–DAD–ESI–MS analysis of the constituents of aqueous preparations of verbena and lemon verbena and evaluation of the antioxidant activity. J Pharm Biomed Anal 2008;46:463–70. [DOI] [PubMed] [Google Scholar]
- 102.Gradolatto A, Basly J-P, Berges R, Teyssier C, Chagnon M-C, Siess M-H, Canivenc-Lavier M-C. Pharmacokinetics and metabolism of apigenin in female and male rats after a single oral administration. Drug Metab Dispos 2005;33:49–54. [DOI] [PubMed] [Google Scholar]
- 103.Cai H, Boocock D, Steward W, Gescher A. Tissue distribution in mice and metabolism in murine and human liver of apigenin and tricin, flavones with putative cancer chemopreventive properties. Cancer Chemother Pharmacol 2007;60:257–66. [DOI] [PubMed] [Google Scholar]
- 104.Hostetler G, Riedl K, Cardenas H, Diosa-Toro M, Arango D, Schwartz S, Doseff AI. Flavone deglycosylation increases their anti-inflammatory activity and absorption. Mol Nutr Food Res 2012;56:558–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Meyer H, Bolarinwa A, Wolfram G, Linseisen J. Bioavailability of apigenin from apiin-rich parsley in humans. Ann Nutr Metab 2006;50:167–72. [DOI] [PubMed] [Google Scholar]
- 106.Nielsen SE, Young JF, Daneshvar B, Lauridsen ST, Knuthsen P, Sandström B, Dragsted LO. Effect of parsley (Petroselinum crispum) intake on urinary apigenin excretion, blood antioxidant enzymes and biomarkers for oxidative stress in human subjects. Br J Nutr 1999;81:447–55. [DOI] [PubMed] [Google Scholar]
- 107.Janssen K, Mensink RP, Cox FJ, Harryvan JL, Hovenier R, Hollman PC, Katan MB. Effects of the flavonoids quercetin and apigenin on hemostasis in healthy volunteers: results from an in vitro and a dietary supplement study. Am J Clin Nutr 1998;67:255–62. [DOI] [PubMed] [Google Scholar]
- 108.Cao J, Zhang Y, Chen W, Zhao X. The relationship between fasting plasma concentrations of selected flavonoids and their ordinary dietary intake. Br J Nutr 2010;103:249–55. [DOI] [PubMed] [Google Scholar]
- 109.Tschiersch K, Hölzl J. Absorption and excretion of apigenin, apigenin-7-glycoside and herniarin after oral administration of extracts of Matricaria recutita (L.) (syn. Chamomilla recutita (L.) Rauschert). Pharmazie 1993;48:554–5. [PubMed] [Google Scholar]
- 110.Li L-P, Jiang H-D. Determination and assay validation of luteolin and apigenin in human urine after oral administration of tablet of Chrysanthemum morifolium extract by HPLC. J Pharm Biomed Anal 2006;41:261–5. [DOI] [PubMed] [Google Scholar]
- 111.Wittemer SM, Ploch M, Windeck T, Müller SC, Drewelow B, Derendorf H, Veit M. Bioavailability and pharmacokinetics of caffeoylquinic acids and flavonoids after oral administration of Artichoke leaf extracts in humans. Phytomedicine 2005;12:28–38. [DOI] [PubMed] [Google Scholar]
- 112.Spanakis M, Kasmas S, Niopas I. Simultaneous determination of the flavonoid aglycones diosmetin and hesperetin in human plasma and urine by a validated GC/MS method: in vivo metabolic reduction of diosmetin to hesperetin. Biomed Chromatogr 2009;23:124–31. [DOI] [PubMed] [Google Scholar]
- 113.Cova D, De Angelis L, Giavarini F, Palladini G, Perego R. Pharmacokinetics and metabolism of oral diosmin in healthy volunteers. Int J Clin Pharmacol Ther Toxicol 1992;30:29–33. [PubMed] [Google Scholar]
- 114.Lin L-Z, Lu S, Harnly JM. Detection and quantification of glycosylated flavonoid malonates in celery, chinese celery, and celery seed by LC-DAD-ESI/MS. J Agric Food Chem 2007;55:1321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nielsen SE, Dragsted LO. Column-switching high-performance liquid chromatographic assay for determination of apigenin and acacetin in human urine with ultraviolet absorbance detection. J Chromatogr B Biomed Sci Appl 1998;713:379–86. [DOI] [PubMed] [Google Scholar]
- 116.Németh K, Plumb GW, Berrin J-G, Juge N, Jacob R, Naim HY, Williamson G, Swallow DM, Kroon PA. Deglycosylation by small intestinal epithelial cell β-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur J Nutr 2003;42:29–42. [DOI] [PubMed] [Google Scholar]
- 117.Hu M, Chen J, Lin H. Metabolism of flavonoids via enteric recycling: mechanistic studies of disposition of apigenin in the Caco-2 cell culture model. J Pharmacol Exp Ther 2003;307:314–21. [DOI] [PubMed] [Google Scholar]
- 118.Berrin J-G, McLauchlan WR, Needs P, Williamson G, Puigserver A, Kroon PA, Juge N. Functional expression of human liver cytosolic β-glucosidase in Pichia pastoris. Insights into its role in the metabolism of dietary glucosides. Eur J Biochem 2002;269:249–58. [DOI] [PubMed] [Google Scholar]
- 119.Boersma MG, van der Woude H, Bogaards J, Boeren S, Vervoort J, Cnubben NHP, van Iersel MLPS, van Bladeren PJ, Rietjens IMCM. Regioselectivity of phase II metabolism of luteolin and quercetin by UDP-glucuronosyl transferases. Chem Res Toxicol 2002;15:662–70. [DOI] [PubMed] [Google Scholar]
- 120.Kanazawa K, Uehara M, Yanagitani H, Hashimoto T. Bioavailable flavonoids to suppress the formation of 8-OHdG in HepG2 cells. Arch Biochem Biophys 2006;455:197–203. [DOI] [PubMed] [Google Scholar]
- 121.Ung D, Nagar S. Variable sulfation of dietary polyphenols by recombinant human sulfotransferase (SULT) 1A1 genetic variants and SULT1E1. Drug Metab Dispos 2007;35:740–6. [DOI] [PubMed] [Google Scholar]
- 122.Ashford M. The gastrointestinal tract-physiology and drug absorption. In: Aulton ME, editor. Pharmaceutics: the science of dosage form design. London: Churchill Livingstone; 2002. p. 217. [Google Scholar]
- 123.Pforte H, Jacobasch G, Naser T, Buhr HJ. Absorption and modification of rutin in the human stomach. Spec Publ R Soc Chem 2000;255:84–7. [Google Scholar]
- 124.Pforte H, Hempel J, Jacobasch G. Distribution pattern of a flavonoid extract in the gastrointestinal lumen and wall of rats. Nahrung 1999;43:205–8. [DOI] [PubMed] [Google Scholar]
- 125.Gee JM, DuPont MS, Day AJ, Plumb GW, Williamson G, Johnson IT. Intestinal transport of quercetin glycosides in rats involves both deglycosylation and interaction with the hexose transport pathway. J Nutr 2000;130:2765–71. [DOI] [PubMed] [Google Scholar]
- 126.Day AJ, Gee JM, DuPont MS, Johnson IT, Williamson G. Absorption of quercetin-3-glucoside and quercetin-4′-glucoside in the rat small intestine: the role of lactase phlorizin hydrolase and the sodium-dependent glucose transporter. Biochem Pharmacol 2003;65:1199–206. [DOI] [PubMed] [Google Scholar]
- 127.Kottra G, Daniel H. Flavonoid glycosides are not transported by the human Na+/glucose transporter when expressed in Xenopus laevis oocytes, but effectively inhibit electrogenic glucose uptake. J Pharmacol Exp Ther 2007;322:829–35. [DOI] [PubMed] [Google Scholar]
- 128.Chen Z, Chen M, Pan H, Sun S, Li L, Zeng S, Jiang H. Role of catechol-O-methyltransferase in the disposition of luteolin in rats. Drug Metab Dispos 2011;39:667–74. [DOI] [PubMed] [Google Scholar]
- 129.Chen Z, Tu M, Sun S, Kong S, Wang Y, Ye J, Li L, Zeng S, Jiang H. The exposure of luteolin is much lower than that of apigenin in oral administration of Flos chrysanthemi extract to rats. Drug Metab Pharmacokinet 2012;27:162–8. [DOI] [PubMed] [Google Scholar]
- 130.Gradolatto A, Canivenc-Lavier M-C, Basly J-P, Siess M-H, Teyssier C. Metabolism of apigenin by rat liver phase I and phase II enzymes and by isolated perfused rat liver. Drug Metab Dispos 2004;32:58–65. [DOI] [PubMed] [Google Scholar]
- 131.Breinholt VM, Offord EA, Brouwer C, Nielsen SE, Brøsen K, Friedberg T. In vitro investigation of cytochrome P450-mediated metabolism of dietary flavonoids. Food Chem Toxicol 2002;40:609–16. [DOI] [PubMed] [Google Scholar]
- 132.Grace PB, Teale P. Purification of the crude solution from Helix pomatia for use as beta-glucuronidase and aryl sulfatase in phytoestrogen assays. J Chromatogr B Analyt Technol Biomed Life Sci 2006;832:158–61. [DOI] [PubMed] [Google Scholar]
- 133.Braune A, Gütschow M, Engst W, Blaut M. Degradation of quercetin and luteolin by eubacterium ramulus. Appl Environ Microbiol 2001;67:5558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schoefer L, Mohan R, Schwiertz A, Braune A, Blaut M. Anaerobic degradation of flavonoids by Clostridium orbiscindens. Appl Environ Microbiol 2003;69:5849–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liang M, Xu W, Zhang W, Zhang C, Liu R, Shen Y, Li H, Wang X, Wang X, Pan Q, et al. . Quantitative LC/MS/MS method and in vivo pharmacokinetic studies of vitexin rhamnoside, a bioactive constituent on cardiovascular system from hawthorn. Biomed Chromatogr 2007;21:422–9. [DOI] [PubMed] [Google Scholar]
- 136.Ma LY, Liu RH, Xu XD, Yu MQ, Zhang Q, Liu HL. The pharmacokinetics of C-glycosyl flavones of Hawthorn leaf flavonoids in rat after single dose oral administration. Phytomedicine 2010;17:640–5. [DOI] [PubMed] [Google Scholar]
- 137.Ying X, Lu X, Sun X, Li X, Li F. Determination of vitexin-2′’-O-rhamnoside in rat plasma by ultra-performance liquid chromatography electrospray ionization tandem mass spectrometry and its application to pharmacokinetic study. Talanta 2007;72:1500–6. [DOI] [PubMed] [Google Scholar]
- 138.Zhang Y, Tie X, Bao B, Wu X, Zhang Y. Metabolism of flavone C-glucosides and p-coumaric acid from antioxidant of bamboo leaves (AOB) in rats. Br J Nutr 2007;97:484–94. [DOI] [PubMed] [Google Scholar]
- 139.Stalmach A, Mullen W, Pecorari M, Serafini M, Crozier A. Bioavailability of C-linked dihydrochalcone and flavanone glucosides in humans following ingestion of unfermented and fermented rooibos teas. J Agric Food Chem 2009;57:7104–11. [DOI] [PubMed] [Google Scholar]
- 140.Hattori M, Shu YZ, el-Sedawy AI, Namba T, Kobashi K, Tomimori T. Metabolism of homoorientin by human intestinal bacteria. J Nat Prod 1988;51:874–8. [DOI] [PubMed] [Google Scholar]
- 141.Bundy R, Walker AF, Middleton RW, Wallis C, Simpson HCR. Artichoke leaf extract (Cynara scolymus) reduces plasma cholesterol in otherwise healthy hypercholesterolemic adults: a randomized, double blind placebo controlled trial. Phytomedicine 2008;15:668–75. [DOI] [PubMed] [Google Scholar]
- 142.Dalli E, Colomer E, Tormos MC, Cosín-Sales J, Milara J, Esteban E, Sáez G. Crataegus laevigata decreases neutrophil elastase and has hypolipidemic effect: a randomized, double-blind, placebo-controlled trial. Phytomedicine 2011;18:769–75. [DOI] [PubMed] [Google Scholar]
- 143.Guilhou JJ, Dereure O, Marzin L, Ouvry P, Zuccarelli F, Debure C, Van Landuyt H, Gillet-Terver MN, Guillot B, Levesque H, et al. . Efficacy of Daflon 500 mg in venous leg ulcer healing: a double-blind, randomized, controlled versus placebo trial in 107 patients. Angiology 1997;48:77–85. [DOI] [PubMed] [Google Scholar]
- 144.Misra MC, Parshad R. Randomized clinical trial of micronized flavonoids in the early control of bleeding from acute internal haemorrhoids. Br J Surg 2000;87:868–72. [DOI] [PubMed] [Google Scholar]
- 145.Shimoi K, Saka N, Nozawa R, Sato M, Amano I, Nakayama T, Kinae N. Deglucuronidation of a flavonoid, luteolin monoglucuronide, during inflammation. Drug Metab Dispos 2001;29:1521–4. [PubMed] [Google Scholar]
- 146.Ruiz PA, Haller D. Functional diversity of flavonoids in the inhibition of the proinflammatory NF-κB, IRF, and Akt signaling pathways in murine intestinal epithelial cells. J Nutr 2006;136:664–71. [DOI] [PubMed] [Google Scholar]
- 147.Kim J-A, Kim D-K, Kang O-H, Choi Y-A, Park H-J, Choi S-C, Kim T-H, Yun K-J, Nah Y-H, Lee Y-M. Inhibitory effect of luteolin on TNF-alpha-induced IL-8 production in human colon epithelial cells. Int Immunopharmacol 2005;5:209–17. [DOI] [PubMed] [Google Scholar]