Abstract
Brown adipose tissue (BAT) is a specialized fat tissue that has a high capacity to dissociate cellular respiration from ATP utilization, resulting in the release of stored energy as heat. Adult humans possess a substantial amount of BAT in the form of constitutively active brown fat or inducible beige fat. BAT activity in humans is inversely correlated with adiposity, blood glucose concentrations, and insulin sensitivity; this suggests that strategies aimed at BAT-mediated bioenergetics are an attractive therapeutic target in combating the continuing epidemic of obesity and diabetes. Despite advances in knowledge regarding the developmental lineage and transcriptional regulators of brown and beige adipocytes, our current understanding of environmental modifiers of BAT thermogenesis, such as diet, is limited. In this review, we consolidated the latest research on dietary molecules that may serve to promote BAT thermogenesis. Here, we summarized the thermogenic function of selected phytochemicals (e.g., capsaicin, resveratrol, curcumin, green tea, and berberine), dietary fatty acids (e.g., fish oil and conjugated linoleic acids), and all-trans retinoic acid, a vitamin A metabolite. We also delineated the proposed mechanisms whereby these dietary molecules promote BAT activity and/or browning of white adipose tissue. Characterizing thermogenic dietary factors may offer novel insight into revising nutritional intervention strategies aimed at obesity and diabetes prevention and management.
Keywords: WAT browning, brown adipocyte, beige adipocytes, dietary molecule, thermogenesis, UCP1
Introduction
At least 3 metabolically distinct adipocyte types are found in humans: white, beige, and classical brown adipocytes. Unlike white adipocytes that deposit extra energy into TGs, beige and classical brown adipocytes are distinguished by their unique ability to dissipate mitochondrial energy into heat via uncoupling protein 1 (UCP1)5. Classical human brown adipocytes possess molecular attributes similar to the interscapular brown adipose tissue (iBAT) of rodents, such as constitutive UCP1 expression, homogeneous multilocular morphology, and a myogenic origin (Myf5+) (1, 2). Conversely, beige adipocytes originate from nonmyogenic (Myf5−) progenitors upon environmental stimuli (such as cold and exercise) (3), and exhibit low levels of UCP1 expression under unstimulated conditions. Despite some uncertainties and controversies surrounding the cellular identity, recruitment, and bidirectional transdifferentiation of beige fat (4, 5), the metabolic significance of both classical brown and beige adipocytes in maintaining energy homeostasis has been unanimously established over the last decade (6–8). It is important to note that the majority of brown adipose tissue (BAT) studies have been conducted in response to cold exposure. Cold exposure causes the sympathetic nervous system (SNS) to release norepinephrine and induces human BAT thermogenesis through β3-adrenergic receptor (ADRB3) activation.
These studies have allowed the identification of numerous biochemical factors that control cellular autonomous functions of brown and beige fat formation. However, cold treatment or direct pharmacologic activation of ADRB3 are not suitable approaches for human interventions, because ADRB3 agonist treatments have been shown to increase the risk of cardiovascular diseases (9).
Emerging evidence suggests that dietary factors can serve as critical environmental regulators of BAT activation and thermogenesis. Excessive adiposity that is induced by consuming a high-fat (HF) diet (10) or through chronic PPARγ (PPARG) agonism treatment (11) can induce white adipose tissue (WAT) browning as a part of the body’s natural defense mechanisms to counteract the excessive energy burden. More importantly, a growing body of literature has revealed that some dietary molecules can be effective in potentiating BAT thermogenic functions and improving metabolism. Considering that the nutritional modulation of adaptive thermogenesis is a newly developing area of research, it is required to thoroughly evaluate its potential as a realistic therapeutic target to promote human health. Therefore, it is timely to ask the question, “What are the available dietary options, effective doses, and diet-induced signaling pathways that will promote adaptive thermogenesis?” Answering this question will help us identify the current gap of knowledge and assess the metabolic significance of diet-induced adaptive thermogenesis.
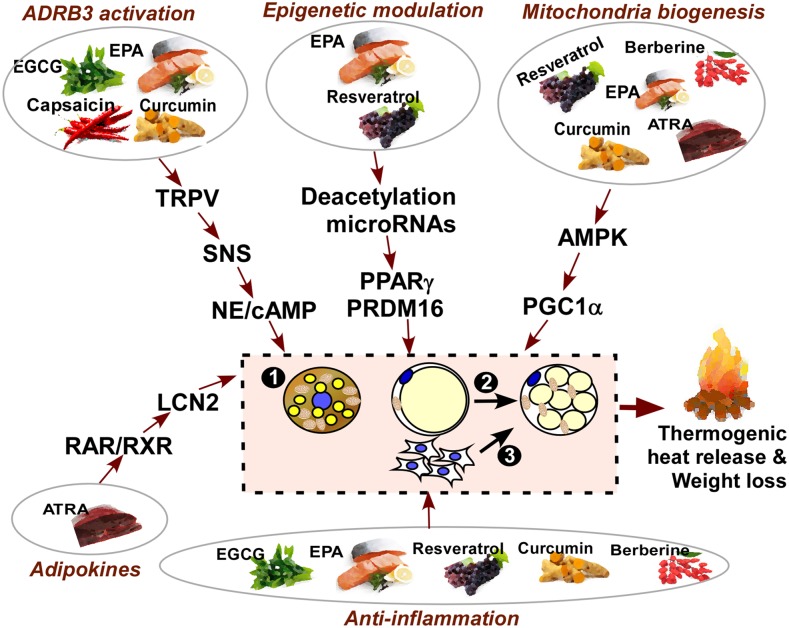
In this review, we assemble the literature that has been reported on dietary molecules with thermogenic activity, and integrate the metabolic pathways whereby these dietary factors mediate BAT thermogenesis. For the purpose of this review, we exclude the thermic effects of food induced by a high-protein diet (12). Nondietary thermogenic agents that act through stimulation of the central nervous system (e.g., ephedrine) (13), or trace elements such as iron, copper, zinc, or selenium that may have some regulatory effects on thermogenesis, are also excluded in this review. Here, we will describe the thermogenic capacity of the following: 1) selective phytochemicals, including capsaicin, resveratrol, curcumin, green tea epigallocatechin gallate (EGCG), and berberine; 2) dietary FAs such as fish oil and conjugated linoleic acids; and 3) all-trans retinoic acid (ATRA), a vitamin A metabolite. We will define the impact of each dietary molecule on cellular responses of brown and beige fat cell formation, including the following: 1) an increase in BAT activity in pre-existing classical brown adipocytes in the iBAT; 2) the metabolic switch of some, if not all, existing white adipocytes to beige adipocytes in the subcutaneous fat (WAT browning); and 3) new beige adipocyte formation from progenitor cells. We schematically categorized the aforementioned dietary molecules based on their potential signaling pathways that trigger brown and beige thermogenesis (i.e., ADRB3 activation, epigenetic regulation, AMP-activated protein kinase (AMPK)/PPARγ coactivator 1-α (PGC1α) signaling, and alternation of immune responses) (Figure 1). We further explain the effects of individual dietary factors on thermogenic activation and their mode of action in the following sections.
FIGURE 1.
Thermogenic dietary molecules and potential mechanisms of action. At least 3 distinct metabolic responses may occur upon thermogenic stimuli, including the following: 1) an increase in BAT activity in pre-existing classical brown adipocytes; 2) the metabolic switch of some, if not all, existing white adipocytes to beige adipocytes in subcutaneous fat (WAT browning); and 3) new beige adipocyte formation from adipogenic progenitor cells. There are multiple and overlapping signaling pathways that are involved in stimulating BAT activation by dietary molecules. In this review, we summarized the several known dietary molecules that function to trigger thermogenic responses via mechanisms that include the following: 1) ADRB3 activation through the TRPV1 channel and the SNS by capsaicin, EGCG, and EPA; 2) epigenetic modification such as histone deacetylation by resveratrol or microRNA biogenesis by EPA; 3) AMPK/PGC1α signaling leading to mitochondrial biogenesis by resveratrol, curcumin, EGCG, EPA, and berberine; 4) thermogenic cytokine secretion of LCN2 by ATRA-RAR signaling; and 5) the anti-inflammatory function of these molecules by resveratrol, EGCG, EPA, and berberine. ADRB3, β3-adrenergic receptor; AMPK, AMP-activated protein kinase; ATRA, all-trans retinoic acid; BAT, brown adipose tissue; EGCG, epigallocatechin gallate; LCN2, lipocalin 2; NE, norepinephrine; PGC1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; PRDM16, PR domain-containing 16; RAR, retinoic acid receptor; RXR, retinoid X receptor; SNS, sympathetic nervous system; TRPV, transient receptor potential vanilloid; WAT, white adipose tissue.
Capsaicin and Capsinoids
One of the most studied food ingredients that cause BAT activation is capsaicin and its analogs capsinoids. Capsinoids are found in a nonpungent type of red pepper, whereas capsaicin is the major pungent component in hot red peppers (14). Both capsaicin and capsinoids are potent in increasing energy expenditure and enhancing fat oxidation in small rodents, as well as humans, especially in high doses (∼10 mg/d) (14, 15).
Capsinoids have been shown to enhance thermogenesis. Yoneshiro et al. (16) examined the acute effect of oral ingestion of a single dose of capsinoids (9 mg) on energy expenditure in relation to BAT activity in humans as measured by cold-induced 18F-2-deoxyglucose uptake. The ingestion of capsinoids resulted in a 3-fold increase in energy expenditure in the BAT-positive group compared with the BAT-negative group (16). In addition, the chronic intake of capsinoids (9 mg/d in capsule form) for 6 wk promoted BAT activity and decreased fat mass upon cold exposure, even in human subjects with low BAT activity (14). Although the direct effect of capsinoids on BAT in humans still needs to be studied (17), the thermogenic effect of capsaicin and capsinoids is well documented in small rodents; the thermogenic effect occurs not only in iBAT, but also in beige adipocyte development. A recent report showed that C57BL/6 mice treated with both mild cold exposure (17°C) and capsinoids (0.3%) resulted in a dramatic increase in beige adipocyte development in inguinal WAT, and ameliorated diet-induced obesity (18).
The mechanisms by which capsinoids stimulate BAT activity include ADRB3 activation, with β-adrenergic blockers inhibiting capsaicin-induced energy metabolism alterations. Capsinoids could exert both direct agonistic β-adrenergic effects and indirect β-adrenergic effects via SNS activation and catecholamine secretion (19). Capsaicin (20–200 mg/kg body weight) has been shown to enhance catecholamine secretion from the adrenal medulla mainly via activation of the SNS (20). In rats, the intragastric administration of capsinoids resulted in time- and dose-dependent (30–300 mg/kg body weight) increases in iBAT sympathetic nerve activity via the activation of transient receptor potential vanilloid (TRPV) 1, a nonselective calcium channel located on primary afferent neurons throughout the body (21), whereas capsinoids failed to increase energy expenditure in Trpv1 knockout mice. In addition, denervation of the extrinsic nerves connected to the jejunum inhibited capsinoid-induced temperature elevation in mice (22), signifying that activation of the gastrointestinal vagus nerve is involved in capsinoid-induced thermogenesis. TRPV1 can activate the SNS, although the mechanisms remain unclear. Baskaran et al. (23) also showed that TRPV1 activation by capsaicin (0.01%, or ∼30 mg/d) is associated with catecholamine production or sirtuin (SIRT) 1–mediated deacetylation of PPARG–PR domain–containing 16 (PRDM16) interaction. In addition to capsinoids, there are other food ingredients that can stimulate transient receptor potential proteins and BAT thermogenesis. For instance, menthol, a cooling and flavor compound in mint, and allyl isothiocyanates and benzyl isothiocyanates, pungent elements in mustard and wasabi (Japanese horseradish), can activate transient receptor potential proteins (14). Also, 6-paradol, a compound responsible for the pungent peppery taste in ginger, has been shown to act as an agonist for TRPV1 and to increase BAT thermogenesis in a dose-dependent manner (1–10 mg/kg body weight) in rats (24).
Collectively, the consumption of a high dose of capsinoids (>10 mg/d) or their structural analogs seems to facilitate cold-induced BAT recruitment and thermogenic activation in humans, presumably via mechanisms of ADRB3 activation through TRPV1-associated SNS activation and norepinephrine release (Figure 1). More work is necessary to determine whether the consumption of dietary capsinoids could achieve magnitudes of BAT activation that are similar to those seen in a cohort study involving pharmacologic doses of capsinoids. In addition, safety concerns regarding the long-term use of food ingredients that can modulate the SNS for the purpose of BAT activation and weight loss in humans should be addressed (17).
Resveratrol
Resveratrol (3,5,4-trihydroxystilbene), a natural polyphenolic compound found in the skin of grapes and other plants, is capable of preventing obesity and related complications (25, 26). In standard diet–fed mice, adding 0.4% resveratrol significantly decreased fat mass and diminished total cholesterol and glucose plasma concentrations (27). In parallel, feeding mice an HF diet supplemented with 0.4% resveratrol protected against obesity and insulin resistance (28). The antiadipogenic and anti-inflammatory effects of resveratrol have been well established in cells. In 3T3-L1 cells, 25 μM resveratrol affected adipogenesis by downregulating the expression of Pparg, CCAAT/enhancer-binding protein α (C/EBPa), sterol regulatory element-binding portein 1c (Srebp1c), fatty acid synthase (Fas), hormone-sensitive lipase (Hsl), and lipoprotein lipase (Lpl), and upregulating the expression of genes regulating mitochondrial activity [Sirt3, Ucp1, and mitofusin 2 (Mfn2)] (29). In obese animals, the addition of 0.4% resveratrol to an HF diet resulted in a lower body weight and lower plasma concentrations of TNFα and monocyte chemoattractant protein (MCP1). In addition, HF diet–induced gene expressions of proinflammatory cytokines and toll-like receptors (i.e., Tlr2 and Tlr4) in epididymal fat were almost completely abolished upon 0.4% resveratrol supplementation (30). However, it remains unclear whether the antiobesogenic and anti-inflammatory effects of resveratrol are a direct consequence of brown and beige adipocyte formation and thermogenic activation.
Resveratrol has been suggested to improve thermogenesis in iBAT. Resveratrol induced UCP1 gene expression and oxygen consumption in the BAT of mice fed either a standard or HF diet supplemented with 0.4% resveratrol for 8–16 wk (27, 28). In rats, the ingestion of an HF diet supplemented with resveratrol (30 mg ⋅ kg body weight−1 ⋅ d−1) for 6 wk led to an increase in mitochondrial transcription factor A (Tfam), cytochrome C oxidase subunit II (Cox2) gene expression, and UCP expression in iBAT and skeletal muscle (31). The effect of resveratrol on thermogenesis is not limited to iBAT. Resveratrol significantly increased brown and beige adipocyte markers, including Prdm16, Ucp1, cell death–inducing DFFA-like effector a (Cidea), fatty acid elongase 3 (Elovl3), Pgc1a, Cd137, and transmembrane portein 26 (Tmem26) in differentiated inguinal WAT-derived stromal vascular cells. In addition, mice receiving a 0.1% resveratrol–supplemented HF diet had increased UCP1 expression and brown-like morphology in inguinal fat compared with HF diet only–fed mice (25). Qiang et al. (32) also showed that the acquisition of brown-like morphology and induction of thermogenesis by WAT (WAT remodeling) is dependent on SIRT1, a NAD-dependent protein deacetylase. The NAD-dependent SIRT1 promotes browning of WAT by deacetylation of PPARG on Lys268 and Lys293, leading to the recruitment of the BAT program coactivator PRDM16. In addition, the effect of resveratrol on beige adipocyte formation is believed to be mediated by AMPKα phosphorylation, because AMPK inhibition or AMPKα deletion eliminate the browning effects of resveratrol (25, 26). Overall, the current evidence strongly suggests that resveratrol possesses thermogenic effects and contributes to increased respiration.
AMPK activation by resveratrol can stimulate mitochondrial biogenesis via SIRT1. Resveratrol is a natural activator of the sirtuin family (27). It induces SIRT1, which can deacetylate and activate PGC1α, a master regulator of mitochondrial biogenesis and oxidative phosphorylation, via increasing NAD+ concentrations (26, 33, 34). However, a causal link between the increase in NAD+ and Sirt1 activation has not been established (33). The activation of the NAD-SIRT1-PGC1α pathway by resveratrol is downstream of AMPK activation, which was shown to increase cellular NAD+ concentrations (25, 35). AMPK-deficient mice were resistant to the metabolic benefits of resveratrol, providing evidence that AMPK is a key mediator of the metabolic benefits produced by resveratrol (25, 26, 33). Park et al. (33) proposed another mechanism whereby resveratrol exerts metabolic benefits. Resveratrol inhibits cAMP-degrading phosphodiesterase and induces cAMP signaling via exchange protein directly activated by cAMP (Epac1), a cAMP effector protein. The resulting activation of Epac1 increases intracellular Ca2+ concentrations and activates the AMPK pathway. As a consequence, resveratrol increases NAD+ and the activity of Sirt1 and PGC1α. Oral gavage of resveratrol increased cAMP concentrations in skeletal muscle and WAT in mice (33). cAMP signaling appears to be essential for resveratrol to activate AMPK. Inhibition of phosphodiesterase 4 by the selective phosphodiesterase 4 inhibitor rolipram reproduces all of the metabolic benefits of resveratrol (33). However, the direct effects of this resveratrol-induced cAMP signaling pathway on WAT browning remain unknown.
There are strong implications that resveratrol supplementation in doses of 0.1–0.4% (diet) induces BAT activation via synergistic mechanisms of mitochondrial biogenesis, histone deacetylation, cAMP signaling, and altered innate immune activity (Figure 1). However, evidence supporting the thermogenic effects of resveratrol in humans is currently lacking. Given the poor bioavailability of polyphenolic compounds including resveratrol (36), the capacity of resveratrol to stimulate BAT observed in rodents may not be attainable by consumption of ordinary resveratrol-containing foods in humans.
Curcumin
Curcumin is a naturally occurring curcuminoid of turmeric, which is a member of the ginger family (Zingiberaceae) and perhaps one of the most studied medicinal herbs. Curcumin is noted for having antiobesity effects. A randomized controlled study evaluated the tolerability and efficacy of 30 d of consumption of a bioavailable form of curcumin (complexed with phosphatidylserine) in overweight human subjects undergoing weight loss during a 30-d diet and lifestyle intervention. The curcumin was tolerable, increased weight loss from 1.9% to 4.9%, and promoted the reduction of fat mass, as well as waist and hip circumference (P < 0.01 for all comparisons) (37).
The browning effect of curcumin in WAT has recently gained attention. The effect of curcumin (10–20 μM) on the induction of beige phenotype in 3T3-L1 and mouse primary white adipocytes was investigated. Curcumin induces brown-specific markers, including Ucp1, Pgc1a, and Prdm16 in these cells via a yet-to-be-clarified mechanism involving AMPK activation (38). By analyzing proteomic changes in cultured white adipocytes isolated from rat inguinal WAT in response to curcumin treatment (20 μM for 6–8 d), hormone-sensitive lipase was reported to be highly correlated with brown-specific markers (39). Consistently, Wang et al. (40) reported that intragastric administration of curcumin (50 or 100 mg/kg) for 50 d led to an increase in thermogenic gene expression (i.e., Ucp1, Pgc1a, Cidea, Prdm16, and Elovl3) and mitochondrial content in inguinal WAT. In addition, curcumin treatment in these mice raised body temperature when challenged for 6 h by cold temperature (4°C). These changes were associated with increases in Adrb3 gene expression and plasma norepinephrine concentrations, although the mechanism for how curcumin induces norepinephrine and ADRB3 remains to be explained (40). Also, effects of curcumin on activating classical brown fat have not been reported yet.
At least in rodents, administration of curcumin (>50 mg/kg body weight) is effective in inducing WAT browning via mechanisms of mitochondrial biogenesis and ADRB3 activation (Figure 1). However, no studies have confirmed the browning potential of curcumin in humans. Therefore, whether the results of curcumin human trials would conform to the WAT browning effects observed in rodents will be of interest.
Green Tea Catechins
Green tea is one of the most widely consumed beverages in the world and is made from the fresh leaves of Camellia sinensis (41). Green tea contains relatively large amounts of polyphenols, mainly tea catechins such as EGCG, epigallocatechin, epicatechin gallate, and epicatechin, which possess antioxidant, antihypertensive, anticarcinogenic, and hypocholesterolemic properties (41–43). Catechins account for ∼10% of the dry weight of green tea, whereas EGCG accounts for ∼50% of the total amount of catechins in green tea (44). In addition to these catechins, green tea extracts contain substantial amounts of caffeine that could, at least partially, contribute to the health benefits of green tea (41, 43).
The consumption of green tea or its components, green tea catechins, is associated with weight loss and the modulation of fat metabolism and energy expenditure. Given to mice for 8 wk, 0.5% tea catechins lowered perirenal WAT mass and increased Ucp1 mRNA expression in iBAT compared with in control mice (42). Yan et al. (45) showed that oral gavage of catechins (100 mg green tea/kg) for 4 wk significantly attenuated fat mass and liver weights and increased serum and liver TGs in rats fed an HF diet. In this study, WAT browning was not observed, but FA oxidation in iBAT increased 2-fold (45). A meta-analysis of human trials revealed that habitual intake of green tea (>300 mg catechins/d), but not caffeine, was effective in decreasing body weight and preventing the relapse of weight gain after weight loss (41). However, thermogenic activation by green tea catechins seems controversial in humans. In obese men, consuming 300 mg EGCG for 3 d (or 200 mg caffeine) increased postprandial fat oxidation, but not energy expenditure (46). In contrast, treating healthy men and women with green tea extracts containing 583 mg catechins for 12 wk resulted in a small (2–3%) but significant reduction in body fat content (47).
In terms of mechanisms, PPARs and related pathways were suggested to play a role in the antiobesity mechanisms of green tea catechins. Catechin supplementation (100 mg/kg body weight) given to rats for 4 wk achieved the following: 1) upregulated concentrations of PPARδ in subcutaneous WAT, visceral WAT, and iBAT; and 2) increased expression of genes involved in FA oxidation in BAT (45). Although the mechanisms whereby green tea and EGCG help treat and prevent obesity have not yet been fully elucidated, several studies suggest that they may work by activating thermogenesis. Short-term thermogenic effects were observed in adult humans after the consumption of a green tea extract (1600 mg EGCG and 600 mg caffeine). In these subjects, shivering activity decreased by 20% and energy expenditure increased by 10% during 3 h of mild cold exposure when compared with placebo (48). In another study, green tea extract (90 mg EGCG and 50 mg caffeine) increased resting energy expenditure over a 24-h period through sympathetic activation, whereas equivalent amounts of caffeine did not affect energy expenditure or urinary catecholamines (48, 49).
It is likely that the thermogenic effect of green tea extract is attributable to noradrenaline and ADRB3 signaling activation, similar to other phytochemicals of curcumin and capsaicin (Figure 1). Catechins in green tea may stimulate thermogenesis and fat oxidation through the inhibition of catechol O-methyltransferase, leading to ADRB3 signaling. The ADRB3 signaling cascade can also be stimulated by caffeine, which has a potent inhibitory activity on phosphodiesterase, a cAMP degrading enzyme (43). Because it contains both tea catechins and caffeine, green tea may exert thermogenic and antiobesogenic effects via cAMP activation by modulating different targets (50). However, the direct evidence supporting the involvement of cAMP in brown and beige adipose tissue development in humans is hitherto fragmented, and more studies are warranted (43). New study designs that quantify adaptive thermogenesis energy expenditure are vital to establish the novel role of green tea in boosting BAT activity.
Berberine
Berberine, an alkaloid compound derived from various herbs such as the medicinal plant Coptis chinensis (Chinese goldthread) and Hydrastis canadensis (goldenseal), prevents weight gain and white fat accumulation in rodents. Berberine attenuated weight gain in olanzapine-treated rats (51) and leptin receptor knockout mice (52). In rats treated with olanzapine for 2 wk, berberine administration (380 mg ⋅ kg−1 ⋅ d−1) protected from olanzapine-induced weight gain and caused changes in genes that control energy expenditure; however, there was no direct measurement of energy expenditure itself (51). In db/db mice, berberine (5 mg ⋅ kg−1 ⋅ d−1 delivered intraperitoneally for 4 wk) decreased weight gain and fat accumulation, suggesting that berberine regulates lipid mobilization and imposes antiobesity effects (52).
It has been reported that berberine plays a role in regulating adaptive thermogenesis. In db/db mice, berberine (5 mg/kg body weight) administration increased whole-body energy expenditure by 20%; the respiratory exchange ratio was significantly decreased, suggesting that berberine shifts fuel preference toward FA oxidation. Moreover, berberine affected BAT in these mice because it achieved the following: 1) decreased lipid accumulation, 2) increased mitochondrial content, 3) increased thermogenic markers (such as Pgc1a, Cidea, and Ucp1), and 4) enhanced BAT activity [as detected by a positron emission tomography (PET)-CT scan], thus demonstrating improved cold tolerance (52).
Berberine stimulated not only BAT activity, but also WAT browning. Also, berberine markedly induced the development of brown-like adipocytes in inguinal, but not epididymal fat in mice. Finally, the expression of Ucp1 and other thermogenic markers increased in inguinal WAT, and mitochondrial biogenesis was enhanced in these mice. The mechanism of thermogenic induction by berberine supplementation is not yet known; however, AMPK and PGC1α activation seem to be involved (52). Although brown adipocyte function can be stimulated by AMPK (53), a key unanswered question is how AMPK activity is induced by berberine. Because various compounds, such as metformin, gallic acid, and resveratrol, have been shown to increase BAT activity via AMPK activation (53), it would be worthwhile to test the synergistic effect of berberine with other compounds on thermogenesis and energy expenditure.
The effect of berberine on inflammation and endoplasmic reticulum (ER) stress was addressed recently. Oral administration of berberine (200 mg/kg body weight) for 5 wk significantly reduced inflammatory markers (such as TNFα and IL-6) and ER stress markers [such as phosphorylated-PKR-like ER kinase (p-PERK), phosphorylated eukaryotic translation initiation factor-2α (p-EIF2α), glucose-regulated protein 78 (GRP78), and C/EBP homologous protein (CHOP)] in the liver. In addition, berberine decreased lipid accumulation and tunicamycin-induced ER stress in primary hepatocytes in vitro (54). Because our group reported that inflammation and ER stress prevention restores thermogenesis in LPS-injected mice (55), it would be interesting to investigate the potential effects of berberine on obesity-associated inflammation and ER stress, especially with respect to enhanced WAT browning and thermogenesis. Although more studies are needed, especially human clinical trials, berberine could have prospective therapeutic implications for the enhancement of thermogenesis (52).
Fish Oil (n–3 PUFAs)
There is accumulating evidence that fish oil, which is rich in the n–3 PUFAs EPA and DHA, stimulates thermogenesis in BAT. For example, an older study showed that rats supplemented with 20% fish oil for 3 wk had increased Ucp1 mRNA levels in iBAT (56). In another study, 27.5% fish oil (a mixture of EPA and DHA) supplementation given to rats for 4 wk stimulated mitochondrial and thermogenic activity in iBAT (57). In addition, short-term feeding with high amounts of PUFAs increased UCP1 expression and nonshivering thermogenesis in mice (58). A more recent study revealed that an HF diet rich in fish oil (12%) given to mice for 8 wk caused an increase in the expression of thermogenic markers (Adrb3, Pgc1a, and Ucp1) in iBAT. Moreover, this diet prevented diet-induced weight gain and adiposity, improved metabolic profiles (glucose tolerance and TG concentrations), and diminished proinflammatory gene expression in these mice (59).
The potential WAT browning properties of n–3 PUFAs has recently gained attention. Zhao et al. (60) tested the effect of EPA on the metabolic activity of adipocytes differentiated from mouse inguinal adipose tissue–derived stem cells. The addition of EPA (200 μM) during adipocyte differentiation enhanced thermogenic and mitochondrial gene expression. However, this effect was not observed when EPA was added to mature white adipocytes for 24 h, suggesting that EPA exerts its browning effect via recruiting beige adipocytes. Kim et al. (61) reported that fish oil supplementation (containing 1.2% pure DHA and 2.4% pure EPA) for 10 wk increased oxygen consumption and core body temperature in mice. In these mice, fish oil induced UCP1 expression not only in iBAT, but also in inguinal WAT. This was likely mediated by the SNS and TRPV1 and catecholamine production, given that EPA-mediated browning effects were abolished in Trpv1 knockout mice (61). However, the effects of WAT browning caused by EPA supplementation are controversial; when mice were fed an HF diet supplemented with pure EPA (3.6% as EPA ethyl ester), the brown marker genes were only elevated in iBAT, but not in inguinal WAT (62).
Although previous work indicates that fish oil intake reduces fat accumulation and induces UCP1 expression, the detailed mechanism underlying these effects remains unclear. Kim et al. (63) recently demonstrated that FFA receptor 4 (FFAR4; also known as G-coupled protein receptor 120) is involved in EPA (100 μM)–induced brown thermogenesis in primary murine brown precursor cells. Furthermore, FFAR4 activation by EPA was linked with clusters of microRNAs that promote brown adipogenesis. This signaling axis (EPA/FFAR4/microRNAs) for brown adipogenesis was confirmed in C57BL/6 mice that were fed fish oil (15%) for 12 wk, suggesting an epigenetic regulation of dietary fish oil for brown thermogenesis (63). Supporting this notion, BAT stimulatory effects by EPA were absent in Ffar4 knockout mice (64).
Despite the increasing body of evidence in rodents, little information is available on whether fish oil–mediated health benefits in humans (i.e., improved plasma lipid profiles, energy expenditure, and improved glucose homeostasis) originate, at least partly, from thermogenic activation (65). It is worth noting that conversion from fully matured white adipocytes to beige-like adipocytes was achievable by EPA in human adipocyte cultures (66); this was not observed in rodents (60); Laiglesia et al. (66) recently reported that EPA treatment facilitates the transdifferentiation of primary white adipocytes into beige fat. These results imply that EPA consumption may activate multiple signaling pathways, including the following: 1) ADRB3/SNS/TRPV, 2) FFAR4 (same as G-coupled protein receptor 120) activation, 3) microRNA modulation, and 4) mitochondrial biogenesis. This leads to the recruitment of beige fat, the transdifferentiation of white fat into beige-like cells, and the facilitation of classical brown fat development (Figure 1). The controversy with WAT browning, presumably in response to the EPA-to-DHA ratio, remains to be identified.
ATRA
Retinoic acid (RA) is an active metabolite of vitamin A and an important regulator of gene transcription (67). RA binds to nuclear hormone receptors, members of the steroid and thyroid receptor superfamily, and ligand-dependent transcription factors (68). Most of the effects of ATRA are mediated by retinoid acid receptors (RARs). Upon dimerization with retinoid X receptors (RXRs), the heterodimer of RAR and RXR controls gene expression through binding to RA response elements in the regulatory regions of target genes (69). RA plays an important role in the development and differentiation of mammalian cells (68). Depending on the dose, RA can promote (70) or inhibit (67) adipogenesis in vitro; high concentrations of ATRA inhibit the differentiation of 3T3-L1 preadipocytes and C3H10T1/2 mesenchymal stem cells via the suppression of CCAAT/enhancer-binding protein β activity and the induction of antiadipogenic genes (69, 71). RA is uniquely effective in suppressing adiposity and insulin resistance. In general, RA treatment in the range of 10–100 mg/kg body weight in obese rodents elicits a 15–50% reduction in fat mass and improves insulin sensitivity by enhancing fat mobilization and energy utilization (67). A controlled RA treatment that used subcutaneous implantation (equivalent to 0.16 mg/d) for 5 wk in obese mice reduced body weight by 50% because of decreased fat mass and adipocyte hypertrophy, despite increased food intake. In addition, RA-treated animals showed a >2-fold increase in brown specific genes, including UCP1 expression and improved insulin sensitivity (72). Although the mechanisms whereby RA improves insulin sensitivity are not fully understood, RA treatment appears to activate RAR and PPARδ, leading to augmented BAT activation and FA oxidation.
RA plays a key role in the development and regulation of BAT thermogenesis. As a transcriptional activation, RA increases Ucp1 gene expression. RA increased Ucp1 mRNA levels 7-fold in differentiated cultures of brown adipocytes, and this induction was independent of adrenergic pathways (68). The stimulatory effect of ATRA on Ucp1 gene expression is mediated by the activation of the heterodimers RAR and RXR, which bind to specific regulatory region in the UCP1 enhancer (69, 73–75). Exposure to ATRA potently induced UCP1 expression at both the mRNA and protein level in mouse embryonic fibroblast–derived adipocytes in a dose-dependent manner. The effect on Ucp1 mRNA was reproduced by retinoid receptor agonists and by retinaldehyde (70). Conversely, consuming a vitamin A–deficient diet increased body weight, whole body fat mass, and leptin expression, and reduced iBAT thermogenic potential (76). Interestingly, Mercader et al. (77) reported that body fat loss after ATRA treatment (50–100 mg/kg body weight) resulted in white adipocyte remodeling into beige, including augmented mitochondrial biogenesis and increased rectal temperature. Tourniaire et al. (78) recently demonstrated the role of ATRA in WAT browning. In their study, transcriptome analysis revealed that 2 μM ATRA upregulated the set of genes linked to mitochondrial DNA replication, transcription, mitochondrial biogenesis, and oxidative phosphorylation in mature white adipocytes. In parallel, oxygen consumption, mitochondrial DNA content, and mitochondrial staining were increased in the ATRA-treated adipocytes. These results were also validated in WAT depots obtained from ATRA-fed mice (50 mg/kg body weight) for 4 d.
ATRA supplementation modulates both iBAT stimulation and WAT remodeling via unique transcriptional regulation, but not ADRB3 activation. Most recently, Guo et al. (79) reported that lipocalin 2 (Lcn2), an adipose-derived novel cytokine, is one of the target genes of RA, and demonstrated with the use of Lcn2 knockout mice that retinoid-mediated thermogenesis is dependent on Lcn2 (Figure 1). Although RA is a potent positive regulator of thermogenesis in rodents, this effect is inhibited or only marginal in human adipocyte cell lines and primary human white adipocytes (69). For this reason, ATRA supplementation does not seem to be an option for brown and beige development in humans.
Other Naturally Occurring Factors for Thermogenesis
In addition to the aforementioned dietary molecules, a vast majority of phytochemicals are claimed to have or are under investigation for thermogenic properties, although not all molecules are derived from edible sources (13, 80). Also, some dietary molecules known to enhance β-oxidation and weight loss have been shown to induce thermogenesis in animal models. For example, Shen et al. (81) demonstrated that supplementing the diet with a mixture or individual geometric isomer of CLAs increases thermogenesis in Sv129 mice (82), although its metabolic significance in humans seems to be lessened because of the proinflammatory action of its trans 10, cis 12 CLA isomer.
Conclusion
Obesity and diabetes have reached epidemic proportions in the United States and globally, and are expected to rise continuously. BAT plays a substantial role in whole-body energy metabolism, and is therefore an attractive target to facilitate weight loss and improve metabolic health in humans. Here, we summarized the recent updates on dietary molecules that could be included as candidates in a dietary regimen for managing obesity and diabetes via BAT-mediated thermogenesis. We have also presented the underlying mechanisms whereby these dietary molecules act on the following: 1) SNS and cAMP production (e.g., capsaicin, green tea EGCG, and EPA), 2) epigenetic modification of adipose tissue by regulating histone deacetylation (e.g., resveratrol) or microRNA biogenesis (e.g., EPA), 3) brown-thermogenic cytokine secretion (e.g., ATRA), and 4) AMPK activation and mitochondrial biogenesis (e.g., resveratrol, curcumin, EGCG, EPA, and berberine). Also, the anti-inflammatory function of these molecules (e.g., resveratrol, EGCG, EPA, and berberine) is speculated to contribute to their thermogenic activity, although it may not be the major mechanism that triggers BAT activation (Figure 1). Apparently, multiple and overlapping signaling pathways are involved in stimulating BAT activation for each dietary molecule. This implies that combinations of these dietary molecules with complementary thermogenic mechanisms could be an option in maximizing BAT thermogenesis, weight reduction, and improvement of metabolic indexes.
One fundamental goal of this review was to attain an unbiased conclusion regarding the potential of dietary modulation of brown and beige fat development as a promising target for obesity and diabetes intervention. In fact, to our knowledge, a substantial number of studies that are needed to identify a general consensus have not yet been conducted. With the exception of capsinoids, to our knowledge, few studies have been done in humans. Therefore, our conclusions are likely tentative.
a. Chronic ingestion of high doses (>10 mg) of capsinoids is effective in promoting thermogenic activation in humans, which has important implications for a preventive and therapeutic approach to control obesity. However, the safety of long-term capsinoid use requires further investigation.
b. Some polyphenolic compounds, such as resveratrol, curcumin, and berberine, activate BAT and beige development at the cellular model and in vivo, at least in rodents. In animal studies, obvious thermogenic activation was only observed at polyphenol supplementation in high doses (>0.1%, or ∼100 mg/kg body weight), which would be considered supraphysiologic for human trials. In addition, overcoming the poor bioavailability of polyphenolic molecules will be another challenge for future human trials.
c. The habitual consumption of green tea (∼100 mg/kg body weight of catechins) seems to trigger thermogenic activation and contributes to the weight reduction properties of green tea, at least in animals. However, it is necessary to revisit human studies to differentiate the browning and thermogenic effects from other metabolic benefits of green tea.
d. The role of fish oil, especially EPA, on BAT and beige development has been extensively investigated. Although some controversy still surrounds effective concentrations, fish oil consumption activates multiple signaling routes to augment BAT activation or WAT browning in rodents. Given that fish oil is one of the most popular dietary supplements, validating these effects in human trials might immensely affect public health.
e. ATRA modulates BAT activation in rodents via transcriptional regulation of RXR and RAR. Human application of ATRA for BAT activation appears to be impractical because of developmental differences between rodents and humans.
Collectively, there is emerging evidence that some dietary molecules can regulate brown and beige fat development. The thermogenic role of these molecules on metabolic improvement and adiposity control in humans has not yet been clearly established. Further research is warranted to determine the efficacy, underlying mechanisms, and safety of these molecules in humans.
One of the caveats of characterizing bioactive molecules as thermogenic agents is that the majority of studies rely on BAT marker gene expression, such as Ucp1 and other signature gene profiles for brown and beige fat [e.g., Prdm16, Cidea, iodothyronine deiodinase 2 (Dio2), and Elovl3]. Given that elevated concentrations of Ucp1 transcript do not necessarily coincide with UCP1-mediated uncoupled respiration and heat production (83), interpretation of Ucp1 mRNA expression data must be made with caution, and concurrent metabolic adaptations should be determined.
The key findings summarized in this review were obtained from studies conducted in rodents. It should be taken into consideration that small animals have a higher propensity to develop WAT browning because of their greater reliance on temperature homeostasis through these mechanisms compared with humans (17). Therefore, determination of the efficacy and safety of the candidate dietary molecules in humans should be addressed before making dietary recommendations for these molecules as thermogenesis-based diet therapy. Also, the 3T3-L1 cells, well-characterized preadipocytes committed to white adipocytes, are widely used to investigate the cellular level of WAT browning. Recent research suggests that WAT browning is initiated by the proliferation and differentiation of beige progenitor cells that are not identical to the committed white progenitor cells (84, 85). Thus, the development of legitimate cell models for beige adipocytes and validation of results obtained from 3T3-L1 is required.
Development of beige fat is tightly associated with the innate immune system (84, 85). Because obesity is associated with chronic and low-grade inflammation (86), a combination of anti-inflammatory bioactive molecules with the aforementioned dietary candidates may pose synergistic effects in promoting brown thermogenesis in humans. Because exercise is an independent external stimulus that activates AMPK and WAT browning, probably through irisin (87), investigation of the synergistic effects of these proposed molecules with exercise would be an interesting topic for future research.
Currently, the gold standard to measure brown and beige fat activation in humans is the PET scan, in conjunction with 18F-2-deoxyglucose uptake as a probe (88). Because 18F-2-deoxyglucose uptake is a surrogate marker for brown adipocytes rather than a direct measure of uncoupled respiration through UCP1, measurement of respiratory exchange ratio and heat release would be complementary to validate brown and beige fat activation. In addition, the PET scan method not only requires expensive equipment, but also exposes humans to radiation. Thus, there is an immense need for developing novel technology that detects human BAT with high sensitivity and convenience, and/or innovative biomarkers that correlate with BAT activity in humans. Recently, serum concentrations of exosomal microRNA-92a have been suggested as a biomarker for BAT activity in humans (89). It would be of interest to determine whether exosomal microRNA-92a could be used as a sensitive and convenient serum marker for assessing thermogenic potential in humans. Future research aimed at the development of new tools for the assessment of BAT activation and innovative biomarkers of human thermogenic activity, will help achieve the following: 1) evaluate whether nutritional modulation BAT and beige development will offer a new therapeutic avenue to promote metabolic health, and 2) identify novel dietary molecules that boost BAT-induced thermogenesis and energy expenditure.
Acknowledgments
We gratefully acknowledge Meri Nantz for editing the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ADRB3, β3-adrenergic receptor; AMPK, AMP-activated protein kinase; ATRA, all-trans retinoic acid; BAT, brown adipose tissue; Cidea, cell death-inducing DFFA-like effector a; EGCG, epigallocatechin gallate; Elovl3, fatty acid elongase 3; ER, endoplasmic reticulum; FFAR4, FFA receptor 4; HF, high-fat; iBAT, interscapular brown adipose tissue; Lcn2, lipocalin 2; PET, positron emission tomography; PGC1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; PRDM16, PR domain–containing 16; RA, retinoic acid; RAR, retinoid acid receptor; RXR, retinoid X receptors; SIRT, sirtuin; SNS, sympathetic nervous system; Srebp1c, sterol regulatory element-binding protein 1c; Tfam, mitochondrial transcription factor A; Tlr, toll-like receptor; Tmem26, transmembrane protein 26; TRPV, transient receptor potential vanilloid; UCP1, uncoupling protein 1; WAT, white adipose tissue.
References
- 1.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al. . PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008;454:961–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. Transcriptional control of brown fat determination by PRDM16. Cell Metab 2007;6:38–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Lans AA, Hoeks J, Brans B, Vijgen GH, Visser MG, Vosselman MJ, Hansen J, Jorgensen JA, Wu J, Mottaghy FM, et al. . Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest 2013;123:3395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenwald M, Perdikari A, Rulicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol 2013;15:659–67. [DOI] [PubMed] [Google Scholar]
- 5.Rosenwald M, Wolfrum C. The origin and definition of brite versus white and classical brown adipocytes. Adipocyte 2014;3:4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeremic N, Chaturvedi P, Tyagi SC. Browning of white fat: novel insight into factors, mechanisms, and therapeutics. J Cell Physiol 2017;232:61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulya A, Kirwan JP. Brown and beige adipose tissue: therapy for obesity and its comorbidities? Endocrinol Metab Clin North Am 2016;45:605–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schrauwen P, van Marken Lichtenbelt WD. Combatting type 2 diabetes by turning up the heat. Diabetologia 2016;59:2269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santulli G, Iaccarino G. Adrenergic signaling in heart failure and cardiovascular aging. Maturitas 2016;93:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.García-Ruiz E, Reynés B, Díaz-Rúa R, Ceresi E, Oliver P, Palou A. The intake of high-fat diets induces the acquisition of brown adipocyte gene expression features in white adipose tissue. Int J Obes 2015;39:1619–29. [DOI] [PubMed] [Google Scholar]
- 11.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 2010;285:7153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pesta DH, Samuel VT. A high-protein diet for reducing body fat: mechanisms and possible caveats. Nutr Metab (Lond) 2014;11:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stohs SJ, Badmaev V. A review of natural stimulant and non-stimulant thermogenic agents. Phytother Res 2016;30:732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, Iwanaga T, Saito M. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest 2013;123:3404–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ludy MJ, Moore GE, Mattes RD. The effects of capsaicin and capsiate on energy balance: critical review and meta-analyses of studies in humans. Chem Senses 2012;37:103–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoneshiro T, Aita S, Kawai Y, Iwanaga T, Saito M. Nonpungent capsaicin analogs (capsinoids) increase energy expenditure through the activation of brown adipose tissue in humans. Am J Clin Nutr 2012;95:845–50. [DOI] [PubMed] [Google Scholar]
- 17.Vosselman MJ, van Marken Lichtenbelt WD, Schrauwen P. Energy dissipation in brown adipose tissue: from mice to men. Mol Cell Endocrinol 2013;379:43–50. [DOI] [PubMed] [Google Scholar]
- 18.Ohyama K, Nogusa Y, Shinoda K, Suzuki K, Bannai M, Kajimura S. A synergistic anti-obesity effect by a combination of capsinoids and cold temperature through promoting beige adipocyte biogenesis. Diabetes 2016;65:1410–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawada T, Watanabe T, Takaishi T, Tanaka T, Iwai K. Capsaicin-induced beta-adrenergic action on energy metabolism in rats: influence of capsaicin on oxygen consumption, the respiratory quotient, and substrate utilization. Proc Soc Exp Biol Med 1986;183:250–6. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe T, Kawada T, Kurosawa M, Sato A, Iwai K. Adrenal sympathetic efferent nerve and catecholamine secretion excitation caused by capsaicin in rats. Am J Physiol 1988;255:E23–7. [DOI] [PubMed] [Google Scholar]
- 21.Ono K, Tsukamoto-Yasui M, Hara-Kimura Y, Inoue N, Nogusa Y, Okabe Y, Nagashima K, Kato F. Intragastric administration of capsiate, a transient receptor potential channel agonist, triggers thermogenic sympathetic responses. J Appl Physiol 1985;2011:789–98. [DOI] [PubMed] [Google Scholar]
- 22.Kawabata F, Inoue N, Masamoto Y, Matsumura S, Kimura W, Kadowaki M, Higashi T, Tominaga M, Inoue K, Fushiki T. Non-pungent capsaicin analogs (capsinoids) increase metabolic rate and enhance thermogenesis via gastrointestinal TRPV1 in mice. Biosci Biotechnol Biochem 2009;73:2690–7. [DOI] [PubMed] [Google Scholar]
- 23.Baskaran P, Krishnan V, Ren J, Thyagarajan B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br J Pharmacol 2016;173:2369–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwami M, Mahmoud FA, Shiina T, Hirayama H, Shima T, Sugita J, Shimizu Y. Extract of grains of paradise and its active principle 6-paradol trigger thermogenesis of brown adipose tissue in rats. Auton Neurosci 2011;161:63–7. [DOI] [PubMed] [Google Scholar]
- 25.Wang S, Liang X, Yang Q, Fu X, Rogers CJ, Zhu M, Rodgers BD, Jiang Q, Dodson MV, Du M. Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) alpha1. Int J Obes 2015;39:967–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, Kim MK, Viollet B, Chung JH. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes 2010;59:554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrade JM, Frade AC, Guimaraes JB, Freitas KM, Lopes MT, Guimaraes AL, de Paula AM, Coimbra CC, Santos SH. Resveratrol increases brown adipose tissue thermogenesis markers by increasing SIRT1 and energy expenditure and decreasing fat accumulation in adipose tissue of mice fed a standard diet. Eur J Nutr 2014;53:1503–10. [DOI] [PubMed] [Google Scholar]
- 28.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, et al. . Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006;127:1109–22. [DOI] [PubMed] [Google Scholar]
- 29.Rayalam S, Yang JY, Ambati S, Della-Fera MA, Baile CA. Resveratrol induces apoptosis and inhibits adipogenesis in 3T3–L1 adipocytes. Phytother Res 2008;22:1367–71. [DOI] [PubMed] [Google Scholar]
- 30.Kim S, Jin Y, Choi Y, Park T. Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochem Pharmacol 2011;81:1343–51. [DOI] [PubMed] [Google Scholar]
- 31.Alberdi G, Rodriguez VM, Miranda J, Macarulla MT, Churruca I, Portillo MP. Thermogenesis is involved in the body-fat lowering effects of resveratrol in rats. Food Chem 2013;141:1530–5. [DOI] [PubMed] [Google Scholar]
- 32.Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y, Rosenbaum M, Zhao Y, Gu W, Farmer SR, et al. . Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Ppargamma. Cell 2012;150:620–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, et al. . Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 2012;148:421–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, et al. . Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006;444:337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009;458:1056–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr 2005;81:230S–42S. [DOI] [PubMed] [Google Scholar]
- 37.Di Pierro F, Bressan A, Ranaldi D, Rapacioli G, Giacomelli L, Bertuccioli A. Potential role of bioavailable curcumin in weight loss and omental adipose tissue decrease: preliminary data of a randomized, controlled trial in overweight people with metabolic syndrome. Preliminary study. Eur Rev Med Pharmacol Sci 2015;19:4195–202. [PubMed] [Google Scholar]
- 38.Lone J, Choi JH, Kim SW, Yun JW. Curcumin induces brown fat-like phenotype in 3T3–L1 and primary white adipocytes. J Nutr Biochem 2016;27:193–202. [DOI] [PubMed] [Google Scholar]
- 39.Kim SW, Choi JH, Mukherjee R, Hwang KC, Yun JW. Proteomic identification of fat-browning markers in cultured white adipocytes treated with curcumin. Mol Cell Biochem 2016;415:51–66. [DOI] [PubMed] [Google Scholar]
- 40.Wang S, Wang X, Ye Z, Xu C, Zhang M, Ruan B, Wei M, Jiang Y, Zhang Y, Wang L, et al. . Curcumin promotes browning of white adipose tissue in a norepinephrine-dependent way. Biochem Biophys Res Commun 2015;466:247–53. [DOI] [PubMed] [Google Scholar]
- 41.Hursel R, Viechtbauer W, Dulloo AG, Tremblay A, Tappy L, Rumpler W, Westerterp-Plantenga MS. The effects of catechin rich teas and caffeine on energy expenditure and fat oxidation: a meta-analysis. Obes Rev 2011;12:e573–81. [DOI] [PubMed] [Google Scholar]
- 42.Nomura S, Ichinose T, Jinde M, Kawashima Y, Tachiyashiki K, Imaizumi K. Tea catechins enhance the mRNA expression of uncoupling protein 1 in rat brown adipose tissue. J Nutr Biochem 2008;19:840–7. [DOI] [PubMed] [Google Scholar]
- 43.Saito M. Capsaicin and related food ingredients reducing body fat through the activation of TRP and brown fat thermogenesis. Adv Food Nutr Res 2015;76:1–28. [DOI] [PubMed] [Google Scholar]
- 44.Lee MS, Kim Y. (-)-Epigallocatechin-3-gallate enhances uncoupling protein 2 gene expression in 3T3–L1 adipocytes. Biosci Biotechnol Biochem 2009;73:434–6. [DOI] [PubMed] [Google Scholar]
- 45.Yan J, Zhao Y, Zhao B. Green tea catechins prevent obesity through modulation of peroxisome proliferator-activated receptors. Sci China Life Sci 2013;56:804–10. [DOI] [PubMed] [Google Scholar]
- 46.Thielecke F, Rahn G, Bohnke J, Adams F, Birkenfeld AL, Jordan J, Boschmann M. Epigallocatechin-3-gallate and postprandial fat oxidation in overweight/obese male volunteers: a pilot study. Eur J Clin Nutr 2010;64:704–13. [DOI] [PubMed] [Google Scholar]
- 47.Nagao T, Hase T, Tokimitsu I. A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity (Silver Spring) 2007;15:1473–83. [DOI] [PubMed] [Google Scholar]
- 48.Gosselin C, Haman F. Effects of green tea extracts on non-shivering thermogenesis during mild cold exposure in young men. Br J Nutr 2013;110:282–8. [DOI] [PubMed] [Google Scholar]
- 49.Dulloo AG, Duret C, Rohrer D, Girardier L, Mensi N, Fathi M, Chantre P, Vandermander J. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am J Clin Nutr 1999;70:1040–5. [DOI] [PubMed] [Google Scholar]
- 50.Diepvens K, Westerterp KR, Westerterp-Plantenga MS. Obesity and thermogenesis related to the consumption of caffeine, ephedrine, capsaicin, and green tea. Am J Physiol Regul Integr Comp Physiol 2007;292:R77–85. [DOI] [PubMed] [Google Scholar]
- 51.Hu Y, Young AJ, Ehli EA, Nowotny D, Davies PS, Droke EA, Soundy TJ, Davies GE. Metformin and berberine prevent olanzapine-induced weight gain in rats. PLoS One 2014;9:e93310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Z, Zhang H, Li B, Meng X, Wang J, Zhang Y, Yao S, Ma Q, Jin L, Yang J, et al. . Berberine activates thermogenesis in white and brown adipose tissue. Nat Commun 2014;5:5493. [DOI] [PubMed] [Google Scholar]
- 53.van Dam AD, Kooijman S, Schilperoort M, Rensen PC, Boon MR. Regulation of brown fat by AMP-activated protein kinase. Trends Mol Med 2015;21:571–9. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Z, Li B, Meng X, Yao S, Jin L, Yang J, Wang J, Zhang H, Zhang Z, Cai D, et al. . Berberine prevents progression from hepatic steatosis to steatohepatitis and fibrosis by reducing endoplasmic reticulum stress. Sci Rep 2016;6:20848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Okla M, Wang W, Kang I, Pashaj A, Carr T, Chung S. Activation of Toll-like receptor 4 (TLR4) attenuates adaptive thermogenesis via endoplasmic reticulum stress. J Biol Chem 2015;290:26476–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takahashi Y, Ide T. Dietary n-3 fatty acids affect mRNA level of brown adipose tissue uncoupling protein 1, and white adipose tissue leptin and glucose transporter 4 in the rat. Br J Nutr 2000;84:175–84. [PubMed] [Google Scholar]
- 57.Oudart H, Groscolas R, Calgari C, Nibbelink M, Leray C, Le Maho Y, Malan A. Brown fat thermogenesis in rats fed high-fat diets enriched with n-3 polyunsaturated fatty acids. Int J Obes Relat Metab Disord 1997;21:955–62. [DOI] [PubMed] [Google Scholar]
- 58.Sadurskis A, Dicker A, Cannon B, Nedergaard J. Polyunsaturated fatty acids recruit brown adipose tissue: increased UCP content and NST capacity. Am J Physiol 1995;269:E351–60. [DOI] [PubMed] [Google Scholar]
- 59.Bargut TC, Silva ESAC, Souza-Mello V, Mandarim-de-Lacerda CA, Aguila MB. Mice fed fish oil diet and upregulation of brown adipose tissue thermogenic markers. Eur J Nutr 2016;55:159–69. [DOI] [PubMed] [Google Scholar]
- 60.Zhao M, Chen X. Eicosapentaenoic acid promotes thermogenic and fatty acid storage capacity in mouse subcutaneous adipocytes. Biochem Biophys Res Commun 2014;450:1446–51. [DOI] [PubMed] [Google Scholar]
- 61.Kim M, Goto T, Yu R, Uchida K, Tominaga M, Kano Y, Takahashi N, Kawada T. Fish oil intake induces UCP1 upregulation in brown and white adipose tissue via the sympathetic nervous system. Sci Rep 2015;5:18013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pahlavani M, Razafimanjato F, Ramalingam L, Kalupahana NS, Moussa H, Scoggin S, Moustaid-Moussa N. Eicosapentaenoic acid regulates brown adipose tissue metabolism in high-fat-fed mice and in clonal brown adipocytes. J Nutr Biochem 2017;39:101–9. [DOI] [PubMed] [Google Scholar]
- 63.Kim J, Okla M, Erickson A, Carr T, Natarajan SK, Chung S. Eicosapentaenoic acid potentiates brown thermogenesis through FFAR4-dependent up-regulation of miR-30b and miR-378. J Biol Chem 2016;291:20551–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Quesada-López T, Cereijo R, Turatsinze JV, Planavila A, Cairó M, Gavaldà-Navarro A, Peyrou M, Moure R, Iglesias R, Giralt M, et al. . The lipid sensor GPR120 promotes brown fat activation and FGF21 release from adipocytes. Nat Commun 2016;7:13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martínez-Fernández L, Laiglesia LM, Huerta AE, Martínez JA, Moreno-Aliaga MJ. Omega-3 fatty acids and adipose tissue function in obesity and metabolic syndrome. Prostaglandins Other Lipid Mediat 2015;121:24–41. [DOI] [PubMed] [Google Scholar]
- 66.Laiglesia LM, Lorente-Cebrian S, Prieto-Hontoria PL, Fernandez-Galilea M, Ribeiro SM, Sainz N, Martinez JA, Moreno-Aliaga MJ. Eicosapentaenoic acid promotes mitochondrial biogenesis and beige-like features in subcutaneous adipocytes from overweight subjects. J Nutr Biochem 2016;37:76–82. [DOI] [PubMed] [Google Scholar]
- 67.Bonet ML, Ribot J, Palou A. Lipid metabolism in mammalian tissues and its control by retinoic acid. Biochim Biophys Acta 2012;1821:177–89. [DOI] [PubMed] [Google Scholar]
- 68.Alvarez R, de Andres J, Yubero P, Vinas O, Mampel T, Iglesias R, Giralt M, Villarroya F. A novel regulatory pathway of brown fat thermogenesis. Retinoic acid is a transcriptional activator of the mitochondrial uncoupling protein gene. J Biol Chem 1995;270:5666–73. [DOI] [PubMed] [Google Scholar]
- 69.Murholm M, Isidor MS, Basse AL, Winther S, Sorensen C, Skovgaard-Petersen J, Nielsen MM, Hansen AS, Quistorff B, Hansen JB. Retinoic acid has different effects on UCP1 expression in mouse and human adipocytes. BMC Cell Biol 2013;14:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mercader J, Palou A, Bonet ML. Induction of uncoupling protein-1 in mouse embryonic fibroblast-derived adipocytes by retinoic acid. Obesity (Silver Spring) 2010;18:655–62. [DOI] [PubMed] [Google Scholar]
- 71.Felipe F, Bonet ML, Ribot J, Palou A. Modulation of resistin expression by retinoic acid and vitamin A status. Diabetes 2004;53:882–9. [DOI] [PubMed] [Google Scholar]
- 72.Berry DC, Noy N. All-trans-retinoic acid represses obesity and insulin resistance by activating both peroxisome proliferation-activated receptor beta/delta and retinoic acid receptor. Mol Cell Biol 2009;29:3286–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature 2000;404:652–60. [DOI] [PubMed] [Google Scholar]
- 74.Teruel T, Hernandez R, Benito M, Lorenzo M. Rosiglitazone and retinoic acid induce uncoupling protein-1 (UCP-1) in a p38 mitogen-activated protein kinase-dependent manner in fetal primary brown adipocytes. J Biol Chem 2003;278:263–9. [DOI] [PubMed] [Google Scholar]
- 75.Rial E, Gonzalez-Barroso M, Fleury C, Iturrizaga S, Sanchis D, Jimenez-Jimenez J, Ricquier D, Goubern M, Bouillaud F. Retinoids activate proton transport by the uncoupling proteins UCP1 and UCP2. EMBO J 1999;18:5827–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bonet ML, Oliver J, Pico C, Felipe F, Ribot J, Cinti S, Palou A. Opposite effects of feeding a vitamin A-deficient diet and retinoic acid treatment on brown adipose tissue uncoupling protein 1 (UCP1), UCP2 and leptin expression. J Endocrinol 2000;166:511–7. [DOI] [PubMed] [Google Scholar]
- 77.Mercader J, Ribot J, Murano I, Felipe F, Cinti S, Bonet ML, Palou A. Remodeling of white adipose tissue after retinoic acid administration in mice. Endocrinology 2006;147:5325–32. [DOI] [PubMed] [Google Scholar]
- 78.Tourniaire F, Musinovic H, Gouranton E, Astier J, Marcotorchino J, Arreguin A, Bernot D, Palou A, Bonet ML, Ribot J, et al. . All-trans retinoic acid induces oxidative phosphorylation and mitochondria biogenesis in adipocytes. J Lipid Res 2015;56:1100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guo H, Foncea R, O’Byrne SM, Jiang H, Zhang Y, Deis JA, Blaner WS, Bernlohr DA, Chen X. Lipocalin 2, a regulator of retinoid homeostasis and retinoid-mediated thermogenic activation in adipose tissue. J Biol Chem 2016;291:11216–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rupasinghe HP, Sekhon-Loodu S, Mantso T, Panayiotidis MI. Phytochemicals in regulating fatty acid beta-oxidation: potential underlying mechanisms and their involvement in obesity and weight loss. Pharmacol Ther 2016;165:153–63. [DOI] [PubMed] [Google Scholar]
- 81.Shen W, Baldwin J, Collins B, Hixson L, Lee KT, Herberg T, Starnes J, Cooney P, Chuang CC, Hopkins R, et al. . Low level of trans-10, cis-12 conjugated linoleic acid decreases adiposity and increases browning independent of inflammatory signaling in overweight Sv129 mice. J Nutr Biochem 2015;26:616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ribot J, Portillo MP, Pico C, Macarulla MT, Palou A. Effects of trans-10, cis-12 conjugated linoleic acid on the expression of uncoupling proteins in hamsters fed an atherogenic diet. Br J Nutr 2007;97:1074–82. [DOI] [PubMed] [Google Scholar]
- 83.Nedergaard J, Cannon B., UCP1 mRNA does not produce heat. Biochim Biophys Acta 2013;1831:943–9. [DOI] [PubMed] [Google Scholar]
- 84.Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, Locksley RM, Chawla A. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell 2015;160:74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Uhm M, Saltiel AR. White, brown, and beige; type 2 immunity gets hot. Immunity 2015;42:15–7. [DOI] [PubMed] [Google Scholar]
- 86.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. . Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003;112:1821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, et al. . A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012;481:463–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Borga M, Virtanen KA, Romu T, Leinhard OD, Persson A, Nuutila P, Enerback S. Brown adipose tissue in humans: detection and functional analysis using PET (positron emission tomography), MRI (magnetic resonance imaging), and DECT (dual energy computed tomography). Methods Enzymol 2014;537:141–59. [DOI] [PubMed] [Google Scholar]
- 89.Chen Y, Buyel JJ, Hanssen MJ, Siegel F, Pan R, Naumann J, Schell M, van der Lans A, Schlein C, Froehlich H, et al. . Exosomal microRNA miR-92a concentration in serum reflects human brown fat activity. Nat Commun 2016;7:11420. [DOI] [PMC free article] [PubMed] [Google Scholar]