Abstract
Constipation is a common and burdensome gastrointestinal disorder that may result from altered gastrointestinal motility. The effect of probiotics on constipation has been increasingly investigated in both animal and human studies, showing promising results. However, there is still uncertainty regarding the mechanisms of action of probiotics on gut motility and constipation. Several factors are vital to normal gut motility, including immune and nervous system function, bile acid metabolism and mucus secretion, and the gastrointestinal microbiota and fermentation; an imbalance or dysfunction in any of these components may contribute to aberrant gut motility and, consequently, symptoms of constipation. For example, adults with functional constipation have significantly decreased numbers of bifidobacteria (with one study showing a mean difference of 1 log10/g) and lactobacilli (mean difference, 1.4 log10/g) in stool samples, as well as higher breath methane, compared with control subjects. Modifying the gut luminal environment with certain probiotic strains may affect motility and secretion in the gut and, hence, provide a benefit for patients with constipation. Therefore, this review explores the mechanisms through which probiotics may exert an effect on gut motility and constipation. Nevertheless, the majority of current evidence is derived from animal studies, and therefore, further human studies are needed to determine the mechanisms through specific probiotic strains that might be effective in constipation.
Keywords: constipation, gut motility, mechanisms, probiotics, gastrointestinal microbiome
Introduction
Motility of the gastrointestinal tract is an imprecise term embracing several measurable phenomena, including enteric contractile activity, gut wall biomechanical functions, and intraluminal flow responsible for the propulsion of gut contents. Sensitivity of the gastrointestinal tract, which refers both to conscious perception of gut stimuli and to afferent input within gastrointestinal sensory pathways, is inextricably linked, and hence, gut motility can be the consequence of interrelated sensory motor functions.
Gut transit is the functional consequence of tonic and phasic gut contractions and refers to the time taken for intraluminal contents to traverse the gastrointestinal tract (1). Despite a wide variation between individuals, normal whole-gut transit time is considered to be 30–40 h (2).
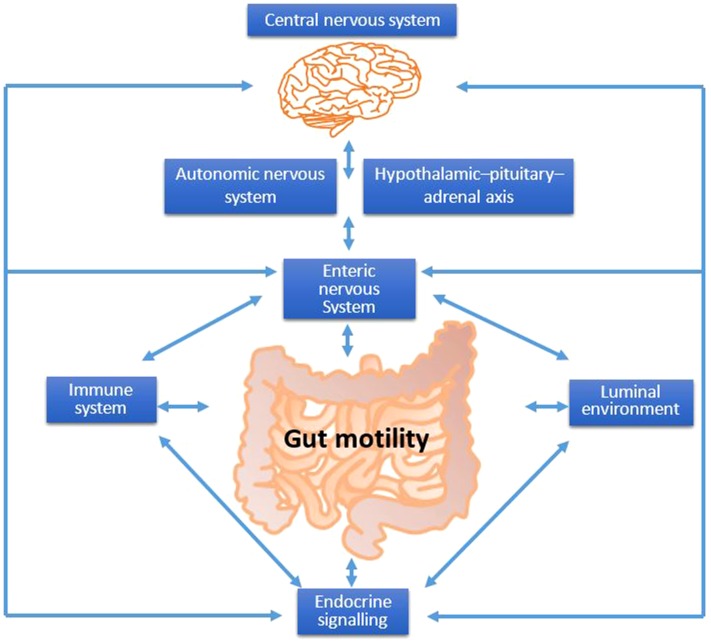
Several factors regulate gut motility (Figure 1). Afferent and efferent neural control is provided through interaction of the gut with the central nervous system (CNS)5 via somatic or autonomic [autonomic nervous system (ANS)] neurons, and communication between different parts of the gut is achieved by the transmission of myogenic and neurogenic signals along the gut via the enteric nervous system (ENS) and by reflex arcs through autonomic neurons (3). The hierarchy of neural control of gut motility is as follows: the primary regulator is ENS, followed by ANS and then CNS. Simultaneously, the immune system, gut secretions, gastrointestinal microbiota, and products of fermentation interact and modulate gut motility.
FIGURE 1.
Factors that control gut motility. The gut luminal environment (including the gastrointestinal microbiota and fermentation), immune system, enteric nervous system, and central nervous system are highly interrelated and control gut motility; disturbance in any of these overlapping systems may contribute to symptoms of constipation.
Constipation may, in some cases, be regarded as a colonic motility disorder (4). Slow-transit constipation may be caused by the dysfunction of colonic smooth muscle or neural innervation, resulting in neural colonic motor abnormalities (5); the principal pathophysiological mechanism is believed to be dysregulated or deficient colonic propulsive motor patterns (5, 6), although abnormal visceral sensitivity may also be involved in some cases.
Patients with constipation have an increased gut transit time compared with healthy controls, with the upper limit of normal considered to be 70 h (2). Patients use a variety of treatments, including fiber supplements, laxatives, and prescription medication (7). However, nearly half of patients are dissatisfied with current treatments, mainly because of a lack of efficacy and concerns about adverse effects (7).
There has been increasing research regarding the importance of the gastrointestinal microbiota to gut function and the effect of probiotics on gut motility and constipation (Table 1). Probiotics are “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” (13). Studies have shown that specific probiotics may help decrease gut transit time in people with or without constipation (Table 1). A recent systematic review and meta-analysis also showed that administration of 108 to 3 × 1010 CFU/d of specific probiotic species and strains decreased gut transit time by 12 h, increased stool frequency by 1.5 stools/wk, and improved some constipation-related symptoms (14). The aim of this review was to summarize existing evidence on the mechanisms through which probiotics may exert an effect on gut motility and constipation.
TABLE 1.
A selection of human studies investigating the effect of probiotics specifically on gut transit time in healthy and constipated individuals1
| Reference | N | Population | Intervention | Comparator | Duration | Method of gut transit time assessment | Outcome |
| Marteau et al., 2002 (8) | 36 | Healthy women | B. animalis DN-173010 (maximum 3.75 × 1010 CFU/d) | Fermented milk without probiotics | 10 d | ROM technique (20 ROM/d for 3 d, X-ray on day 4) | Gut transit time was significantly lower in the probiotic group than in the placebo group (52 h vs. 61 h, respectively; P < 0.005). |
| Agrawal et al., 2009 (9) | 41 | Constipation (Rome III for IBS-C) | B. lactis DN-173 010 (2.5 × 1010 CFU/d) | Nonfermented dairy product | 4 wk | ROM technique (24 ROM/d for 3 d, X-ray on day 4) | Gut transit time was significantly reduced in the probiotic group compared with the placebo group (mean difference: −12 h; P = 0.026). |
| Krammer et al., 2011 (10) | 24 | Constipation (gut transit time >72 h) | L. casei Shirota (6.5 × 109 CFU/d) | Milk drink without probiotics | 4 wk | ROM technique (20 ROM over 6 d, X-ray on day 7) | Gut transit time was decreased from 96 h at baseline to 77 h after the probiotic consumption (P = 0.05). No statistical comparisons were performed between the probiotic and placebo groups at the end of the treatment period. |
| Waller et al., 2011 (11) | 88 | Constipation (2–47 stool type at Bristol stool chart and 1–3 stool/wk) | B. lactis HN019 (17.2 × 109 CFU/d or 1.8 × 109 CFU/d) | Capsules with rice maltodextrin | 14 d | ROM technique (24 ROM/d for 6 d, X-ray on day 7) | Change in WGTT was statistically significant across study groups (high dose: −28 h, low dose: −19 h, placebo: +1 h; P < 0.001). |
| Merenstein et al., 2014 (12) | 68 | Healthy women | B. lactis Bf-6 (5.6 × 1010 CFU/d) | Yogurt without probiotics | 2 wk | ROM technique (24 ROM/d for 3 d, X-ray on day 4 and 8) | Gut transit time was not different between the probiotic and placebo periods (42 h vs. 43 h, respectively; P > 0.69). |
B., Bifidobacterium; IBS-C, Constipation-predominant irritable bowel syndrome; L., Lactobacillus; ROM, radio-opaque marker; WGTT, whole-gut transit time.
Current Status of Knowledge
In order to understand the mechanisms through which probiotics exert an effect on gut motility and constipation, first the physiology of gut motility and its pathophysiology in constipation must be reviewed, including the role of the central and enteric nervous systems, the gastrointestinal microbiota and fermentation and immune system function.
CNS and ENS
ENS, CNS, gut motility, and constipation.
The ENS can function independently of the CNS and contains the reflex pathways associated with normal motor and sensory function of the gut (15, 16). Studies in germ-free mice have shown that bacterial colonization of the gut is key to the development and maturation of the ENS (17). Furthermore, metabolic products from gastrointestinal microbiota fermentation, such as SCFAs, or peptides can stimulate the ENS and affect gut transit (17). The neuroendocrine system of the gut has also been shown to interact with the microbiota (18) via serotonin [5-hydroxytryptamine (5-HT)] (19). 5-HT is produced in both the ENS and CNS and is a key neurotransmitter that plays a pivotal role in mediating motor and secretory responses in the ENS (20). 5-HT stimulates local enteric nervous reflexes to initiate secretion and propulsive motility and acts on vagal afferents to modulate contractile activities (20).
CNS control of gastrointestinal tract motility is modulated via the ANS and the hypothalamus-pituitary-adrenal axis (21). Both the sympathetic and parasympathetic branches of the ANS regulate gut motility via influences on the circuits of the ENS (22). The gastrointestinal microbiota plays a crucial role in the normal development of the CNS (23) and seems to interact with both the CNS and gut (24) through microbiota–enterochromaffin cells–vagal afferent nerve signaling (25). There is now increasing evidence to support the existence of the bidirectional “microbiota-gut-brain axis” (26), which has a key role in regulating gut motility (27).
Impaired gut motility can develop through dysfunction of the control mechanisms of the ENS (1), potentially leading to gut symptoms, including constipation. Abnormal gastrointestinal microbiota composition may cause disruption in microbiota-gut-brain axis signaling, leading to changes in gut motility (23, 24). Alteration in gut motility can also be a result of a primary defect in CNS modulation (28), although impaired gut motility can develop through dysfunction of control mechanisms at any level from the gut to the CNS (1).
ENS, CNS, gut motility, and constipation: effects of probiotics.
Modulation of microbiota gut-brain interactions with probiotics has been proposed as a novel therapeutic tool for the treatment of gut motility disorders (29). Administration of Lactobacillus reuteri has been shown to modulate neural-dependent motility reflexes that communicate with the brain in the mouse (30). Furthermore, L. reuteri has been shown to interact with the gut-brain axis in rats through the modulation of afferent sensory nerves that influence gut motility (31). However, although certain probiotic species and strains have been shown to modulate brain activity in humans (29), their effect on gut motility via CNS modulation has yet to be investigated in humans (22). L. reuteri has also been shown to selectively increase the excitability of myenteric neurons in rats, indicating that the mechanism of action of probiotics involves the ENS. Furthermore, supernatant from Escherichia coli Nissle increased the maximal tension forces of smooth muscle from the human colon in an in vitro study, although blockage of enteric nerves abolished these effects, suggesting that E. coli Nissle may potentially influence contractility by direct stimulation of smooth muscle cells (32). This effect was not attributed to fermentation end products, such as SCFAs, but to other unidentified contractility enhancing agents (32).
In summary, although the ENS appears to be the primary regulator of gut motility, both the ENS and CNS are involved in its control, and both interact with the gastrointestinal microbiota. Dysfunction or dysregulation of the ENS or CNS can lead to symptoms of constipation. A small number of studies have now shown that the beneficial effects of probiotics on gut motility are mediated through the nervous system, providing evidence that probiotics may help regulate the ENS or CNS to normalize gut motility.
Luminal factors
Microbiota, gut fermentation, and gut motility.
The gastrointestinal microbiota play a vital role in gut motility, as highlighted by studies in germ-free mice showing that, in the absence of a gastrointestinal microbiota, gastric emptying and gut transit time are increased compared with in wild-type mice (33, 34). Colonization with a specific pathogen-free microbiota normalizes small-bowel migrating motor complexes (35), and colonization with L. acidophilus, Bifidobacterium bifidum, or Clostridium tabificum in germ-free rats also normalized the small-bowel migrating motor complexes and gut transit time, whereas colonization with E. coli inhibited intestinal myoelectric activity (36). In vitro and in vivo studies have shown that colonization with microbiota in conventionally raised and germ-free mice results in a 2- to 5-fold increase in mRNAs encoding l-glutamate transporter, l-glutamate decarboxylase, γ-aminobutyric acid (neuromodulator in enteric nerves), vesicle-associated protein 33 (protein involved in neurotransmitter release), enteric γ-actin, and cysteine-rich protein-2, indicating that the gastrointestinal microbiota affects ENS components crucial to motility (37–39). A murine study has also shown that colonic contractility was higher and gut transit time significantly decreased in mice colonized with gastrointestinal microbiota compared with germ-free controls (286 min compared with 457 min, respectively; P < 0.005) (40). In the same study, the administration of polyethylene glycol (a laxative) further decreased gut transit time and caused a decrease in the relative abundance of Peptococcaceae, Eubacteriaceae, and Anaeroplasmataceae, whereas the administration of loperamide (a constipating agent) increased gut transit time and resulted in an increase in the ratio of Firmicutes to Bacteroides and a decrease in relative abundance of Lachnospiraceae (40). Administration of cellulose (insoluble dietary fiber) led to a decrease in gastrointestinal transit, an effect that was independent of the presence of a gastrointestinal microbiota (277 min compared with 502 min in germ-free controls and 225 min compared with 300 min in colonized mice; P < 0.05). The authors concluded that dietary-induced changes in microbial composition may be partly mediated by changes in gut transit time and that the effect of diet on gut transit time may be in part caused by altered functionality of the gastrointestinal microbiota resulting from dietary change (40). It is important to note that there is no perfect animal model that exhibits anatomical and functional defects consistent with constipation. Therefore, caution is needed when extrapolating conclusions from animal studies to humans (41) but also when extrapolating from in vitro studies to in vivo effects.
End products of bacterial fermentation can also affect gut motility. For example, the chemotactic peptides produced by the gastrointestinal microbiota, such as formyl-methionyl-leucyl-phenylalanine, stimulate the ENS and primary afferent nerves (42), whereas the bacterial endotoxin LPS may promote gut dysmotility via the ENS and intestinal smooth muscle contractions (43). SCFAs also affect gut contractility, with an in vitro study in the rat colon showing that propionate, butyrate, and valerate induced concentration-dependent phasic contractions in the middle and distal colon (44), although other studies contradict these findings (45), possibly because of variations in animal models, experimental methods, and the nature and form of SCFA used. There are several mechanisms for the effect of SCFAs on gut motility, which are not completely understood; for example, in vitro studies have shown that propionate, butyrate, and valerate stimulate the mucosal receptors connected to enteric and/or vagal nerves (44) and act directly on the colonic smooth muscle (46). Intraluminal administration of a blend of acetate, propionate, and butyrate in rats was also shown to lead to increased 5-HT concentrations and, hence, decreased colonic transit time (47).
SCFAs have been shown to affect gut motility independent of pH. Colonic infusion of acids does not impact gut motility, whereas infusion of a solution containing acetate, propionate, and butyrate (while maintaining an intraluminal pH 6.2–6.4) reduced gut transit time (45). Furthermore, human in vivo studies have shown that infusion of boluses containing a blend of SCFAs stimulate ileal motility to a greater degree than air or saline (48), although these effects have not been consistently demonstrated (49).
Other products of microbiota fermentation are methane and hydrogen (50). Methane is a gas produced by gastrointestinal microbiota and acts as a neuromuscular transmitter affecting gut motility (50). Methane increased small intestinal transit time in a canine in vivo model, whereas exposure to methane increased in vitro ileal circular muscle contractility in guinea pigs (51). Conversely, another ex vivo study showed that methane decreased contractility of ileal muscle, whereas a hydrogen infusion increased it and decreased colonic transit time (52). The effects of microbiota-derived fermentation gases on contractility and gut motility thus remain uncertain.
The microbiota also indirectly influence gut motility via gut immune responses, mediated through pattern-recognition receptors, such as toll-like receptors (Figure 2). In mice, antibiotic-induced depletion of the microbiota resulted in low-grade gut inflammation, decreased gut transit time, and reduced amplitude of spontaneous contractions (58). More specifically, activation of toll-like receptor 4 expression by LPSs restored these effects, suggesting that the presence of bacteria containing LPSs (such as proteobacteria) may contribute to maintaining normal gut motility (58).
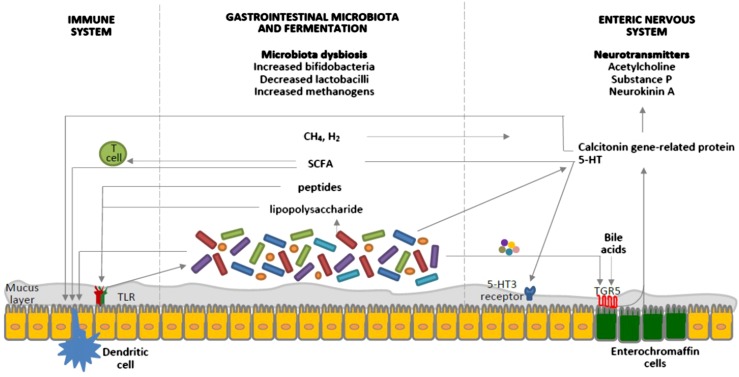
FIGURE 2.
Interrelated factors involved in the pathophysiology of constipation as potential targets for the therapeutic role of probiotics. Probiotics affect the gastrointestinal microbiota composition, the byproducts of which interact with pattern-recognition receptors, such as TLRs, as well as with dendritic cells. SCFAs increase intestinal regulatory T cells, which limit intestinal inflammation, by reducing histone deacetylase 9 gene expression (53). The gastrointestinal microbiota regulates 5-HT production by elevating its synthesis by host enterochromaffin cells via the release of metabolites, such as deoxycholate, which activates TGR5, expressed by enterochromaffin cells (54). 5-HT is also released from enterochromaffin cells in response to SCFAs produced by the gastrointestinal microbiota and stimulates 5-HT3 receptors located on the vagal afferent fibers, resulting in muscle contractions (47). Gases produced by the gastrointestinal microbiota seem to affect gut motility via the ENS, rather than the brain-gut axis; however, the exact mechanisms are still unknown (50). Moreover, the gastrointestinal microbiota is key to the development of the ENS, which is the primary regulator of gut motility, and certain bacteria are known to produce 5-HT. Calcitonin gene–related protein, a sensory neuropeptide, modulates dendritic cell function and may signal the presence of gastrointestinal microbiota to the brain (55). Components of the gastrointestinal microbiota also act via intestinal dendritic cells to influence the inflammatory process (56). TLRs signaling controls ENS structure and neuromuscular function and hence motility (57). Bile acids activate TGR5 expressed by enterochromaffin cells and myenteric neurons and release 5-HT and calcitonin genre–related peptide. Furthermore, probiotics appear to interact with the gut-brain axis via the modulation of afferent sensory nerves that influence gut motility. CH4, methane; ENS, enteric nervous system; H2, hydrogen; TGR5, a G protein–coupled receptor; TLR, toll-like receptor; 5-HT, 5-hydroxytryptamine; 5-HT3, 5-hydroxytryptamine type 3.
Altered microbiota and gut fermentation in constipation.
Several studies have investigated the gastrointestinal microbiota in constipation, as well as in constipation-predominant irritable bowel syndrome (IBS) (Table 2). In adults, these studies consistently demonstrate decreased bifidobacteria and lactobacilli and increased Bacteroidetes compared with controls (61, 63, 65, 66), although this has not been confirmed in pediatric studies (60, 64). Parthasarathy et al. (66), using a case-control study design, showed that fecal microbiota composition was correlated with colonic transit time, and the colonic mucosal microbiota composition was associated with constipation status even after adjusting for age, BMI (in kg/m2), diet, and transit time (Table 2). More specifically, the abundance of Actinobacteria, Bacteroides, Lactococcus, and Roseburia were correlated with faster gut transit time, whereas Faecalibacterium was directly correlated to slower gut transit time (66). However, whether dysbiosis causes constipation or is merely a consequence of it remains unclear.
TABLE 2.
Summary of human case-control studies investigating the gastrointestinal microbiota composition and fermentation profiles in constipation1
| Population group |
Comparison group |
|||||
| Study | N | Characteristics | N | Characteristics | Method of microbiota characterization | Summary of main findings in constipation (compared to comparison groups) |
| Shimoyama et al., 1984 (59) | 25 | Patients with chronic constipation with several other diseases (not specified if adults) | 37 | Healthy controls without constipation | Unclear | Lower Bifidobacteria, Bacteroidaceae, and Clostridia |
| Higher Micrococcaceae | ||||||
| Zoppi et al., 1998 (60) | 42 | Children with functional constipation | 14 | Healthy children without constipation | Culture methods | Higher Clostridia and Bifidobacteria |
| Clostridia outnumber Bacteroides and E. coli in constipated children, whereas healthy children had similar mean counts for clostridia, Bacteroides, and Bifidobacteria | ||||||
| Khalif et al., 2005 (61) | 57 | Adults with chronic constipation | 25 | Healthy adults without constipation | Culture methods | Lower Bifidobacterium and Lactobacillus |
| Higher E. coli, Enterobacteria, and Staphylococcus aureus | ||||||
| Attaluri et al., 2010 (62) | 96 | Adults with functional constipation (n = 48 with STC, n = 48 with normal-transit time) | 106 | Adults without constipation | Glucose breath test, which measured H2 and CH4 (used to define presence of methanogenic flora) | Significantly higher concentrations of methane in STC; the prevalence of methanogenic microbiota was 75%, 44%, and 28% in participants with STC and normal-transit constipation and in control participants, respectively |
| Chassard et al., 2012 (63) | 14 | Women with IBS-C | 12 | Healthy women without constipation | Strictly anaerobic cultural evaluation of functional groups of microbes and FISH | Lower Bifidobacteria (6.8 log10/g stool vs. 7.8 log10/g; P < 0.001), Lactobacilli (5.5 log10/g vs. 6.9 log10/g; P = 0.0007), and lactate-utilizing bacteria (7.9 log10/g vs. 9.3 log10/g; P = 0.0046) and the number of H2-consuming populations, methanogens (8.3 log10/g vs. 9.7 log10/g; P = 0.03) and reductive acetogens |
| Higher number of lactate- and H2-utilizing sulfate-reducing population | ||||||
| Lower Roseburia in the E. rectale group | ||||||
| No difference in F. prausnitzii concentration | ||||||
| Zhu et al., 2014 (64) | 8 | Obese adolescents with constipation | 14 | Obese adolescents without constipation | 16S rRNA pyrosequencing | Lower Prevotella, Bacteroides |
| Higher Firmicutes (i.e., Lachnospiraceae and Ruminococcaceae) | ||||||
| No difference in Lactobacillus and Bifidobacteria | ||||||
| Absence of Acidobacteria, OP3 and TM7 | ||||||
| Kim et al., 2015 (65) | 30 | Adults with functional constipation | 30 | Healthy controls without constipation | Real-time qPCR | Lower Bifidobacterium and Bacteroides |
| Parthasarathy et al., 2016 (66) | 25 | Adults with functional constipation, IBS-C, and mixed IBS | 25 | Healthy controls | 16S-based sequencing | Higher abundance of Bacteroidetes (7% vs. 0.001%; P < 0.01), Flavobacteriaceae (7% vs. 0.001%; P < 0.01), and Caulobacteraceae (0.2% vs. 0.1%; P < 0.04) |
| Lower Odoribacteraceae (0.01% vs. 1%; P < 0.01) and Comamonadaceae (0.2% vs. 12%; P < 0.01) | ||||||
CH4, methane; E., Escherichia; F., Faecalibacterium; FISH, fluorescent in situ hybridization; H2, hydrogen; IBS, irritable bowel syndrome; IBS-C, constipation-predominant irritable bowel syndrome; rRNA, ribosomal ribonucleic acid; STC, slow-transit constipation.
Little evidence exists regarding differences in SCFAs and stool pH between constipated and healthy subjects; SCFAs have been shown to be lower in constipation-predominant IBS than in healthy controls (63); however, this may result from slower gut transit time increasing SCFA absorption (67). No difference was observed in stool pH between constipated children and controls (60).
One study showed that more individuals with slow-transit constipation (59%) have a positive methane breath test compared with normal-transit constipation (13%) and healthy controls (12%) (P < 0.01) (68). It has also been shown that methane production is higher in functional constipation than in constipation-predominant IBS (69). A recent case-control study showed that breath methane production was associated with the composition of the fecal microbiota but not with colonic transit time (66). In an attempt to investigate the mechanisms of action of methane on gut motility, a small study of 18 patients with IBS showed that the postprandial blood 5-HT concentration in methane-producing patients was decreased compared with hydrogen producers, suggesting a possible, as yet unclear, interaction between methane and the enteric nervous function (70).
Altered microbiota, fermentation, gut motility, and constipation: effects of probiotics.
The effect of probiotics on the microbiota in constipation is still relatively poorly understood. Some clinical trials that demonstrated improvements in stool frequency in constipation during supplementation with some probiotics have also reported alterations in gastrointestinal microbiota. Examples include B. lactis (1010 CFU/d for 2 wk) increasing total bifidobateria (71) and L. casei Shirota (4 × 109 CFU/d for 2 wk) increasing Bifidobacteria, Lactobacilli, and Atopobium (72); however, there are examples where little impact on the microbiota occurred, including VSL#3 (9 × 1011 CFU/d for 2 wk) having no impact on bifibdobacteria and Bacteroides despite affecting stool frequency (65).
One of the most popular theories on the mechanistic actions of probiotics in constipation is that some may increase SCFA concentration, thus normalizing gut motility (73). Several studies of various probiotic species and strains that demonstrate improvements in constipated-related outcomes (stool frequency, stool consistency) provide conflicting results regarding the effect of these probiotics on SCFA production; some show a change in acetate, propionate, and butyrate (72, 74, 75), and others show no change (76, 77), which could be attributed to the different strains tested. Nevertheless, these studies measured SCFA concentrations in stool samples (rather than the colonic lumen), which are not predictive of those found proximally (78) because >95% of SCFAs are absorbed during colonic transit (79).
In summary, the gastrointestinal microbiota and SCFAs, which influence gut motility through interaction with the ENS, have been shown to be imbalanced in patients with constipation. Preliminary results show that the administration of some probiotics affects the composition of some microbiota and SCFA production, but the mechanisms of the subsequent effect on motility and constipation are not yet fully understood.
Other luminal factors, gut motility, and constipation: effects of probiotics.
In addition to the gastrointestinal microbiota and SCFAs, other luminal factors such as bile acids and mucus may also play a role in regulating gut motility. Bile acids act as physiological laxatives, altering luminal electrolyte and water transport (80, 81). Several decades ago, administration of deoxycholic acid in the rabbit colon was shown to increase circular smooth muscle contractile activity (81). In vitro studies have shown that bile acids influence gut motility via the ENS (82), as well as through endocrine and paracrine mechanisms (83); they appear to activate TGR5, a G protein-coupled receptor expressed by enterochromaffin cells and myenteric neurons, and release 5-HT and calcitonin gene–related peptide (84) (Figure 2). With regard to bile acid metabolism, reduced synthesis and/or concentrations of specific bile acids have been shown in patients with constipation (85), and administration of chenodeoxycholic acid or ileal bile acid–transporter inhibitors decrease gut transit time compared with placebo and improve constipation symptoms (86). Notably, bile acid metabolism and the gastrointestinal microbiota have been shown to interact because the gastrointestinal microbiota regulates hepatic bile acid synthesis and promotes deconjugation, dehydrogenation, and dihydroxylation of primary bile acids, increasing the chemical diversity of bile acids (87, 88).
The enterocytes of the gut are lined with a surface mucus gel that serves as a lubricant to protect the mucosa (89) but that also facilitates the passage of stools. A study in rats with drug-induced constipation reported a decreased mucus layer thickness, which might impede the stool passage (90). Reduction in mucus may be a consequence of cholinergic dysfunction, which has been recognized in some constipated patients (91). Conversely, a study of loperamide-induced constipation in a rat model showed that inducing gut motility with sulfated polysaccharides increased epithelial mucin production and mucus layer thickness, which is linked to an increase in stool excretion (92).
No effect of probiotic supplementation on stool bile acid concentrations (8) or stool water (72, 77) has been documented thus far in constipation. VSL#3 has been shown to induce colonic mucin production via upregulation of the MUC2 gene (93). The same study also included in vitro experiments assessing the effect of VSL#3, as well as that of its constituent individual bacterial species, on mucin secretion. Interestingly, Lactobacillus was as effective as VSL#3 in exerting a mucin secretion effect, whereas Bifidobacterium and Streptococcus salivarius had minimal effects (93). Furthermore, a human study (elderly nursing home residents) showed no effect of 4-wk supplementation of L. reuteri and Propionibacterium freudenreichii on mucin excretion (94). These data suggest that different probiotic species and strains have different effects on mucin production and may in part explain the variation in the effectiveness of probiotics in constipation reported in the literature.
In summary, bile acid metabolism and the mucus layer contribute to normal gut motility, and both appear to be altered in constipation, although the true cause-and-effect relation, or whether this represents an epiphenomenon, remains unclear. There is limited evidence supporting any impact of probiotics on these factors.
Immune System
Immune system, gut motility, and constipation
Evidence exists regarding a causal relation between gut mucosal inflammation and altered sensory and motor functions (95). The effect of enteropathogenic infection on the intestinal mucosal immune system and the increased gut motility and induction of diarrhea is well described. For example, infection of rats with Nippostrongylus brasiliensis resulted in an increase in the contractile responses of the gut longitudinal muscle to agonists (96). This increased muscular contractile activity was diminished by suppressing the inflammatory response with the use of a corticosteroid (97). A study in mice infected with Trichinella spiralis showed an increased expression of the cytokine receptors IL-4Rα and TGF-β1, which mediate the effect of IL-4 or IL-13 and of TGF-1, respectively, and a subsequent upregulation of cyclooxygenase-2 (COX-2) and PGE2 at the level of the smooth muscle cell, indicating that cytokines and their upregulation of COX-2 and PGE2 are involved in the increased contractility (98). Furthermore, a strong correlation between the activation of mast cells via the immune response and the development of gut dysmotility in T. spiralis rats has been shown (99). In rats, intestinal mast cells have been shown to modify nervous reflexes and to modulate endocrine responses induced by intraluminal stimulus (100). It has also been suggested that the altered motility caused by inflammation may result from changes in the afferent nerve input into intrinsic and extrinsic neural circuits, as well as by imbalance of the ANS (95). Intestinal inflammation has been suggested to be linked to neurological changes (101). Indeed, in rectal biopsies from patients with slow-transit constipation decreased levels of the excretory substance P of the ENS of mucosa and submucosa have been found (102).
A microscopy study using surgically resected jejunal, ileal, or colonic specimens from patients with Crohn’s disease showed that inflammation can result in morphological and functional changes in enteric nerves even at noninflamed sites (103), suggesting that inflammation at one site of the gut may possibly alter gut motility at another noninflamed site.
There are limited studies of immune system manifestations of constipation. One study revealed a disturbance in intestinal permeability as measured by ovalbumin serum concentration; whereas normalization of stool frequency with the use of bisacodyl (a stimulant laxative that increases water absorption and gut motility) resulted in restitution of normal intestinal permeability (61). The same study provided evidence of immune activation in functional constipation, with increased CD3+, CD4+, CD8+, and CD25+ T cells, as well as proliferation of lymphocytes, indicating the activation of T cell immunity (61).
Immune system, gut motility, and constipation: effect of probiotics
It has been shown that some probiotics, such as L. rhamnosus GG, can modulate the mucosal immune barrier and/or systemic immune barrier and normalize inflammation-related dysmotility in a small number of studies, albeit not specifically in constipation (104, 105). For example, L. paracasei has been shown to produce antagonistic metabolites, such as glutathionine, to stimulate immune cells in vitro (106), whereas Saccharomyces boulardii can improve gut epithelial cell restitution (107). Furthermore, subjects who consumed B. lactis (3 × 1011 CFU/d for 6 wk) had enhanced concentrations of IFN-α and polymorphonuclear cell phagocytic capacity compared with those who consumed a placebo (108).
In summary, it is well established that the immune system influences gut motility, and there is emerging evidence of an inflammatory response in some patients with constipation. Probiotics may have beneficial effects regarding some components of the immune system that could potentially influence gut motility, but the effect regarding constipation has not been investigated.
Clinical implication of probiotics in constipation
To this point, animal studies have suggested that various probiotic species and strains may have beneficial effects on gut motility and constipation; however, there are still limited data for human studies, and hence, it is difficult to extrapolate which probiotic strain is likely the most clinically efficacious. A systematic review and meta-analysis of 14 randomized controlled trials (n = 1182 participants) investigated the effect of probiotics in adults with functional constipation and revealed that overall probiotics reduced whole-gut transit time by 12 h and increased stool frequency by 1.3 bowel movements/wk (14). Most importantly, this study showed that the effect of probiotics were species- and strain-specific, with various B. lactis strains improving gut transit time, stool frequency and consistency, and flatulence, whereas the strain L. casei Shirota did not confer any beneficial results (14). However, caution is needed with the interpretation of these results because of the high heterogeneity and high levels of bias among the individual studies. Although there is no clear consensus to date on using probiotics for symptoms of constipation, a recent survey of 1830 health professionals in primary care showed that 18% recommend probiotics to patients with constipation, showing that clinicians have started incorporating probiotics as a management option in clinical practice (109).
Conclusion
The gut luminal environment, immune system, ENS, and CNS are highly interrelated and control gut motility; disturbance in any of these overlapping systems may contribute to symptoms of constipation. Modifying the gut luminal environment with certain probiotic species and strains may affect motility and secretion in the gut and hence provide benefit for patients with constipation. However, the majority of the current evidence is derived from animal studies, as their effect in humans is unclear because of a paucity of human studies. Further research of high methodological quality is required to fully establish the complex interactions of the luminal environment, immune system, and nervous system on gut motility and constipation and how different probiotic species and strains affect them. Further studies are needed to determine which probiotic species and strains, dose, and treatment duration are particularly effective in constipation, as well as to examine any potential probiotic-diet interactions and interindividual variability that may lead to differential responses to probiotics.
Acknowledgments
The authors' responsibilities were as follows—ED: wrote the initial manuscript; SC, SMS, and KW: contributed to the writing of the manuscript; SMS and KW: contributed to critical revision of the manuscript; and all authors: read and approved the final manuscript.
Footnotes
Abbreviations used: ANS, autonomic nervous system; CNS, central nervous system; ENS, enteric nervous system; IBS, irritable bowel syndrome; 5-HT, 5-hydroxytryptamine.
References
- 1.Kellow JE, Azpiroz F, Delvaux M, Gebhart GF, Mertz HR, Quigley EM, Smout AJ. Applied principles of neurogastroenterology: physiology/motility sensation. Gastroenterology 2006;130:1412–20. [DOI] [PubMed] [Google Scholar]
- 2.Kim ER, Rhee PL. How to interpret a functional or motility test - colon transit study. J Neurogastroenterol Motil 2012;18:94–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kellow JE, Delvaux M, Azpiroz F, Camilleri M, Quigley EM, Thompson DG. Principles of applied neurogastroenterology: physiology/motility-sensation. Gut 1999;45(Suppl 2):II17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassotti G, Stanghellini V, Chiarioni G, Germani U, De Giorgio R, Vantini I, Morelli A, Corinaldesi R. Upper gastrointestinal motor activity in patients with slow-transit constipation. Further evidence for an enteric neuropathy. Dig Dis Sci 1996;41:1999–2005. [DOI] [PubMed] [Google Scholar]
- 5.Dinning PG, Zarate N, Hunt LM, Fuentealba SE, Mohammed SD, Szczesniak MM, Lubowski DZ, Preston SL, Fairclough PD, Lunniss PJ, et al. . Pancolonic spatiotemporal mapping reveals regional deficiencies in, and disorganization of colonic propagating pressure waves in severe constipation. Neurogastroenterol Motil 2010;22:e340–9. [DOI] [PubMed] [Google Scholar]
- 6.Dinning PG, Wiklendt L, Maslen L, Patton V, Lewis H, Arkwright JW, Wattchow DA, Lubowski DZ, Costa M, Bampton PA. Colonic motor abnormalities in slow transit constipation defined by high resolution, fibre-optic manometry. Neurogastroenterol Motil 2015;27:379–88. [DOI] [PubMed] [Google Scholar]
- 7.Johanson JF, Kralstein J. Chronic constipation: a survey of the patient perspective. Aliment Pharmacol Ther 2007;25:599–608. [DOI] [PubMed] [Google Scholar]
- 8.Marteau P, Cuillerier E, Meance S, Gerhardt MF, Myara A, Bouvier M, Bouley C, Tondu F, Bommelaer G, Grimaud JC. Bifidobacterium animalis strain DN-173 010 shortens the colonic transit time in healthy women: a double-blind, randomized, controlled study. Aliment Pharmacol Ther 2002;16:587–93. [DOI] [PubMed] [Google Scholar]
- 9.Agrawal A, Houghton LA, Morris J, Reilly B, Guyonnet D, Goupil Feuillerat N, Schlumberger A, Jakob S, Whorwell PJ. Clinical trial: the effects of a fermented milk product containing Bifidobacterium lactis DN-173 010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Aliment Pharmacol Ther 2009;29:104–14. [DOI] [PubMed] [Google Scholar]
- 10.Krammer H-J, von Seggern H, Schaumburg J, Neumer F. Effect of Lactobacillus casei Shirota on colonic transit time in patients with chronic constipation. Coloproctology 2011;33:109–13. [Google Scholar]
- 11.Waller PA, Gopal PK, Leyer GJ, Ouwehand AC, Reifer C, Stewart ME, Miller LE. Dose-response effect of Bifidobacterium lactis HN019 on whole gut transit time and functional gastrointestinal symptoms in adults. Scand J Gastroenterol 2011;46:1057–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merenstein DJ, D’Amico F, Palese C, Hahn A, Sparenborg J, Tan T, Scott H, Polzin K, Kolberg L, Roberts R. Short-term, daily intake of yogurt containing Bifidobacterium animalis ssp. lactis Bf-6 (LMG 24384) does not affect colonic transit time in women. Br J Nutr 2014;111:279–86. [DOI] [PubMed] [Google Scholar]
- 13.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, et al. . Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 2014;11:506–14. [DOI] [PubMed] [Google Scholar]
- 14.Dimidi E, Christodoulides S, Fragkos KC, Scott SM, Whelan K. The effect of probiotics on functional constipation in adults: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr 2014;100:1075–84. [DOI] [PubMed] [Google Scholar]
- 15.Blackshaw LA, Brookes SJ, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil 2007;19(1 Suppl):1–19. [DOI] [PubMed] [Google Scholar]
- 16.Bunnett NW. The stressed gut: contributions of intestinal stress peptides to inflammation and motility. Proc Natl Acad Sci USA 2005;102:7409–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbara G, Stanghellini V, Brandi G, Cremon C, Di Nardo G, De Giorgio R, Corinaldesi R. Interactions between commensal bacteria and gut sensorimotor function in health and disease. Am J Gastroenterol 2005;100:2560–8. [DOI] [PubMed] [Google Scholar]
- 18.Lyte M. Microbial endocrinology and infectious disease in the 21st century. Trends Microbiol 2004;12:14–20. [DOI] [PubMed] [Google Scholar]
- 19.Uribe A, Alam M, Johansson O, Midtvedt T, Theodorsson E. Microflora modulates endocrine cells in the gastrointestinal mucosa of the rat. Gastroenterology 1994;107:1259–69. [DOI] [PubMed] [Google Scholar]
- 20.Spiller R. Recent advances in understanding the role of serotonin in gastrointestinal motility in functional bowel disorders: alterations in 5-HT signalling and metabolism in human disease. Neurogastroenterol Motil 2007;19(Suppl 2):25–31. [DOI] [PubMed] [Google Scholar]
- 21.Mayer EA, Savidge T, Shulman RJ. Brain-gut microbiome interactions and functional bowel disorders. Gastroenterology 2014;146:1500–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest 2015;125:926–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kennedy PJ, Clarke G, Quigley EM, Groeger JA, Dinan TG, Cryan JF. Gut memories: towards a cognitive neurobiology of irritable bowel syndrome. Neurosci Biobehav Rev 2012;36:310–40. [DOI] [PubMed] [Google Scholar]
- 24.Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 2015;28:203–9. [PMC free article] [PubMed] [Google Scholar]
- 25.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol 2009;6:306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012;13:701–12. [DOI] [PubMed] [Google Scholar]
- 27.Bercik P, Collins SM, Verdu EF. Microbes and the gut-brain axis. Neurogastroenterol Motil 2012;24:405–13. [DOI] [PubMed] [Google Scholar]
- 28.Altaf MA, Sood MR. The nervous system and gastrointestinal function. Dev Disabil Res Rev 2008;14:87–95. [DOI] [PubMed] [Google Scholar]
- 29.Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, Guyonnet D, Legrain-Raspaud S, Trotin B, Naliboff B, et al. . Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013;144:1394–401, e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang B, Mao YK, Diorio C, Pasyk M, Wu RY, Bienenstock J, Kunze WA. Luminal administration ex vivo of a live Lactobacillus species moderates mouse jejunal motility within minutes. FASEB J 2010;24:4078–88. [DOI] [PubMed] [Google Scholar]
- 31.Kunze WA, Mao YK, Wang B, Huizinga JD, Ma X, Forsythe P, Bienenstock J. Lactobacillus reuteri enhances excitability of colonic AH neurons by inhibiting calcium-dependent potassium channel opening. J Cell Mol Med 2009;13 8B:2261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bar F, Von Koschitzky H, Roblick U, Bruch HP, Schulze L, Sonnenborn U, Bottner M, Wedel T. Cell-free supernatants of Escherichia coli Nissle 1917 modulate human colonic motility: evidence from an in vitro organ bath study. Neurogastroenterol Motil 2009;21:559–66, e16–7. [DOI] [PubMed] [Google Scholar]
- 33.Abrams GD, Bishop JE. Effect of the normal microbial flora on gastrointestinal motility. Proc Soc Exp Biol Med 1967;126:301–4. [DOI] [PubMed] [Google Scholar]
- 34.Iwai H, Ishihara Y, Yamanaka J, Ito T. Effects of bacterial flora on cecal size and transit rate of intestinal contents in mice. Jpn J Exp Med 1973;43:297–305. [PubMed] [Google Scholar]
- 35.Husebye E, Hellstrom PM, Midtvedt T. Intestinal microflora stimulates myoelectric activity of rat small intestine by promoting cyclic initiation and aboral propagation of migrating myoelectric complex. Dig Dis Sci 1994;39:946–56. [DOI] [PubMed] [Google Scholar]
- 36.Husebye E, Hellstrom PM, Sundler F, Chen J, Midtvedt T. Influence of microbial species on small intestinal myoelectric activity and transit in germ-free rats. Am J Physiol Gastrointest Liver Physiol 2001;280:G368–80. [DOI] [PubMed] [Google Scholar]
- 37.Liu MT, Rothstein JD, Gershon MD, Kirchgessner AL. Glutamatergic enteric neurons. J Neurosci 1997;17:4764–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science 2001;291:881–4. [DOI] [PubMed] [Google Scholar]
- 39.Skehel PA, Fabian-Fine R, Kandel ER. Mouse VAP33 is associated with the endoplasmic reticulum and microtubules. Proc Natl Acad Sci USA 2000;97:1101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kashyap PC, Marcobal A, Ursell LK, Larauche M, Duboc H, Earle KA, Sonnenburg ED, Ferreyra JA, Higginbottom SK, Million M, et al. . Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology 2013;144:967–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zarate N, Spencer NJ. Chronic constipation: lessons from animal studies. Best Pract Res Clin Gastroenterol 2011;25:59–71. [DOI] [PubMed] [Google Scholar]
- 42.Giuliani S, Santicioli P, Tramontana M, Geppetti P, Maggi CA. Peptide N-formyl-methionyl-leucyl-phenylalanine (FMLP) activates capsaicin-sensitive primary afferent nerves in guinea-pig atria and urinary bladder. Br J Pharmacol 1991;102:730–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rebollar E, Arruebo MP, Plaza MA, Murillo MD. Effect of lipopolysaccharide on rabbit small intestine muscle contractility in vitro: role of prostaglandins. Neurogastroenterol Motil 2002;14:633–42. [DOI] [PubMed] [Google Scholar]
- 44.Yajima T. Contractile effect of short-chain fatty-acids on the isolated colon of the rat. J Physiol 1985;368:667–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cherbut C, Ferrier L, Roze C, Anini Y, Blottiere H, Lecannu G, Galimiche JP. Short-chain fatty acids modify colonic motility through nerves and polypeptide YY release in the rat. Am J Physiol 1998;275:G1415–22. [DOI] [PubMed] [Google Scholar]
- 46.Rondeau MP, Meltzer K, Michel KE, McManus CM, Washabau RJ. Short chain fatty acids stimulate feline colonic smooth muscle contraction. J Feline Med Surg 2003;5:167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M, Eubanks S, Harris M, Pappas TN, Takahashi T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol 2003;284:R1269–76. [DOI] [PubMed] [Google Scholar]
- 48.Kamath PS, Phillips SF, Zinsmeister AR. Short-chain fatty acids stimulate ileal motility in humans. Gastroenterology 1988;95:1496–502. [DOI] [PubMed] [Google Scholar]
- 49.Jouët P, Moussata D, Duboc H, Boschetti G, Attar A, Gorbatchef C, Sabaté JM, Coffin B, Flourié B. Effect of short-chain fatty acids and acidification on the phasic and tonic motor activity of the human colon. Neurogastroenterol Motil 2013;25:943–9. [DOI] [PubMed] [Google Scholar]
- 50.Triantafyllou K, Chang C, Pimentel M. Methanogens, methane and gastrointestinal motility. J Neurogastroenterol Motil 2014;20:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pimentel M, Lin HC, Enayati P, van den Burg B, Lee HR, Chen JH, Park S, Kong Y, Conklin J. Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. Am J Physiol Gastrointest Liver Physiol 2006;290:G1089–95. [DOI] [PubMed] [Google Scholar]
- 52.Jahng J, Jung IS, Choi EJ, Conklin JL, Park H. The effects of methane and hydrogen gases produced by enteric bacteria on ileal motility and colonic transit time. Neurogastroenterol Motil 2012;24:185–90, e92. [DOI] [PubMed] [Google Scholar]
- 53.Kieffer DA, Martin RJ, Adams SH. Impact of dietary fibers on nutrient management and detoxification organs: gut, liver, and kidneys. Adv Nutr 2016;7:1111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015;161:264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Collins SM, Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology 2009;136:2003–14. [DOI] [PubMed] [Google Scholar]
- 56.Ng SC, Benjamin JL, McCarthy NE, Hedin CRH, Koutsoumpas A, Plamondon S, Price CL, Hart AL, Kamm MA, Forbes A, et al. . Relationship between human intestinal dendritic cells, gut microbiota, and disease activity in Crohn’s disease. Inflamm Bowel Dis 2011;17:2027–37. [DOI] [PubMed] [Google Scholar]
- 57.Brun P, Giron MC, Qesari M, Porzionato A, Caputi V, Zoppellaro C, Banzato S, Grillo AR, Spagnol L, De Caro R, et al. . Toll-like receptor 2 regulates intestinal inflammation by controlling integrity of the enteric nervous system. Gastroenterology 2013;145:1323–33. [DOI] [PubMed] [Google Scholar]
- 58.Grasa L, Abecia L, Forcen R, Castro M, de Jalon JA, Latorre E, Alcalde AI, Murillo MD. Antibiotic-induced depletion of murine microbiota induces mild inflammation and changes in toll-like receptor patterns and intestinal motility. Microb Ecol 2015;70:835–48. [DOI] [PubMed] [Google Scholar]
- 59.Shimoyama T, Hori S, Tamura K, Yamamurama M, Tanaka M, Yamazaki K. Microflora of patients with stool abnormality. Bifidobact Microflora 1984;3:35–42. [Google Scholar]
- 60.Zoppi G, Cinquetti M, Luciano A, Benini A, Muner A, Bertazzoni Minelli E. The intestinal ecosystem in chronic functional constipation. Acta Paediatr 1998;87:836–41. [DOI] [PubMed] [Google Scholar]
- 61.Khalif IL, Quigley EM, Konovitch EA, Maximova ID. Alterations in the colonic flora and intestinal permeability and evidence of immune activation in chronic constipation. Dig Liver Dis 2005;37(11):838–49. [DOI] [PubMed] [Google Scholar]
- 62.Attaluri A, Jackson M, Valestin J, Rao SS. Methanogenic flora is associated with altered colonic transit but not stool characteristics in constipation without IBS. Am J Gastroenterol 2010;105:1407–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chassard C, Dapoigny M, Scott KP, Crouzet L, Del’homme C, Marquet P, Martin JC, Pickering G, Ardid D, Eschalier A, et al. . Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment Pharmacol Ther 2012;35:828–38. [DOI] [PubMed] [Google Scholar]
- 64.Zhu L, Liu W, Alkhouri R, Baker RD, Bard JE, Quigley EM, Baker SS. Structural changes in the gut microbiome of constipated patients. Physiol Genomics 2014;46:679–86. [DOI] [PubMed] [Google Scholar]
- 65.Kim SE, Choi SC, Park KS, Park MI, Shin JE, Lee TH, Jung KW, Koo HS, Myung SJ; Constipation Research group of Korean Society of Neurogastroenterology and Motility. Change of fecal flora and effectiveness of the short-term VSL#3 probiotic treatment in patients with functional constipation. J Neurogastroenterol Motil 2015;21:111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parthasarathy G, Chen J, Chen X, Chia N, O'Connor HM, Wolf PG, Gaskins HR, Bharucha AE. Relationship between microbiota of the colonic mucosa vs feces and symptoms, colonic transit, and methane production in female patients with chronic constipation. Gastroenterology 2016;150:367–79. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lewis SJ, Heaton KW. Increasing butyrate concentration in the distal colon by accelerating intestinal transit. Gut 1997;41:245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee KM, Paik CN, Chung WC, Yang JM, Choi MG. Breath methane positivity is more common and higher in patients with objectively proven delayed transit constipation. Eur J Gastroenterol Hepatol 2013;25:726–32. [DOI] [PubMed] [Google Scholar]
- 69.Furnari M, Savarino E, Bruzzone L, Moscatelli A, Gemignani L, Giannini EG, Zentilin P, Dulbecco P, Savarino V. Reassessment of the role of methane production between irritable bowel syndrome and functional constipation. J Gastrointestin Liver Dis 2012;21:157–63. [PubMed] [Google Scholar]
- 70.Pimentel M, Kong YT, Park S. IBS subjects with methane on lactulose breath test have lower postprandial serotonin levels than subjects with hydrogen. Dig Dis Sci 2004;49:84–7. [DOI] [PubMed] [Google Scholar]
- 71.Ishizuka A, Tomizuka K, Aoki R, Nishijima T, Saito Y, Inoue R, Ushida K, Mawatari T, Ikeda T. Effects of administration of Bifidobacterium animalis subsp. lactis GCL2505 on defecation frequency and bifidobacterial microbiota composition in humans. J Biosci Bioeng 2012;113:587–91. [DOI] [PubMed] [Google Scholar]
- 72.Matsumoto K, Takada T, Shimizu K, Kado Y, Kawakami K, Makino I, Yamaoka Y, Hirano K, Nishimura A, Kajimoto O, et al. . The effects of a probiotic milk product containing Lactobacillus casei strain Shirota on the defecation frequency and the intestinal microflora of sub-optimal health state volunteers: a randomized placebo-controlled cross-over study. Biosci Microflora 2006;25:39–48. [Google Scholar]
- 73.Salminen S, Salminen E. Lactulose, lactic acid bacteria, intestinal microecology and mucosal protection. Scand J Gastroenterol Suppl 1997;222:45–8. [DOI] [PubMed] [Google Scholar]
- 74.Veiga P, Pons N, Agrawal A, Oozeer R, Guyonnet D, Brazeilles R, Faurie JM, van Hylckama Vlieg JE, Houghton LA, Whorwell PJ, et al. . Changes of the human gut microbiome induced by a fermented milk product. Sci Rep 2014;4:6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu ZM, Xu ZY, Han M, Guo BH. Efficacy of pasteurised yoghurt in improving chronic constipation: a randomised, double-blind, placebo-controlled trial. Int Dairy J 2015;40:1–5. [Google Scholar]
- 76.Riezzo G, Orlando A, D’Attoma B, Guerra V, Valerio F, Lavermicocca P, De Candia S, Russo F. Randomised clinical trial: efficacy of Lactobacillus paracasei-enriched artichokes in the treatment of patients with functional constipation–a double-blind, controlled, crossover study. Aliment Pharmacol Ther 2012;35:441–50. [DOI] [PubMed] [Google Scholar]
- 77.Sakai T, Makino H, Ishikawa E, Oishi K, Kushiro A. Fermented milk containing Lactobacillus casei strain Shirota reduces incidence of hard or lumpy stools in healthy population. Int J Food Sci Nutr 2011;62:423–30. [DOI] [PubMed] [Google Scholar]
- 78.Marsono Y, Illman RJ, Clarke JM, Trimble RP, Topping DL. Plasma lipids and large bowel volatile fatty acids in pigs fed on white rice, brown rice and rice bran. Br J Nutr 1993;70:503–13. [DOI] [PubMed] [Google Scholar]
- 79.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev 2001;81:1031–64. [DOI] [PubMed] [Google Scholar]
- 80.Keely SJ, Scharl MM, Bertelsen LS, Hagey LR, Barrett KE, Hofmann AF. Bile acid-induced secretion in polarized monolayers of T84 colonic epithelial cells: structure-activity relationships. Am J Physiol Gastrointest Liver Physiol 2007;292:G290–7. [DOI] [PubMed] [Google Scholar]
- 81.Snape WJ Jr., Shiff S, Cohen S. Effect of deoxycholic acid on colonic motility in the rabbit. Am J Physiol 1980;238:G321–5. [DOI] [PubMed] [Google Scholar]
- 82.Karlström L, Cassuto J, Jodal M, Lundgren O. Involvement of the enteric nervous system in the intestinal secretion induced by sodium deoxycholate and sodium ricinoleate. Scand J Gastroenterol 1986;21:331–40. [DOI] [PubMed] [Google Scholar]
- 83.Kidd M, Modlin IM, Gustafsson BI, Drozdov I, Hauso O, Pfragner R. Luminal regulation of normal and neoplastic human EC cell serotonin release is mediated by bile salts, amines, tastants, and olfactants. Am J Physiol Gastrointest Liver Physiol 2008;295:G260–72. [DOI] [PubMed] [Google Scholar]
- 84.Alemi F, Poole DP, Chiu J, Schoonjans K, Cattaruzza F, Grider JR, Bunnett NW, Corvera CU. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology 2013;144:145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abrahamsson H, Ostlund-Lindqvist AM, Nilsson R, Simren M, Gillberg PG. Altered bile acid metabolism in patients with constipation-predominant irritable bowel syndrome and functional constipation. Scand J Gastroenterol 2008;43:1483–8. [DOI] [PubMed] [Google Scholar]
- 86.Wong BS, Camilleri M, McKinzie S, Burton D, Graffner H, Zinsmeister AR. Effects of A3309, an ileal bile acid transporter inhibitor, on colonic transit and symptoms in females with functional constipation. Am J Gastroenterol 2011;106:2154–64. [DOI] [PubMed] [Google Scholar]
- 87.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res 2006;47:241–59. [DOI] [PubMed] [Google Scholar]
- 88.Sayin SI, Wahlstrom A, Felin J, Jantti S, Marschall HU, Bamberg K, Angelin B, Hyotylainen T, Oresic M, Backhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab 2013;17:225–35. [DOI] [PubMed] [Google Scholar]
- 89.Matsuo K, Ota H, Akamatsu T, Sugiyama A, Katsuyama T. Histochemistry of the surface mucous gel layer of the human colon. Gut 1997;40:782–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shimotoyodome A, Meguro S, Hase T, Tokimitsu I, Sakata T. Decreased colonic mucus in rats with loperamide-induced constipation. Comp Biochem Physiol A Mol Integr Physiol 2000;126:203–12. [DOI] [PubMed] [Google Scholar]
- 91.Burleigh DE. Evidence for a functional cholinergic deficit in human colonic tissue resected for constipation. J Pharm Pharmacol 1988;40:55–7. [DOI] [PubMed] [Google Scholar]
- 92.Shimotoyodome A, Meguro S, Hase T, Tokimitsu I, Sakata T. Sulfated polysaccharides, but not cellulose, increase colonic mucus in rats with loperamide-induced constipation. Dig Dis Sci 2001;46:1482–9. [DOI] [PubMed] [Google Scholar]
- 93.Caballero-Franco C, Keller K, De Simone C, Chadee K. The VSL#3 probiotic formula induces mucin gene expression and secretion in colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol 2007;292:G315–22. [DOI] [PubMed] [Google Scholar]
- 94.Ouwehand AC, Lagstrom H, Suomalainen T, Salminen S. Effect of probiotics on constipation, fecal azoreductase activity and fecal mucin content in the elderly. Ann Nutr Metab 2002;46:159–62. [DOI] [PubMed] [Google Scholar]
- 95.Collins SM. The immunomodulation of enteric neuromuscular function: implications for motility and inflammatory disorders. Gastroenterology 1996;111:1683–99. [DOI] [PubMed] [Google Scholar]
- 96.Farmer SG, Brown JM, Pollock D. Increased responsiveness of intestinal and vascular smooth muscle to agonists in rats infected with Nippostrongylus brasiliensis. Arch Int Pharmacodyn Ther 1983;263:217–27. [PubMed] [Google Scholar]
- 97.Farmer SG. The effect of betamethasone on altered responsiveness of isolated intestine from rats infected with Nippostrongylus brasiliensis (abstr). Br J Pharmacol 1982;76:192P. [Google Scholar]
- 98.Akiho H, Deng Y, Blennerhassett P, Kanbayashi H, Collins SM. Mechanisms underlying the maintenance of muscle hypercontractility in a model of postinfective gut dysfunction. Gastroenterology 2005;129:131–41. [DOI] [PubMed] [Google Scholar]
- 99.Serna H, Porras M, Vergara P. Mast cell stabilizer ketotifen [4-(1-methyl-4-piperidylidene)-4h-benzo[4,5]cyclohepta[1,2-b]thiophen-10(9H)-one fumarate] prevents mucosal mast cell hyperplasia and intestinal dysmotility in experimental Trichinella spiralis inflammation in the rat. J Pharmacol Exp Ther 2006;319:1104–11. [DOI] [PubMed] [Google Scholar]
- 100.Vergara P, Saavedra Y, Juanola C. Neuroendocrine control of intestinal mucosal mast cells under physiological conditions. Neurogastroenterol Motil 2002;14:35–42. [DOI] [PubMed] [Google Scholar]
- 101.O’Morain C, Bishop AE, McGregor GP, Levi AJ, Bloom SR, Polak JM, Peters TJ. Vasoactive intestinal peptide concentrations and immunocytochemical studies in rectal biopsies from patients with inflammatory bowel disease. Gut 1984;25:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tzavella K, Riepl RL, Klauser AG, Voderholzer WA, Schindlbeck NE, Muller-Lissner SA. Decreased substance P levels in rectal biopsies from patients with slow transit constipation. Eur J Gastroenterol Hepatol 1996;8:1207–11. [DOI] [PubMed] [Google Scholar]
- 103.Dvorak AM, Connell AB, Dickersin GR. Crohn’s disease: a scanning electron microscopic study. Hum Pathol 1979;10:165–77. [DOI] [PubMed] [Google Scholar]
- 104.Guarino MP, Altomare A, Stasi E, Marignani M, Severi C, Alloni R, Dicuonzo G, Morelli L, Coppola R, Cicala M. Effect of acute mucosal exposure to Lactobacillus rhamnosus GG on human colonic smooth muscle cells. J Clin Gastroenterol 2008;42 Suppl 3 Pt 2:S185–90. [DOI] [PubMed] [Google Scholar]
- 105.Isolauri E, Sutas Y, Kankaanpaa P, Arvilommi H, Salminen S. Probiotics: effects on immunity. Am J Clin Nutr 2001;73(2 Suppl)444S–50S. [DOI] [PubMed] [Google Scholar]
- 106.Ibnou-Zekri N, Blum S, Schiffrin EJ, von der Weid T. Divergent patterns of colonization and immune response elicited from two intestinal Lactobacillus strains that display similar properties in vitro. Infect Immun 2003;71:428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Canonici A, Pellegrino E, Siret C, Terciolo C, Czerucka D, Bastonero S, Marvaldi J, Lombardo D, Rigot V, Andre F. Saccharomyces boulardii improves intestinal epithelial cell restitution by inhibiting alphavbeta5 integrin activation state. PLoS One 2012;7:e45047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Arunachalam K, Gill HS, Chandra RK. Enhancement of natural immune function by dietary consumption of Bifidobacterium lactis (HN019). Eur J Clin Nutr 2000;54:263–7. [DOI] [PubMed] [Google Scholar]
- 109.Johnson N, Thomas L, Jordan D. Probiotics: assessing health professionals’ knowledge and understanding. Gastrointestinal Nursing. 2016;14:26–33. [Google Scholar]