Abstract
Fructose-containing added sugars, such as sucrose and high-fructose corn syrup, have been experimentally, epidemiologically, and clinically shown to be involved in the current epidemics of obesity and diabetes. Here we track this history of intake of sugar as it relates to these epidemics. Key experimental studies that have identified mechanisms by which fructose causes obesity and diabetes are reviewed, as well as the evidence that the uricase mutation that occurred in the mid-Miocene in ancestral humans acted as a “thrifty gene” that increases our susceptibility for fructose-associated obesity today. We briefly review recent evidence that obesity can also be induced by nondietary sources of fructose, such as from the metabolism of glucose (from high-glycemic carbohydrates) through the polyol pathway. These studies suggest that fructose-induced obesity is driven by engagement of a “fat switch” and provide novel insights into new approaches for the prevention and treatment of these important diseases.
Keywords: fructose, sucrose, uric acid, obesity, diabetes, thrifty gene, added sugar, metabolic syndrome
Introduction
Obesity and metabolic syndrome are complex conditions that involve many factors, including diet, exercise, genetics, and environment. Nevertheless, the evidence that the intake of sugary beverages containing sugar (sucrose) or high-fructose corn syrup (HFCS) has a role in metabolic syndrome is now well established (1–3). Indeed, experimental evidence (4–7), epidemiological studies (1–3, 8), and clinical studies (9–13) have provided convincing evidence that sugary beverages increase the risk not only for obesity but also for features of metabolic syndrome, including elevated blood pressure, insulin resistance, fatty liver, and dyslipidemia.
Experimental evidence suggests the key component of sugar and HFCS responsible for the predisposition for metabolic syndrome is fructose. Fructose intake appears to drive excessive food intake by inducing leptin resistance (14, 15) and by stimulating neural and hedonic responses in the brain (16–18). Even when excessive caloric intake is controlled, fructose has been shown in experimental models to have metabolic effects independent of weight gain, including the ability to induce fatty liver, insulin resistance, and elevated blood pressure (6, 19). These mechanisms are not mediated by the caloric effects of fructose but rather by the ability of fructose to induce a decrease in intracellular ATP levels and adenine nt turnover (4, 20–25). This biochemical pathway is associated with mitochondrial oxidative stress that leads to increased lipogenesis as well as a block in FA oxidation; it also stimulates gluconeogenesis (20–24, 26, 27). Blood pressure rises because of an inhibition of endothelial NO and activation of the renin angiotensin system, driven by the intracellular uric acid generated during fructose metabolism (6, 28). A summary of these biochemical pathways has been previously published (29).
Benner et al. (30) argued that insights into causation can be enhanced if one incorporates other fields besides classical epidemiology, genetics, and physiology. One area that is often ignored is the evolutionary biology, anthropology, and historical record as it relates to a disease process. In this regard, obesity and diabetes were exceptionally rare in the 1800s but have emerged as major health conditions of the 20th and 21st centuries. We contend that much can be learned when a disease emerges, because when it is rare it is easier to identify associated risk factors. One can also obtain insights into how current scientific thought about the etiology of the disease process developed over time. Given the knowledge of today, one can identify where assumptions may have been made in the past that may have led the field astray. Given these considerations, in this article we review the emergence of the obesity and diabetes epidemic beginning in the early 20th century and discuss its relation with the intake of fructose-laden sugars.
A Historical Perspective: The Quiet before the Storm
On 1 May 1893, a record-breaking world’s fair opened in Chicago (IL) with 46 countries represented. Celebrating the 400th anniversary of Columbus’s discovery of America, the World’s Columbian Exposition boasted full-size replicas of the Niña, the Pinta, and the Santa Maria, the ships Columbus sailed when he discovered America. However, although there was commemoration of the past, as exemplified by the presence of Buffalo Bill Cody’s Wild West Show adjacent to the fair, the 27 million visitors that summer came to witness the new innovation and technology that would transform the future. The first American automobile company opened that year using the new gasoline powered engine. The night lit up with electric lights provided by Westinghouse. Telephone service had just been established between Chicago and New York, nearly 1000 miles away. Phonograph parlors could now be visited within the city, and the first commercial disk record was just released. The fair introduced the moving walkway (so one did not have to walk) and the Ferris Wheel (so you could see the whole world without leaving your seat) as well as caramel- and sugar-coated popcorn, juicy fruit gum, and Pabst beer. And a young doctor by the name of Arthur Conan Doyle won the heart of the public with his novel, The Adventures of Sherlock Holmes, that chronicled a brilliant detective who could outfox Scotland Yard.
Back at Johns Hopkins, another physician, Sir William Osler, was publishing the first edition of his classic textbook, The Principles and Practice of Medicine (31). Here there was also much to celebrate. The tubercle bacillus had been discovered by Robert Koch only 10 y before, and Louis Pasteur had introduced a rabies vaccine in addition to the small pox vaccine already available. Even more exciting was the recent introduction of antisera for the treatment of tetanus and diphtheria by Emil von Behring, who would receive the first Nobel Prize in Medicine (or Physiology) for this discovery. It seemed like the diseases that had been the scourge of mankind, such as tuberculosis, pneumonia, and puerperal sepsis, would soon be eliminated, and a golden age in human health would begin.
Little did the world know what diseases the future would bring, yet within the 1130-page book Osler wrote (31), the diseases that would take over the 20th century were just being recognized. Obesity (corpulence) affected only 1 in 30 adults (32) and was given 3 pages, and high blood pressure (elevated pulse tension) still could not be measured easily but would be reported 14 y later to affect <1% of the adult population aged <65 y (33). Diabetes, which had recently been shown to consist of a lean (type 1) or obese (type 2) phenotype (34), affected just 2 individuals per 100,000 population but was of sufficient interest to receive 10 pages (31). Angina, a type of strangling chest pain, received 4 pages because it was only just being recognized as a symptom of heart disease and would have to wait an additional 20 y before it was shown to be caused by disease of the coronary arteries (35).
The Beginning of the Epidemics: Sugar Intake or Overnutrition?
In 1907 Sir Richard Havelock Charles, a British physician stationed in India, made the alarming observation that type 2 diabetes was increasing rapidly among the wealthy Bengal Indians living in Calcutta, whereas it was still rare among the poor Punjabi, and he linked this with an increasing intake of sugar (sucrose) (36). At a symposium held that year, other physicians from other tropical countries made similar observations (37–40). New York City Public Health Commissioner Haven Emerson also became concerned with the 10-fold rise in diabetes that had occurred in New York City that then afflicted 1 in 10,000 individuals, and in one of the finest epidemiological studies Emerson and Larimore (41) found a strong linkage of refined sugar intake with diabetes. Other world experts, including Nobel laureate Sir Frederick Banting, also suggested that refined sugar may be a major cause of adult-onset diabetes (42).
Although sugar intake was the original risk factor identified for obesity, this was challenged by Elliott Joslin, who suggested a simpler explanation. Joslin coined the word, “overnutrition,” to suggest that the cause of both obesity and diabetes was simply the fact that as food became more plentiful, that it was easier to overeat, and likewise, that the introduction of elevators and cars had made it easier to avoid exercise (43, 44). Thus, the simple law of thermodynamics could explain the problem. Too much food in, too little exercise out, and the excess energy has to be stored as fat. Indeed, because fat contained 9 kcal/g compared with 4 kcal/g for sugar, fat was likely the primary source, and after all, diseased atherosclerotic vessels were filled with fat and cholesterol. This simple concept became the central philosophy for what would drive dietary and clinical thinking about the etiology of the epidemics of obesity, diabetes, and heart disease for the next 75 y. However, if obesity simply was the result of the personal choice to eat more and exercise less, as Joslin implied, then why was it so hard for obese subjects to maintain weight loss? It seemed like something was missing in our understanding of obesity.
Insights into the Mechanisms Driving Obesity and Diabetes
It is well known that most animals, including hibernating mammals and long-distance migratory birds, regulate their weight throughout the year and that transient manipulations to increase or reduce weight are followed by rapid correction to the desired weight (45–47). Similarly, studies in humans have also shown that overfeeding or underfeeding results in compensatory changes in food intake and energy metabolism that tend to correct subjects back to their baseline weight (48–50).
Several biological mechanisms that regulate weight have subsequently been identified (51, 52). One of the major mechanisms is by the release of leptin from adipose tissue after food ingestion, which induces satiety by acting on hypothalamic centers in the brain (51). When leptin is inactivated by mutation, animals do not regulate their food intake, and massive obesity results. This led to the idea that obesity might be related to leptin deficiency. However, instead of low leptin concentrations, obese subjects usually have high concentrations because the hypothalamic centers are resistant to the effects of leptin, thus resulting in “leptin resistance” and the ineffective stimulation of the satiety response by leptin (53).
In addition to hormonal mechanisms, some foods, especially sugar, can induce pleasure responses in the brain by stimulating dopamine in the nucleus accumbens and other sites of the midbrain (54). Repeated stimulation of dopamine by sugar in mice results in a downregulation of the dopamine receptors (especially dopamine 1 and dopamine 2) that similarly occurs in animals that are addicted to cocaine or opiates, and signs of withdrawal can be elicited on removal of the sugar (54–56). Volkow et al. (57) reported that subjects with obesity also show a downregulation of dopamine 2 receptors in their midbrain based on neural imaging, suggesting that obesity can also be associated with an addictive response to food(s).
Thus, obesity is associated with leptin resistance that would result in an impaired satiety response and with reduced dopamine receptors in the nucleus accumbens that are associated with an impaired control related to food intake (57, 58). Other mechanisms regulating food intake have also been identified that appear altered in obesity, such as alterations in ghrelin and glucagon-like peptide-1 (59).
Obesity is also associated with being sedentary, and as an example the risk for obesity increases with the amount of time one spends watching television (60, 61). However, it remains unclear if it is the television that is leading to the sedentary behavior or whether subjects with obesity are simply more tired and hence reduce their physical activity. Consistent with the latter are reports that fatigue is more common in obese children than lean children and that fatigue is increased in obese adults independent of sleep apnea, especially after the age of 40 (62–64). Obese women also show less exercise capacity than lean women, fatigue earlier, walk slower, and achieve only half of the maximum oxygen consumption (65–67). Indeed, a study that randomly assigned children, who were watching television a mean of 28 h/wk, to half of their normal viewing time failed to show any increase in physical activity with the reduction in viewing time (68).
One potential mechanism for causing fatigue may relate to mitochondrial function and/or ATP concentrations. Certain foods, such as fructose, are known to reduce ATP concentrations in the liver, even after oral ingestion of an amount of fructose equivalent to being in a soft drink (69, 70). Fructose intake also induces mitochondrial oxidative stress that inactivates enoyl coenzyme A hydratase and leads to an impairment in fat oxidation that may lead to a reduction in hepatic ATP concentrations (20). Obesity itself is also associated with impaired FA oxidation not only in the liver but in muscle and fat tissues (71–73). In turn, when ATP concentrations are reduced in the liver by blocking FA oxidation, hunger is stimulated (74–76). The increased energy intake may help restore ATP concentrations but at the expense of a greater accumulation of fat (77). In contrast, high-fat foods increase substrate delivery to mitochondria, resulting in mitochondrial oxidative stress and normal or high ATP concentrations, at least initially (78).
Although not all studies show a reduction in ATP concentrations in subjects with obesity, there are several reports that hepatic ATP concentrations correlate inversely with BMI (in kg/m2) and are lower in subjects with nonalcoholic fatty liver disease, especially in those with a history of a high fructose intake (79, 80). ATP concentrations also tend to be lower in the muscle of obese subjects (81, 82) and are lower in the brain of obese subjects than in the brain of lean subjects (82). Mitochondrial dysfunction and oxidative stress have also been reported to be present in obese subjects (83).
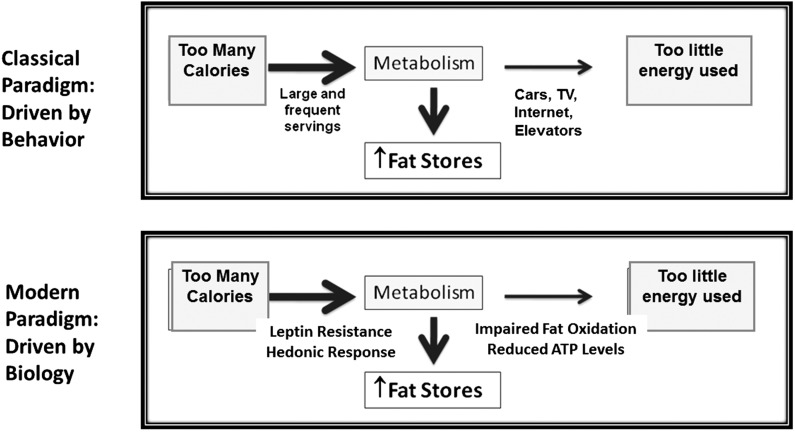
These data are consistent with the concept that obesity is driven by an increased food intake and sedentary behavior as originally proposed by Joslin. However, the emerging data are that increased energy intake and sedentary behavior may not simply reflect the consequence of choice, but that there are hormonal and hedonic pathways driving food intake, as well as alterations in mitochondrial function that may adversely affect energy production. Of course, culture remains important, and advertisement agencies know how to encourage individuals to be sedentary by providing attractive television shows and the internet and by encouraging the intake of processed foods, high in calories and low in nutrients (84). Yet the basis of obesity likely involves an underlying biology (Figure 1).
FIGURE 1.
Obesity and the law of thermodynamics. Elliott Joslin proposed that obesity and diabetes resulted from overnutrition and a reduction in exercise. The concept was simple: we are taking in more calories than we expend, and the rest is stored in fat. The theory was transformed into the idea that the increased ingestion of calories and the reduction in exercise were the result of behavior and a lack of will power. However, over time it became apparent that the process of both gaining and losing weight involves a lot of metabolic adaptations. The modern paradigm is based on the fact that there are multiple mechanisms driving weight gain, of which one of the more important is increased food intake because of the inability to suppress satiety responses as a result of leptin resistance and because certain foods induce a pleasure (hedonic) response associated with dopamine release. Likewise, most obese subjects show defects in mitochondrial function with an impairment in fat oxidation and reduced ATP concentrations that correlate with fatigue. Although cultural influences are still important, the basis of obesity relates to biological changes that result in fat storage and weight gain. TV, television.
Indeed, the process of storing fat is also associated with features of the metabolic syndrome. For example, fatty liver, insulin resistance, and increased adiposity are observed in many animals that have activated the switch to store fat, including long-distance migrating birds in the weeks before their flight or hibernating mammals in the late autumn as they prepare for their winter sleep (45, 85). This is why we have suggested that the metabolic syndrome not be viewed as a pathophysiological condition but rather as fat storage syndrome (86).
Revisiting Fructose as a Driver of Fat Storage Syndrome
As discussed earlier, sugar was discarded as a cause of obesity and diabetes in the 1930s, and although the nutritional content was acknowledged to be poor (for which it was described as an “empty calorie”), it was viewed as a risk factor solely because of its caloric content. However, in the last 15 y fructose or sugar has been shown to induce leptin resistance and hedonic responses in animals, to block FA oxidation, and to reduce energy output, leading to increasing fat stores (14, 20, 21, 55). Studies in humans have also shown that fructose increases energy intake, reduces insulin sensitivity, increases circulating TGs and visceral fat stores, reduces fat oxidation, and reduces energy metabolism compared with other foods such as glucose or starch (11, 87, 88). Thus, fructose activates a process that leads to fat storage, and indeed fructose (or sugar) intake is strongly linked with the development of obesity, diabetes, fatty liver, and heart disease (2, 3, 89, 90).
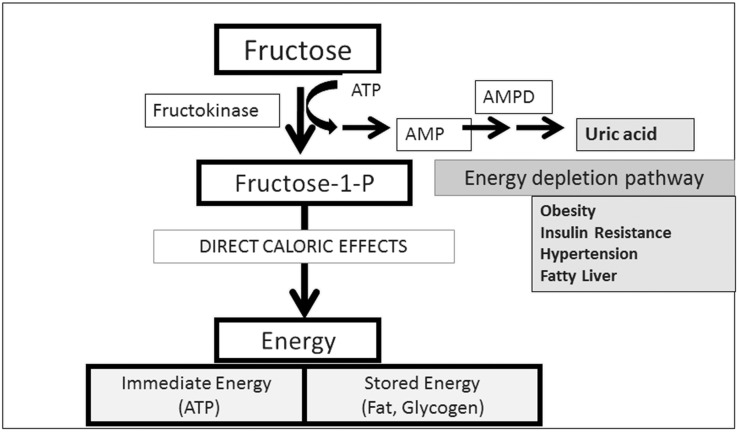
The reason fructose is distinct from other foods in its ability to cause fat storage was shown to be a unique enzyme (fructokinase C) in fructose metabolism that phosphorylates fructose so rapidly that intracellular phosphate and ATP depletion result, leading to activation of AMP deaminase and the stepwise degradation of AMP to uric acid (Figure 2) (4). The activation of AMP deaminase generates uric acid and mitochondrial oxidative stress that result in increased lipogenesis (from reduced aconitase and increased activation of ATP citrate lyase), reduced FA oxidation (from the reduction of enoyl coenzyme A hydratase), an inhibition of AMP-activated protein kinase, and a reduction in ATP generation (20–22). This same process also results in local oxidative stress and inflammation via activation of NAD(P)H oxidase, nuclear factor-κB, and other cellular signaling systems (21, 92).
FIGURE 2.
The unique metabolism of fructose. Fructose is the only nutrient that reduces energy in liver cells before generating energy. The mechanism is caused by the rapid phosphorylation of fructose by fructokinase C that leads to intracellular phosphate depletion, the activation of AMPD, and the stepwise breakdown of AMP to uric acid. This side-chain reaction leads to intracellular inflammation in and oxidative stress to the mitochondria, resulting in an impairment of FA oxidation and reduction in ATP. There is also inhibition of AMP-activated protein kinase and a stimulation of hepatic gluconeogenesis. Studies of this side chain suggest it is responsible for activating a “fat switch” that leads to fat storage, insulin resistance, and metabolic syndrome (20, 21, 91). AMPD, AMP deaminase.
Although the effects of fructose on leptin and hedonic responses encourage increased food intake, the mitochondrial effects of fructose are likely the reason fructose can also induce features of metabolic syndrome compared with other nutrients when total caloric intake is identical (6, 7, 93). In experimental studies in rats and mice, one cannot show a difference in weight gain because the duration of the study is usually too short for the subtle differences in resting metabolism to manifest. However, differences in body composition are relatively easier to show. We have found, for example, that fructose (or sugar) will induce fatty liver and insulin resistance (or diabetes) even in calorically restricted rats (7). Thus, the metabolic effects tend to reduce ATP generation while shifting the incoming energy to fat or glycogen stores.
Uric Acid, the Thrifty Gene, and the Predisposition of Humans to Fructose-Containing Sugars
As mentioned, uric acid is produced during the side-chain noncaloric reaction in fructose metabolism (Figure 2), and fructose intake also stimulates de novo uric acid synthesis from amino acid precursors (94, 95). Although the role of uric acid in obesity and metabolic disease remains controversial, there is experimental evidence that uric acid may have a role in mediating the mitochondrial oxidative stress induced by fructose, and that it may have a contributory role by which fructose induces metabolic syndrome (21). Soluble uric acid, for example, can induce mitochondrial oxidative stress, reduce ATP production in endothelial cells, and stimulate lipogenesis and block fat oxidation in liver (HepG2) cells (20, 21, 27). Lowering uric acid can also improve metabolic syndrome in both fructose-dependent and -independent animal models (6, 96), and some studies suggest similar improvements in blood pressure and insulin resistance in humans (97–99).
It is of interest that humans have higher concentrations of serum uric acid than almost all other mammals because of a mutation that occurred in the mid–Miocene Epoch (100, 101). The mutation occurred in the gene uricase, which codes for an enzyme that breaks down uric acid. Studies have shown that there was a stepwise series of mutations that progressively reduced the activity of the enzyme until it was completely knocked out ∼15 million years ago in an ancestor of both humans and great apes (100). A similar mutation knocked out uricase in ancestors of the lesser apes during the same period, which suggests a natural selection advantage to having higher uric acid levels (101).
The Miocene corresponds to a period of time in which the first apes populated Africa. These tree-living apes lived primarily on fruits that were present in the forests throughout the year. Initially, the apes were very successful (102), and indeed, many apes migrated into Eurasia ∼17 million years ago when global cooling resulted in a land bridge between Eurasia and Africa as sea levels fell (103). Unfortunately, as global cooling continued the Eurasian apes began to suffer seasonal starvation during the cooler months when fruits were less available, and during this time these apes had to forage for other fallback foods, such as tubers and roots, forcing them to walk on the ground and to change their dentition to eat harder foods, such as nuts (102, 103). Although the apes eventually died out in Europe, the fossil record strongly suggests that some of these European apes returned to Africa and are the common ancestor to humans and great apes (102–105).
In collaboration with Peter Andrews at the Museum of Natural History in London, we have postulated that the uricase mutation may have acted to enhance the effect of fructose to increase fat stores and thereby may have provided a survival advantage (106, 107), similar to the thrifty gene as proposed by James Neel >50 y ago (108). Indeed, blocking uricase in rats increases their sensitivity to the metabolic effects of fructose. One mechanism may be a positive feedback by which uric acid upregulates fructokinase (22). To further address this pathway, we collaborated with Eric Gaucher who resurrected the ancestral uricase from early hominoids (100). When this uricase was expressed in human liver (HepG2) cells, we could show a blunted effect of fructose both on lipogenesis and gluconeogenesis, thereby strongly suggesting that the loss of uricase was a mechanism that could enhance the ability of fructose to generate fat (91, 100). Of note, other benefits of the uricase mutation have also been proposed and are not mutually exclusive, including effects on reaction time and foraging or effects to block systemic oxidative stress (109, 110).
The Rise of Sugar Intake Parallels the Rise in Obesity and Diabetes
Early humans were hunters and gatherers who could not uncommonly face periods of food shortage. Adequate fat stores not only allowed survival through these difficult periods but were particularly critical for successful pregnancy (111) and have been proposed as the reason why the fertility (Venus) figurines of the Neolithic Period commonly depict women who were obese. However, obesity remained relatively rare, although there is indirect evidence that the production of honey after the introduction of apiaries in the Egyptian Old Kingdom may have led to a rise in obesity and caries among the noblemen and royalty of that period (112).
This all changed with the discovery of sugarcane in the Ganges River Valley in ∼400 BC. The great physician Sushruta was the first to link obesity and diabetes with the intake of sugary liquids (113). Subsequently sugarcane was brought to China (100 AD), Persia (500 AD), and Egypt (600 AD) where it was grown. Maimonides, a physician from the 12th century, spent much of his life in Spain where he noted diabetes was absent (and sugar had not yet been introduced), whereas he found >20 cases after he moved to Egypt (where sugar had entered the diet) (114). Sugar was then taken to Venice, where it was so expensive that 1 pound (0.5 kg) was worth the equivalent of 28 pounds (12.7 kg) of cheese (115, 116). As such, it was the kings and royalty who could afford sugar, and many kings became severely obese, including William the Conqueror, who was accused of being pregnant because of his obesity (112).
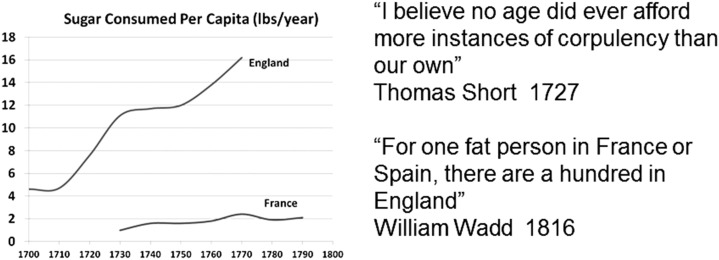
Sugar remained expensive until the plantations in the Americas began mass producing sugar. England initially horded the sugar, resulting in a dramatic rise in sugar intake compared with other countries, such as France (Figure 3) (112, 117). Perhaps not surprisingly, obesity, diabetes, hypertension, and cardiovascular disease emerged first in England (118–120). Not far behind was Holland, for the Dutch East Indies Company would bring sugar in from Java, which was linked by Stephen Blankaart and others to the rise of obesity there as well (121–123).
FIGURE 3.
Sugar consumption increases in England. England initially controlled most of the importation of sugar from the Americas, and most of the sugar was consumed in England compared with other European countries, such as France. Shown is the amount of sugar consumed per capita compared with England during the 18th century. Data are originally from Austen and Smith (117), and the quotes are by authors who wrote about the rise in Obesity in England (118, 119). Reproduced from reference 112 with permission.
Sugar production continued, fueled not only by the sugarcane plantations, but by the discovery that sugar beets were also an excellent source of sucrose. Based on disappearance data, the average per capita sugar intake in the United States and England rose from 4 pounds (1.8 kg)/y in 1700 to >150 pounds (68.2 kg)/y in 2000 (124, 125). An inflection point at ∼1975 led to an even greater rise in overall intake of fructose-containing sugars because of the introduction of HFCS (126). HFCS not only was less expensive but was liquid and could be added to a wide variety of foods to enhance its taste.
One of the most important sources of sugar was liquid sugar provided in soft drinks. Soft drink intake increased markedly over the last century and by the year 2000 accounted for ∼9% of overall energy intake in the average American (127, 128). Given that the obesogenic effects of fructose are mediated by intracellular energy depletion, which is a function of the fructose concentration a cell is exposed to, it is readily evident why liquid sugary beverages confer much greater risk than solid foods containing sugar.
Decreasing Intakes of Sugar and HFCS is Leading to a Stabilization of the Obesity and Diabetes Epidemics
During the last decade, there has been a remarkable spreading of knowledge on the role of sugar and HFCS in driving obesity and diabetes (2, 87, 129–132), and this has led to a wide variety of measures to reduce sugar intake. Guidelines established by the AHA have proposed a maximum intake of 37.5 g (∼9 teaspoons) of sugar for men and 25 g (∼6 teaspoons) for women (133). Community-based education programs, such as the ShFat That initiative in Colorado, have not only educated children but led to public health initiatives and state proclamations to focus on children’s health (http://www.shfatthat.com/). Some elementary and middle schools, such as in California, eliminated the sale of sugary beverages from their premises and were able to show a reduction in obesity (134). Taxes on sugary beverages have also been initiated in several countries, including Mexico and Great Britain, as well 18 states in the United States (135).
These measures have also been associated with a leveling and then a steady decrease in the intake of sugary soft drinks. According to some estimates, the intake of dietary fructose and sugars peaked in 1999 in the United States, with data based on sugar dispersed for sales giving a per capita intake of 158 pounds (71.8 kg)/y (136). This overestimates true sugar intake, as it measures the amount of sugar that went to the markets and disappeared from the shelves. Although consumption of carbonated soft drinks was increasing at a rate of ∼3%/y until 1999, the increase slowed, and since 2005 it has been progressively decreasing by 0.5% in 2010, 1% in 2011, 1.2% in 2012, and 3% in 2013 (137, 138). Not surprisingly, the obesity epidemic in the United States has begun to plateau (139, 140), although it is still increasing in adolescent males (141) in whom sugar intake remains extremely high. The prevalence of type 2 diabetes has also plateaued (142). Nevertheless, intake of sugar/HFCS is still very high compared with earlier in the twentieth century, and much more needs to be done.
Limitations and Other Considerations
Challenges to the fructose hypothesis
Not all studies have concluded that the intake of sugary beverages or high-sugar diets increase the risk of obesity. For example, 2 meta-analyses concluded that sugar (or fructose) does not induce weight gain in isocaloric trials in which sugar is exchanged for a nonsugar caloric source (143, 144). However, as discussed earlier, fructose has been shown to induce weight gain by stimulating energy intake, so one would not expect to see differences in weight in an isocaloric study (145). There are also reviews that suggest that the current intake of sugar does not increase the risk of obesity or metabolic syndrome (146). However, the degree of sugar intake varies markedly in the country with some high-risk groups, such as adolescents, young adults, and ethnic groups (Native Americans, African Americans, and Hispanic Americans), having particularly high intakes (3, 147–149). These observations, coupled with the strong epidemiology linking sugar intake with obesity and metabolic syndrome (1), as well as clinical data showing a direct relation of fructose intake with ATP depletion (69, 70), a rise in serum uric acid (88, 150), and the development of fatty liver (12), strongly argue that the current intake is high, and it explains why the AHA (133) and WHO (151) have argued for a reduction in sugar intake.
Other mechanisms driving obesity
Although sugar (fructose) intake appears to have a major role in causing obesity and metabolic syndrome, there are other important factors that regulate weight, such as genetics (152), epigenetics and fetal programming (153), and the host microbiome (154–156). High-glycemic carbohydrates (157), trans-fats (158), ω-6 FAs, high-fat diets (159), and a high salt intake (160, 161) also increase the risk of obesity or metabolic syndrome, whereas ω-3 FAs and dairy fats may be protective (162, 163).
Fructose may also have a role in obesity in response to certain micronutrients or foods that do not contain fructose but can induce endogenous fructose production. For example, we have found that high-glycemic diets can induce fructose production in the liver because of the stimulation of aldose reductase and that the endogenous fructose is responsible for how high-glycemic diets induce fatty liver and insulin resistance and is partly responsibility for the development of obesity (164). Likewise, we have unpublished data that a high-salt diet can induce obesity and metabolic syndrome by increasing serum osmolarity and inducing aldose reductase and endogenous fructose production in the liver (MA Lanaspa, A Andres-Hernando, M Kuwabara, N Li, C Cicerchi, T Jensen, DJ Orlicky, C Roncal-Jimenez, T Ishimoto, T Nakagawa, et al., unpublished results, 2017). Other foods, such as beer, contain purines that may by-pass fructose to enter the AMP deaminase side-chain pathway that results in metabolic syndrome and may provide a mechanism linking umami-based foods with increased diabetic risk (165, 166). Thus, obesity is not simply driven by sugar but may involve mechanisms mediated by endogenously produced fructose and uric acid, as well as other nonfructose pathways.
Role of natural fruit
Our studies suggest natural fruits may also increase the risk of obesity and diabetes. Indeed, it is known that some animals, including orangutans, bears, migrating birds, and the Pacu fish will ingest large amounts of fructose-containing fruit as a means of increasing their fat stores (45, 167–169). However, a single, natural fruit is usually limited in fructose content (4–8 g) and contains fiber that may slow absorption and a variety of antioxidants and flavonols that may counter the effects of fructose. Indeed, a low-fructose diet that included fruits provided greater benefits than a low-fructose diet without fruits (170). Having said this, the intake of fruit juice, which contains high concentrations of fructose, should be limited according to the American Academy for Pediatrics because fruit juice is associated with an increased risk of obesity in children (171).
Importance of exercise
Finally, although fructose-containing sugars provide a great mechanism for stimulating the storage of fat and features of fat storage syndrome, the loss of weight requires not only caloric restriction but exercise. With obesity there is a progressive loss of mitochondria (83), and stimulating mitochondrial biogenesis is likely key to being able to reset to a lower weight. Exercise remains one of the best ways to stimulate mitochondrial biogenesis (172) and is likely key to long-term success for any weight-loss program.
In conclusion, the year 1893 was the starting point for major epidemics of obesity and diabetes. Although multiple factors likely play a role, we suggest that one contributor is the excessive intake of sugar (and HFCS). With education, the better labeling of foods, and taxes, measures to reduce the consumption of sugar and HFCS should be possible and that should not only arrest but eventually help reverse this epidemic that has been costly to human health.
Acknowledgments
The authors’ responsibilities were as follows—RJJ: wrote the first draft of the manuscript; MAL, PA, and LGS-L: edited the manuscript; and all authors: read and approved the final manuscript.
References
- 1.Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr 2013;98:1084–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu FB, Malik VS. Sugar-sweetened beverages and risk of obesity and type 2 diabetes: epidemiologic evidence. Physiol Behav 2010;100:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Q, Zhang Z, Gregg EW, Flanders WD, Merritt R, Hu FB. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern Med 2014;174:516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishimoto T, Lanaspa MA, Le MT, Garcia GE, Diggle CP, Maclean PS, Jackman MR, Asipu A, Roncal-Jimenez CA, Kosugi T, et al. Opposing effects of fructokinase C and A isoforms on fructose-induced metabolic syndrome in mice. Proc Natl Acad Sci USA 2012;109:4320–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishimoto T, Lanaspa MA, Rivard CJ, Roncal-Jimenez CA, Orlicky DJ, Cicerchi C, McMahan RH, Abdelmalek MF, Rosen HR, Jackman MR, et al. High-fat and high-sucrose (western) diet induces steatohepatitis that is dependent on fructokinase. Hepatology 2013;58:1632–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, Ouyang X, Feig DI, Block ER, Herrera-Acosta J, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol 2006;290:F625–31. [DOI] [PubMed] [Google Scholar]
- 7.Roncal-Jimenez CA, Lanaspa MA, Rivard CJ, Nakagawa T, Sanchez-Lozada LG, Jalal D, Andres-Hernando A, Tanabe K, Madero M, Li N, et al. Sucrose induces fatty liver and pancreatic inflammation in male breeder rats independent of excess energy intake. Metabolism 2011;60:1259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jalal DI, Smits G, Johnson RJ, Chonchol M. Increased fructose associates with elevated blood pressure. J Am Soc Nephrol 2010;21:1543–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stanhope KL, Bremer AA, Medici V, Nakajima K, Ito Y, Nakano T, Chen G, Fong TH, Lee V, Menorca RI, et al. Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-B in young men and women. J Clin Endocrinol Metab 2011;96:E1596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanhope KL, Griffen SC, Keim NL, Ai M, Otokozawa S, Nakajima K, Schaefer E, Havel PJ. Consumption of fructose-, but not glucose-sweetened beverages produces an atherogenic lipid profile in overweight/obese men and women. Diabetes 2007;56(Suppl 1):A16 (abstr). [Google Scholar]
- 11.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 2009;119:1322–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maersk M, Belza A, Stodkilde-Jorgensen H, Ringgaard S, Chabanova E, Thomsen H, Pedersen SB, Astrup A, Richelsen B. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr 2012;95:283–9. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Pozo SE, Schold J, Nakagawa T, Sanchez-Lozada LG, Johnson RJ, Lillo JL. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: role of uric acid in the hypertensive response. Int J Obes (Lond) 2010;34:454–61. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro A, Mu W, Roncal C, Cheng KY, Johnson RJ, Scarpace PJ. Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. Am J Physiol Regul Integr Comp Physiol 2008;295:R1370–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shapiro A, Tumer N, Gao Y, Cheng KY, Scarpace PJ. Prevention and reversal of diet-induced leptin resistance with a sugar-free diet despite high fat content. Br J Nutr 2011;106:390–7. [DOI] [PubMed] [Google Scholar]
- 16.Bernal SY, Dostova I, Kest A, Abayev Y, Kandova E, Touzani K, Sclafani A, Bodnar RJ. Role of dopamine D1 and D2 receptors in the nucleus accumbens shell on the acquisition and expression of fructose-conditioned flavor-flavor preferences in rats. Behav Brain Res 2008;190:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol 2004;286:R31–7. [DOI] [PubMed] [Google Scholar]
- 18.Lane MD, Cha SH. Effect of glucose and fructose on food intake via malonyl-CoA signaling in the brain. Biochem Biophys Res Commun 2009;382:1–5. [DOI] [PubMed] [Google Scholar]
- 19.Reungjui S, Roncal CA, Mu W, Srinivas TR, Sirivongs D, Johnson RJ, Nakagawa T. Thiazide diuretics exacerbate fructose-induced metabolic syndrome. J Am Soc Nephrol 2007;18:2724–31. [DOI] [PubMed] [Google Scholar]
- 20.Lanaspa MA, Cicerchi C, Garcia G, Li N, Roncal-Jimenez CA, Rivard CJ, Hunter B, Andres-Hernando A, Ishimoto T, Sanchez-Lozada LG, et al. Counteracting roles of AMP deaminase and AMP kinase in the development of fatty liver. PLoS One 2012;7:e48801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanaspa MA, Sanchez-Lozada LG, Choi YJ, Cicerchi C, Kanbay M, Roncal-Jimenez CA, Ishimoto T, Li N, Marek G, Duranay M, et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J Biol Chem 2012;287:40732–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanaspa MA, Sanchez-Lozada LG, Cicerchi C, Li N, Roncal-Jimenez CA, Ishimoto T, Le M, Garcia GE, Thomas JB, Rivard CJ, et al. Uric acid stimulates fructokinase and accelerates fructose metabolism in the development of fatty liver. PLoS One 2012;7:e47948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi YJ, Shin HS, Choi HS, Park JW, Jo I, Oh ES, Lee KY, Lee BH, Johnson RJ, Kang DH. Uric acid induces fat accumulation via generation of endoplasmic reticulum stress and SREBP-1c activation in hepatocytes. Lab Invest 2014;94:1114–25. [DOI] [PubMed] [Google Scholar]
- 24.Choi YJ, Yoon Y, Lee KY, Hien TT, Kang KW, Kim KC, Lee J, Lee MY, Lee SM, Kang DH, et al. Uric acid induces endothelial dysfunction by vascular insulin resistance associated with the impairment of nitric oxide synthesis. FASEB J 2014;28:3197–204. [DOI] [PubMed] [Google Scholar]
- 25.Sánchez-Lozada LG, Tapia E, Bautista-Garcia P, Soto V, Avila-Casado C, Vega-Campos IP, Nakagawa T, Zhao L, Franco M, Johnson RJ. Effects of febuxostat on metabolic and renal alterations in rats with fructose-induced metabolic syndrome. Am J Physiol Renal Physiol 2008;294:F710–8. [DOI] [PubMed] [Google Scholar]
- 26.Lanaspa MA, Epperson LE, Li N, Cicerchi C, Garcia GE, Roncal-Jimenez CA, Trostel J, Jain S, Mant CT, Rivard CJ, et al. Opposing activity changes in AMP deaminase and AMP-activated protein kinase in the hibernating ground squirrel. PLoS One 2015;10:e0123509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez-Lozada LG, Lanaspa MA, Cristobal-Garcia M, Garcia-Arroyo F, Soto V, Cruz-Robles D, Nakagawa T, Yu MA, Kang DH, Johnson RJ. Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron Exp Nephrol 2012;121:e71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, Krotova K, Block ER, Prabhakar S, Johnson RJ. Hyperuricemia induces endothelial dysfunction. Kidney Int 2005;67:1739–42. [DOI] [PubMed] [Google Scholar]
- 29.Johnson RJ, Nakagawa T, Sanchez-Lozada LG, Shafiu M, Sundaram S, Le M, Ishimoto T, Sautin YY, Lanaspa MA. Sugar, uric Acid, and the etiology of diabetes and obesity. Diabetes 2013;62:3307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benner SA, Caraco MD, Thomson JM, Gaucher EA. Planetary biology–paleontological, geological, and molecular histories of life. Science 2002;296:864–8. [DOI] [PubMed] [Google Scholar]
- 31.Osler W. The principles and practice of medicine. New York: D. Appleton and Co.; 1893. [Google Scholar]
- 32.Helmchen LA, Henderson RM. Changes in the distribution of body mass index of white US men, 1890–2000. Ann Hum Biol 2004;31:174–81. [DOI] [PubMed] [Google Scholar]
- 33.Janeway TC. The clinical study of blood pressure. New York: D. Appleton and Company; 1907. [Google Scholar]
- 34.Lancereaux E. Le Diabete maigre et le Diabete gras. [Lean diabetes and diabetes.] L’ Union Mid 1880;29:161–7. [Google Scholar]
- 35.Herrick JB. Landmark article (JAMA 1912). Clinical features of sudden obstruction of the coronary arteries. JAMA 1983;250:1757–65. [PubMed] [Google Scholar]
- 36.Charles R. Diabetes in the tropics. BMJ 1907;19:1051–64. [Google Scholar]
- 37.Fernando HM. Discussion on diabetes in the tropics. BMJ 1907;19:1060; discussion. [Google Scholar]
- 38.Mallick IM. Discussion on diabetes in the tropics. BMJ 1907;19:1061–2; discussion. [Google Scholar]
- 39.Sandwith PM. Discussion on diabetes in the tropics. VI. BMJ 1907;19:1059–60. [Google Scholar]
- 40.Ziemann H. Discussion on diabetes in the tropics. BMJ 1907;19:1061; discussion. [Google Scholar]
- 41.Emerson H, Larimore LD. Diabetes mellitus: a contribution to its epidemiology based chiefly on mortality statistics. Arch Intern Med (Chic) 1924;34:585–630. [Google Scholar]
- 42.Banting F. The history of insulin. Edinburgh Med J 1929;36:2. [Google Scholar]
- 43.Joslin EP, Dublin LI, Marks HH. Studies in diabetes mellitus. II. Its incidence and the factors underlying its variations. Am J Med Sci 1934;187:433–57. [Google Scholar]
- 44.Joslin EP, Dublin LI, Marks HH. Studies in diabetes mellitus. III. Interpretation of the variations in diabetes incidence. Am J Med Sci 1935;189:163–91. [Google Scholar]
- 45.Bairlein F. How to get fat: nutritional mechanisms of seasonal fat accumulation in migratory songbirds. Naturwissenschaften 2002;89:1–10. [DOI] [PubMed] [Google Scholar]
- 46.Mrosovsky N, Sherry DF. Animal anorexias. Science 1980;207:837–42. [DOI] [PubMed] [Google Scholar]
- 47.Keesey RE, Hirvonen MD. Body weight set-points: determination and adjustment. J Nutr 1997;127:1875S–83S. [DOI] [PubMed] [Google Scholar]
- 48.Sims EA, Goldman RF, Gluck CM, Horton ES, Kelleher PC, Rowe DW. Experimental obesity in man. Trans Assoc Am Physicians 1968;81:153–70. [PubMed] [Google Scholar]
- 49.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med 1995;332:621–8. [DOI] [PubMed] [Google Scholar]
- 50.Roberts SB, Young VR, Fuss P, Fiatarone MA, Richard B, Rasmussen H, Wagner D, Joseph L, Holehouse E, Evans WJ. Energy expenditure and subsequent nutrient intakes in overfed young men. Am J Physiol 1990;259:R461–9. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Proenca R, Maffei M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994;372:425–32. [DOI] [PubMed] [Google Scholar]
- 52.Pradhan G, Samson SL, Sun Y. Ghrelin: much more than a hunger hormone. Curr Opin Clin Nutr Metab Care 2013;16:619–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sahu A. Leptin signaling in the hypothalamus: emphasis on energy homeostasis and leptin resistance. Front Neuroendocrinol 2003;24:225–53. [DOI] [PubMed] [Google Scholar]
- 54.Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience 2005;134:737–44. [DOI] [PubMed] [Google Scholar]
- 55.Avena NM, Bocarsly ME, Rada P, Kim A, Hoebel BG. After daily bingeing on a sucrose solution, food deprivation induces anxiety and accumbens dopamine/acetylcholine imbalance. Physiol Behav 2008;94:309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spangler R, Wittkowski KM, Goddard NL, Avena NM, Hoebel BG, Leibowitz SF. Opiate-like effects of sugar on gene expression in reward areas of the rat brain. Brain Res Mol Brain Res 2004;124:134–42. [DOI] [PubMed] [Google Scholar]
- 57.Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, Alexoff D, Ding YS, Wong C, Ma Y, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage 2008;42:1537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Volkow ND, Wang GJ, Maynard L, Jayne M, Fowler JS, Zhu W, Logan J, Gatley SJ, Ding YS, Wong C, et al. Brain dopamine is associated with eating behaviors in humans. Int J Eat Disord 2003;33:136–42. [DOI] [PubMed] [Google Scholar]
- 59.Hopkins M, Blundell JE. Energy balance, body composition, sedentariness and appetite regulation: pathways to obesity. Clin Sci (Lond) 2016;130:1615–28. [DOI] [PubMed] [Google Scholar]
- 60.Gortmaker SL, Must A, Sobol AM, Peterson K, Colditz GA, Dietz WH. Television viewing as a cause of increasing obesity among children in the United States, 1986–1990. Arch Pediatr Adolesc Med 1996;150:356–62. [DOI] [PubMed] [Google Scholar]
- 61.Hernández B, Gortmaker SL, Colditz GA, Peterson KE, Laird NM, Parra-Cabrera S. Association of obesity with physical activity, television programs and other forms of video viewing among children in Mexico city. Int J Obes Relat Metab Disord 1999;23:845–54. [DOI] [PubMed] [Google Scholar]
- 62.Lean ME. Pathophysiology of obesity. Proc Nutr Soc 2000;59:331–6. [DOI] [PubMed] [Google Scholar]
- 63.Vgontzas AN, Bixler EO, Tan TL, Kantner D, Martin LF, Kales A. Obesity without sleep apnea is associated with daytime sleepiness. Arch Intern Med 1998;158:1333–7. [DOI] [PubMed] [Google Scholar]
- 64.Okamoto M, Tan F, Suyama A, Okada H, Miyamoto T, Kishimoto T. The characteristics of fatigue symptoms and their association with the life style and the health status in school children. J Epidemiol 2000;10:241–8. [DOI] [PubMed] [Google Scholar]
- 65.Hulens M, Vansant G, Lysens R, Claessens AL, Muls E. Exercise capacity in lean versus obese women. Scand J Med Sci Sports 2001;11:305–9. [DOI] [PubMed] [Google Scholar]
- 66.Ardévol A, Adán C, Franco L, García-Lorda P, Rubio F, Remesar X, Fernández-López JA, Salas-Salvadó J, Alemany M. During intense exercise, obese women rely more than lean women on aerobic energy. Pflugers Arch 1998;435:495–502. [DOI] [PubMed] [Google Scholar]
- 67.Mattsson E, Larsson UE, Rossner S. Is walking for exercise too exhausting for obese women? Int J Obes Relat Metab Disord 1997;21:380–6. [DOI] [PubMed] [Google Scholar]
- 68.Robinson TN. Reducing children's television viewing to prevent obesity: a randomized controlled trial. JAMA 1999;282:1561–7. [DOI] [PubMed] [Google Scholar]
- 69.Bawden SJ, Stephenson MC, Ciampi E, Hunter K, Marciani L, Macdonald IA, Aithal GP, Morris PG, Gowland PA. Investigating the effects of an oral fructose challenge on hepatic ATP reserves in healthy volunteers: a P MRS study. Clin Nutr 2016;35:645–9. [DOI] [PubMed] [Google Scholar]
- 70.Bawden SJ, Stephenson MC, Marciani L, Aithal GP, Macdonald IA, Gowland PA, Morris PA. Investigating alterations in hepatic ATP levels following fructose and fructose+glucose ingestion: a simple non-invasive technique to assess liver function using 31P MRS. Proc Intl Soc Magn Reson Med Sci Meet Exhib 2012;20:1369. [Google Scholar]
- 71.Schutz Y, Flatt JP, Jéquier E. Failure of dietary fat intake to promote fat oxidation: a factor favoring the development of obesity. Am J Clin Nutr 1989;50:307–14. [DOI] [PubMed] [Google Scholar]
- 72.Westerterp KR. Dietary fat oxidation as a function of body fat. Curr Opin Lipidol 2009;20:45–9. [DOI] [PubMed] [Google Scholar]
- 73.Blaak EE. Basic disturbances in skeletal muscle fatty acid metabolism in obesity and type 2 diabetes mellitus. Proc Nutr Soc 2004;63:323–30. [DOI] [PubMed] [Google Scholar]
- 74.Koch JE, Ji H, Osbakken MD, Friedman MI. Temporal relationships between eating behavior and liver adenine nucleotides in rats treated with 2,5-AM. Am J Physiol 1998;274:R610–7. [DOI] [PubMed] [Google Scholar]
- 75.Ji H, Graczyk-Milbrandt G, Friedman MI. Metabolic inhibitors synergistically decrease hepatic energy status and increase food intake. Am J Physiol Regul Integr Comp Physiol 2000;278:R1579–82. [DOI] [PubMed] [Google Scholar]
- 76.Friedman MI, Harris RB, Ji H, Ramirez I, Tordoff MG. Fatty acid oxidation affects food intake by altering hepatic energy status. Am J Physiol 1999;276:R1046–53. [DOI] [PubMed] [Google Scholar]
- 77.Wlodek D, Gonzales M. Decreased energy levels can cause and sustain obesity. J Theor Biol 2003;225:33–44. [DOI] [PubMed] [Google Scholar]
- 78.Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW 3rd, Kang L, Rabinovitch PS, Szeto HH, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 2009;119:573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nair S, Chacko VP, Arnold C, Diehl AM. Hepatic ATP reserve and efficiency of replenishing: comparison between obese and nonobese normal individuals. Am J Gastroenterol 2003;98:466–70. [DOI] [PubMed] [Google Scholar]
- 80.Abdelmalek MF, Lazo M, Horska A, Bonekamp S, Lipkin EW, Balasubramanyam A, Bantle JP, Johnson RJ, Diehl AM, Clark JM. Higher dietary fructose is associated with impaired hepatic adenosine triphosphate homeostasis in obese individuals with type 2 diabetes. Hepatology 2012;56:952–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lennmarken C, Sandstedt S, von Schenck H, Larsson J. Skeletal muscle function and metabolism in obese women. JPEN J Parenter Enteral Nutr 1986;10:583–7. [DOI] [PubMed] [Google Scholar]
- 82.Schmoller A, Hass T, Strugovshchikova O, Melchert UH, Scholand-Engler HG, Peters A, Schweiger U, Hohagen F, Oltmanns KM. Evidence for a relationship between body mass and energy metabolism in the human brain. J Cereb Blood Flow Metab 2010;30:1403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nisoli E, Clementi E, Carruba MO, Moncada S. Defective mitochondrial biogenesis: a hallmark of the high cardiovascular risk in the metabolic syndrome? Circ Res 2007;100:795–806. [DOI] [PubMed] [Google Scholar]
- 84.Kunkel D. Children and television advertising. In: Singer DG, Singer JL, editors. Handbook of children and the media. Thousand Oaks (CA): Sage; 2001. p. 375–93. [Google Scholar]
- 85.Florant GL, Lawrence AK, Williams K, Bauman WA. Seasonal changes in pancreatic B-cell function in euthermic yellow-bellied marmots. Am J Physiol 1985;249:R159–65. [DOI] [PubMed] [Google Scholar]
- 86.Johnson RJ, Stenvinkel P, Martin SL, Jani A, Sanchez-Lozada LG, Hill JO, Lanaspa MA. Redefining metabolic syndrome as a fat storage condition based on studies of comparative physiology. Obesity (Silver Spring) 2013;21:659–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cox CL, Stanhope KL, Schwarz JM, Graham JL, Hatcher B, Griffen SC, Bremer AA, Berglund L, McGahan JP, Havel PJ, et al. Consumption of fructose-sweetened beverages for 10 weeks reduces net fat oxidation and energy expenditure in overweight/obese men and women. Eur J Clin Nutr 2012;66:201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cox CL, Stanhope KL, Schwarz JM, Graham JL, Hatcher B, Griffen SC, Bremer AA, Berglund L, McGahan JP, Keim NL, et al. Consumption of fructose- but not glucose-sweetened beverages for 10 weeks increases circulating concentrations of uric acid, retinol binding protein-4, and gamma-glutamyl transferase activity in overweight/obese humans. Nutr Metab (Lond) 2012;9:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, Johnson RJ, Abdelmalek MF. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol 2008;48:993–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 2010;33:2477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cicerchi C, Li N, Kratzer J, Garcia G, Roncal-Jimenez CA, Tanabe K, Hunter B, Rivard CJ, Sautin YY, Gaucher EA, et al. Uric acid-dependent inhibition of AMP kinase induces hepatic glucose production in diabetes and starvation: evolutionary implications of the uricase loss in hominids. FASEB J 2014;28:3339–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cirillo P, Gersch MS, Mu W, Scherer PM, Kim KM, Gesualdo L, Henderson GN, Johnson RJ, Sautin YY. Ketohexokinase-dependent metabolism of fructose induces proinflammatory mediators in proximal tubular cells. J Am Soc Nephrol 2009;20:545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reungjui S, Pratipanawatr T, Johnson RJ, Nakagawa T. Do thiazides worsen metabolic syndrome and renal disease? The pivotal roles for hyperuricemia and hypokalemia. Curr Opin Nephrol Hypertens 2008;17:470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mäenpää PH, Raivio KO, Kekomaki MP. Liver adenine nucleotides: fructose-induced depletion and its effect on protein synthesis. Science 1968;161:1253–4. [DOI] [PubMed] [Google Scholar]
- 95.Raivio KO, Becker A, Meyer LJ, Greene ML, Nuki G, Seegmiller JE. Stimulation of human purine synthesis de novo by fructose infusion. Metabolism 1975;24:861–9. [DOI] [PubMed] [Google Scholar]
- 96.Baldwin W, McRae S, Marek G, Wymer D, Pannu V, Baylis C, Johnson RJ, Sautin YY. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome.Diabetes 2011;60:1258–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA 2008;300:924–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Soletsky B, Feig DI. Uric acid reduction rectifies prehypertension in obese adolescents. Hypertension 2012;60:1148–56. [DOI] [PubMed] [Google Scholar]
- 99.Takir M, Kostek O, Ozkok A, Elcioglu OC, Bakan A, Erek A, Mutlu HH, Telci O, Semerci A, Odabas AR, et al. Lowering uric acid with allopurinol improves insulin resistance and systemic inflammation in asymptomatic hyperuricemia. J Investig Med 2015;63:924–9. [DOI] [PubMed] [Google Scholar]
- 100.Kratzer JT, Lanaspa MA, Murphy MN, Cicerchi C, Graves CL, Tipton PA, Ortlund EA, Johnson RJ, Gaucher EA. Evolutionary history and metabolic insights of ancient mammalian uricases. Proc Natl Acad Sci USA 2014;111:3763–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Oda M, Satta Y, Takenaka O, Takahata N. Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol Biol Evol 2002;19:640–53. [DOI] [PubMed] [Google Scholar]
- 102.Andrews P. Species diversity and diet in monkeys and apes during the Miocene. In: Ciochan RL, Fleagle JG, editors. Primate evolution and human origins. Menlo Park (CA): Benjamin Publishing Co.; 1985. p. 194–204. [Google Scholar]
- 103.Andrews P, Kelley J. Middle Miocene dispersals of apes. Folia Primatol (Basel) 2007;78:328–43. [DOI] [PubMed] [Google Scholar]
- 104.Begun DR. Middle Miocene hominoid origins. Science 2000;287:2375. [PubMed] [Google Scholar]
- 105.Begun DR. African and Eurasian Miocene hominoids and the origins of the Hominidae. Andrews P, Koufos G, DeBonis L, editors. Hominoid evolution and environmental change in the neogene of Europe. Cambridge (United Kingdom): Cambridge University Press; 2001. p. 231–53. [Google Scholar]
- 106.Johnson RJ, Andrews P. Fructose, uricase, and the back-to-Africa hypothesis. Evol Anthropol 2010;19:250–7. [Google Scholar]
- 107.Johnson RJ, Andrews P. The fat gene: a genetic mutation in prehistoric apes may underlie today’s pandemic of obesity and diabetes. Sci Am 2015;313:64–9. [Google Scholar]
- 108.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet 1962;14:353–62. [PMC free article] [PubMed] [Google Scholar]
- 109.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA 1981;78:6858–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sutin AR, Cutler RG, Camandola S, Uda M, Feldman NH, Cucca F, Zonderman AB, Mattson MP, Ferrucci L, Schlessinger D, et al. Impulsivity is associated with uric acid: evidence from humans and mice. Biol Psychiatry 2014;75:31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Frisch RE. Critical fatness hypothesis. Am J Physiol 1997;273:E231–2. [DOI] [PubMed] [Google Scholar]
- 112.Johnson RJ. The fat switch. Hoffman Estates (IL): Mercola; 2012. [Google Scholar]
- 113.Bhishagranta KKL. An English translation of the sushruta samhita. Calcutta (India) Sutrasthanam; 1907. [Google Scholar]
- 114.Rosner F. The life of Moses Maimonides, a prominent medieval physician. Einstein Q J Biol Med 2002;19:125–8. [Google Scholar]
- 115.Deer N. The history of sugar. London: Chapman and Hall; 1949–50. [Google Scholar]
- 116.Galloway JH. The sugar cane industry. Cambridge (United Kingdom): Cambridge University Press; 1989. [Google Scholar]
- 117.Austen RA, Smith WD. Private tooth decay as public economic virtue: the slave-sugar triangle, consumerism, and European industrialization. Durham (NC): Duke University Press; 1990. p. 95–115. [Google Scholar]
- 118.Short T. A discourse concerning the causes and effects of corpulency together with its method for prevention and cure. London: J Roberts; 1727. [Google Scholar]
- 119.Wadd W. Cursory remarks on corpulence or obesity considered as a disease with critical examination of ancient and modern opinions relative to the causes and cure. 3rd ed. London: Smith and Davy; 1816. [Google Scholar]
- 120.Venner T. Vita Recta ad Longum vitam [The right way to a long life.] London: Abel Roper; 1660. [Google Scholar]
- 121.Blankaart S. Borgerlyke tafe: Om lang gesond sonder ziekten te leven [Accurate treatise on the podagra and the running gout, with whose true causes and certain curiosity thoroughly presented]. Amsterdam: Jan Claesz ten Hoorn; 1683. [Google Scholar]
- 122.Blankaart S. Accurate abhandlung von dem Podagra und der lauffenden Gicht [Treatise on podagra and migratory gout.] Leipzig (Germany): J.G. Gleditsch; 1690. [Google Scholar]
- 123.Flemyng M. A discourse on the nature, causes and cures of corpulency. London: printed for L Davis and C Reymers, Royal Society; 1760. [Google Scholar]
- 124.Johnson RJ, Perez-Pozo SE, Sautin YY, Manitius J, Sanchez-Lozada LG, Feig DI, Shafiu M, Segal M, Glassock RJ, Shimada M, et al. Hypothesis: could excessive fructose intake and uric acid cause type 2 diabetes? Endocr Rev 2009;30:96–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH, Gersch MS, Benner S, Sanchez-Lozada LG. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr 2007;86:899–906. [DOI] [PubMed] [Google Scholar]
- 126.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 2004;79:537–43. [DOI] [PubMed] [Google Scholar]
- 127.Anderson TA. Recent trends in carbohydrate consumption. Annu Rev Nutr 1982;2:113–32. [DOI] [PubMed] [Google Scholar]
- 128.Nielsen SJ, Popkin BM. Changes in beverage intake between 1977 and 2001. Am J Prev Med 2004;27:205–10. [DOI] [PubMed] [Google Scholar]
- 129.Lustig RH, Mulligan K, Noworolski SM, Tai VW, Wen MJ, Erkin-Cakmak A, Gugliucci A, Schwarz JM. Isocaloric fructose restriction and metabolic improvement in children with obesity and metabolic syndrome. Obesity (Silver Spring) 2016;24:453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Goran MI, Ulijaszek SJ, Ventura EE. High fructose corn syrup and diabetes prevalence: a global perspective. Glob Public Health 2013;8:55–64. [DOI] [PubMed] [Google Scholar]
- 131.Taubes G. Good calories, bad calories. New York: Alfred A Knopf; 2007. [Google Scholar]
- 132.Malik VS, Popkin BM, Bray GA, Despres JP, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation 2010;121:1356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Johnson RK, Appel LJ, Brands M, Howard BV, Lefevre M, Lustig RH, Sacks F, Steffen LM, Wylie-Rosett J. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation 2009;120:1011–20. [DOI] [PubMed] [Google Scholar]
- 134.Shi L, van Meijgaard J. Substantial decline in sugar-sweetened beverage consumption among California’s children and adolescents. Int J Gen Med 2010;3:221–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jacobson MF, Brownell KD. Small taxes on soft drinks and snack foods to promote health. Am J Public Health 2000;90:854–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Marriott BP, Cole N, Lee E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr 2009;139:1228S–35S. [DOI] [PubMed] [Google Scholar]
- 137.Special Issue. U.S. beverage results for 2011. Beverage Digest 2012;61:2. [Google Scholar]
- 138.Special Issue. U.S. beverage results for 2013. Beverage Digest 2014;65:1–3. [Google Scholar]
- 139.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010;303:235–41. [DOI] [PubMed] [Google Scholar]
- 140.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012;307:491–7. [DOI] [PubMed] [Google Scholar]
- 141.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA 2012;307:483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Geiss LS, Wang J, Cheng YJ, Thompson TJ, Barker L, Li Y, Albright AL, Gregg EW. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA 2014;312:1218–26. [DOI] [PubMed] [Google Scholar]
- 143.Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ 2012;346:e7492. [DOI] [PubMed] [Google Scholar]
- 144.Sievenpiper JL, de Souza RJ, Mirrahimi A, Yu ME, Carleton AJ, Beyene J, Chiavaroli L, Di Buono M, Jenkins AL, Leiter LA, et al. Effect of fructose on body weight in controlled feeding trials: a systematic review and meta-analysis. Ann Intern Med 2012;156:291–304. [DOI] [PubMed] [Google Scholar]
- 145.Johnson RJ, Lanaspa MA, Roncal-Jimenez C, Sanchez-Lozada LG. Effects of excessive fructose intake on health. Ann Intern Med 2012;156:905. [DOI] [PubMed] [Google Scholar]
- 146.Rippe JM, Angelopoulos TJ. Added sugars and risk factors for obesity, diabetes and heart disease. Int J Obes (Lond) 2016;40(Suppl 1):S22–7. [DOI] [PubMed] [Google Scholar]
- 147.Yracheta JM, Alfonso J, Lanaspa MA, Roncal-Jimenez C, Johnson SB, Sanchez-Lozada LG, Johnson RJ. Hispanic Americans living in the United States and their risk for obesity, diabetes and kidney disease: genetic and environmental considerations. Postgrad Med 2015;127:503–10. [DOI] [PubMed] [Google Scholar]
- 148.Yracheta JM, Lanaspa MA, Le MT, Abdelmalak MF, Alfonso J, Sanchez-Lozada LG, Johnson RJ. Diabetes and kidney disease in American Indians: potential role of sugar-sweetened beverages. Mayo Clin Proc 2015;90:813–23. [DOI] [PubMed] [Google Scholar]
- 149.Saab KR, Kendrick J, Yracheta JM, Lanaspa MA, Pollard M, Johnson RJ. New insights on the risk for cardiovascular disease in African Americans: the role of added sugars. J Am Soc Nephrol 2015;26:247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Le MT, Frye RF, Rivard CJ, Cheng J, McFann KK, Segal MS, Johnson RJ, Johnson JA. Effects of high-fructose corn syrup and sucrose on the pharmacokinetics of fructose and acute metabolic and hemodynamic responses in healthy subjects. Metabolism 2012;61:641–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. WHO . Guideline: sugars intake for adults and children . Geneva (Switzerland) : WHO ; 2015 . p. 1 – 50 . [PubMed] [Google Scholar]
- 152.Smemo S, Tena JJ, Kim KH, Gamazon ER, Sakabe NJ, Gomez-Marin C, Aneas I, Credidio FL, Sobreira DR, Wasserman NF, et al. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature 2014;507:371–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Heerwagen MJ, Miller MR, Barbour LA, Friedman JE. Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. Am J Physiol Regul Integr Comp Physiol 2010;299:R711–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 2004;101:15718–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA 2005;102:11070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature 2006;444:1022–3. [DOI] [PubMed] [Google Scholar]
- 157.Bhupathiraju SN, Tobias DK, Malik VS, Pan A, Hruby A, Manson JE, Willett WC, Hu FB. Glycemic index, glycemic load, and risk of type 2 diabetes: results from 3 large US cohorts and an updated meta-analysis. Am J Clin Nutr 2014;100:218–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med 2006;354:1601–13. [DOI] [PubMed] [Google Scholar]
- 159.van Dam RM, Willett WC, Rimm EB, Stampfer MJ, Hu FB. Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care 2002;25:417–24. [DOI] [PubMed] [Google Scholar]
- 160.Libuda L, Kersting M, Alexy U. Consumption of dietary salt measured by urinary sodium excretion and its association with body weight status in healthy children and adolescents. Public Health Nutr 2012;15:433–41. [DOI] [PubMed] [Google Scholar]
- 161.Larsen SC, Angquist L, Sorensen TI, Heitmann BL. 24h urinary sodium excretion and subsequent change in weight, waist circumference and body composition. PLoS One 2013;8:e69689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Simopoulos AP. Dietary omega-3 fatty acid deficiency and high fructose intake in the development of metabolic syndrome, brain metabolic abnormalities, and non-alcoholic fatty liver disease. Nutrients 2013;5:2901–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kratz M, Marcovina S, Nelson JE, Yeh MM, Kowdley KV, Callahan HS, Song X, Di C, Utzschneider KM. Dairy fat intake is associated with glucose tolerance, hepatic and systemic insulin sensitivity, and liver fat but not beta-cell function in humans. Am J Clin Nutr 2014;99:1385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lanaspa MA, Ishimoto T, Li N, Cicerchi C, Orlicky DJ, Ruzicky P, Rivard C, Inaba S, Roncal-Jimenez CA, Bales ES, et al. Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nat Commun 2013;4:2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Johnson RJ, Nakagawa T, Sanchez-Lozada LG, Lanaspa MA, Tamura Y, Tanabe K, Ishimoto T, Thomas J, Inaba S, Kitagawa W, et al. Umami: the taste that drives purine intake. J Rheumatol 2013;40:1794–6. [DOI] [PubMed] [Google Scholar]
- 166.Minami S, Sato M, Shiraiwa Y, Iwamoto K. Molecular characterization of adenosine 5′-monophosphate deaminase–the key enzyme responsible for the umami taste of nori (Porphyra yezoensis Ueda, Rhodophyta). Mar Biotechnol (NY) 2011;13:1140–7. [DOI] [PubMed] [Google Scholar]
- 167.Knott CD. Changes in orangutan caloric intake, energy balance, and ketones in response to fluctuating fruit availability. Int J Primatol 1998;19:1061–79. [Google Scholar]
- 168.Junk WJ. Temporary fat storage, an adaptation of some fish species to the waterlevel fluctuations and related environmental changes of the Amazon river. Amazoniana 1985;9:315–51. [Google Scholar]
- 169.Auger J, Meyer SE, Black HL. Are American black bears (Ursus americanus) legitimate seed dispersers for fleshy-fruited shrubs? Am Midl Nat 2002;147:352–67. [Google Scholar]
- 170.Madero M, Arriaga JC, Jalal D, Rivard C, McFann K, Perez-Mendez O, Vazquez A, Ruiz A, Lanaspa MA, Jimenez CR, et al. The effect of two energy-restricted diets, a low-fructose diet versus a moderate natural fructose diet, on weight loss and metabolic syndrome parameters: a randomized controlled trial. Metabolism 2011;60:1551–9. [DOI] [PubMed] [Google Scholar]
- 171.American Academy of Pediatrics. The use and misuse of fruit juice in pediatrics. Pediatrics 2001;107:1210–3. [DOI] [PubMed] [Google Scholar]
- 172.Trevellin E, Scorzeto M, Olivieri M, Granzotto M, Valerio A, Tedesco L, Fabris R, Serra R, Quarta M, Reggiani C, et al. Exercise training induces mitochondrial biogenesis and glucose uptake in subcutaneous adipose tissue through eNOS-dependent mechanisms. Diabetes 2014;63:2800–11. [DOI] [PubMed] [Google Scholar]