Abstract
Primary membranous nephropathy (also known as idiopathic membranous nephropathy, IMN) is an organ specific autoimmune kidney disease characterized by the development of immune complex deposits in the sub-epithelial spaces, podocyte effacement and glomerular capillary wall thickening in the later stages. Clinical studies have demonstrated that over 70% of patients with IMN possess circulating autoimmune antibodies specifically targeting the phospholipase A2 receptor (PLA2R) on the surface of podocytes. The autoantibodies only bind to the extracellular portion of PLA2R under the non-reducing condition, indicating that the epitope in PLA2R is conformational requiring specific disulfide bonds to maintain its structure. We recently have successfully located the dominant epitope in PLA2R to the extreme N-terminus of the receptor. This finding has opened a new direction for understanding the pathogenesis of anti-PLA2R autoantibody induced IMN and offered a strong basis for developing sensitive clinical assays for IMN diagnosis and prognosis, and potentially, new therapeutic approaches for IMN treatment.
Keywords: Apheresis column, enzyme-linked immunosorbent assay (ELISA), epitope, idiopathic membranous nephropathy (IMN), immunotolerance therapy, integrin α3β1, kidney, mechanism, pathogenesis, phospholipase A2 receptor (PLA2R)
INTRODUCTION
Membranous nephropathy (MN) is a common cause of nephrotic syndrome, accounting for ~20-40% of clinical cases in adults over the age of 40 [1-7]. It can be a primary form without identified causes (also known as “idiopathic MN”, IMN), or a secondary form associated with various autoimmune diseases, infections, cancers and exposure to drugs or toxic agents [1, 7]. IMN is an organ specific autoimmune disease. The major antigen responsible for the autoantibody binding in IMN patients was identified to be the phospholipase A2 receptor (PLA2R) [8], an integral transmembrane receptor that binds and removes the secreted phospholipase A2 enzyme (sPLA2) from circulation. Clinical studies have demonstrated that over 70% of IMN patients possess circulating autoantibodies that specifically target the PLA2R expressed on the surface of human podocytes [8] and moreover, the level of autoantibodies in circulation correlates with the severity of proteinuria in patients [9-11]. We recently successfully identified the location of the dominant epitope in PLA2R in IMN [12]. This finding has opened a new direction for understanding the pathogenesis of anti-PLA2R autoantibody induced IMN and offered a strong basis for developing sensitive clinical assays for IMN diagnosis and prognosis, and potentially, new therapeutic approaches for IMN treatment.
Pathophysiology of IMN
IMN is an autoimmune kidney disease that accounts for ~80% of all MN cases in adults [1, 13]. It affects 10-12 people per million population. Clinically, about a third of IMN patients undergo spontaneous remission and about 40% progress to the end-stage renal disease in ~10-15 years [14-16]. The common clinical manifestation of IMN is edema and proteinuria. Pathologically, IMN is characterized by the development of immune complex deposits in the subepithelial spaces causing a membrane-like thickening of the capillary wall and eventually the glomerular basement membrane (GBM). The name of membranous nephropathy derives from the prominent thickened capillary wall that is visible under light microscope in the later stages of the disease. Currently, the diagnosis of IMN is strictly dependent on immunofluorescence and electron microscopy of the renal biopsies, which detects antibody deposition in the peripheral capillary loop, electron dense deposits in the subepithelial spaces and podocyte foot process effacement, respectively. However, such pathology cannot distinguish between the primary and the secondary forms of MN easily.
The immune deposits in the capillary wall in MN consist of IgG, antigens, and complement components including the membrane attack complex (complement components C5b–9) [17]. IgG4 is usually the dominant IgG subclass in the immune deposits in IMN, although variable amounts of IgG1, IgG2 and IgG3 are also detected. In contrast, IgG1, IgG2 and IgG3 are usually the dominant depositions in the secondary membranous nephropathy [18-21]. The formation of subepithelial immune deposits and complement activation is believed to be the cause of glomerular capillary wall impairment that leads to proteinuria in patients.
Three mechanisms have been proposed for immune complexes formation and deposition in the lesion of MN [4, 22]: 1) immune complexes are formed in circulation and then deposited in the glomerular subepithelial spaces; 2) immune complexes are formed directly in situ with local antigens; and 3) immune complexes are formed locally with antigens that are planted in the glomerular subepithelial spaces. These mechanisms have been successfully demonstrated in the animal models such as rabbit chronic serum sickness [23, 24], Heymann nephritis [25] and cationic bovine serum albumin induced rabbit MN [26]. In humans, MN cases with all three of these mechanisms have been demonstrated.
The current understanding of IMN pathogenesis largely derives from an experimental rat model of MN, the Heymann nephritis. In this model, antibodies directly bind to megalin [27, 28], a membrane receptor located on the basal surface of the rat podocytes that forms immune deposits in situ, which activates the complement pathway that damages podocyte biology leading to proteinuria [17, 29, 30]. Complement activation and C5b-9 membrane attack complex formation play a key role in causing sublethal podocyte injury and proteinuria in the Heymann nephritis. The sublytic level of C5b-9 complex activates various mediators including phospholipases, protein kinases, cyclooxygenases, transcription factors and cytokines that alter podocyte biology over a period of time. The signals generated by these pathways interfere with podocyte metabolism, structure and function of cytoskeletal proteins, expression and localization of nephrin, turnover of extracellular matrix, and DNA integrity. The assembly of the sublytic C5b-9 complex on podocytes was also shown to up-regulate NADPH oxidoreductase expression and translocation to the cell surfaces. Subsequently, reactive oxygen species are produced and accumulate locally, which leads to oxidation of podocyte membrane lipids, membrane proteins and glomerular basement membrane components [4, 13, 17, 29-31].
Although megalin was identified to be the local podocyte antigen in Heymann nephritis, it is not found in the human podocytes [6, 32]. Two membrane proteins, the neutral endopeptidase (NEP) [33] and PLA2R [8] expressed on the basal surface of podocytes have been identified to serve as the local antigens for the neonatal alloimmune and adult IMN respectively. More recently, a new antigen, thrombospondin type-1 domain-containing 7A was also identified to serve as a local antigen in ~ 2.5-5% of adult patients with IMN [34].
Anti-PLA2R Autoantibody and IMN
In 2009, Beck and coworkers made a seminal discovery that, about 70% of patients in the United States with IMN, but not in those with secondary MN or in controls, possess anti-PLA2R autoantibodies in circulation that specifically bind to the PLA2R expressed in podocytes [8]. The autoantibodies were found to be deposited within the subepithelial immune complexes in patients with IMN, and the eluted autoantibodies from biopsy specimen reacted with PLA2R on the Western-blot. The antigen, PLA2R was also found in the immune complex deposits at a very high level. Importantly, the autoantibody only recognized the non-reduced form of PLA2R, indicating that the epitope in PLA2R is conformational requiring specific disulfide bonds to maintain its structure.
Since this initial discovery, multiple cohort studies have been performed across the world to determine the prevalence of anti-PLA2R autoantibodies in IMN patients [9-11, 35-44]. Four techniques have been used to detect the presence of autoantibodies in patients: Western-blotting of PLA2R protein under the nonreducing condition (WB), recombinant cell-based indirect immunofluorescence assay (IFA), enzyme-linked immunosorbent assay (ELISA) and immunofluorescence staining of renal biopsies. The available results indicated that the prevalence of anti-PLA2R autoantibodies in IMN patients varies significantly in different regions. Specifically, cohort studies showed that the prevalence of anti-PLA2R autoantibodies in IMN patients in China [36], Korea [37], Japan [38], Iran [39], Germany [40], UK [9] and Netherlands [41] is 81.7%, 69%, 53%, 75%, 69%, 75% and 74% respectively. Of all the cohort studies, 5 had a sample size over 100 cases, and the average prevalence of anti-PLA2R autoantibodies across the world in IMN patients is about 70%. Results from 3 European studies indicated that the level of autoantibodies in circulation correlates with the severity of proteinuria and the clinical outcomes in patients [9-11]; yet results from 2 Asian studies indicated that no significant correlations could be detected [37, 38]. This discrepancy may be caused by different techniques used in the cohort studies. Occasionally, the anti-PLA2R antibody was detected in the secondary MN (cancer and hepatitis B) [36] but it is unknown if this is merely a chance occurrence.
The pathogenic role of anti-PLA2R autoantibody in IMN was also studied in the kidney transplant recipients. It was reported that IMN could recur one week after kidney transplantation when circulating anti-PLA2R antibodies were present at the time of transplantation [45, 46]. It was also reported that some patients with high titers of autoantibodies at the time of kidney transplantation did not have recurrence [45]. Moreover, the recurrence of anti-PLA2R autoantibody in IMN patients after kidney transplant [47, 48] was found to be associated strongly with the disease relapse, supporting that the autoantibody serves as the cause of IMN in patients.
Unlike the anti-NEP autoantibody in neonatal alloimmune MN [33], transfer of anti-PLA2R patient sera to animals failed to reproduce nephrotic syndrome as that in human. This is likely due to the fact that PLA2R is not expressed in the glomerulus of rabbit, rat or mouse [8]. A recent study successfully generated a PLA2R knock-in mouse model with human PLA2R specifically expressed in the mouse podocytes [49]. Transfusion of human anti-PLA2R sera into the PLA2R knock-in mice indeed triggered immune complex deposition in the glomerular subepithelial spaces; however, no proteinuria was detected in these animals. This unexpected finding seems to indicate that formation of the immune deposit itself is not sufficient enough to cause proteinuria in patients, and that complement activation or additional mechanisms must be involved, as shown in the Heymann nephritis.
In Heymann nephritis, immune deposits formation and complement activation, as well as a T cell (CD8) involvement are critically required for developing proteinuria in the animals. Depletion of complement components obviated proteinuria in the animal models despite the presence of IgG containing immune deposits in the GBM [50]. The dominant anti-PLA2R autoantibody detected in the renal biopsies of IMN patients is IgG4 (other subclasses are also detected at relatively low levels). IgG4 is known to be ineffective to activate the classical complement pathway, suggesting that the mechanism of anti-PLA2R autoantibody induced proteinuria in patients may differ significantly from that in the Heymann nephritis. A recent study suggested that aberrant glycosylated IgG4 may play an important role in activating complements via the mannose-binding lectin (MBL) pathway [51], and indeed, MBL components can be found in the immune complex deposits of IMN [52]. In a special clinical case where a patient had recurrent IMN after kidney transplantation, the anti-PLA2R autoantibody was identified to be IgG3k, which is capable of activating the classical complement pathway directly [53].
Similar to the type I autoimmune pancreatitis [54], human IMN is an IgG4-related disease with the glomerular immune deposits containing predominantly IgG4-subclass antibodies, indicating the involvement of T helper 2 cells (CD4) in immune response [55, 56]. Clinical studies indeed showed that in IMN patients, the CD4+/CD8+ ratio of peripheral T cells and the level of IL-10 and IL-13 mRNA expression in peripheral blood mononuclear cells significantly increased [56]. It is likely that in IMN patients, the antigen primed T helper 2 cells engaged with specific B cells secret IL-10 and IL-13 that activates B cells and promotes IgG4 autoantibody production.
Structure and Function of PLA2R
Molecular Structure
Two forms of PLA2R exist in the body: a membrane associated form (PLA2R) and a circulative form (cPLA2R) [57, 58]. The membrane associated PLA2R is a type I transmembrane glycoprotein (MW ~180 kDa) consisting of a large glycosylated extracellular portion, a single transmembrane helix and a short cytoplasmic tail. The circulating PLA2R is a truncated form of the membrane associated PLA2R that only contains the extracellular portion due to alternative splicing [57]. In the kidney, both forms of PLA2R are present with a ratio of 1.6:1 (PLA2R:cPLA2R) at the mRNA level [57]. Surprisingly, the anti-PLA2R autoantibody only recognizes and binds to the membrane associated but not the circulative PLA2R [8], suggesting that the two forms of PLA2R may fold differently. The current review focuses on the structure and function of the membrane associated PLA2R.
PLA2R belongs to the mannose receptor family that consists of four members: the mannose receptor (CD206), Endo180 (also known as the urokinase type plasminogen activator receptor-associated protein uPARAP or CD280), the dendritic cell receptor DEC205 (CD205) and PLA2R [59, 60]. All the 4 receptors share a similar three-dimensional protein structure, especially in the extracellular portion, which contains an N-terminal cysteine-rich domain (CysR) followed by a single fibronectin-like type II domain (FnII), and eight to ten C-type lectin-like domains (CTLD), Fig. (1). The protein structure of the individual CysR, FnII and CTLD domain has been solved at the atomic level which showed the critical roles of disulfide bonding in the proper folding of each of the domains [61-63]. Single particle electron microscopy images of the extracellular portion of mannose receptor and Endo180 are also available [64], which indicated that both molecules have two distinctive configurations: a bent conformation with the N-terminal domains folding back toward the middle of the molecule, and an extended conformation with the N-terminal CysR domain pointing outwards from the cell membrane [59]. Transitions between the “bent” and “extended” conformation have been predicted to serve as a general structural mechanism for the mannose receptor family to regulate their ligands binding.
Fig. (1).
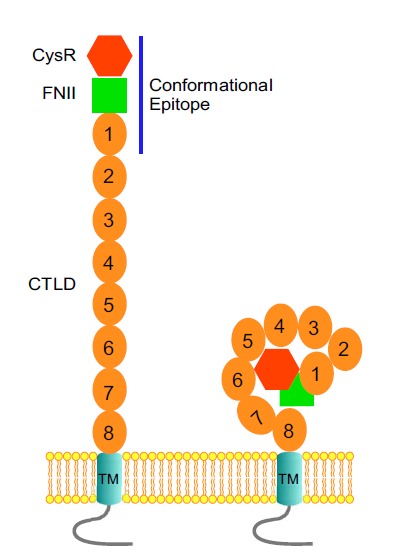
The extended (left) and the bent (right) configurations of PLA2R in the cell membranes. The immunodominant epitope region for autoantibody recognition is indicated as a solid line.
As a member of the mannose receptor family, the extracellular portion of PLA2R has also been predicted to have a bent and an extended conformation on the cell surfaces [8] Fig. (1). Whether each of the domains in PLA2R is arranged in a similar configuration to that of the mannose receptor in three-dimensional structure is not clear. The difference in autoantibody recognition between the circulating and the membrane associated PLA2R seems to indicate that a conformational mechanism is involved in exposing the epitope in PLA2R for the autoantibody binding.
Biological Function
Of all the mannose receptor family members, the mannose receptor is known to be involved in the pathogen recognition in the innate immune system; DEC-205 involved in antigen presentation in the dendritic cells; Endo 180/uPARAP involved in regulating extracellular matrix degradation and remodeling. However, the biological function of PLA2R has remained largely unclear. PLA2R was identified two decades ago based on its high affinity for the venom secreted phospholipase A2 [65]. Following its cloning, the affinity of PLA2R for different sPLA2 family members was analyzed, and a pancreatic form of sPLA2 (sPLA2-IB) was shown to have the highest affinity [58]. Binding of sPLA2 to PLA2R triggers rapid receptor endocytosis, indicating that PLA2R functions as a scavenger receptor for removal of the bound sPLA2 from circulation [66]. Binding of sPLA2 to PLA2R was also found to activate signaling pathways that triggers cellular responses [67, 68]. Indeed, studies from cultured cells as well as experimental animal models both supported the contention that PLA2R is a signaling receptor in cell membranes. Recent reports also suggested that PLA2R plays an important role in cell senescence [69] and tumor suppression [70], further supporting the concept that PLA2R functions as a signaling molecule in the cell membranes.
PLA2R has also been reported to function as an endogenous inhibitor for sPLA2 in the lung with elevated expression levels during lung infection [71], suggesting its important role in inflammation. However, the biological role of PLA2R in the kidney has remained completely unknown despite it being highly expressed in the basal membrane of podocytes [8]. PLA2R was predicted to have a protective role for podocyte plasma membranes against being attacked by sPLA2 enzymes. Intriguingly, in vitro biochemical assays showed that although sPLA2-IB actively digests phospholipids when mixed with bile, it has minimal activity in digesting membrane phospholipids [72]. sPLA2-IB has a molecular weight of 14 kDa and is readily filtered, so it is unlikely to cause damage to the podocyte plasma membranes. Surprisingly, when the PLA2R gene was knocked out, mice appeared completely normal with no disease phenotype [73]. These observations seem to suggest that PLA2R has a minimal role in podocyte function; however, clinical evidence clearly demonstrated its involvement in mediating podocyte injury in IMN when bound with the anti-PLA2R autoantibodies, suggesting that additional physiological roles other than being a functional inhibitor for sPLA2 in human kidney might be involved.
Tissue Distribution
After its cloning, the distribution of PLA2R in human tissue was analyzed by RNA blotting, which showed that PLA2R is highly expressed in the pancreas [74], kidney [57, 74], placenta [57, 74], lung [57] and skeletal muscle [57]. Interestingly, PLA2R mRNA was only detected in the kidney at a very high level in the human fetus [74]. Lately, PLA2R expression was also detected in the human neutrophils [75], gestational tissues [76], the primary human mammary epithelial cells [69], human diploid cells [69] and leukemic cells [77]. Based on the mRNA expression pattern of PLA2R in GeneAtlas (provided by Genomics Institute of the Novartis Research Foundation), PLA2R is widely expressed throughout the body.
Epitope in PLA2R for Autoantibody Binding in IMN
The anti-PLA2R autoantibody specifically binds to the extracellular portion of PLA2R under the nonreducing condition [8], indicating that the epitope is conformational and requires specific disulfide bonds to maintain its structure. A disulfide bond is formed by sulfhydryl groups of two adjacently located cysteine amino acids in a folded protein. PLA2R contains 66 endogenous cysteines throughout the extracellular portion, and disulfide bonds are likely to be present in every one of the 10 domains. The conformational epitope in PLA2R could be formed either by a single or by multiple extracellular domains in a folded conformation. Genetic studies have determined that several polymorphisms exist in the extracellular portion of PLA2R in IMN patients, especially, M292V and H300D in the CTLD1 domain and G1106S in the linker region between CTLD6 and CTLD7 domain may correlate with the occurrence of IMN in patients with susceptible genetic backgrounds [78, 79]. By using small peptide mapping approach, a number of regions in the PLA2R extracellular portion have been predicted to bind to the autoantibodies, suggesting that the epitope in PLA2R could potentially be distributed throughout the extracellular portion [80].
Since PLA2R is widely expressed yet IMN is an organ specific disease, we predicted that the PLA2R expressed in human podocytes adopt a unique conformation with a specific epitope region highly exposed for the autoantibody binding. To test this hypothesis, we sequentially truncated the extracellular portion of PLA2R into 10 protein fragments that contain CysR, CysR and FnII, CysR, FnII and CTLD1-10 domains, Fig. (2). These protein fragments were selectively truncated in the linker regions between each of the domains, to minimize the potential structural disturbance. By probing the truncated PLA2R domains expressed in the HEK 293 cells, we successfully determined that the dominant epitope for autoantibody binding is exclusively located in the extreme N-terminus of the receptor, specifically, a region encompassing the CysR-FnII-CTLD1 domain [12]. This epitope was recognized strongly by the sera from various patients that contained anti-PLA2R autoantibodies, but not by the sera that were negative of the autoantibodies. Importantly, the isolated epitope region completely blocked the autoantibody binding to the full length PLA2R protein on the Western-blot, indicating that the epitope in its native conformation binds strongly to the autoantibody. Our data also demonstrated that all the three domains are required to form an integrated structure for the autoantibody recognition, and neither of the individual domains alone could serve as the epitope on the Western-blot. Therefore, we propose that the epitope in PLA2R is formed by regions from the CysR and the CTLD1 domain with FnII domain serves as a critical structural component to bring the two domains in a close proximity. Indeed, the EM structures of both mannoses receptor and Endo180 showed that the CysR-FnII-CTLD1 domains are compactly folded, indicating that regions in the CysR and CTLD1 domains are close enough to form a conformational epitope [64, 81].
Fig. (2).
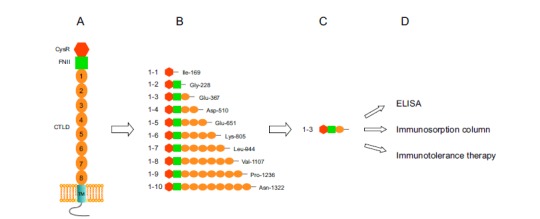
Identification of the dominant epitope in PLA2R in IMN and its clinical applications. A. Topological model of PLA2R in the cell membranes. B. Truncated PLA2R extracellular domains for IMN patient sera probing on the Western-blot. C. The identified dominant epitope region. D. Clinical applications of the identified epitope in PLA2R.
Shortly after our discovery, a similar study on identifying the epitope in PLA2R in IMN patients was reported [82]. This study initially determined that the epitope was exclusively located in the CTLD3 domain based on the results using small-angle X-ray scattering, electron microscopy and surface plasmon resonance approaches [83], and in the final published manuscript [82], the epitope was relocated to the CysR domain that corroborated our finding. Specifically, the study determined that a 31 amino acid peptide in the CysR domain forms a major epitope that is recognized by the anti-PLA2R autoantibodies. Moreover, the study determined that this epitope could only be maintained under the non-denaturing condition, and that the CTLD3 domain is essentially required for keeping the epitope conformation under the denaturing condition. In our study, the region encompassing the CysR-FnII-CTLD1 domain was well recognized by the autoantibodies from various patients on the Western-blot under the denaturing condition. This discrepancy requires further investigation. Nevertheless, this study clearly supported our conclusion that the dominant epitope in PLA2R in IMN is located at the extreme N-terminus of the receptor.
Pathophysiological and Clinical Significance of Epitope Identification
Pathophysiological Significance
IMN is an organ specific autoimmune disease. The endogenous antigen, PLA2R, is not only expressed in the human kidney, but also highly expressed in the lung, pancreas, placenta, neutrophils, cardiomyocytes and many other tissues. Why the autoantibodies only target the PLA2R expressed on the surface of podocytes in the kidney, but not in other organs, has remained completely unclear. Based on the NMR structure of the mannose receptor, PLA2R is likely to have an extended and a bent conformation on the cell surface, and the autoantibodies were thought to bind only one of the conformations. Therefore, IMN was predicted to be an autoimmune conformational disease, termed as “conformerapathy” [84], which means the antigen is formed due to a specific conformation of the receptor.
Our finding that the dominant epitope in PLA2R is exclusively located at the extreme N-terminus has offered a new level of understanding of the disease pathogenesis and potentially a plausible explanation why IMN is an organ specific autoimmune disease. We predict that the PLA2R expressed on the basal surface of the podocytes adopts an extended conformation towards the GBM facing the incoming plasma fluid and as a result is highly accessible to autoantibody binding; yet in other organs, PLA2R adopts a bent conformation that precludes the epitope region being recognized by the autoantibodies. In supporting of this hypothesis, we have determined that the extracellular domains of PLA2R associate with integrin α3β1 [3, 85], an adhesion molecule in the podocytes. Integrin α3β1 is highly expressed in the basal surface of the podocytes, where it mediates podocytes attachment to the GBM. Integrin molecule is known to have a bent conformation in the inactive state, and become extended in the active state when interacting with its ligand [86]. Association of PLA2R extracellular domains with the active form integrin α3β1 is likely to induce the receptor to adopt an extended conformational that highly exposes the N-terminal dominant epitope for autoantibody binding.
Although the anti-PLA2R autoantibody is now considered to be the cause of IMN in patients, how the autoantibody is produced initially in the body has remained unclear. In human glomeruli, PLA2R is only expressed in the podocytes [8], and podocytes are epithelial cells separated from circulation by the GBM. Therefore, it is unlikely that exposure of the dominant epitopes in PLA2R on the surface of podocytes can serve as the trigger for the autoantibody production since they are inaccessible to the circulating T cells, which help to activate the antigen specific B cells and promote antibody formation and secretion. Identification of the dominant epitope in PLA2R has offered an important clue in searching for unknown environmental agents that are potentially to serve as the trigger for the autoantibody production in the body, and subsequently the produced antibodies cross-reacting with the PLA2R epitope on the surface of human podocytes through molecular mimicry mechanism.
Comparison of the 31 amino acids in CysR domain against the microbial protein database revealed that a bacterial cell wall enzyme, D-alanyl-D alanine carboxypeptidase, shares a sequence of “LTLENCK” completely with the CysR domain [82], suggesting its potential role to serve as an environmental trigger for IMN. Our study indicated that the CTLD1 domain is critically required for forming the conformational epitope [12], and therefore we compared the amino acid sequence of CTLD1 domain against the NCBI database of non-redundant protein sequences. We found surprisingly that a bacterial subgroup of the C-type lectin-like domain and an invertebrate C-type lectin, CEL-I in the secreted toxin of two ocean organisms Table 1 are about 20-25% homologous to CTLD1. We postulate that exposure to these environmental agents might induce the production of pathogenic anti-PLA2R autoantibodies in a genetically susceptible population.
Table 1.
Human PLA2R CTLD-1 domain homologous protein in a group of bacterial and two ocean organisms.
| Human PLA2R CTLD-1 homologous domain in a subgroup of bacterial C-type lectin-like domain proteins | |||
|---|---|---|---|
| Gene ID | Definition | Source organism | Alignment score |
| gi 78171192 | C-type lectin | Chlorobium chlorochromatii CaD3 | 21.58 |
| gi 33863107 | C-type lectin domain-containing protein |
Prochlorococcus marinus str. MIT 9313 | 15.83 |
| gi 86135736 | Hypothetical protein MED193_16474 | Roseobacter sp. MED193 | 15.11 |
| gi 87308660 | Hypothetical protein WH7805_05191 | Synechococcus sp. WH 7805 | 10.79/17.99 |
| gi 32471540 | Hypothetical protein RB1661 | Rhodopirellula baltica SH 1 | 12.23 |
| gi 32472568 | Heme/hemopexin utilization protein huxA | Rhodopirellula baltica SH 1 | 12.23 |
| gi 57506013 | TraN protein, homolog | Campylobacter upsaliensis RM3195 | 12.95 |
| gi 59802590 | Cyclin-dependent kinase-activating kinase | Prosthecobacter dejongeii | 13.67 |
| gi 72003075 | C-type lectin | Prochlorococcus marinus str. NATL2A | 15.11 |
| gi 78166157 | VCBS protei | Chlorobium luteolum DSM 273 | 15.83 |
| gi 86134618 | VCBS | Tenacibaculum sp. MED152 | 15.83 |
| gi 86749366 | VCBS | Rhodopseudomonas palustris HaA2 | 9.35 |
| gi 87124836 | Hypothetical protein RS9917_01402 | Synechococcus sp. RS9917 | 10.79 |
| gi 32477673 | Mannan-binding protein MBP (lectin) | Rhodopirellula baltica SH 1 | 15.83 |
| gi 87310299 | Serine/threonine protein kinase | Blastopirellula marina DSM 3645 | 15.11 |
| gi 67925505 | YD repeat | Crocosphaera watsonii WH 8501 | 19.42 |
| Human PLA2R CTLD-1 homologous domain in CEL-1 from Cucumaria echinata and Echinoidin from Anthocidaris crassispina | |||
| Gene ID | Definition | Source organism | Alignment Score |
| gi 126127 | Echinoidin | Heliocidaris crassispina | 25.18 |
| gi 3378108 | secreted lectin homolog | Heliocidaris erythrogramma | 20.86 |
| gi 17385630 | GalNAc-specific lectin | Asterina pectinifera | 19.42 |
| gi 21637389 | C-type lectin domain protein | Strongylocentrotus purpuratus | 20.14 |
| gi 37732133 | spEchinoidin | Strongylocentrotus purpuratus | 19.42 |
| gi 68357792 | PREDICTED: similar to hCG1657150 | Danio rerio | 12.23 |
| gi 72010149 | PREDICTED: similar to secreted lectin homolog; HeEL-1 |
Strongylocentrotus purpuratus | 19.42 |
| gi 72015052 | PREDICTED: aggrecan core protein-like | Strongylocentrotus purpuratus | 25.18 |
| gi 72085529 | PREDICTED: echinoidin-like | Strongylocentrotus purpuratus | 22.3 |
| gi 72125841 | PREDICTED: aggrecan core protein-like isoform 2 | Strongylocentrotus purpuratus | 24.46 |
| gi 73959018 | PREDICTED: similar to Neurocan core protein precursor (Chondroitin sulfate proteoglycan 3) | Canis familiaris | 12.96 |
Clinical Significance
Currently, clinical diagnosis of MN is strictly dependent on renal biopsies that requires signify cant sample processing time, and the method itself potentially poses a risk of kidney bleeding. The discovery of PLA2R as the major antigen in IMN has opened new avenues into the diagnosis and treatment of IMN. Serological tests, such as indirect IFA [35] and ELISA [87, 88], are available on the market with sensitivity for anti-PLA2R autoantibodies around 70%. IFA utilizes formalin fixed PLA2R expressing HEK 293 cells coated on a coverslip, and ELISA utilizes purified full extracellular portion of PLA2R protein, as the target antigens for detecting the presence of autoantibodies in patient sera. It is known that human sera are a mixture containing variety of antibodies; application of such mixture on the fixed human cells will inevitably produce high background. Indeed, IFA could not be used to monitor the autoantibody titer in patient sera and analysis of IFA results is subjective depending on experiences [89]. Identification of the antigenic epitope in PLA2R has now made it possible to develop a sensitive and cost-effective epitope specific ELISA assay for detecting the autoantibodies in patient sera. We coated the identified epitope region on an ELISA plate and tested its efficiency for the anti-PLA2R autoantibody recognition. Our preliminary data showed that the new assay can detect the presence of autoantibodies up to 1:6,400 dilutions, and moreover, has a ratio of ~10-15 fold of the autoantibody positive vs. negative sera. Our assay has offered a new level of sensitivity and cost effectiveness for detecting the autoantibodies in patient sera, monitoring the disease prognosis, and differentiating the primary and the secondary MN in patients.
Identification of the dominant epitope in PLA2R has also offered a strong basis for developing specific treatments for patients with anti-PLA2R autoantibody induced IMN. Clinically, IMN patients are treated routinely with high doses of steroids and immunosuppressive agents, which are non-specific and have significant side effects. In some patients, these treatments are not effective. Moreover, the slow decline in circulating autoantibodies after treatment exposes the podocytes to the risk for ongoing injury [90, 91]. If the autoantibodies can be removed selectively from the patient circulation, it may effectively stop the disease progress in the early stage, which would reverse the abnormalities in kidney function with minimal side-effects [92]. We have successfully built an epitope coated apheresis column and tested its effectiveness for autoantibody removal from the patient sera. Our obtained results have demonstrated that the column has a very high capacity and efficiency to bind and remove the autoantibodies from patient sera, indicating its future potential for IMN treatment. We envision this new treatment will specifically remove the anti-PLA2R autoantibodies from circulation without affecting the level of normal antibodies and therefore poses no undesirable immunosuppressive side-effects to the patients.
Alternatively, the identified epitope could be used as a reagent for developing immunotolerance therapies [93, 94] for IMN patients. Immunotolerance is a state of immune unresponsiveness toward a particular antigen due to repeated antigen exposure that results in peripheral T cell tolerance. Immune tolerance therapy intends to reprogram the immune system so that it stops producing autoantibodies. We predict that IMN patients could be exposed to the purified epitope region repeatedly over a period of time until the body tolerates the PLA2R epitope without reacting to it. This treatment may potentially stop the anti-PLA2R autoantibody production in IMN patients and have long lasting effects.
Conclusion
For the past half century, the pathogenesis of primary membranous nephropathy has been uncertain, and it was referred to as “idiopathic”. With the application of modern technologies in renal disease research, especially protein mass spectrometry, we now have entered into a new era of elucidating the pathogenesis of renal autoimmune diseases. In regard to IMN research, we expect in a near future that a panel of antigens responsible for IMN in patients will be discovered. As a result, we believe that the strict dependence of IMN diagnosis on renal biopsies will one day be fully replaced by the laboratory tests.
ACKNOWLEDGEMENTS
We thank Dr. Richard Glassock for critical reading of the manuscript. This work was supported in part by the Norman S. Coplon Grant from Satellite Healthcare and funding from the UCLA Division of Nephrology.
CONFLICT OF INTEREST
The author confirms that this article content has no conflict of interest.
REFERENCES
- 1.Glassock R.J. The pathogenesis of idiopathic membranous nephropathy: A 50-year odyssey. Am. J. Kidney Dis. 2010;56(1):157–167. doi: 10.1053/j.ajkd.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Glassock R.J. The pathogenesis of membranous nephropathy: Evolution and revolution. Curr. Opin. Nephrol. Hypertens. 2012;21(3):235–242. doi: 10.1097/MNH.0b013e3283522ea8. [DOI] [PubMed] [Google Scholar]
- 3.Glassock R.J. Pathogenesis of membranous nephropathy: A new paradigm in evolution. Contrib. Nephrol. 2013;181:131–142. doi: 10.1159/000348472. [DOI] [PubMed] [Google Scholar]
- 4.Ronco P., Debiec H. Pathogenesis of membranous nephropathy: Recent advances and future challenges. Nat. Rev. Nephrol. 2012;8(4):203–213. doi: 10.1038/nrneph.2012.35. [DOI] [PubMed] [Google Scholar]
- 5.Ronco P., Debiec H. Anti-phospholipase A2 receptor antibodies and the pathogenesis of membranous nephropathy. Nephron Clin. Pract. 2014;128(3-4):232–237. doi: 10.1159/000368588. [DOI] [PubMed] [Google Scholar]
- 6.Beck L.H., Jr, Salant D.J. Membranous nephropathy: Recent travels and new roads ahead. Kidney Int. 2010;77(9):765–770. doi: 10.1038/ki.2010.34. [DOI] [PubMed] [Google Scholar]
- 7.Beck L.H., Jr, Salant D.J. Membranous nephropathy: From models to man. J. Clin. Invest. 2014;124(6):2307–2314. doi: 10.1172/JCI72270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck L.H., Jr, Bonegio R.G., Lambeau G., Beck D.M., Powell D.W., Cummins T.D., Klein J.B., Salant D.J. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N. Engl. J. Med. 2009;361(1):11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanigicherla D., Gummadova J., McKenzie E.A., Roberts S.A., Harris S., Nikam M., Poulton K., McWilliam L., Short C.D., Venning M., Brenchley P.E. Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int. 2013;83(5):940–948. doi: 10.1038/ki.2012.486. [DOI] [PubMed] [Google Scholar]
- 10.Hofstra J.M., Beck L.H., Jr, Beck D.M., Wetzels J.F., Salant D.J. Anti-phospholipase A receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin. J. Am. Soc. Nephrol. 2011;6(6):1286–1291. doi: 10.2215/CJN.07210810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoxha E., Thiele I., Zahner G., Panzer U., Harendza S., Stahl R.A. Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. J. Am. Soc. Nephrol. 2014;25(6):1357–1366. doi: 10.1681/ASN.2013040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kao L., Lam V., Waldman M., Glassock R.J., Zhu Q. Identification of the immunodominant epitope region in phospholipase A2 receptor-mediating autoantibody binding in idiopathic membranous nephropathy. J. Am. Soc. Nephrol. 2015;26(2):291–301. doi: 10.1681/ASN.2013121315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerjaschki D. Pathogenetic concepts of membranous glomerulopathy (MGN). J. Nephrol. 2000;13(Suppl. 3):S96–S100. [PubMed] [Google Scholar]
- 14.Polanco N., Gutierrez E., Covarsi A., Ariza F., Carreno A., Vigil A., Baltar J., Fernandez-Fresnedo G., Martin C., Pons S., Lorenzo D., Bernis C., Arrizabalaga P., Fernandez-Juarez G., Barrio V., Sierra M., Castellanos I., Espinosa M., Rivera F., Oliet A., Fernandez-Vega F., Praga M. Grupo de Estudio de las Enfermedades Glomerulares de la Sociedad Espanola de, N. Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J. Am. Soc. Nephrol. 2010;21(4):697–704. doi: 10.1681/ASN.2009080861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polanco N., Gutierrez E., Rivera F., Castellanos I., Baltar J., Lorenzo D., Praga M. Grupo de Estudio de las Enfermedades Glomerulares de la Sociedad Espanola de, N. Spontaneous remission of nephrotic syndrome in membranous nephropathy with chronic renal impairment. Nephrol. Dial. Transplant. 2012;27(1):231–234. doi: 10.1093/ndt/gfr285. [DOI] [PubMed] [Google Scholar]
- 16.Glassock R.J. Diagnosis and natural course of membranous nephropathy. Semin. Nephrol. 2003;23(4):324–332. doi: 10.1016/s0270-9295(03)00049-4. [DOI] [PubMed] [Google Scholar]
- 17.Nangaku M., Shankland S.J., Couser W.G. Cellular response to injury in membranous nephropathy. J. Am. Soc. Nephrol. 2005;16(5):1195–1204. doi: 10.1681/ASN.2004121098. [DOI] [PubMed] [Google Scholar]
- 18.Imai H., Hamai K., Komatsuda A., Ohtani H., Miura A.B. IgG subclasses in patients with membranoproliferative glomerulonephritis, membranous nephropathy, and lupus nephritis. Kidney Int. 1997;51(1):270–276. doi: 10.1038/ki.1997.32. [DOI] [PubMed] [Google Scholar]
- 19.Kuroki A., Shibata T., Honda H., Totsuka D., Kobayashi K., Sugisaki T. Glomerular and serum IgG subclasses in diffuse proliferative lupus nephritis, membranous lupus nephritis, and idiopathic membranous nephropathy. Intern. Med. 2002;41(11):936–942. doi: 10.2169/internalmedicine.41.936. [DOI] [PubMed] [Google Scholar]
- 20.Ohtani H., Wakui H., Komatsuda A., Okuyama S., Masai R., Maki N., Kigawa A., Sawada K., Imai H. Distribution of glomerular IgG subclass deposits in malignancy-associated membranous nephropathy. Nephrol. Dial. Transplant. 2004;19(3):574–579. doi: 10.1093/ndt/gfg616. [DOI] [PubMed] [Google Scholar]
- 21.Segawa Y., Hisano S., Matsushita M., Fujita T., Hirose S., Takeshita M., Iwasaki H. IgG subclasses and complement pathway in segmental and global membranous nephropathy. Pediatr. Nephrol. 2010;25(6):1091–1099. doi: 10.1007/s00467-009-1439-8. [DOI] [PubMed] [Google Scholar]
- 22.Glassock R.J. Human idiopathic membranous nephropathy--a mystery solved? N. Engl. J. Med. 2009;361(1):81–83. doi: 10.1056/NEJMe0903343. [DOI] [PubMed] [Google Scholar]
- 23.Dixon F.J., Feldman J.D., Vazquez J.J. Experimental glomerulonephritis. The pathogenesis of a laboratory model resembling the spectrum of human glomerulonephritis. J. Exp. Med. 1961;113:899–920. doi: 10.1084/jem.113.5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson C.B., Dixon F.J. Quantitation of acute and chronic serum sickness in the rabbit. J. Exp. Med. 1971;134(3 Pt 2):7s–8s. [PubMed] [Google Scholar]
- 25.Heymann W., Hackel D.B., Harwood S., Wilson S.G., Hunter J.L. Production of nephrotic syndrome in rats by Freund's adjuvants and rat kidney suspensions. Proc. Soc. Exp. Biol. Med. 1959;100(4):660–664. doi: 10.3181/00379727-100-24736. [DOI] [PubMed] [Google Scholar]
- 26.Border W.A., Ward H.J., Kamil E.S., Cohen A.H. Induction of membranous nephropathy in rabbits by administration of an exogenous cationic antigen. J. Clin. Invest. 1982;69(2):451–461. doi: 10.1172/JCI110469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerjaschki D., Farquhar M.G. The pathogenic antigen of Heymann nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proc. Natl. Acad. Sci. USA. 1982;79(18):5557–5561. doi: 10.1073/pnas.79.18.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerjaschki D., Farquhar M.G. Immunocytochemical localization of the Heymann nephritis antigen (GP330) in glomerular epithelial cells of normal Lewis rats. J. Exp. Med. 1983;157(2):667–686. doi: 10.1084/jem.157.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cybulsky A.V., Quigg R.J., Salant D.J. Experimental membranous nephropathy redux. Am. J. Physiol. Renal Physiol. 2005;289(4):F660–F671. doi: 10.1152/ajprenal.00437.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cunningham P.N., Quigg R.J. Contrasting roles of complement activation and its regulation in membranous nephropathy. J. Am. Soc. Nephrol. 2005;16(5):1214–1222. doi: 10.1681/ASN.2005010096. [DOI] [PubMed] [Google Scholar]
- 31.Neale T.J., Ojha P.P., Exner M., Poczewski H., Ruger B., Witztum J.L., Davis P., Kerjaschki D. Proteinuria in passive Heymann nephritis is associated with lipid peroxidation and formation of adducts on type IV collagen. J. Clin. Invest. 1994;94(4):1577–1584. doi: 10.1172/JCI117499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ronco P., Debiec H. Target antigens and nephritogenic antibodies in membranous nephropathy: of rats and men. Semin. Immunopathol. 2007;29(4):445–458. doi: 10.1007/s00281-007-0091-2. [DOI] [PubMed] [Google Scholar]
- 33.Debiec H., Guigonis V., Mougenot B., Decobert F., Haymann J.P., Bensman A., Deschenes G., Ronco P.M. Antenatal membranous glomerulonephritis due to anti-neutral endopeptidase antibodies. N. Engl. J. Med. 2002;346(26):2053–2060. doi: 10.1056/NEJMoa012895. [DOI] [PubMed] [Google Scholar]
- 34.Tomas N.M., Beck L.H., Jr, Meyer-Schwesinger C., Seitz-Polski B., Ma H., Zahner G., Dolla G., Hoxha E., Helmchen U., Dabert-Gay A.S., Debayle D., Merchant M., Klein J., Salant D.J., Stahl R.A., Lambeau G. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N. Engl. J. Med. 2014;371(24):2277–2287. doi: 10.1056/NEJMoa1409354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoxha E., Harendza S., Zahner G., Panzer U., Steinmetz O., Fechner K., Helmchen U., Stahl R.A. An immunofluorescence test for phospholipase-A(2)-receptor antibodies and its clinical usefulness in patients with membranous glomerulonephritis. Nephrol. Dial. Transplant. 2011;26(8):2526–2532. doi: 10.1093/ndt/gfr247. [DOI] [PubMed] [Google Scholar]
- 36.Qin W., Beck L.H., Jr, Zeng C., Chen Z., Li S., Zuo K., Salant D.J., Liu Z. Anti-phospholipase A2 receptor antibody in membranous nephropathy. J. Am. Soc. Nephrol. 2011;22(6):1137–1143. doi: 10.1681/ASN.2010090967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh Y.J., Yang S.H., Kim D.K., Kang S.W., Kim Y.S. Autoantibodies against phospholipase A2 receptor in Korean patients with membranous nephropathy. PLoS One. 2013;8(4):e62151. doi: 10.1371/journal.pone.0062151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akiyama S., Akiyama M., Imai E., Ozaki T., Matsuo S., Maruyama S. Prevalence of anti-phospholipase A2 receptor antibodies in Japanese patients with membranous nephropathy. Clin. Exp. Nephrol. 2014 doi: 10.1007/s10157-014-1054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ardalan M., Ghafari A., Hamzavi F., Nasri H., Baradaran B., Majidi J., Nikbin B. Anti-phospholipase A2 receptor antibody in idiopathic membranous nephropathy: A report from Iranian population. J. Nephropathol. 2013;2(4):241–248. doi: 10.12860/JNP.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoxha E., Kneissler U., Stege G., Zahner G., Thiele I., Panzer U., Harendza S., Helmchen U.M., Stahl R.A. Enhanced expression of the M-type phospholipase A2 receptor in glomeruli correlates with serum receptor antibodies in primary membranous nephropathy. Kidney Int. 2012;82(7):797–804. doi: 10.1038/ki.2012.209. [DOI] [PubMed] [Google Scholar]
- 41.Hofstra J.M., Debiec H., Short C.D., Pelle T., Kleta R., Mathieson P.W., Ronco P., Brenchley P.E., Wetzels J.F. Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J. Am. Soc. Nephrol. 2012;23(10):1735–1743. doi: 10.1681/ASN.2012030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Svobodova B., Honsova E., Ronco P., Tesar V., Debiec H. Kidney biopsy is a sensitive tool for retrospective diagnosis of PLA2R-related membranous nephropathy. Nephrol. Dial. Transplant. 2013;28(7):1839–1844. doi: 10.1093/ndt/gfs439. [DOI] [PubMed] [Google Scholar]
- 43.Larsen C.P., Messias N.C., Silva F.G., Messias E., Walker P.D. Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor staining in renal biopsies. Mod. Pathol. 2013;26(5):709–715. doi: 10.1038/modpathol.2012.207. [DOI] [PubMed] [Google Scholar]
- 44.Barrett C.M., Troxell M.L., Larsen C.P., Houghton D.C. Membranous glomerulonephritis with crescents. Int. Urol. Nephrol. 2014;46(5):963–971. doi: 10.1007/s11255-013-0593-x. [DOI] [PubMed] [Google Scholar]
- 45.Debiec H., Martin L., Jouanneau C., Dautin G., Mesnard L., Rondeau E., Mousson C., Ronco P. Autoantibodies specific for the phospholipase A2 receptor in recurrent and De Novo membranous nephropathy. Am. J. Transplant. 2011;11(10):2144–2152. doi: 10.1111/j.1600-6143.2011.03643.x. [DOI] [PubMed] [Google Scholar]
- 46.Stahl R., Hoxha E., Fechner K. PLA2R autoantibodies and recurrent membranous nephropathy after transplantation. N. Engl. J. Med. 2010;363(5):496–498. doi: 10.1056/NEJMc1003066. [DOI] [PubMed] [Google Scholar]
- 47.Quintana L.F., Blasco M., Seras M., Perez N.S., Lopez-Hoyos M., Villarroel P., Rodrigo E., Vinas O., Ercilla G., Diekmann F., Gomez-Roman J.J., Fernandez-Fresnedo G., Oppenheimer F., Arias M., Campistol J.M. Antiphospholipase A2 receptor antibody levels predict the risk of posttransplantation recurrence of membranous nephropathy. Transplantation. 2015 doi: 10.1097/TP.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 48.Larsen C.P., Walker P.D. Phospholipase A2 receptor (PLA2R) staining is useful in the determination of de novo versus recurrent membranous glomerulopathy. Transplantation. 2013;95(10):1259–1262. doi: 10.1097/TP.0b013e31828a947b. [DOI] [PubMed] [Google Scholar]
- 49.Zahner G., Meyer-Schwesinger C., Tomas N.M., Hoxha E., Wiech T., Stahl R.A. In Development, and Morphologic Characteriziation of a Mouse Model of Membranous Nephropathy Involving the Human Phospholipase A2 Receptor, Kidney Week 2014, Philadelphia, PA, Nov. 11-16, 2014; American Society of Nephrology: Florida, U.S.A., 2014; pp. 66A. ; 2014. [Google Scholar]
- 50.Baker P.J., Ochi R.F., Schulze M., Johnson R.J., Campbell C., Couser W.G. Depletion of C6 prevents development of proteinuria in experimental membranous nephropathy in rats. Am. J. Pathol. 1989;135(1):185–194. [PMC free article] [PubMed] [Google Scholar]
- 51.Ma H., Sandor D.G., Beck L.H., Jr The role of complement in membranous nephropathy. Semin. Nephrol. 2013;33(6):531–542. doi: 10.1016/j.semnephrol.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lhotta K., Wurzner R., Konig P. Glomerular deposition of mannose-binding lectin in human glomerulonephritis. Nephrol. Dial. Transplant. 1999;14(4):881–886. doi: 10.1093/ndt/14.4.881. [DOI] [PubMed] [Google Scholar]
- 53.Debiec H., Hanoy M., Francois A., Guerrot D., Ferlicot S., Johanet C., Aucouturier P., Godin M., Ronco P. Recurrent membranous nephropathy in an allograft caused by IgG3kappa targeting the PLA2 receptor. J. Am. Soc. Nephrol. 2012;23(12):1949–1954. doi: 10.1681/ASN.2012060577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okazaki K., Uchida K., Sumimoto K., Mitsuyama T., Ikeura T., Takaoka M. Autoimmune pancreatitis: pathogenesis, latest developments and clinical guidance. Ther. Adv. Chronic Dis. 2014;5(3):104–111. doi: 10.1177/2040622314527120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hirayama K., Ebihara I., Yamamoto S., Kai H., Muro K., Yamagata K., Kobayashi M., Koyama A. Predominance of type-2 immune response in idiopathic membranous nephropathy. Cytoplasmic cytokine analysis. Nephron. 2002;91(2):255–261. doi: 10.1159/000058401. [DOI] [PubMed] [Google Scholar]
- 56.Kuroki A., Iyoda M., Shibata T., Sugisaki T. Th2 cytokines increase and stimulate B cells to produce IgG4 in idiopathic membranous nephropathy. Kidney Int. 2005;68(1):302–310. doi: 10.1111/j.1523-1755.2005.00415.x. [DOI] [PubMed] [Google Scholar]
- 57.Ancian P., Lambeau G., Mattei M.G., Lazdunski M. The human 180-kDa receptor for secretory phospholipases A2. Molecular cloning, identification of a secreted soluble form, expression, and chromosomal localization. J. Biol. Chem. 1995;270(15):8963–8970. doi: 10.1074/jbc.270.15.8963. [DOI] [PubMed] [Google Scholar]
- 58.Ishizaki J., Hanasaki K., Higashino K., Kishino J., Kikuchi N., Ohara O., Arita H. Molecular cloning of pancreatic group I phospholipase A2 receptor. J. Biol. Chem. 1994;269(8):5897–5904. [PubMed] [Google Scholar]
- 59.Llorca O. Extended and bent conformations of the mannose receptor family. Cell. Mol. Life Sci. 2008;65(9):1302–1310. doi: 10.1007/s00018-007-7497-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.East L., Isacke C.M. The mannose receptor family. Biochim. Biophys. Acta. 2002;1572(2-3):364–386. doi: 10.1016/s0304-4165(02)00319-7. [DOI] [PubMed] [Google Scholar]
- 61.Liu Y., Chirino A.J., Misulovin Z., Leteux C., Feizi T., Nussenzweig M.C., Bjorkman P.J. Crystal structure of the cysteine-rich domain of mannose receptor complexed with a sulfated carbohydrate ligand. J. Exp. Med. 2000;191(7):1105–1116. doi: 10.1084/jem.191.7.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sticht H., Pickford A.R., Potts J.R., Campbell I.D. Solution structure of the glycosylated second type 2 module of fibronectin. J. Mol. Biol. 1998;276(1):177–187. doi: 10.1006/jmbi.1997.1528. [DOI] [PubMed] [Google Scholar]
- 63.Weis W.I., Kahn R., Fourme R., Drickamer K., Hendrickson W.A. Structure of the calcium-dependent lectin domain from a rat mannose-binding protein determined by MAD phasing. Science. 1991;254(5038):1608–1615. doi: 10.1126/science.1721241. [DOI] [PubMed] [Google Scholar]
- 64.Boskovic J., Arnold J.N., Stilion R., Gordon S., Sim R.B., Rivera-Calzada A., Wienke D., Isacke C.M., Martinez-Pomares L., Llorca O. Structural model for the mannose receptor family uncovered by electron microscopy of Endo180 and the mannose receptor. J. Biol. Chem. 2006;281(13):8780–8787. doi: 10.1074/jbc.M513277200. [DOI] [PubMed] [Google Scholar]
- 65.Hanasaki K., Arita H. Purification and characterization of a high-affinity binding protein for pancreatic-type phospholipase A2. Biochim. Biophys. Acta. 1992;1127(3):233–241. doi: 10.1016/0005-2760(92)90226-l. [DOI] [PubMed] [Google Scholar]
- 66.Zvaritch E., Lambeau G., Lazdunski M. Endocytic properties of the M-type 180-kDa receptor for secretory phospholipases A2. J. Biol. Chem. 1996;271(1):250–257. doi: 10.1074/jbc.271.1.250. [DOI] [PubMed] [Google Scholar]
- 67.Hanasaki K., Arita H. Phospholipase A2 receptor: a regulator of biological functions of secretory phospholipase A2. Prostag. Oth. Lipid M. 2002;68-69:71–82. doi: 10.1016/s0090-6980(02)00022-9. [DOI] [PubMed] [Google Scholar]
- 68.Murakami M., Taketomi Y., Girard C., Yamamoto K., Lambeau G. Emerging roles of secreted phospholipase A2 enzymes: Lessons from transgenic and knockout mice. Biochimie. 2010;92(6):561–582. doi: 10.1016/j.biochi.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 69.Augert A., Payre C., de Launoit Y., Gil J., Lambeau G., Bernard D. The M-type receptor PLA2R regulates senescence through the p53 pathway. EMBO Rep. 2009;10(3):271–277. doi: 10.1038/embor.2008.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vindrieux D., Augert A., Girard C.A., Gitenay D., Lallet-Daher H., Wiel C., Le Calve B., Gras B., Ferrand M., Verbeke S., de Launoit Y., Leroy X., Puisieux A., Aubert S., Perrais M., Gelb M., Simonnet H., Lambeau G., Bernard D. PLA2R1 mediates tumor suppression by activating JAK2. Cancer Res. 2013;73(20):6334–6345. doi: 10.1158/0008-5472.CAN-13-0318. [DOI] [PubMed] [Google Scholar]
- 71.Granata F., Staiano R.I., Loffredo S., Petraroli A., Genovese A., Marone G., Triggiani M. The role of mast cell-derived secreted phospholipases A2 in respiratory allergy. Biochimie. 2010;92(6):588–593. doi: 10.1016/j.biochi.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 72.Hanasaki K., Ono T., Saiga A., Morioka Y., Ikeda M., Kawamoto K., Higashino K., Nakano K., Yamada K., Ishizaki J., Arita H. Purified group X secretory phospholipase A(2) induced prominent release of arachidonic acid from human myeloid leukemia cells. J. Biol. Chem. 1999;274(48):34203–34211. doi: 10.1074/jbc.274.48.34203. [DOI] [PubMed] [Google Scholar]
- 73.Hanasaki K., Yokota Y., Ishizaki J., Itoh T., Arita H. Resistance to endotoxic shock in phospholipase A2 receptor-deficient mice. J. Biol. Chem. 1997;272(52):32792–32797. doi: 10.1074/jbc.272.52.32792. [DOI] [PubMed] [Google Scholar]
- 74.Higashino K., Ishizaki J., Kishino J., Ohara O., Arita H. Structural comparison of phospholipase-A2-binding regions in phospholipase-A2 receptors from various mammals. Eur. J. Biochem. 1994;225(1):375–382. doi: 10.1111/j.1432-1033.1994.00375.x. [DOI] [PubMed] [Google Scholar]
- 75.Silliman C.C., Moore E.E., Zallen G., Gonzalez R., Johnson J.L., Elzi D.J., Meng X., Hanasaki K., Ishizaki J., Arita H., Ao L., England K.M., Banerjee A. Presence of the M-type sPLA(2) receptor on neutrophils and its role in elastase release and adhesion. Am. J. Physiol. Cell Physiol. 2002;283(4):C1102–C1113. doi: 10.1152/ajpcell.00608.2001. [DOI] [PubMed] [Google Scholar]
- 76.Moses E.K., Freed K.A., Brennecke S.P., Rice G.E. Distribution of the phospholipase A2 receptor messenger RNA in human gestational tissues. Placenta. 1998;19(1):35–40. doi: 10.1016/s0143-4004(98)90096-0. [DOI] [PubMed] [Google Scholar]
- 77.Menschikowski M., Platzbecker U., Hagelgans A., Vogel M., Thiede C., Schonefeldt C., Lehnert R., Eisenhofer G., Siegert G. Aberrant methylation of the M-type phospholipase A(2) receptor gene in leukemic cells. BMC Cancer. 2012;12:576. doi: 10.1186/1471-2407-12-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stanescu H.C., Arcos-Burgos M., Medlar A., Bockenhauer D., Kottgen A., Dragomirescu L., Voinescu C., Patel N., Pearce K., Hubank M., Stephens H.A., Laundy V., Padmanabhan S., Zawadzka A., Hofstra J.M., Coenen M.J., den Heijer M., Kiemeney L.A., Bacq-Daian D., Stengel B., Powis S.H., Brenchley P., Feehally J., Rees A.J., Debiec H., Wetzels J.F., Ronco P., Mathieson P.W., Kleta R. Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. N. Engl. J. Med. 2011;364(7):616–626. doi: 10.1056/NEJMoa1009742. [DOI] [PubMed] [Google Scholar]
- 79.Coenen M.J., Hofstra J.M., Debiec H., Stanescu H.C., Medlar A.J., Stengel B., Boland-Auge A., Groothuismink J.M., Bockenhauer D., Powis S.H., Mathieson P.W., Brenchley P.E., Kleta R., Wetzels J.F., Ronco P. Phospholipase A2 receptor (PLA2R1) sequence variants in idiopathic membranous nephropathy. J. Am. Soc. Nephrol. 2013;24(4):677–683. doi: 10.1681/ASN.2012070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Behnert A., Fritzler M.J., Teng B., Zhang M., Bollig F., Haller H., Skoberne A., Mahler M., Schiffer M. An anti-phospholipase A2 receptor quantitative immunoassay and epitope analysis in membranous nephropathy reveals different antigenic domains of the receptor. PLoS One. 2013;8(4):e61669. doi: 10.1371/journal.pone.0061669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rivera-Calzada A., Robertson D., MacFadyen J.R., Boskovic J., Isacke C.M., Llorca O. Three-dimensional interplay among the ligand-binding domains of the urokinase-plasminogen-activator-receptor-associated protein, Endo180. EMBO Rep. 2003;4(8):807–812. doi: 10.1038/sj.embor.embor898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fresquet M., Jowitt T.A., Gummadova J., Collins R., O'Cualain R., McKenzie E.A., Lennon R., Brenchley P.E. Identification of a major epitope recognized by PLA2R autoantibodies in primary membranous nephropathy. J. Am. Soc. Nephrol. 2015;26(2):302–313. doi: 10.1681/ASN.2014050502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fresquet M., Jowitt T.A., Gummadova J.O., McKenzie E.A., Lennon R., Brenchley P.E. In Understanding the Structure of PLA2R: Insights into Formation of the Immunodominant Epitope Recognized by Autoantibodies in Patients with Idiopathic Membranous Nephropathy, ASN Kidney Week 2013, Atlanta, GA, Nov. 5-10, 2013; American Society of Nephrology: Florida, U.S.A., 2013; pp. 21A. ; 2013. [Google Scholar]
- 84.Salant D.J. Genetic variants in membranous nephropathy: perhaps a perfect storm rather than a straightforward conformeropathy? J. Am. Soc. Nephrol. 2013;24(4):525–528. doi: 10.1681/ASN.2013020166. [DOI] [PubMed] [Google Scholar]
- 85.Zhu Q., Kao L. In PLA2R tightly associates with integrin a3b1 in the podocyte membrane: A new insight into IMN pathogenesis, Kidney Week 2013, Atlanta, GA, Nov. 5-10, 2013; American Society of Nephrology: Florida, U.S.A., 2013; pp. 567A. ; 2013. [Google Scholar]
- 86.Luo B.H., Carman C.V., Springer T.A. Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dahnrich C., Komorowski L., Probst C., Seitz-Polski B., Esnault V., Wetzels J.F., Hofstra J.M., Hoxha E., Stahl R.A., Lambeau G., Stocker W., Schlumberger W. Development of a standardized ELISA for the determination of autoantibodies against human M-type phospholipase A2 receptor in primary membranous nephropathy. Clin. Chim. Acta. 2013;421:213–218. doi: 10.1016/j.cca.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 88.Timmermans S.A., Damoiseaux J.G., Heerings-Rewinkel P.T., Ayalon R., Beck L.H., Jr, Schlumberger W., Salant D.J., van Paassen P., Tervaert J.W., Limburg Renal R. Evaluation of anti-PLA2R1 as measured by a novel ELISA in patients with idiopathic membranous nephropathy: a cohort study. Am. J. Clin. Pathol. 2014;142(1):29–34. doi: 10.1309/AJCP8QMOY5GLRSFP. [DOI] [PubMed] [Google Scholar]
- 89.Behnert A., Schiffer M., Muller-Deile J., Beck L.H., Jr, Mahler M., Fritzler M.J. Antiphospholipase A(2) receptor autoantibodies: a comparison of three different immunoassays for the diagnosis of idiopathic membranous nephropathy. . J. Immunol. Res. 2014. [DOI] [PMC free article] [PubMed]
- 90.Beck L.H., Jr, Fervenza F.C., Beck D.M., Bonegio R.G., Malik F.A., Erickson S.B., Cosio F.G., Cattran D.C., Salant D.J. Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J. Am. Soc. Nephrol. 2011;22(8):1543–1550. doi: 10.1681/ASN.2010111125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ruggenenti P., Debiec H., Ruggiero B., Chianca A., Pelle T., Gaspari F., Suardi F., Gagliardini E., Orisio S., Benigni A., Ronco P., Remuzzi G. Anti-Phospholipase A2 Receptor Antibody Titer Predicts Post-Rituximab Outcome of Membranous Nephropathy. J. Am. Soc. Nephrol. 2015 doi: 10.1681/ASN.2014070640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Meyrier A., Ronco P. Allotransplantation using a diseased kidney: when a swallow makes a summer. Nephrol. Dial. Transplant. 2014;29(12):2164–2166. doi: 10.1093/ndt/gfu336. [DOI] [PubMed] [Google Scholar]
- 93.Harrison L.C. The prospect of vaccination to prevent type 1 diabetes. Hum. Vaccin. 2005;1(4):143–150. doi: 10.4161/hv.1.4.1923. [DOI] [PubMed] [Google Scholar]
- 94.Harrison L.C., Wentworth J.M., Zhang Y., Bandala-Sanchez E., Bohmer R.M., Neale A.M., Stone N.L., Naselli G., Bosco J.J., Auyeung P., Rashidi M., Augstein P., Morahan G. Antigen-based vaccination and prevention of type 1 diabetes. Curr. Diab. Rep. 2013;13(5):616–623. doi: 10.1007/s11892-013-0415-7. [DOI] [PubMed] [Google Scholar]


