Abstract
Background:
Patients suffering from diabetes mellitus (DM) may experience an increased risk of cancer; however, it is not certain whether this effect is due to diabetes per se.
Objective:
To examine the association between DM and cancers by a systematic review and meta-analysis according to the PRISMA guidelines.
Data Sources:
The systematic literature search includes Medline at PubMed, Embase, Cinahl, Bibliotek.dk, Cochrane library, Web of Science and SveMed+ with the search terms: “Diabetes mellitus”, “Neoplasms”, and “Risk of cancer”.
Study Eligibility Criteria:
The included studies compared the risk of cancer in diabetic patients versus non-diabetic patients. All types of observational study designs were included.
Results:
Diabetes patients were at a substantially increased risk of liver (RR=2.1), and pancreas (RR=2.2) cancer. Modestly elevated significant risks were also found for ovary (RR=1.2), breast (RR=1.1), cervix (RR=1.3), endometrial (RR=1.4), several digestive tract (RR=1.1-1.5), kidney (RR=1.4), and bladder cancer (RR=1.1). The findings were similar for men and women, and unrelated to study design. Meta-regression analyses showed limited effect modification of body mass index, and possible effect modification of age, gender, with some influence of study characteristics (population source, cancer- and diabetes ascertainment).
Limitations:
Publication bias seemed to be present. Only published data were used in the analyses.
Conclusions:
The systematic review and meta-analysis confirm the previous results of increased cancer risk in diabetes and extend this to additional cancer sites. Physicians in contact with patients with diabetes should be aware that diabetes patients are at an increased risk of cancer.
Keywords: Cancer risk, diabetes mellitus, meta-analysis, neoplasm, systematic review
Introduction
Rationale
The CAncer Risk and INsulin analoGues (CARING) project aims to assess the possible carcinogenic effect of
insulin. As part of this project evaluation of the background risk of developing cancer in diabetes patients was performed in this systematic review and meta-analysis.
Diabetes Mellitus is associated with increased morbidity and mortality. Diabetes is the 8th leading cause of mortality in high-income countries; whereas colorectal and breast cancer are the 7th and 10th leading causes, respectively [1]. Associations between diabetes and cancer have already been established for specific cancer sites in several meta-analyses [2-23], however it is not known whether the observed associations were due to diabetes per sé or caused by competing risks.
Associations between diabetes and cancer have been established by several meta-analyses including only studies of an observational design (case control and/or cohort). All meta-analyses reporting a significant increased risk among diabetes patients for pancreatic cancer between 1.8 to 2.1 [2-4] and liver cancer between 1.8 to 3.6 [5-8, 24]. Subgroup analyses stratified by gender or statistical adjustment for Body Mass Index (BMI), smoking and alcohol did not influence the risk for pancreatic cancer [2]. However, results were conflicting on whether a duration of diabetes of 10 years was associated with an increased risk of pancreatic cancer [2, 3], while duration of diabetes appeared not to influence risk of liver cancer [5]. Furthermore, diabetes treatment modulated the risk of liver cancer with greater risk estimates for insulin or sulfonylurea users than for metformin users [5]. Several observational studies have examined the relationship between diabetes and gastrointestinal cancers. Results were conflicting in the meta-analyses on gastric cancer [15,16], while an increased risk of esophageal cancer was reported [13]. In addition, diabetes has been associated with an increased risk of colorectal cancer [17-19, 21-23] after adjustment for BMI and smoking [20]. Both endometrial cancer [7] and breast cancer [25-28] were reported to be increased in diabetes, while prostate cancer was found to be decreased in men with diabetes by 10% [9,10]. The association with prostate cancer was independent of BMI [10]. Diabetes was also associated with increased risk of kidney cancer [11] and bladder cancer [12]; however this last association was not significant when using estimates adjusted for BMI due to wider confidence intervals. Last of all an increased risk among diabetes patients for non-Hodgkin lymphoma and leukemia but not multiple myeloma has also been reported in a meta-analysis [14].
It is uncertain whether the relationship between diabetes and cancer is direct (e.g., due to hyperglycemia), whether diabetes is a marker of underlying biologic factors that alter cancer risk (e.g., insulin resistance and hyperinsulinemia), or whether the association between diabetes and cancer is indirect and due to common risk factors such as obesity. Duration of diabetes has been found to be of importance in the development of cancer among insulin using type 2 diabetes (T2D) patients [29]; however whether cancer risk was influenced by the duration of diabetes is a critical and complex issue and may be complicated further by the multidrug therapy often necessary for diabetes treatment. The incidence of cancer increases with age, and as age increases with duration of diabetes, this may confound the association between diabetes and cancer. However, an association between diabetes and cancer was present for several cancer sites. Few studies take into account duration of diabetes, medication use or age of the participants. Furthermore, a meta-analysis reported an association between obesity and several cancer types including colorectal, kidney, breast and endometrial cancer, and also an independent association between obesity and T2D [30]. Therefore, it is important both to take obesity into account and to distinguish between type 1 diabetes (T1D) and T2D, which have not been done in previous reports. Except from Ge et al. [16] (using three databases), none of the meta-analyses described in the introduction have used more than two databases in their search (Medline at PubMed and Embase/ Medline at PubMed and Cochrane database of systematic reviews), and many only used Medline at PubMed leaving them with a possible publication bias.
Objectives
In an attempt to evaluate the risk of cancer in diabetes patients and taking possible determinants into account this thorough systematic review and meta-analysis was conducted. The primary objective was to study the effects of diabetes per sé, by collating observational studies that compared diabetes patients to non-diabetes. A secondary objective was to examine the effects that type of diabetes, body weight, metabolic control, diet as well as study design had on the risk of cancer.
Methods
Protocol and Registration
The systematic review and meta-analysis was developed according to the Cochrane Collaboration (http://www.cochr ane.org/training/cochrane-handbook), and PRISMA guidelines [31] (http://www.prisma-statement.org/) and was registered on PROSPERO (http://www.crd.york.ac.uk/prospero/) with the registration number: CRD42012002310.
Eligibility Criteria
The eligibility criteria for the studies were those studies that evaluated the association between diabetes and cancer (incidence, odds or prevalence) as the outcomes. Studies evaluating solely cancer mortality were excluded. The studies needed to compare diabetes patients with a non-diabetes reference group. All types of observational study designs (e.g. case control, cohort and cross-sectional studies) were included. Studies assessing the effect of a specific intervention compared to no intervention were excluded. Studies only published as conference abstracts were excluded. Studies were not excluded due to language or publication year.
Information Sources
The systematic literature search included 7 databases: Medline at PubMed, Embase, Cinahl, Bibliotek.dk, Cochrane library, Web of Science, and SveMed+. The first search was performed 11th of January 2012, and updated with the last search on the 9th November 2012. Additional studies were added after assessment of the reference list in meta-analyses and reviews found in the search. Furthermore, studies were retrieved from the literature search of a systematic review of insulin use and cancer risk also performed by the CARING project group (PROSPERO registration number: CRD42012002428).
Search
The search terms included: “Diabetes mellitus”, “diabetes”, “Neoplasms”, “cancer”, “Prospective study”, “statistics”, “cancer statistics”, and “Risk of cancer”. Other search terms such as statistics and cancer statistics were also used but gave to few results and were not used as the final result. The search was performed using the thesaurus if available in the respective databases. Limitations were used to refine the search if available in the databases (“biochemistry”, “cancer”, ”physiology and endocrinology”, ”cochrane review”, “controlled clinical trial”, “systematic review”, “clinical trial”, “randomized controlled trial”, “review”, “meta-analysis”), qualifiers (“analysis”, “blood”, “classification”, “epidemiology”, “statistics and numerical data”), categories (“endocrinology metabolism”, “oncology”) and research areas (“endocrinology metabolism”, “oncology”, “biochemistry molecular biology”). Search terms, limitations, qualifiers, categories and research areas used differently by database dependent on the functions available at the database. The search from Embase is listed below. The results from #9 in the Embase search were used in this study.
Search from the 09th of November 2012
No. No. Query Results
#1 ‘Diabetes Mellitus’/exp 526,730
#2 ‘Neoplasm’/exp 3,165,370
#3 #1 AND #2 34,949
#4 #1 AND #2 ([biochemistry]/lim 15,064
OR [cancer]/lim
OR [physiology and endocrinology]/lim)
#5 ‘Cancer statistics’/exp 2,034
#6 #4 AND #5 7
#7 ‘Cancer statistics’/exp 272,379
#8 #4 AND #5 36
#9 #1 AND #2([biochemistry]/lim 634
OR [cancer]/lim
OR [physiology and endocrinology]/lim)
AND ([cochrane review]/lim
OR [controlled clinical trial]/lim
OR [systematic review]/lim))
Study Selection and Data Collection Process
Studies were assessed for eligibility using the criteria above. Reviewer one (JSL) performed the literature search in collaboration with a research librarian. Reviewer one and reviewer two (ØK) added additional studies from the insulin and cancer literature search, and studies were added from other meta-analyses and reviews by reviewer one. Reviewer one and reviewer two examined all studies by screening title and abstract. Studies passing this round were retrieved in full text and independently assessed for eligibility by reviewer one and two. Records for which both reviewers agreed on were included in the systematic review and meta-analysis. Disagreement were settled by discussion or if necessary by reviewer three (PV). No supplementary data were collected from the authors of the studies.
Data Items
From each study information was extracted on cancer risk (prevalence ratio, risk ratio, odds ratio, incidence ratio, standardized incidence ratio, hazard ratio), cancer site, patient characteristics including type of diabetes mellitus (type 1, type 2 or unspecified), age (mean/median/not reported), duration of diabetes (mean/median/not reported), HbA1c level (mean/median/not reported), BMI (mean/median/not reported), follow up years (mean/median/not reported), and on study design (case control/cohort/cross-sectional), population (population based/hospital based), confounders used to adjust for, and specific comorbidities. Data was extracted by reviewer one and validated by reviewer two. Any disagreement was solved by discussion. Studies that used the same study population as other studies were excluded by reviewer one and reviewer two to secure that no duplicate estimates were used in the meta-analysis.
Risk of Bias in Individual Studies
The risk of bias in individual studies was assessed using the Newcastle Ottawa Scale (NOS) [32]. The user-defined items required in the NOS score were defined as follows: age was the most important adjustment factor; the exposed patients in cohorts should be representative of the average “diabetic population”, minimum follow up time as 5 years, and loss to follow-up less than 10%. A scale modified for cross-sectional studies were produced for the quality score of these studies (the NOS are available in the Supplementary Material 1).
Reviewer one and reviewer two scored the studies based on the NOS. If the reviewers scored differently it was solved by discussion and if this was not possible reviewer three decided the score.
Summary Measures and Synthesis of Results
Prevalence ratios, risk ratios (RR), odds ratios, incidence ratios, standardized incidence ratios (in general standardized by age and sex using a reference population from same cancer registry, same district or the entire population of a country) and hazard ratios including 95% CI comparing the risk of cancer in diabetes patients compared to a non-diabetes group were the summary measures. A random effects model, Der Simonian and Laird, was used in all analyses [33]. The random effects model considers both in study and between study variability. As all the measures are common effect estimates the pooled result can be interpreted as a risk ratio. Only estimates based on two or more populations were included in the meta-analysis. χ2 tests were used to test for heterogeneity across studies. All analyses were performed in STATA 8 (StataCorp. 2003. Stata Statistical Software: Release 8. College Station, TX: StataCorp LP).
Risk of Bias Across Studies
Risk of publication bias across studies was assessed using Egger’s regression analysis [34] in STATA 8.
Additional Analyses
Subgroup analyses were performed for cancer sites, study design and gender. Meta-regression analyses were performed to assess whether any of the extracted characteristics were determinants of cancer risk. For the meta-regression the covariates were coded as follows: gender (0 = female, 1 = male), diabetes type (0 = unspecified, 1 = T1D, 2 = T2D), study design (0= case control, 1 = cohort, 2 = cross-sectional), source (1=population, 2=hospital, 3=other), adjustment factor (0= no age adjustment, 1= age + other, 2= BMI / obesity / waist hip ratio + other, 3 = Age, BMI +other, 4= Age, Sex, BMI, Smoking + other) 5= Age, BMI and duration of diabetes), diabetes ascertainment (1 = registry, 2 = questionnaire / interview, 3 = biochemical analysis or criteria, 4 = other), cancer ascertainment (1 = registry with confirmation, 2 = questionnaire / interview, 3 = pathology / histology/ imaging / criteria, 4=other) and NOS (0-9). Other covers mixed ascertainments and other types of ascertainment. Age (years) was calculated as the difference of the age between cases and controls in case control studies and between diabetes cohort and non-diabetes cohort in cohort studies. The same applied for BMI (kg/m2). Sub analysis for age and BMI were performed by study design. For age, BMI and follow up years only mean or median estimates were used in the meta-regression. Age BMI and follow up years where treated as numerical outcomes in the meta-regression, whereas other variables were treated as categorical outcomes. Only analyses with the use of three or more populations were included in the meta-regression. HbA1c and duration of diabetes were extracted from the records, but too few values (two studies report on mean HbA1c and 4 studies report mean duration of diabetes) were available to perform a meaningful analysis.
Results
Study Selection
The selection process is depicted in Fig. (1). 1,849 records were identified from the database search. An additional 172 records were identified from the reference list in meta-analyses and reviews identified in the search, and from the systematic literature search (PROSPERO registration number: CRD42012002428) on insulin and cancer also performed by the CARING group. In total 2,021 records were identified. The RefWorks (RefWorks, RefWorks-COS, ProQuest RefWorks 2.0, 2010) functions exact duplicates and close duplicates were used to remove duplicates. In total 1,785 unique records were retrieved. Screening by title and abstract by reviewer one and two excluded 1,534 records, thus 251 records remained. Of these records, 193 records (106 cohort studies, 80 case-control studies, 6 cross-sectional studies and 1 combined case-control and cross-sectional study [35]) were included in the systematic review, while 66 records were excluded after assessing for full text eligibility (21 were excluded due to duplicate data with other studies, 3 were excluded due to lack of data, 11 were excluded because diabetes was not the exposure, 4 were excluded because they did not compare to a non-diabetes reference, 1 record was excluded due to interventional study design and 16 studies were excluded because the outcome was not incident or prevalent cancer). 190 records were included in the meta-analysis. Two studies were excluded from this analysis due to lack of information on the outcome to an extent that made analysis impossible [36, 37]. One study was the only to report on head and neck cancer [38] and was not included in the meta-analysis.
Fig. (1).
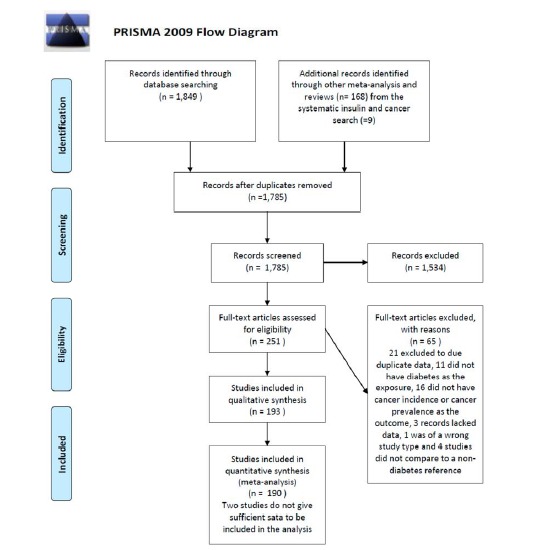
PRISMA flow diagram.
Study Characteristics and Risk of Bias within Studies
Tables 1-3 present the study characteristics and NOS score of the included studies in the systematic review for cohort and cross-sectional studies and case-control studies, respectively. Additional study information is available in the electronic Supplementary Material 2. The study quality ranged from as low as 3 to the highest score of 9, although most of studies (84%) were of fair quality (NOS 6-9). NOS is part of the meta-regression presented below.
Table 1.
Study Table of Included Cohort Studies Divided by Diabetes Type
| Authors | Data Source | Cancer Site | Follow Up Years | Source | DM (n) | Age | BMI | Non- DM (n) | Age | BMI | Co Morbidity | NOS-Score (0-9) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort Studies | ||||||||||||
| Type 1 diabetes | ||||||||||||
| Zendehdel 2003 Sweden [39] | Swedish inpatient registry | Several | 14.4 | Population | 29,187 | 17.1 | - | External standard population | - | - | - | 8 |
| Type 2 diabetes | ||||||||||||
| Kao 2012 Taiwan [40] | NHIRD | All | 2001-2009 | Population | 22,910 | 56.5 | - | 91,636 | 56.5 | - | - | 8 |
| Bowker 2011 Canada [41] | BCLHD (1996-2006) | Breast | 4.4 | Population | 84,506 | 61.8 | - | 84,506 | 61.8 | - | - | 6 |
| Michels 2003 US [42] | Nurses health study (1976-1998) | Breast | 22 (total) | Nurses | 6,120 | 59.1 | 30.7 | 110,368 | 52.1 | 25.0 | 6 | |
| Campbell 2010 [43] | Cancer prevention study II Nutrition cohort | Colorectal | 1992-2007 | Population | 11,335 | 63 | - | 143,640 | 64 | - | - | 7 |
| Ren 2009 China [44] | Nan-Hu district | Colorectal | - | Population | 7,938 | 61.1 | 23.6 | External standard population | - | - | - | 6 |
| Lai 2006 Taiwan [45] | KCIS (1999-2003) | Liver | 2.78 | Population | 5,732 | - | - | 49,184 | - | - | - | 5 |
| Wang 2009 Taiwan [46] | A-Lein Township | Liver | 8 (total) | Viral hepatitis screened. | 352 | - | - | 5,377 | 53.9 (total) | - | - | 9 |
| Joh 2011 US [47] | Nurses Health study | Kidney | 1976-2008 | Nurses | 6,424 | 57,0 | 30,5 | 107,714 | 56.8 | 25,5 | 6 | |
| Hemminiki 2010 Sweden [48] | Nationwide hospital discharge 1964-2007 | Several | 13 median | Population | 125,126 | - | - | External standard population | - | - | - | 9 |
| Hense 2011 Germany [49] | SHI 2003-2008 | Several | 3.5 median | Disease management programme | 26,742 | 64 | ♂ 29.7 ♀ 31.0 |
External standard population | - | - | - | 6 |
| Lee 2012 Taiwan [50] | NHI programme (1999-2009) | Several | 11 (total) | Population | 104,343 | - | - | 985,815 (Total) | - | - | - | 9 |
| Ogunleye 2009 UK [51] | Tayside | Several | 3.9 | Population (RISCH primary care) |
9,577 | - | - | 19,154 | 62 (total) | - | - | 6 |
| Diabetes type unspecified | ||||||||||||
| Fillenbaum 2000 US [52] | EPESE | Any | 6 (total) | Population | 4034 total | 73.4 | 5 | |||||
| Larsson 2008 Sweden [53] | COSM | Bladder | 9.3 | Population | 2,835 | 64.5 | 27.4 | 43,071 | 60.1 | 25.7 | - | 8 |
| Tripathi 2002 US [54] | IWHS | Bladder | 13 (total) | Population | 6% | - | - | 37,459 (total) | - | - | - | 7 |
| Bosco 2012 US [55] | Black women’s health study | Breast | 10.5 | Population | 1,900 | 49,172 (total) | 6 | |||||
| Chlebowski 2012 US [56] | WHI | Breast | 11.8 | Postmenopausal Population based sample |
3,401 | 62.6 | - | 64,618 | 64.0 | - | - | 7 |
| De Waard 1974 (36) | GP Netherlands | Breast | 5,4 | Population | 7,259 women | 4 | ||||||
| Goodman 1997 Japan [57] | LSS Cohort | Breast | 8.31 | Population (atomic bomb survivors) | - | - | - | 22,200 (total) | - | - | - | 6 |
| Lipscombe 2006 [58] Canada | Ontario 1995-2002 | Breast | 4.5 median | Population | 73,796 | 66.2 | - | 391,714 | 64.9 | - | - | 7 |
| Mink 2002 US [59] | ARIC | Breast | 7.1 | Population | - | - | - | 7,894 (total) | - | - | - | 8 |
| Reeves 2012 US [60] | SOF | Breast | 14,4 | Population | 607 | 7,772 | 7 | |||||
| Sellers 1994 US [61] | IWHS | Breast | 5 (total) | Population | - | - | - | 36,603(total) | - | - | - | 5 |
| Weiderpass 1997 Sweden [62] | Swedish in patient registry | Breast, endometrial | 6.7 | Population | ♂ 63,988 ♀ 70,110 |
♂ 59.2 ♀ 64.2 |
- | External standard population | - | - | - | 6 |
| Lambe 2011 Sweden [63] | AMORIS | Breast, endometrial, ovarian | 11.7 | Population | 5,615 | 58.5 | 26.7 | 225,122 | 46.6 | 23.9 | - | 7 |
| Bowers 2006 Finland [64] | Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study |
Colorectal | 14.1 median | Population | 1,226 | - | - | 27,757 | - | - | - | 7 |
| Flood 2010 UK [65] | BCDDP | Colorectal | 8.4 | Individuals with breast affection and matched healthy individuals (gives no statement on how these were determined). | - | 64.3 | 28.0 | 43,078 | 61.8 | 24.5 | - | 6 |
| Hartz 2012 US [66] | WHI | Colorectal | 8 median | Postmenopausal Population based sample |
4.5% of total | - | - | 150,912 (total) |
63.11 (Total) |
- | - | 7 |
| He 2010 US [67] | Multiethnic cohort | Colorectal | 1993-2006 | Population | ♂15,060 ♀ 16,271 |
- | - | ♂74,418 ♀93,393 |
♂60.2 ♀ 59.7 |
- | - | 7 |
| Hu 1999 US [68] | NHS | Colorectal | 18 (total) | Nurses | 7,069 | 45 | 28 | 111,003 | 42 | 24 | - | 5 |
| Khaw 2004 UK [69] | Norfolk | Colorectal | 6 | Population | - | - | - | 25,623 (total) | 45-79 | - | - | 7 |
| Larsson 2005 [70] Sweden | COSM | Colorectal | 6.2 | Population | 45,550 men (total) | 6 | ||||||
| Limburg 2005 US [71] | IWHS | Colorectal | 14 (total) | Population | 1,900 | 62.3 | 30.6 | 33,072 | 61.5 | 26.8 | - | 7 |
| Nilsen 2001, Norway [72] | Nord-trøndelag | Colorectal | 10.8 median | Population | - | - | - | 75,219 (total) | ♂48.5 ♀49.8 |
- | - | 7 |
| Schoen 1999 US [73] | CHS 1989-1990 | Colorectal | 6.6 | Population | - | - | - | 5,201 | 72.8 (without cancer) |
- | - | 6 |
| Seow 2006 Singapore [74] | Singapore Chinese health study | Colorectal | 7.1 | Population | 5,469 | 60 | 24.1 | 55,851 | 56.0 | 23 | - | 7 |
| Sturmer 2006 US [75] | The Physicians’ Health Study | Colorectal | 19 median | physicians | 9% | - | - | 22,701 | 54 | - | - | 6 |
| Will 1998 US [76] | Cancer prevention study | Colorectal | 1959-1972 | 25 states | 15,487 | ♂57.4 ♀57.8 |
♂25.4 ♀26.2 |
850,946 | ♂52.9 ♀51.7 |
♂25.2 ♀24.2 |
- | 6 |
| Anderson 2001 US [77] | IWHS | Endometrial | 1986-1997 | Population | 1,325 | 62.6 | 30.5 | 23,150 | 61.8 | 26.8 | - | 7 |
| Friberg 2007 Sweden [78] | Uppsala | Endometrial | 7 | Population | 1,628 | 66.5 | 27.5 | 35,145 | 61.7 | 24.9 | - | 8 |
| Lindemann 2008 Norway [79] | HUNT study | Endometrial | 15.7 | Population | 1,010 | - | - | 35,751 | 49 (total) | - | - | 7 |
| Lin 2011 US [80] | NIH-AARP study | Esophagus, gastric | 7.96 | Population | 41,388 | 62.81 | 29.83 | 428,060 | 61.90 | 26.83 | - | 8 |
| Chuma 2008 Japan [81] | Hokkaido University hospital | Liver | 10.2 | Hospital | 19 | 104 (total) | 50.5 (median) | Chronic hepatitis or cirrhosis. Hepatitis C virus positive | 7 | |||
| Di constanzo 2008 Italy [82] | Naples (1994-2004) | Liver | 7 median | Hospital | 41 | - | - | 138 (total) | 63.3 | - | Hepatitis C virus cirrhosis | 4 |
| El-Serag 2004 US [83] | PTF 1985-1990 | Liver | 8.6 | Hospital (Veteran Affairs (VA)) | 173,643 | 61.7 | - | 650,620 | 54.5 | - | - | 6 |
| Hung 2011 Taiwan [84] | Chang Gung Memorial Hospital | Liver | 4.4 | Hospital | 253 (T2D) | 56 median | 25 median | 1,217 | 52 median | 24 median | Inteferon therapy for hepatitis c | 7 |
| Ionnau 2007 US [85] | VA 1994-2005 | Liver | 3.6 | Veterans | 452 | - | - | 1,668 | - | - | Cirrhosis | 6 |
| Kavamura 2010 Japan [86] | Toronamon hospital, Tokyo | Liver | 6.7 median | Hospital | 104 | - | - | 1,954 | 50 (total) | - | Inteferon therapy for hepatitis c | 7 |
| N’kontchou2006 France [87] | - | Liver | 4.2 | Screened for HCC | 231 | - | - | 540 | 61.4 total | 25.4 total | alcoholic or viral C cirrhosis | 6 |
| Ohata 2003 Japan [88] | Nagasaki university hospital | Liver | 6.4 | Hospital | 26 | - | - | 161 (total) | 53 | 22.7 0.24 |
Chronic HCV infection | 7 |
| Ohki 2008 Japan [89] | University of Tokyo Hospital 1994-2004 | Liver | 6.1 | Hospital | - | - | - | 1,431 | 60.1 | - | Chronic HCV infection | 7 |
| Tazawa 2002 Japan [90] | Tsuchiura Kyodo General Hospital | Liver | 5.4 | Hospital | 23 | - | - | 279 (all) | 49.4 | - | Hepatitis C infection | 5 |
| Veldt 2008 Europe and Canada [91] | Hepatology Units | Liver | 4.0 | Hospital | 85 | 51 (median) | 27 (median) | 456 | 49 median | 25 (median) | Hepatitis C and fibrosis or cirrhosis | 6 |
| Adami 1996 Sweden [92] | Swedish in patient register | Liver and biliary tract | 6.7 | Hospital | 153,852 | ♂60.5 ♀65.2 |
- | External standard population | - | - | - | 8 |
| Chen 2010 Taiwan [93] | NHI | Liver and biliary tract | 6.9 median | Population | 615,532 | 60.1 | - | 614,871 | 60.0 | - | - | 9 |
| Ehrlich 2010 US [94] | Kaiser Permanente Medical Care Program Northern California |
Lung | 1996- 2005 | Medical Care Program | 70,645 | 60 median | 29.80 | 51,241 | 51 median | 26.06 | - | 7 |
| Hall 2005 UK [95] | GPRD | Lung | 3.95 | Population (primary care) | 66,848 | 60.8 | - | 267,272 | 60.7 | - | - | 8 |
| Lai 2012 Taiwan [96] | NHI Taiwan 1 million random sample cohort | Lung | 2000-2008 | Population | 19624 | 56.4 | 78,496 | 56.5 | 8 | |||
| Luo 2012 US [97] | WHI | Lung | 11 | Postmenopausal Population |
8,154 | 64.3 | 32.1 | 137,611 | 63.0 | 27.7 | - | 7 |
| Cerhan 1997 US [98] | IWHS | NHL | 1986-1992 | Population | - | - | - | 37, 934 (total) | 61.5 (total) | - | - | 6 |
| Erber 2009 US [99] | Multiethnic cohort (MEC) study | NHL | 10 median | Population | 13% | - | - | ♂87,078 ♀105,972 (total) |
- | - | - | 6 |
| Khan 2008 Europe [100] | EPIC | NHL and multiple myeloma | 8.5 | Population | ♂5,111 ♀6,028 |
♂58% ♀56.8% |
- | ♂134,320 ♀248,018 |
♂51.9% ♀50.1% |
- | - | 7 |
| Gapstur 2012 US [101] | Cancer Prevention Study-II Nutrition Cohort |
Ovary | 1992-2007 | Population | 3,577 | 63.6 | - | 59,863 | 62.2 | - | - | 7 |
| Chen 2011 Taiwan [102] | NHI | Pancreas | 6.9 median | Population | 615,532 | 60.1 | - | 614,871 | 60.0 | - | - | 9 |
| Chow 1995 Sweden [103] | Swedish in patient register | Pancreas | 6.8 | Hospital | ♂63,987 ♀70,109 |
- | - | External standard population | - | - | - | 5 |
| Gupta 2006 US [104] | Veterans Health Administration | Pancreas | 1999-2004 | Veterans (developed diabetes) | 36,631 | 61.8 | - | 1,385,163 | 63.6 | - | - | 7 |
| Larsson 2005 Sweden [105] | COSM and SMC | Pancreas | 1997-2004 | Population | - | - | - | ♀37,147 ♂ 45,906 (total) |
♀62 ♂ 60 (total) |
♀25 ♂25.8 (total) |
- | 8 |
| Liao 2012 Taiwan [106] | NHI | Pancreas | 1998-2007 | Population | 49,803 | 55.92 | - | 199,212 | 55.92 | - | - | 8 |
| Shibata 1994 US [107] | Laguna Hills | Pancreas | 9 (total) | Retirement community | - | - | - | 13,976 (total) | 74 | - | - | 6 |
| Stevens 2009 UK [108] | Breast cancer screening | Pancreas | 7.2 | Population | 2,7% | - | - | 1,290,000 | 55.9 | 26.2 | - | 7 |
| Stolzenberg-Solomon 2002 Finland [109] | ATBC | Pancreas | 10.2 median | Population | - | - | - | 29,048 | 57 | 26.0 | Smokers | 8 |
| Yun 2006 Korea [110] | NHIC | Pancreas | 10 (total) | Population | - | - | - | 446407 | - | - | - | 8 |
| Jamal 2009 US [111] | VA | Pancreas and gallbladder | 1990-2000 | Hospital | 278,761 (diabetes patients) | 65.8 | - | 836,283 (non diabetes patients) | 64.8 | - | - | 7 |
| Giovannuci 1998 US [112] | Health professionals follow up study | Prostate | 1986-1994 | Health professionals | 2,551 | - | - | 45,230 | - | - | - | 6 |
| Leitzmann 2008 US [113] | PLCO | Prostate | 8.9 (total) | From a randomized controlled trial, where participants were randomized to cancer screening. | 3,024 | 64.0 | 28.7 | 30,064 | 62.0 | 26.8 | - | 7 |
| Li 2010 Japan [114] | Ohsaki Cohort | Prostate | 1995-2003 | Population | 1,645 | 62.41 | 23.74 | 20,813 | 59.07 | 23.32 | 7 | |
| Rodriguez 2005 US [115] | Cancer prevention study II nutrition cohort | Prostate | 1992-2001 | Population | 10,053 | 62,617 | 7 | |||||
| Thompson 1989 US [116] | Rancho bernardo | Prostate | 14 (total) | Population | - | - | - | 1,776 (all) | 65.9 | 25.62 | - | 7 |
| Velicer 2007 US [117] | VITAL | Prostate | 2000-2004 | Population (mailing list) | 2,878 | 64.3 | 30.5 | 32,361 | 61.5 | 27.4 | - | 6 |
| Waters 2009 US [118] | The Multiethnic Cohort | Prostate | 1993-2005 | Population | 10,825 | - | - | 86,303 total | 59.9 | - | - | 7 |
| Weiderpass 2002 Sweden [119] | Swedish In-Patient Register | Prostate | 5.6 | Population | 135,950 | 61.7 | - | External standard population | - | - | - | 6 |
| Will 1999 US [120] | 25 states 1959-1972 |
Prostate | 13 (total) | Population | 6,086 | - | - | 298,979 | - | - | - | 5 |
| Nicodemus 2004 US [121] | IWHS 1986-2000 | Kidney | 15 total | Population (drivers license list) | - | - | - | 34,637 (total) | - | - | - | 6 |
| Adami 1991 Sweden [122] | Uppsala In patient registry | Several | 1965-1984 | Hospital | 51,008 | - | - | Expected | - | - | - | 5 |
| Atchison 2010, US [123] | VA hospitals | Several | 10.5 (median) | Veterans (male) | 594,815 | 57.5 | - | 3,906,763 | 51.5 | - | - | 7 |
| Carstensen 2012 Denmark [124] | Central personal register | Several | 1995-2009 | The entire Danish Population | - | - | - | - | - | - | - | 7 |
| Chodick 2010 Israel [125] | Maccabi Healthcare Services |
Several | 8 | Population | 16,721 | 61.6 | - | 83,874 | 61.6 | - | - | 8 |
| Dankner 2007 Israel [126] | Population registry | Several | 20 (total) | Population | 437 | 57.6 | - | 1,740 | 51.9 | - | - | 8 |
| Folsom 2008 [127] | ARIC 1987-1989 | Several | 1987-2000 | Population | - | - | - | 13,117 (total) | - | - | - | 8 |
| Hjalgrim 1997 Denmark [128] | All men born1949-1964 with DM before age 20 | Several | 1968-1992 | Population | 1,659 | - | - | External standard population | - | - | - | 3 |
| Hjalgrim 1997 Denmark [128] | funen county | Several | 1973-1992 | Population | 1,499 | - | - | External standard population | - | - | - | 4 |
| Inoue 2006 Japan [129] | Japan Public Health Center–Based Prospective Study |
Several | 10.7 | Population | ♂3,097♀ 1,571 | ♂54 ♀ 56 |
- | ♂43,451 ♀ 49,652 |
♂51.2 ♀ 51.6 |
- | - | 7 |
| Jee 2005 Korea [130] | NHIC | Several | 10 total | government employees, teachers and dependents (10.7% of total population) | ♂5.1% ♀4.5% |
- | - | ♂829,770 ♀468,615 (total) |
♂45.3 ♀49.6 |
♂23.2 ♀23.2 |
- | 7 |
| Johnson 2011 [131] Canada | BCLHD | Several | 4,3 | Population | 185,100 | 60.7 | 185,100 | 60.7 | 8 | |||
| Joshu 2012 US [132] | ARIC (1990-2006) | Several | 15 median | Population | ♀ 626 ♂ 499 |
♀ 58.5 ♂ 58.8 |
♀ 31.5 ♂ 30.0 |
11,667 | - | - | - | 6 |
| Khan 2006 Japan [133] | JACC | Several | 1988-1997 | Population | 3,307 | 40-79 | - | 53,574 | 40-79 | - | - | 7 |
| Ragozzino 1982 US [134] | Rochester, Minnesota | Several | - | Population | 1,135 | - | - | External standard population | - | - | - | 4 |
| Rapp 2006 Austria [135] | VHM&PP | Several | 8.4 | Population | 3.4% | - | - | 140,813 | 43 | - | - | 8 |
| Steenland 1995 US [136] | NHANES I | Several | 7.7 | Civilian population | - | - | - | 14,407 | 60 (cases) 48 (non-cases) | - | - | 7 |
| Swerdlow 2005 UK [137] | The diabetes uk cohort | Several | 1972-2003 | Population | 29,701 | 0-49 | - | External standard population | - | - | - | 6 |
| Wideroff 1997 [138] Denmark | Danish Central Hospital Discharge Register |
Several | 1977-1993 | Hospital | 109, 581 | ♂ 64 ♀69 median |
- | External standard population | - | - | - | 6 |
| Wotton 2011 UK [139] | ORLS 1 | Several | 1963-1998 | Hospital | 15,898 | - | - | 275,564 | - | - | - | 7 |
| Wotton 2011 UK [139] | ORLS 2 | Several | 1999-2008 | Hospital | 7,771 | - | - | 185,123 | - | - | - | 7 |
| Yeh 2012 US [140] | CLUE II | Several | 1989-2006 | Population | 599 | 61.8 | 29.5 | 17,681 | 51.5 | 26.3 | - | 7 |
| Aschebrook-kilfoy 2011 US [141] | NIH-AARP study | Thyroid | 10 | Population | 44,693 | 62.9 | 29.9 | 451,855 | 61.9 | 26.8 | - | 7 |
| Kitahara 2012 US [142] | Pooled analysis of 5 cohort studies | Thyroid | 10.5 median | Previous studies | 8% | - | - | 674,491 (total) | 59.8 | - | - | 7 |
| Meinhold 2010 US [143] | US Radiologic Technologists Study |
Thyroid | 15.8 | Radiologic technologists | - | - | - | ♀69,50 6 ♂21,207 (total) |
♂43.3 ♀39.3 | - | - | 6 |
Table 3.
Study Table of Case Control Studies by Diabetes Type
| Authors | Data Source | Cancer Site | Source | Cases (n) | Age | BMI | Controls (n) | Age | BMI | Co Morbidity* | NOS-Score (0-9) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case Control Studies | ||||||||||||
| Type 2 diabetes | ||||||||||||
| Khachatryan 2011 Armenia [150] | - | Breast | Population | 150 | 55.79 | 29.03 | 152 | 51.11 | 27.67 | - | 6 | |
| Rollison 20078US [151] | 4 corners breast cancer study | Breast | Population | 2,324 | - | - | 2,523 | 56 | - | - | 6 | |
| Li 2012 China [152] | - | Liver | Hospital | 1,105 | 53.8 | - | 5,170 | 44.9 | - | Chronic hepatitis B | 6 | |
| Diabetes type unspecified | ||||||||||||
| Grainge 2009 UK [153] | GPRD 1987-2002 | Biliary tract | Population | 611 | 71.3 (at diagnosis) |
- | 5,760 | - | - | - | 8 | |
| Khan 1999 US [154] | CPMC 1980-1994 | Biliary tract | Hospital | 69 | - | - | 138 | - | - | - | 6 | |
| Shaib 2007 US [155] | M.D. Anderson Cancer Center |
Biliary tract | Hospital | 83 ICC 163 ECC |
ICC 59.8 ECC 61.1 |
- | 236 | 58.1 | - | - | 5 | |
| Shebl 2010 China | Shanghai, China | Biliary tract | Population | 627 | - | - | 959 | - | - | - | 7 | |
| Tao 2010 China [156] | PUMCH | Biliary tract | Hospital | 190(total) 61 ICC 129 ECC |
58.6 ECC 58.7 ICC |
380 | 59.7 | 5 | ||||
| Welzel 2007 US [157] | SEER | Biliary tract | Population | ECC 549 ICC 535 |
ECC 78.7 ICC 79 |
- | 102,782 | 77.1 | - | - | 7 | |
| Kantor 1984 US [158] | SEER | Bladder | Population | 2,982 | - | - | 5,782 | - | - | - | 6 | |
| Kravchick 2001, Israel [159] | Bladder | Hospital | 252 | ♂71.5 ♀ 73 |
- | 549 | - | - | - | 4 | ||
| Mackenzie 2012 US [160] | New England | Bladder | Population | 331 | 62 | 28.0 | 263 | 60 | 27,0 | - | 6 | |
| Ng 2003 UK [161] | Bedford General Hospital | Bladder | Hospital | 125 | - | - | 80 | - | - | - | 5 | |
| Risch 1988 Canada [162] | Edmonton, Calgary, Toronto, and Kingston | Bladder | Population | 835 | 35-79 | - | 792 | - | - | - | 6 | |
| Baron 2001 US [163] | Wisconsin and New Hampshire | Breast | Population | 5,659 | 65.3 | - | 5,928 | 64.1 | - | - | 6 | |
| Beji 2007 Turkey [164] | Breast | Hospital | 405 | - | - | 1050 | - | - | - | 3 | ||
| Cleveland 2012 US [165] | Long Island Breast Cancer study project | Breast | Population | 1,495 | 63.6 | 30.9 | 1,543 | 57.4 | 26.1 | 6 | ||
| Garmendia 2007 Chile [166] | Breast | Hospital (mammography service) | 170 | 56.5 | 28.59 | 170 | 55.18 | 29.23 | - | 5 | ||
| Jordan 2009 Thailand [167] | Thai Cohort | Breast | University students | 43 | 39 median | - | 860 | - | - | - | 4 | |
| Weiss 1999 US [168] | New Jersey, Atlanta, Seattle | Breast | Population | 2,173 | - | - | 1,990 | - | - | - | 5 | |
| Wu 2007 US [169] | Los Angeles County Cancer Surveillance Program |
Breast | Population | 1,248 | - | - | 1,148 | - | - | - | 6 | |
| Kune 1988 Australia [170] | Melbourne 1980-1981 | Colorectal | Population | 715 | 65 | - | 727 | 65 | - | - | 6 | |
| Le Marchand 1997 US [171] | Colorectal | Population | 1,192 | ♂67 ♀65 (median) | - | 1,192 | ♂65 ♀65 (median) | - | - | 7 | ||
| Rinaldi 2008 European countries [172] | EPIC (8 countries) | Colorectal | Population | 1,026 | 59.1 (CC) 58.2 (RC) | 27.3 (CC) 27.0 (RC) | 1,026 | 59.1 (Control CC) 58.2 (control RC) | 26.9 (Control CC) 26.6 (control RC) | 8 | ||
| Safaee 2009 Iran [173] | Shahid Beheshti University of Medical Sciences, Tehran, Iran |
Colorectal | Population (cases: cancer registry. Controls: health survey) | 862 | - | - | 862 | - | - | - | 4 | |
| Vinikoor 2009 US [174] | NCCCS1 | Colorectal | Population | 637 | 63.69 | 1,044 | 66.06 | 6 | ||||
| Vinikoor 2009 US [174] | NCCCS2 | Colorectal | Population | 1,007 | 61.88 | 988 | 63.86 | 6 | ||||
| Yang 2005 UK [175] | GPRD | Colorectal | Population | 10,447 | - | - | 104,429 | - | - | - | 8 | |
| Fortuny 2009 US [176] | EDGE study | Endometrial | Population | 469 | 61.7 | - | 467 | 63.6 (all) | - | - | 6 | |
| Inoue 1994 Japan [177] | Osaka University Medical School | Endometrial | Hospital | 143 | 53.6 | - | 143 | 53.2 | - | - | 5 | |
| Saltzman 2008 US [178] | Washington State | Endometrial | Population | 1,303 | 1,779 | 7 | ||||||
| Yamazawa 2003 Japan [179] | Chiba University Hospital |
Endometrial | Hospital | 41 | - | - | 123 | - | - | - | 5 | |
| Neale 2009 Australia [180] | Queensland 2001-2005 | Esophagus | Population | 1,102 | - | - | 1,580 | - | - | - | 6 | |
| Reavis 2004 US [181] | Portland VA Medical Center | Esophagus | Hospital (and dental clinic) | 63 | 69.6 | - | 50 + 50 + 56 |
63.7 64.7 58.9 |
- | - | 5 | |
| Rubenstein 2005 US [182] | Veterans database 1995-2003 | Esophagus and gastric cardia | Veterans | 311 | 71.2 median | - | 10,154 | 66.3 median | - | GERD | 7 | |
| Vineis 2000 Italy [183] | 11 Italian areas | Haemapoietic | Population | 2,669 | 56.1 | - | 1,718 | 54.9 | - | - | 6 | |
| Stott-Miller 2012 (38) | Pooled analysis | Head and neck | Mixed | 6.448 | - | - | 13.747 | - | - | - | 4 | |
| Davila 2005 US [184] | Surveillance Epidemiology and End-Results Program (SEER) | Liver | Population | 2,061 | 76.1 | - | 6,183 | 76.4 | - | - | 8 | |
| El-Serag 2001 US [185] | 1997-1999 VA | Liver | Hospital (veterans) | 823 | 62 | - | 3,459 | 60 | - | - | 4 | |
| Hassan 2002 US [186] | MD Anderson Cancer center 1994-1995 | Liver | Hospital | 115 | 59.5 | - | 230 | 59.1 | - | - | 7 | |
| Hassan 2010 US [187] | MD Anderson Cancer center 2000-2008 | Liver | Hospital | 420 | 63 | - | 1,104 | 60 | - | - | 7 | |
| Matsuo 2003 Japan [188] | Kyushu | Liver | Population | 222 | ♂63.6. ♀ 64.3 |
222 | ♂ 63.5. ♀ 64.1 |
6 | ||||
| Tung 2010 Taiwan [35] | Tainan | Liver | Population | 72 | 68.4 | - | 144 | 68.2 | - | - | 7 | |
| Tung 2010 Taiwan [35] | Tainan | Liver | Population | 72 | 68.4 | - | 144 | 67.7 | - | Hepatitis C infection | 7 | |
| Yuan 2004 US [189] | Los Angeles county 1984-2001 | Liver | Population | 295 | 60.6 | - | 435 | 60.1 | - | - | 5 | |
| Fortuny 2005 Spain [190] | Lymphoma | Hospital | 565 | - | - | 601 | 59 (total) | - | - | 6 | ||
| Cartwright 1988 UK [191] | 1979-1984 Yorkshire | NHL | Hospital | 437 | - | - | 724 | - | - | - | 4 | |
| Cerhan 2005 US [192] | Detroit, LA, Seattle 1998-2000 | NHL | Population | 759 | 56.6 | 27.7 | 589 | 56.9 | 27.7 | - | 6 | |
| Lin 2007 Taiwan [193] | CGMH | NHL | Population | 242 | 59 median at diagnosis | - | 71,379 | - | - | - | 6 | |
| Smedby 2006 Denmark, Sweden [194] | SCALE | NHL | Population | 3,055 | 60 median | - | 3,187 | 59 median | - | - | 6 | |
| Bonelli 2003 Italy [195] | Northern Italy 1992-1996 | Pancreas | Hospital | 202 | - | - | 404 | - | - | - | 6 | |
| Bueno de mesquita 1992 Netherlands [196] | 1984-1987 | Pancreas | Population | 174 | 35-79 | 487 | 35-79 | 6 | ||||
| Cuzick 1989 UK [197] | Leeds, London, Oxford (1983-1986) | Pancreas | Hospital | 216 | - | - | 279 | - | - | - | 7 | |
| Ekoe 1992 Canada [198] | Quebec | Pancreas | Population | 179 | 63,9 | 239 | 62,1 | 7 | ||||
| Friedman 1993 US [199] | Kaiser Permanente Medical Care Program | Pancreas | Kaiser Permanente Medical Care Program (inpatient and outpatient) | 450 | 54.6 | - | 2,687 | 54.4 | - | - | 6 | |
| Frye 2000 New Zeeland [200] | Canterbury Health case mix database | Pancreas | Hospital | 116 | 70.1 | - | 116 | 70.2 | - | Controls: Fracture of femur neck | 6 | |
| Grote 2011 [201] | EPIC (10 countries) | Pancreas | Population | 466 | 58 | 26.6 | 466 | 58 | 25.9 | 5 | ||
| Gullo 1994 Italy [202] | 14 Italian university and community hospitals (1987-1989) | pancreas | Hospital | 720 | 62.6 | - | 720 | - | - | - | 6 | |
| Hassan 2007 US [203] | Pancreas | Hospital | 808 | 61.9 | - | 808 | 60.2 | - | - | 7 | ||
| Hiatt 1988 US [204] | KPMPC (1960-1984) | Pancreas | Member of medical care program | 49 | 67.6 | - | 12,104 | - | - | - | 6 | |
| Jain 1991 Canada [205] | Toronto 1983-1986 | Pancreas | Population | 249 | 64.6 | - | 505 | 64.8 | - | - | 6 | |
| Kalapothaki 1993 Greece [206] | Athens 1991-1992 | Pancreas | Population | 181 | - | - | 181; 818 (2 control groups) |
- | - | - | 5 | |
| Li 2011 US [207] | Three previous studies- pooled analysis | Pancreas | Population | 2,192 | 63 | 5,113 | 63 | 7 | ||||
| Maisonnueve 2010 Australia, Canada, Poland [208] Netherlands | Multicenter (pooled analysis) | Pancreas | Population | 823 | - | - | 1,679 | - | - | - | 5 | |
| Matsubayashi 2011 Japan [209] | Shizuoka Cancer center | Pancreas | Hospital | 577 | 64.9 | 577 | 64.9 | 5 | ||||
| Mizuno 1992 Japan [210] | Japanese university hospitals | Pancreas | Hospital | 124 | - | - | 124 | - | - | - | 6 | |
| Baradaran 2009 Iran [211] | Multicenter | Prostate | Hospital | 194 | 71.06 | 26.3 | 317 | 66.5 | 26.8 | - | 4 | |
| Coker 2004 US [212] | South Carolina Central Cancer Registry (SCCCR) (1999-2001) |
Prostate | Population | 407 | 65-79 | - | 393 | 65-79 | - | - | 6 | |
| Gong 2006 [213] | PCPT | Prostate | Previous study | 1,936 | 63.7 | 27.6 | 8,322 | 62.6 | 27.7 | 7 | ||
| Gonzales-Perez 2005 UK [214] | GPRD 1995-2001 | Prostate | Population | 2,183 | 72 median | - | 10,000 | 72 median | - | - | 8 | |
| Lightfoot 2004 Canada [215] | Ontario 1995-1999 | Prostate | Population | 760 | - | - | 1,632 | - | - | - | 5 | |
| Rosenberg 2002 US [216] | University Medical Center in New York City |
Prostate | Hospital | 320 | 69.6 | - | 189 | 68.1 | - | - | 6 | |
| Tavani 2002 Italy and Greece [217] | Milan Pordenone and Athens, Greece |
Prostate | hospital | 608 | - | - | 1,008 | - | - | - | 6 | |
| Turner 2011 UK [218] | Protect study | Prostate | Population | 1,291 | 62.2 | 26.7 | 6,479 | 62.0 | 26.9 | - | 7 | |
| Zhu 2004 US [219] | US Physicians’ Health Study | Prostate | Physicians | 1,110 | - | 24.9 | 1,110 | - | 24.9 | - | 6 | |
| Attner 2012 Sweden [220] | Swedish cancer registry (2003-2007) | Several | Population | 19,756 | 45-84 | - | 147,324 | 45-84 | - | - | 7 | |
| Bosetti 2011 Italy and Switzerland [221] | 1991-2009 | Several | Hospital | 230- 2390 depending on cancer type | 56-66 depending on cancer type | 12,060 | 56-65 depending on cancer type | 7 | ||||
| Jorgensen 2012 Denmark [222] | Funen county | Several | Population | 6,325 | 78 median | - | 25,299 | 78 Median | - | - | 7 | |
| Kuriki 2007 Japan [223] | HERPACC 1989-2000 | Several | Hospital | ♂5,341 ♀6,331 |
♂65.3 ♀60.6 |
♂22.7 ♀22.4 |
♂14,199 ♀ 33,569 |
♂60.6 ♀57.0 |
♂23.0 ♀22.1 |
- | 6 | |
| La Vecchia 1994 Italy [224] | Milan 1983-1992 | Several | Hospital | 9,991 | - | - | 7,834 | - | - | - | 5 | |
| O Mara 1985 US(37) |
Roswell Park Memorial Institute (RPMI) (1957-1965) | Several | Hospital | 14,910 | - | - | 4,838 | - | - | - | 3 | |
| Rousseau 2006 Canada [225] | Montreal 1979-1985 | Several | Population | 3,107 | - | - | 509 | 59.6 | 58.2% BMI>25 |
- | 6 | |
(β =-0.23) and colorectal (β =-0.23) cancer and otherwise not a determinant like follow up years was not a determinant. Compared to adjustment of age, adjustment of both age and BMI was a significantly positive determinant in the risk of biliary tract and gallbladder cancer (β =0.79), cervix cancer (β =0.37), myeloma (β =0.49), non Hodgkin lymphoma (β =0.39), ovary-(β =0.52), prostate cancer (β =0.11), rectum cancer (β =0.40) and thyroid cancer (β =0.47), while it was a significantly negative determinant of larynx cancer (β =-0.23). In addition adjustment of age, diabetes and smoking was a positive determinant of risk ratio in colorectal- (β =0.11), ovary-(β =0.51), and skin cancer (β =0.69) compared to adjustment by age. Furthermore some specific cancer risks may be determined by diabetes ascertainment, cancer ascertainment, and data source. In the electronic Supplementary Material 4 the results of the meta-regression are available.
Results of Individual Studies
The results of the individual studies are presented in the electronic Supplementary Material 3. Stott-Miller et al. [38] was the only study specifically addressing head and neck cancer, and they presented an odds ratio of 1.09 (0.95-1.24) for head and neck cancer for diabetes patients compared to a non-diabetes reference. Thus it was not used in the included in the meta-analysis.
Synthesis of Results
Table 4 presents the pooled analysis of the studies and the pooled results are depicted in Fig. (2). All available cancer types were included. Diabetes patients have a significant increased risk of any cancer, biliary and gallbladder cancer, bladder cancer, bone cancer, breast cancer, colon cancer, colorectal cancer, rectal cancer, esophagus cancer, liver cancer, lung cancer, leukemia, lymphoma, non-Hodgkin lymphoma, pancreas cancer, kidney cancer, small intestine cancer, stomach cancer and thyroid cancer. Female diabetes patients were also at increased risk for breast, cervix, endometrial and ovary
Table 4.
Results of the Pooled Analysis by Random Effects Model for All Included Studies on Any Cancer and Specific Cancer Sites
| Cancer Site | RR (95% Confidence Interval) | Number of Populations | Test for Heterogeneity |
|---|---|---|---|
| Any | 1.15 (1.06-1.25) | 42 | P < 0.001 |
| Biliary tract and gall bladder* | 1.69 (1.41-2.03) | 26 | P < 0.001 |
| Bladder | 1.14 (1.05-1.22) | 35 | P < 0.001 |
| Bone | 1.00 (0.69-1.45) | 7 | P = 0.895 |
| Breast | 1.14 (1.08-1.19) | 62 | P < 0.001 |
| Cervix | 1.34 (1.10-1.63) | 19 | P < 0.001 |
| Colon | 1.29 (1.21-1.36) | 41 | P < 0.001 |
| Colorectal | 1.27 (1.21-1.34) | 51 | P < 0.001 |
| Endometrial | 1.81 (1.63-2.01) | 29 | P < 0.001 |
| Esophagus | 1.20 (1.02-1.41) | 29 | P < 0.001 |
| Kidney | 1.37 (1.18-1.59) | 33 | P < 0.001 |
| Larynx | 1.10 (0.84-1.43) | 11 | P < 0.001 |
| Leukemia | 1.25 (1.08-1.45) | 20 | P < 0.001 |
| Liver | 2.13 (1.81-2.50) | 61 | P < 0.001 |
| Lung | 1.07 (0.97-1.17) | 44 | P < 0.001 |
| Lymphoma** | 1.39 (1.17-1.64) | 18 | P < 0.001 |
| Melanoma | 1.00 (0.91-1.10) | 18 | P = 0.002 |
| Myeloma | 1.11 (0.92-1.34) | 11 | P < 0.001 |
| Nervous system | 1.19 (0.97-1.46) | 19 | P < 0.001 |
| Non-Hodgkin lymfoma | 1.19 (1.05-1.36) | 28 | P < 0.001 |
| Ovary | 1.20 (1.03-1.40) | 21 | P < 0.001 |
| Pancreas | 2.21 (1.93-2.54) | 65 | P < 0.001 |
| Prostate | 0.85 (0.80-0.91) | 27 | P < 0.001 |
| Rectum | 1.17 (1.08-1.27) | 37 | P < 0.001 |
| Skin*** | 0.91 (0.83-0.99) | 18 | P < 0.001 |
| Small intestine | 1.47 (1.03-2.11) | 6 | P = 0.005 |
| Stomach | 1.13 (1.02-1.24) | 37 | P < 0.001 |
| Testes | 0.88 (0.71-1.09) | 6 | P = 0.924 |
| Thyroid | 1.27 (1.12-1.43) | 21 | P = 0.076 |
Significance is indicated by bold. Number of populations covers the number of populations used in the pooled analysis, this may not be the same as the number of records used in the analysis, thus some records have multiple populations. RR: Risk ratio, CI: Confidence interval. * In this category studies estimating the risk of biliary tract extra- and intra hepatic, gallbladder cancer and cholangiocarcinoma were pooled ** In this category estimates of lymphoma, Hodgkin lymphoma and combined estimates of lymphoma including Non-Hodgkin lymphoma were pooled. *** Some estimates used in skin cancer cover both non-melanoma skin cancer and melanoma.
Fig. (2).
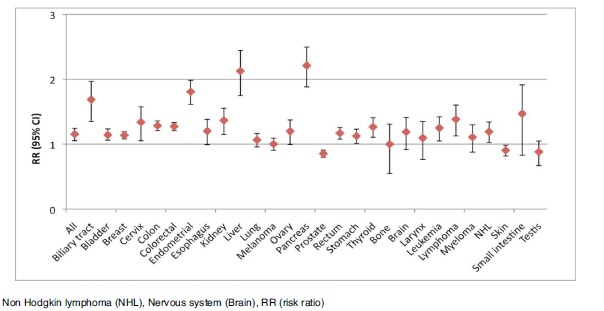
Plot of the pooled analysis of all populations of the risk of cancer among diabetes patients compared to a non-diabetes population.
cancer. However; diabetes patients have a lower risk of prostate cancer and skin cancer than non-diabetic subjects. In these analyses, only bone and thyroid cancer did not display significant heterogeneity by chi square testing. For the remaining cancer types (testes cancer, myeloma, melanoma, lung, larynx, bone cancer and nervous system cancers) no significantly in- or decreased association between diabetes patients and non-diabetes was observed.
Subgroup analyses were performed on study design (cohort/case control) and gender (male/female). Figs. (3-6) illustrates the results of the analyses. Cohort studies found among diabetes patients an increased risk of any, biliary, breast, cervix, colon, colorectal, endometrial, kidney, liver, ovary, pancreas, rectum, small intestine, stomach, and thyroid cancer, as well as leukemia, all lymphomas, and non-Hodgkin lymphoma, while the risks of prostate, and skin cancer were decreased. Case control studies show similar results as cohort studies including an increased risk of larynx cancer; however the pooled estimates for cervix-, kidney-, leukemia-, non Hodgkin lymphoma-, prostate-, stomach-, and thyroid cancer were without significance. Males with diabetes were at an increased risk of all cancers combined, biliary, colon, colorectal, kidney, liver, pancreas, rectum, small intestine, and thyroid cancer and leukemia, while the risk of prostate cancer was decreased. Females with diabetes were at an increased risk of any, breast, cervix, colon, colorectal, endometrial, kidney, leukemia, liver, ovary, and pancreas cancer.
Fig. (3).
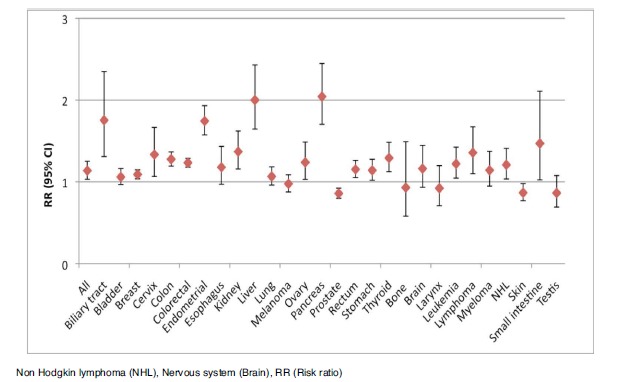
Plot of the pooled analysis of all cohort populations of the risk of cancer among diabetes patients compared to a non-diabetes population.
Fig. (6).
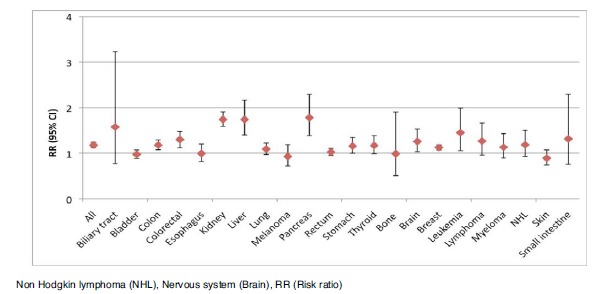
Plot of the pooled analysis of all populations only consisting of females of the risk of cancer among diabetes patients compared to a non-diabetes population.
Risk of Bias Across Studies
Egger’s regression test revealed significant publication bias for any cancer (p=0.048), colorectal cancer (p=0.024), esophagus cancer (p=0.022), larynx cancer (p=0.041), lymphoma (p=0.041) and lung cancer (p=0.015). The graphical depictions of the bias test for these cancer types are available in the electronic Supplementary Material 5. All these publication biases have a positive intercept value indicating higher effect size in smaller studies. None of the other cancer types displayed publication bias.
Meta-Regression
Table 5 present results from the meta-analysis. These results reflect the effect modification of the variables on the measured cancer risk in the studies. A positive determinant increases the risk ratio for cancer among diabetes patients, whereas a negative determinant decreases the risk ratio for cancer among diabetes patients. The coefficient is the beta-coefficient from the regression. Not all variables were available for all of the specific cancer analyses. In the following only specific parts will be highlighted. Male gender was a significant negative determinant of the risk of leukemia in (β = -1.52) and reduces the risk of leukemia among diabetes patients. Age difference may both be a significantly positive, negative and no determinant depending on cancer type. BMI differences was no determinant of breast-, colorectal-, endometrial-, kidney-, liver-, pancreas-, and prostate-cancer risk, however it was a negative determinant (β = -0.08) for lung cancer. Diabetes type was only a significantly negative determinant in colon
Table 5.
Results of the Meta-Regression on the Specific Cancer Types
| Cancer Site | Gender* | Age (Years)* | BMI (kg/m2)* | Diabetes Type* | Follow Up (Years) * | Source£ | Adjustment (Adjustment for Age vs Adjustment for Age and BMI)£ | Diabetes Ascertainment£ | Cancer Ascertainment£ | NOS* |
|---|---|---|---|---|---|---|---|---|---|---|
| Any | 0 (5) | - (5) | 0 (42) | 0 (42) | 0 (42) | 0 (42) | - (5) | |||
| Biliary tract and gall bladder | 0 (7) | 0 (7) | - (25) | + (25) | 0 (25) | 0 (25) | 0 (7) | |||
| Bladder | 0 (5) | - (5) | 0 (33) | 0 (33) | 0 (33) | 0 (33) | 0 (5) | |||
| Bone | 0 (4) | 0 (7) | 0 (7) | 0 (4) | ||||||
| Breast | 0 (7) | 0 (7) | 0 (7) | 0 (60) | 0 (60) | 0 (60) | 0 (60) | 0 (7) | ||
| Cervix | 0 (6) | 0 (6) | 0 (17) | + (17) | 0 (17) | 0 (17) | 0 (6) | |||
| Colon | 0 (13) | 0 (13) | - (13) | 0 (40) | 0 (40) | 0 (40) | 0 (40) | 0 (13) | ||
| Colorectal | 0 (7) | 0 (7) | 0 (7) | - (9)** | 0 (47) | 0 (47) | 0 (47) | 0 (47) | 0 (7) | |
| Endometrial | 0 (5)** | 0 (4) | 0 (5)** | 0 (26) | 0 (26) | 0 (26) | 0 (26) | 0 (5)** | ||
| Esophagus | 0 (5) | - (5) C | 0 (27) | 0 (27) | 0 (27) | 0 (27) | - (5) | |||
| Kidney | 0 (5) | 0 (5) | 0 (4) | 0 (31) | 0 (31) | + (31) | 0 (31) | 0 (5) | ||
| Larynx | 0 (6) | 0 (10) | - (10) | + (10) | 0 (10) | 0 (6) | ||||
| Leukemia | - (5) | 0 (5) | 0 (18) | 0 (18) | 0 (18) | 0 (18) | 0 (5) | |||
| Liver | 0 (7)** | - (10)**CC | 0 (4) | 0 (58) | 0 (58) | 0 (58) | 0 (58) | 0 (7)** | ||
| Lung | 0 (6)** | 0 (6)** | - (5) | 0 (42) | 0 (42) | 0 (42) | 0 (42) | - (6)** | ||
| Lymphoma | 0 (9) | - (18) | 0 (18) | 0 (18) | 0 (18) | 0 (9) | ||||
| Melanoma | 0 (12) | 0 (16) | 0 (16) | 0 (16) | 0 (12) | |||||
| Myeloma | 0 (6) | - (11) | + (11) | 0(11) | 0 (11) | 0 (6) | ||||
| Nervous system | 0 (12) | 0 (17) | 0 (17) | 0 (17) | 0 (17) | 0 (12) | ||||
| NHL | 0 (13) | - (26) | + (26) | 0 (26) | 0 (26) | 0 (13) | ||||
| Ovary | 0 (7) | 0 (19) | + (19) | 0 (19) | - (19) | 0 (7) | ||||
| Pancreas | 0 (7)** | 0 (7)** | 0 (4) | 0 (63) | 0 (63) | 0 (63) | 0 (63) | 0 (7)** | ||
| Prostate | + (5)***0 | 0 (5)*** | 0 (12) | 0 (26) | + (26) | 0 (26) | - (26) | + (5)*** | ||
| Rectum | 0 (11)** | 0 (11)** | 0 (3) | 0 (11)** | 0 (35) | + (35) | 0 (35) | 0 (35) | 0 (11)** | |
| Skin | 0 (10) | 0 (18) | 0 (18) | 0 (18) | 0 (18) | - (10) | ||||
| Small intestine | 0 (5) | - (6) | 0 (6) | 0 (5) | ||||||
| Stomach | 0 (7) | - (7) C | 0 (6) | 0 (35) | 0 (35) | 0 (35) | 0 (35) | 0 (7) | ||
| Testes | 0 (6) | |||||||||
| Thyroid | 0 (6) | 0 (6) | 0 (5) | 0 (20) | + (20) | 0 (20) | 0 (20) | 0 (6) |
+: statistically significant positive determinant, -: statistically significant negative determinant, 0: no statistical significance, blank: could not be performed and not included in the meta-regression). The () marks how many populations were available for the regression results. Number of populations covers the number of populations used in the pooled analysis, this may not be the same as the number of records used in the analysis, thus some records have multiple populations. Some estimates used in skin cancer cover both non melanoma skin cancer and melanoma. Gender, diabetes type, source, diabetes ascertainment, cancer ascertainment, adjustment and NOS were all coded as categorical values, * regression analysis included age, gender, NOS and BMI if available. £ regression analyses included study design, source, diabetes ascertainment, adjustment factors and cancer ascertainment. ** Regression performed without BMI. *** Regression performed without diabetes type. BMI: Body Mass Index, NHL: Non Hodgkin lymphoma, NOS: Newcastle Ottawa Scale score. C: significance only applies to cohort studies not case control studies. CC: significance only apply to case control studies. Variables were entered in the categories as described in the methods section.
Age and BMI are provided by means if nothing else is specified. Follow up years are provided by means, medians or follow up period. *Comobidity in the population examined. Body mass index (BMI), diabetes mellitus (DM), digital rectal examination (DRE), general practicioner (GP), hepatitis C virus (HCV),. Total: For the whole group or the complete study period.
Age and BMI are provided by means if nothing else is specified. Follow up years are provided by means, medians or follow up period. *Comobidity in the population examined. Body mass index (BMI), diabetes mellitus (DM), digital rectal examination (DRE), general practicioner (GP), Total: For the whole group or the complete study period.
Age and BMI are provided by means if nothing else is specified. Follow up years are provided by means, medians or follow up period. *Comobidity in the population examined. Body mass index (BMI), diabetes mellitus (DM), digital rectal examination (DRE), general practicioner (GP), hepatitis C virus (HCV),. Total: For the whole group or the complete study period.
Non Hodgkin lymphoma (NHL), Nervous system (Brain), RR (risk ratio)
Non Hodgkin lymphoma (NHL), Nervous system (Brain), RR (Risk ratio)
Non Hodgkin lymphoma (NHL), RR (Risk ratio)
Non Hodgkin lymphoma (NHL), RR (Risk ratio)
Non Hodgkin lymphoma (NHL), Nervous system (Brain), RR (Risk ratio)
Discussion
Summary of Evidence
This systematic review and meta-analysis confirms the previous findings of an increased cancer risk among diabetes patients. The addition of several databases to the literature search compared to previous meta-analyses did not change the associations previously found. Diabetes patients were especially susceptible to liver cancer (RR= 2.13; 95% CI 1.81-2.50), pancreas cancer (RR= 2.21; 95%CI 1.93-2.54), and endometrial cancer (RR= 1.81; 95% CI 1.63-2.01). In addition, new cancer sites have been investigated: risks of cervix (RR=1.34; 95% CI 1.10-1.63)), ovary cancer (RR= 1.20; 95% CI 1.03-1.40), and small intestinal cancer was reported (RR=1.47; 95% CI 1.03-2.11) were also slightly increased in diabetes patients. In addition female diabetes patients were at increased risk of breast (RR= 1.13 95% CI 1.07-1.18). Thus females with diabetes were at increased risk of gender specific and hormone related cancers compared to their non-diabetic counterparts. However, male diabetes patients seem to be have a reduced risk of prostate cancer (RR= 0.85; 95% CI 0.80-0.91), which support the previous findings [9,10]. Furthermore, our findings support an increased risk of gastric and stomach cancer (RR=1.13; 95% CI 1.02-1.24), whereas former reports have been conflicting [15,16]. An elevation in thyroid cancer (RR=1.27; 95% CI 1.12-1.43) was also present among diabetes patients. A single study reported on head and neck cancer, which found that cancer risk, was not significantly increased among diabetes patients [38].
Neither study design nor gender appears to modulate the overall increase in cancer risk among diabetes patients. Duration of diabetes was not available for analyses, which may influence results. The increased risk of pancreas cancer in diabetes may be due to cancer diagnosis in the following years after diabetes diagnosis, where the risk especially was increased [207]. Normalization of the cancer risk occurs10 years after diabetes diagnosis [207], and may be a result of detection bias or indicate that diabetes diagnosis was a symptom of pancreatic cancer. Johnson et al. [131] investigated time dependent factors in cancer risk and diabetes and conclude that the increased cancer risk may be due to increased ascertainment after diabetes diagnosis.
Obesity may be a confounder when assessing cancer risk in diabetes patients [30]. This was not supported by the meta-regression conducted. BMI was a negative determinant for risk of lung cancer, while no other cancer risk was determined by BMI; hence effect modification was only apparent when looking at lung cancer. When looking at the adjustment performed by the studies in the meta-analysis; adjustment by BMI and age were positive determinants of cancer risk in comparison to adjustment for age alone. These results indicate that obesity among diabetes patients was not an effect modifier on the risk of cancer in diabetes, and obesity may not be the explanation for the increased cancer risk for the types rectum, thyroid, biliary tract and gallbladder, ovary, non-Hodgkin lymphoma, myeloma and cervix cancer (adjustment by BMI and age was a positive determinant for these cancer types). Unsurprisingly, age differences may also affect the outcome (Table 5). The limited analyses on follow up time were inconclusive. Male gender was a significantly negative determinant of risk of leukemia, which was in accordance with the fact that risk of leukemia was increased in female diabetes patients (RR= 1.45, 95% CI 1.06-1.99) and only slightly increased in male diabetes patients (RR= 1.12, 95% CI 1.00-1.26).
From the present literature, it was impossible to distinguish the cancer risk between T1D and T2D. Only a single study report of T1D [39], whereas some studies report of T2D. Some of the studies classified as diabetes unspecified in Table 1-3 claim to report only of T2D, however exclude T1D by age at diagnosis: excluding diabetes diagnosed at younger age than 18 [45], 20 [97], 21 [56], 25 [65] or 30 [68,71,115,139]. Nevertheless, the investigated population may consist of both T1D and T2D.
Diabetes ascertainment and cancer ascertainment (available in the electronic Supplementary Material 2) varied between studies and may, based on the meta-regression, be a determinant of the study outcome. Whether the study was hospital or population based may also affect the outcome (Table 5). These methodological differences, which may bias the results, raise the question of the necessity of uniform standards to reduce bias. In general the study quality did not determine the outcome of the pooled analysis (Table 5), however study quality based on NOS score was a significantly negative determinant risk of lung cancer and a significantly positive determinant of prostate cancer; meaning that the risk ratios drew closer towards 1 for both cancers. Adjustment for the NOS score only changed the outcome little. Some publication bias was present, with an underreporting of non-significant results from small studies. This may also affect the outcomes. Also only published data as age and BMI were collected, whereas not all studies reported these factors. This may affect the results of the meta-regression. These restrictions and limitations may affect the results, but it is implausible to be the explanation of the increased risk of cancer among diabetes patients.
Conclusion
The present systematic review and meta-analysis confirms the previous findings of an increased cancer risk in diabetes and extends these findings to additional cancer types. The results indicate that the risk was not modified by obesity and was thus either due to diabetes per se or other confounders. Unfortunately, important covariates as HbA1c and duration of diabetes were not available in a sufficient number of studies. It is thus difficult to determine whether the increased cancer risk was due to diabetes per se or other prognostic factors like anti-diabetic treatment.
Nevertheless, the clinical implications of this and previous studies are of importance. It is recommendable that physicians in contact with patients with diabetes are attentive to the increased cancer risk associated with diabetes. Whether the awareness should be aimed at a diabetes group receiving a specific treatment is unknown and the future results of the CARING project are awaited.
Patient consent
Declared none.
Fig. (4).
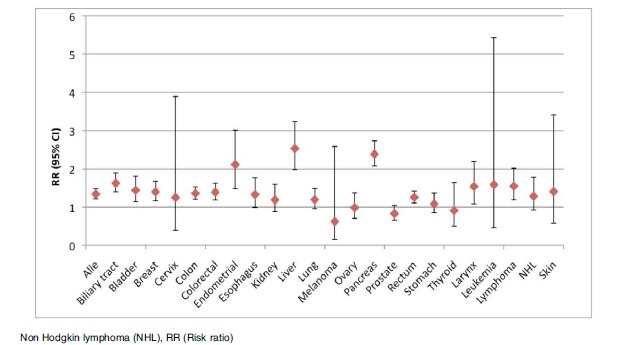
Plot of the pooled analysis of all case control populations of the risk of cancer among diabetes patients compared to a non-diabetes population.
Fig. (5).
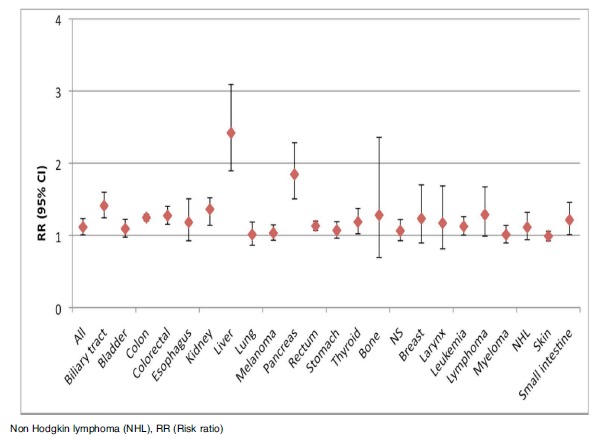
Plot of the pooled analysis of all populations only consisting of males of the risk of cancer among diabetes patients compared to a non-diabetes population.
Table 2.
Study Table of Cross-Sectional Studies by Diabetes Type
| Authors | Data Source | Cancer Site | Follow Up Years | Source | DM (n) | Age | BMI | Non- DM (n) | Age | BMI | Co Morbidity | NOS-Score (0-9) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cross-Sectional Studies | ||||||||||||
| Type 2 diabetes | ||||||||||||
| Baur 2011 Germany [144] | DETECT study | Any | - | Population | 1,308 | 70.4(with cancer) 66.6(without cancer) |
28.3 (with cancer) 29.8 (without cancer) |
6,211 | 65.5 (with cancer) 55.5 (without cancer) |
26.7 (with cancer) 26.6 (without cancer) |
- | 7 |
| Diabetes type unspecified | ||||||||||||
| Lawlor 2004 UK [145] | The British Women’s Heart and Health Study | Breast | - | Randomly from GP | 147 women with cancer | 68.5 | 28.1 | 3,890 women without cancer | 68.9 | 27.6 | - | 6 |
| Sandhu 2001 UK [146] | Norfolk | Colorectal | - | Population (GP lists) | 561 | 45-74 | - | 28,782 | 45-74 | - | - | 6 |
| Tung 2010 Taiwan [35] | Tainan | Liver | Population | 72 | 68.4 | - | 56,193 | - | - | - | 6 | |
| Moreira 2011 US [147] | Durham VA | Prostate | - | Hospital (performed prostate biopsy, high risk patient population (referred for biopsy because of elevated PSA or Abnomral DRE))) | 284 | 64 | 30,4 | 714 | 63 | 27,7 | - | 5 |
| Moses 2012 US [148] | Hospital (high risk population (referred to biopsy for elevated PSA or abnormal DRE)) |
Prostate | Hospital | 1,045 | - | - | 1,265 | - | - | - | 5 | |
| Li 2011US [149] | BRFSS | Several | - | Population | - | - | - | 397,783 total | 46.8 | - | - | 4 |
ACKNOWLEDGEMENTS
The research leading to the results of this study has received funding from the European Community’s Seventh Framework Programme (FP-7) under grant agreement number 282526, the CARING project. The funding source had no role in study design, data collection, data analysis, data interpretation or writing of the report. Research librarian Ms. Edith Clausen is acknowledged for great help during the searches. Without her help, the work would not have been possible.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s web site along with the published article.
CONFLICT OF INTEREST
Frank de Vries and Anthonius de Boer are employed by Utrecht University and are conducting research under the umbrella of the Centre for Research Methods. This Centre has received unrestricted funding from the Netherlands Organisation for Health Research and Development (ZonMW), the Dutch Health Care Insurance Board (CVZ), the Royal Dutch Pharmacists Association (KNMP), the private-public funded Top Institute Pharma (www.tipharma.nl, includes co-funding from universities, government, and industry), the EU Innovative Medicines Initiative (IMI), the EU 7th Framework Program (FP7), the Dutch Ministry of Health and industry (including GlaxoSmithKline, Pfizer, and others). ML De Bruin is employed by Utrecht University and is conducting research under the umbrella of the WHO Collaborating Centre for pharmaceutical policy and regulation. This Centre receives no direct funding or donations from private parties, including pharma industry. Research funding from public-private partnerships, e.g. IMI, TI Pharma (www.tipharma.nl) is accepted under the condition that no company-specific product or company related study is conducted. The Centre has received unrestricted research funding from public sources, e.g. Netherlands Organisation for Health Research and Development (ZonMW), the Dutch Health Care Insurance Board (CVZ), EU 7th Framework Program (FP7), Dutch Medicines Evaluation Board (MEB), and Dutch Ministry of Health. None of the abovementioned companies was involved in the preparation of this manuscript. Other authors had no conflicts of interest
REFERENCES
- 1.WHO The top 10 causes of death. Accessed 10/03,Available from: http://who.int/mediacentre/factsheets/fs310/en/inde x1.html. 2013.
- 2.Ben Q., Xu M., Ning X., et al. Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. Eur. J. Cancer. 2011;47(13):1928–1937. doi: 10.1016/j.ejca.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Huxley R., Ansary-Moghaddam A., Berrington De Gonzalez A., Barzi F., Woodward M. Type-II diabetes and pancreatic cancer: A meta-analysis of 36 studies. Br. J. Cancer. 2005;92(11):2076–2083. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Everhart J., Wright D. Diabetes mellitus as a risk factor for pancreatic cancer: A meta-analysis. JAMA. 1995;273(20):1605–1609. [PubMed] [Google Scholar]
- 5.Wang P., Kang D., Cao W., Wang Y., Liu Z. Diabetes mellitus and risk of hepatocellular carcinoma: A systematic review and meta-analysis. Diabetes Metab. Res. Rev. 2012;28(2):109–122. doi: 10.1002/dmrr.1291. [DOI] [PubMed] [Google Scholar]
- 6.Wang C., Wang X., Gong G., et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int. J. Cancer. 2012;130(7):1639–1648. doi: 10.1002/ijc.26165. [DOI] [PubMed] [Google Scholar]
- 7.Noto H., Osame K., Sasazuki T., Noda M. Substantially increased risk of cancer in patients with diabetes mellitus: a systematic review and meta-analysis of epidemiologic evidence in Japan. J. Diabetes Complications. 2010;24(5):345–353. doi: 10.1016/j.jdiacomp.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 8.El-Serag H.B., Hampel H., Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin. Gastroenterol. Hepatol. 2006;4(3):369–380. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Bonovas S., Filioussi K., Tsantes A. Diabetes mellitus and risk of prostate cancer: A meta-analysis. Diabetologia. 2004;47(6):1071–1078. doi: 10.1007/s00125-004-1415-6. [DOI] [PubMed] [Google Scholar]
- 10.Kasper J.S., Giovannucci E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol. Biomarkers Prev. 2006;15(11):2056–2062. doi: 10.1158/1055-9965.EPI-06-0410. [DOI] [PubMed] [Google Scholar]
- 11.Larsson S.C., Wolk A. Diabetes mellitus and incidence of kidney cancer: a meta-analysis of cohort studies. Diabetologia. 2011;54(5):1013–1018. doi: 10.1007/s00125-011-2051-6. [DOI] [PubMed] [Google Scholar]
- 12.Larsson S.C., Orsini N., Brismar K., Wolk A. Diabetes mellitus and risk of bladder cancer: A meta-analysis. Diabetologia. 2006;49(12):2819–2823. doi: 10.1007/s00125-006-0468-0. [DOI] [PubMed] [Google Scholar]
- 13.Huang W., Ren H., Ben Q., Cai Q., Zhu W., Li Z. Risk of esophageal cancer in diabetes mellitus: a meta-analysis of observational studies. Cancer Causes Control. 2012;23(2):263–272. doi: 10.1007/s10552-011-9874-9. [DOI] [PubMed] [Google Scholar]
- 14.Castillo J.J., Mull N., Reagan J.L., Nemr S., Mitri J. Increased incidence of non-Hodgkin lymphoma, leukemia, and myeloma in patients with diabetes mellitus type 2: a meta-analysis of observational studies. Blood. 2012;119(21):4845–4850. doi: 10.1182/blood-2011-06-362830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian T., Zhang L.Q., Ma X.H., Zhou J.N., Shen J. Diabetes mellitus and incidence and mortality of gastric cancer: a meta-analysis. Exp. Clin. Endocrinol. Diabetes. 2012;120(4):217–223. doi: 10.1055/s-0031-1297969. [DOI] [PubMed] [Google Scholar]
- 16.Ge Z., Ben Q., Qian J., Wang Y., Li Y. Diabetes mellitus and risk of gastric cancer: A systematic review and meta-analysis of observational studies. Eur. J. Gastroenterol. Hepatol. 2011;23(12):1127–1135. doi: 10.1097/MEG.0b013e32834b8d73. [DOI] [PubMed] [Google Scholar]
- 17.Larsson S.C., Orsini N., Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J. Natl. Cancer Inst. 2005;97(22):1679–1687. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 18.Kramer H.U., Schottker B., Raum E., Brenner H. Type 2 diabetes mellitus and colorectal cancer: Meta-analysis on sex-specific differences. Eur. J. Cancer. 2012;48(9):1269–1282. doi: 10.1016/j.ejca.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Deng L., Gui Z., Zhao L., Wang J., Shen L. Diabetes mellitus and the incidence of colorectal cancer: an updated systematic review and meta-analysis. Dig. Dis. Sci. 2012;57(6):1576–1585. doi: 10.1007/s10620-012-2055-1. [DOI] [PubMed] [Google Scholar]
- 20.Jiang Y., Ben Q., Shen H., Lu W., Zhang Y., Zhu J. Diabetes mellitus and incidence and mortality of colorectal cancer: A systematic review and meta-analysis of cohort studies. Eur. J. Epidemiol. 2011;26(11):863–876. doi: 10.1007/s10654-011-9617-y. [DOI] [PubMed] [Google Scholar]
- 21.Luo W., Cao Y., Liao C., Gao F. Diabetes mellitus and the incidence and mortality of colorectal cancer: A meta-analysis of 24 cohort studies. Colorectal Dis. 2012;14(11):1307–1312. doi: 10.1111/j.1463-1318.2012.02875.x. [DOI] [PubMed] [Google Scholar]
- 22.Sun L., Yu S. Diabetes mellitus is an independent risk factor for colorectal cancer. Dig. Dis. Sci. 2012;57(6):1586–1597. doi: 10.1007/s10620-012-2059-x. [DOI] [PubMed] [Google Scholar]
- 23.Yuhara H., Steinmaus C., Cohen S.E., Corley D.A., Tei Y., Buffler P.A. Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer. Am. J. Gastroenterol. 2011;106(11):1911–1921. doi: 10.1038/ajg.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang W., Va P., Bray F., et al. The role of pre-existing diabetes mellitus on hepatocellular carcinoma occurrence and prognosis: A meta-analysis of prospective cohort studies. PLoS One. 2011;6(12):e27326. doi: 10.1371/journal.pone.0027326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peairs K.S., Barone B.B., Snyder C.F., et al. Diabetes mellitus and breast cancer outcomes: a systematic review and meta-analysis. J. Clin. Oncol. 2011;29(1):40–46. doi: 10.1200/JCO.2009.27.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsson S.C., Mantzoros C.S., Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int. J. Cancer. 2007;121(4):856–862. doi: 10.1002/ijc.22717. [DOI] [PubMed] [Google Scholar]
- 27.Liao S., Li J., Wei W., et al. Association between diabetes mellitus and breast cancer risk: a meta-analysis of the literature. Asian Pac. J. Cancer Prev. 2011;12(4):1061–1065. [PubMed] [Google Scholar]
- 28.Xue F., Michels K.B. Diabetes, metabolic syndrome, and breast cancer: a review of the current evidence. Am. J. Clin. Nutr. 2007;86(3):s823–s835. doi: 10.1093/ajcn/86.3.823S. [DOI] [PubMed] [Google Scholar]
- 29.Li C., Zhao G., Okoro C.A., Wen X.J., Ford E.S., Balluz L.S. Prevalence of diagnosed cancer according to duration of diagnosed diabetes and current insulin use among U.S. adults with diagnosed diabetes: findings from the 2009 Behavioral Risk Factor Surveillance System. Diabetes Care. 2013;36(6):1569–1576. doi: 10.2337/dc12-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guh D.P., Zhang W., Bansback N., Amarsi Z., Birmingham C.L., Anis A.H. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moher D., Liberati A., Tetzlaff J., Altman D.G. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wells G, Shea B, O’connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in metaanalyses. 2000.
- 33.Hardy R.J., Thompson S.G. A likelihood approach to meta-analysis with random effects. Stat. Med. 1996;15(6):619–629. doi: 10.1002/(SICI)1097-0258(19960330)15:6<619::AID-SIM188>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 34.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tung H.D., Wang J.H., Tseng P.L., et al. Neither diabetes mellitus nor overweight is a risk factor for hepatocellular carcinoma in a dual HBV and HCV endemic area: community cross-sectional and case-control studies. Am. J. Gastroenterol. 2010;105(3):624–631. doi: 10.1038/ajg.2009.711. [DOI] [PubMed] [Google Scholar]
- 36.de Waard F., Baanders-van Halewijn E.A. A prospective study in general practice on breast-cancer risk in postmenopausal women. Int. J. Cancer. 1974;14(2):153–160. doi: 10.1002/ijc.2910140203. [DOI] [PubMed] [Google Scholar]
- 37.O'Mara B.A., Byers T., Schoenfeld E. Diabetes mellitus and cancer risk: a multisite case-control study. J. Chronic Dis. 1985;38(5):435–441. doi: 10.1016/0021-9681(85)90139-0. [DOI] [PubMed] [Google Scholar]
- 38.Stott-Miller M., Chen C., Chuang S.C., et al. History of diabetes and risk of head and neck cancer: a pooled analysis from the international head and neck cancer epidemiology consortium. Cancer Epidemiol. Biomarkers Prev. 2012;21(2):294–304. doi: 10.1158/1055-9965.EPI-11-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zendehdel K, Nyren O, Ostenson C, Adami H, Ekbom A, Ye W. Cancer incidence in patients with type 1 diabetes mellitus: A population-based cohort study in Sweden RID A-5939-2008. . J Natl Cancer Inst . 200; 95 (23):1797–1800. doi: 10.1093/jnci/djg105. [DOI] [PubMed] [Google Scholar]
- 40.Kao C.H., Sun L.M., Chen P.C., et al. A population-based cohort study in Taiwan--use of insulin sensitizers can decrease cancer risk in diabetic patients? Ann. Oncol. 2012 doi: 10.1093/annonc/mds472. [DOI] [PubMed] [Google Scholar]
- 41.Bowker S.L., Richardson K., Marra C.A., Johnson J.A. Risk of Breast Cancer After Onset of Type 2 Diabetes: Evidence of detection bias in postmenopausal women. Diabetes Care. 2011;34(12):2542–2544. doi: 10.2337/dc11-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michels K.B., Solomon C.G., Hu F.B., et al. Type 2 diabetes and subsequent incidence of breast cancer in the Nurses' Health Study. Diabetes Care. 2003;26(6):1752–1758. doi: 10.2337/diacare.26.6.1752. [DOI] [PubMed] [Google Scholar]
- 43.Campbell P.T., Deka A., Jacobs E.J., et al. Prospective study reveals associations between colorectal cancer and type 2 diabetes mellitus or insulin use in men 24. Gastroenterology. 2010;139(4):1138–1146. doi: 10.1053/j.gastro.2010.06.072. [DOI] [PubMed] [Google Scholar]
- 44.Ren X., Zhang X., Zhang X., et al. Type 2 diabetes mellitus associated with increased risk for colorectal cancer: evidence from an international ecological study and population-based risk analysis in China. Public Health. 2009;123(8):540–544. doi: 10.1016/j.puhe.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 45.Lai M.S., Hsieh M.S., Chiu Y.H., Chen T.H. Type 2 diabetes and hepatocellular carcinoma: A cohort study in high prevalence area of hepatitis virus infection. Hepatology. 2006;43(6):1295–1302. doi: 10.1002/hep.21208. [DOI] [PubMed] [Google Scholar]
- 46.Wang C.S., Yao W.J., Chang T.T., Wang S.T., Chou P. The impact of type 2 diabetes on the development of hepatocellular carcinoma in different viral hepatitis statuses. Cancer Epidemiol. Biomarkers Prev. 2009;18(7):2054–2060. doi: 10.1158/1055-9965.EPI-08-1131. [DOI] [PubMed] [Google Scholar]
- 47.Joh H.K., Willett W.C., Cho E. Type 2 diabetes and the risk of renal cell cancer in women. Diabetes Care. 2011;34(7):1552–1556. doi: 10.2337/dc11-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hemminki K., Li X., Sundquist J., Sundquist K. Risk of Cancer Following Hospitalization for Type 2 Diabetes. Oncologist. 2010;15(6):548–555. doi: 10.1634/theoncologist.2009-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hense H., Kajuter H., Wellmann J., Batzler W.U. Cancer incidence in type 2 diabetes patients - First results from a feasibility study of the D2C cohort. Diabetol. Metab. Syndr. 2011;3:15. doi: 10.1186/1758-5996-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee M.Y., Lin K.D., Hsiao P.J., Shin S.J. The association of diabetes mellitus with liver, colon, lung, and prostate cancer is independent of hypertension, hyperlipidemia, and gout in Taiwanese patients. Metabolism. 2012;61(2):242–249. doi: 10.1016/j.metabol.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 51.Ogunleye A.A., Ogston S.A., Morris A.D., Evans J.M. A cohort study of the risk of cancer associated with type 2 diabetes. Br. J. Cancer. 2009;101(7):1199–1201. doi: 10.1038/sj.bjc.6605240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fillenbaum G.G., Pieper C.F., Cohen H.J., Cornoni-Huntley J.C., Guralnik J.M. Comorbidity of five chronic health conditions in elderly community residents: determinants and impact on mortality. J. Gerontol. A Biol. Sci. Med. Sci. 2000;55(2):M84–M89. doi: 10.1093/gerona/55.2.m84. [DOI] [PubMed] [Google Scholar]
- 53.Larsson S.C., Andersson S., Johansson J., Wolk A. Diabetes mellitus, body size and bladder cancer risk in a prospective study of Swedish men. Eur. J. Cancer. 2008;44(17):2655–2660. doi: 10.1016/j.ejca.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 54.Tripathi A., Folsom A.R., Anderson K.E., Iowa Women's Health Study Risk factors for urinary bladder carcinoma in postmenopausal women. The Iowa Women's Health Study. Cancer. 2002;95(11):2316–2323. doi: 10.1002/cncr.10975. [DOI] [PubMed] [Google Scholar]
- 55.Bosco J.L., Palmer J.R., Boggs D.A., Hatch E.E., Rosenberg L. Cardiometabolic factors and breast cancer risk in U.S. black women. Breast Cancer Res. Treat. 2012;134(3):1247–1256. doi: 10.1007/s10549-012-2131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chlebowski R.T., McTiernan A., Wactawski-Wende J., et al. Diabetes, metformin, and breast cancer in postmenopausal women. J. Clin. Oncol. 2012;30(23):2844–2852. doi: 10.1200/JCO.2011.39.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goodman M.T., Cologne J.B., Moriwaki H., Vaeth M., Mabuchi K. Risk factors for primary breast cancer in Japan: 8-year follow-up of atomic bomb survivors. Prev. Med. 1997;26(1):144–153. doi: 10.1006/pmed.1996.9979. [DOI] [PubMed] [Google Scholar]
- 58.Lipscombe L.L., Goodwin P.J., Zinman B., McLaughlin J.R., Hux J.E. Diabetes mellitus and breast cancer: a retrospective population-based cohort study. Breast Cancer Res. Treat. 2006;98(3):349–356. doi: 10.1007/s10549-006-9172-5. [DOI] [PubMed] [Google Scholar]
- 59.Mink P.J., Shahar E., Rosamond W.D., Alberg A.J., Folsom A.R. Serum insulin and glucose levels and breast cancer incidence: the atherosclerosis risk in communities study. Am. J. Epidemiol. 2002;156(4):349–352. doi: 10.1093/aje/kwf050. [DOI] [PubMed] [Google Scholar]
- 60.Reeves K.W., McLaughlin V., Fredman L., Ensrud K., Cauley J.A. Components of metabolic syndrome and risk of breast cancer by prognostic features in the study of osteoporotic fractures cohort. Cancer Causes Control. 2012;23(8):1241–1251. doi: 10.1007/s10552-012-0002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sellers T.A., Sprafka J.M., Gapstur S.M., et al. Does body fat distribution promote familial aggregation of adult onset diabetes mellitus and postmenopausal breast cancer? Epidemiology. 1994;5(1):102–108. doi: 10.1097/00001648-199401000-00015. [DOI] [PubMed] [Google Scholar]
- 62.Weiderpass E., Gridley G., Persson I., Nyren O., Ekbom A., Adami H.O. Risk of endometrial and breast cancer in patients with diabetes mellitus. Int. J. Cancer. 1997;71(3):360–363. doi: 10.1002/(sici)1097-0215(19970502)71:3<360::aid-ijc9>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 63.Lambe M., Wigertz A., Garmo H., Walldius G., Jungner I., Hammar N. Impaired glucose metabolism and diabetes and the risk of breast, endometrial, and ovarian cancer. Cancer Causes Control. 2011;22(8):1163–1171. doi: 10.1007/s10552-011-9794-8. [DOI] [PubMed] [Google Scholar]
- 64.Bowers K., Albanes D., Limburg P., et al. A prospective study of anthropometric and clinical measurements associated with insulin resistance syndrome and colorectal cancer in male smokers. Am. J. Epidemiol. 2006;164(7):652–664. doi: 10.1093/aje/kwj253. [DOI] [PubMed] [Google Scholar]
- 65.Flood A., Strayer L., Schairer C., Schatzkin A. Diabetes and risk of incident colorectal cancer in a prospective cohort of women. Cancer Causes Control. 2010;21(8):1277–1284. doi: 10.1007/s10552-010-9555-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hartz A., He T., Ross J.J. Risk factors for colon cancer in 150,912 postmenopausal women. Cancer Causes Control. 2012;23(10):1599–1605. doi: 10.1007/s10552-012-0037-4. [DOI] [PubMed] [Google Scholar]
- 67.He J., Stram D.O., Kolonel L.N., Henderson B.E., Le Marchand L., Haiman C.A. The association of diabetes with colorectal cancer risk: the Multiethnic Cohort. Br. J. Cancer. 2010;103(1):120–126. doi: 10.1038/sj.bjc.6605721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu F.B., Manson J.E., Liu S., et al. Prospective study of adult onset diabetes mellitus (type 2) and risk of colorectal cancer in women. J. Natl. Cancer Inst. 1999;91(6):542–547. doi: 10.1093/jnci/91.6.542. [DOI] [PubMed] [Google Scholar]
- 69.Khaw K., Wareham N., Bingham S., Luben R., Welch A., Day N. Preliminary communication: Glycated hemoglobin, diabetes, and incident colorectal cancer in men and women: A prospective analysis from the European Prospective Investigation into Cancer-Norfolk Study. Cancer Epidemiol. Biomarkers Prev. 2004;13(6):915–919. [PubMed] [Google Scholar]
- 70.Larsson S.C., Giovannucci E., Wolk A. Diabetes and colorectal cancer incidence in the cohort of Swedish men. Diabetes Care. 2005;28(7):1805–1807. doi: 10.2337/diacare.28.7.1805. [DOI] [PubMed] [Google Scholar]
- 71.Limburg P.J., Anderson K.E., Johnson T.W., et al. Diabetes mellitus and subsite-specific colorectal cancer risks in the Iowa Women's Health Study. Cancer Epidemiol. Biomarkers Prev. 2005;14(1):133–137. [PubMed] [Google Scholar]
- 72.Nilsen T.I., Vatten L.J. Prospective study of colorectal cancer risk and physical activity, diabetes, blood glucose and BMI: exploring the hyperinsulinaemia hypothesis. Br. J. Cancer. 2001;84(3):417–422. doi: 10.1054/bjoc.2000.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schoen R.E., Tangen C.M., Kuller L.H., et al. Increased blood glucose and insulin, body size, and incident colorectal cancer. J. Natl. Cancer Inst. 1999;91(13):1147–1154. doi: 10.1093/jnci/91.13.1147. [DOI] [PubMed] [Google Scholar]
- 74.Seow A., Yuan J.M., Koh W.P., Lee H.P., Yu M.C. Diabetes mellitus and risk of colorectal cancer in the Singapore Chinese Health Study. J. Natl. Cancer Inst. 2006;98(2):135–138. doi: 10.1093/jnci/djj015. [DOI] [PubMed] [Google Scholar]
- 75.Sturmer T., Buring J.E., Lee I.M., Gaziano J.M., Glynn R.J. Metabolic abnormalities and risk for colorectal cancer in the physicians' health study. Cancer Epidemiol. Biomarkers Prev. 2006;15(12):2391–2397. doi: 10.1158/1055-9965.EPI-06-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Will J.C., Galuska D.A., Vinicor F., Calle E.E. Colorectal cancer: another complication of diabetes mellitus? Am. J. Epidemiol. 1998;147(9):816–825. doi: 10.1093/oxfordjournals.aje.a009534. [DOI] [PubMed] [Google Scholar]
- 77.Anderson K.E., Anderson E., Mink P.J., et al. Diabetes and endometrial cancer in the Iowa women's health study. Cancer Epidemiol. Biomarkers Prev. 2001;10(6):611–616. [PubMed] [Google Scholar]
- 78.Friberg E., Mantzoros C.S., Wolk A. Diabetes and risk of endometrial cancer: a population-based prospective cohort study. Cancer Epidemiol. Biomarkers Prev. 2007;16(2):276–280. doi: 10.1158/1055-9965.EPI-06-0751. [DOI] [PubMed] [Google Scholar]
- 79.Lindemann K., Vatten L.J., Ellstrom-Engh M., Eskild A. Body mass, diabetes and smoking, and endometrial cancer risk: a follow-up study. Br. J. Cancer. 2008;98(9):1582–1585. doi: 10.1038/sj.bjc.6604313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin S.W., Freedman N.D., Hollenbeck A.R., Schatzkin A., Abnet C.C. Prospective study of self-reported diabetes and risk of upper gastrointestinal cancers. Cancer Epidemiol. Biomarkers Prev. 2011;20(5):954–961. doi: 10.1158/1055-9965.EPI-10-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chuma M., Hige S., Nakanishi M., et al. 8-Hydroxy-2'-deoxy-guanosine is a risk factor for development of hepatocellular carcinoma in patients with chronic hepatitis C virus infection. J. Gastroenterol. Hepatol. 2008;23(9):1431–1436. doi: 10.1111/j.1440-1746.2008.05502.x. [DOI] [PubMed] [Google Scholar]
- 82.Di Costanzo G.G., De Luca M., Tritto G., et al. Effect of alcohol, cigarette smoking, and diabetes on occurrence of hepatocellular carcinoma in patients with transfusion-acquired hepatitis C virus infection who develop cirrhosis. Eur. J. Gastroenterol. Hepatol. 2008;20(7):674–679. doi: 10.1097/MEG.0b013e3282f762e1. [DOI] [PubMed] [Google Scholar]
- 83.El-Serag H.B., Tran T., Everhart J.E. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126(2):460–468. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 84.Hung C.H., Lee C.M., Wang J.H., et al. Impact of diabetes mellitus on incidence of hepatocellular carcinoma in chronic hepatitis C patients treated with interferon-based antiviral therapy. Int. J. Cancer. 2011;128(10):2344–2352. doi: 10.1002/ijc.25585. [DOI] [PubMed] [Google Scholar]
- 85.Ioannou G.N., Splan M.F., Weiss N.S., McDonald G.B., Beretta L., Lee S.P. Incidence and predictors of hepatocellular carcinoma in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2007;5(8):938–945. doi: 10.1016/j.cgh.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 86.Kawamura Y., Arase Y., Ikeda K., et al. Diabetes enhances hepatocarcinogenesis in noncirrhotic, interferon-treated hepatitis C patients. Am. J. Med. 2010;123(10):951–956. doi: 10.1016/j.amjmed.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 87.N'Kontchou G., Paries J., Htar M.T., et al. Risk factors for hepatocellular carcinoma in patients with alcoholic or viral C cirrhosis. Clin. Gastroenterol. Hepatol. 2006;4(8):1062–1068. doi: 10.1016/j.cgh.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 88.Ohata K., Hamasaki K., Toriyama K., et al. Hepatic steatosis is a risk factor for hepatocellular carcinoma in patients with chronic hepatitis C virus infection. Cancer. 2003;97(12):3036–3043. doi: 10.1002/cncr.11427. [DOI] [PubMed] [Google Scholar]
- 89.Ohki T., Tateishi R., Sato T., et al. Obesity is an independent risk factor for hepatocellular carcinoma development in chronic hepatitis C patients. Clin. Gastroenterol. Hepatol. 2008;6(4):459–464. doi: 10.1016/j.cgh.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 90.Tazawa J., Maeda M., Nakagawa M., et al. Diabetes mellitus may be associated with hepatocarcinogenesis in patients with chronic hepatitis C. Dig. Dis. Sci. 2002;47(4):710–715. doi: 10.1023/a:1014715327729. [DOI] [PubMed] [Google Scholar]
- 91.Veldt B.J., Chen W., Heathcote E.J., et al. Increased risk of hepatocellular carcinoma among patients with hepatitis C cirrhosis and diabetes mellitus. Hepatology. 2008;47(6):1856–1862. doi: 10.1002/hep.22251. [DOI] [PubMed] [Google Scholar]
- 92.Adami H.O., Chow W.H., Nyren O., et al. Excess risk of primary liver cancer in patients with diabetes mellitus. J. Natl. Cancer Inst. 1996;88(20):1472–1477. doi: 10.1093/jnci/88.20.1472. [DOI] [PubMed] [Google Scholar]
- 93.Chen H., Chen P., Li C. Risk of malignant neoplasms of liver and biliary tract in diabetic patients with different age and sex stratifications. Hepatology. 2010;52(1):155–163. doi: 10.1002/hep.23641. [DOI] [PubMed] [Google Scholar]
- 94.Ehrlich S.F., Quesenberry C.P., Van Den E.S., Shan J., Ferrara A. Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care. 2010;33(1):55–60. doi: 10.2337/dc09-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hall G.C., Roberts C.M., Boulis M., Mo J., MacRae K.D. Diabetes and the risk of lung cancer. Diabetes Care. 2005;28(3):590–594. doi: 10.2337/diacare.28.3.590. [DOI] [PubMed] [Google Scholar]
- 96.Lai S.W., Liao K.F., Chen P.C., Tsai P.Y., Hsieh D.P., Chen C.C. Antidiabetes drugs correlate with decreased risk of lung cancer: a population-based observation in Taiwan. Clin. Lung Cancer. 2012;13(2):143–148. doi: 10.1016/j.cllc.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 97.Luo J., Chlebowski R., Wactawski-Wende J., Schlecht N.F., Tinker L., Margolis K.L. Diabetes and lung cancer among postmenopausal women. Diabetes Care. 2012;35(7):1485–1491. doi: 10.2337/dc11-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cerhan J.R., Wallace R.B., Folsom A.R., et al. Medical history risk factors for non-Hodgkin's lymphoma in older women. J. Natl. Cancer Inst. 1997;89(4):314–318. doi: 10.1093/jnci/89.4.314. [DOI] [PubMed] [Google Scholar]
- 99.Erber E., Lim U., Maskarinec G., Kolonel L.N. Common immune-related risk factors and incident non-Hodgkin lymphoma: the multiethnic cohort. Int. J. Cancer. 2009;125(6):1440–1445. doi: 10.1002/ijc.24456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Khan A.E., Gallo V., Linseisen J., et al. Diabetes and the risk of non-Hodgkin's lymphoma and multiple myeloma in the European Prospective Investigation into Cancer and Nutrition. Haematologica. 2008;93(6):842–850. doi: 10.3324/haematol.12297. [DOI] [PubMed] [Google Scholar]
- 101.Gapstur S.M., Patel A.V., Diver W.R., et al. Type II Diabetes Mellitus and the Incidence of Epithelial Ovarian Cancer in the Cancer Prevention Study-II Nutrition Cohort 98 393. Cancer Epidemiol. Biomarkers Prev. 2012;21(11):2000–2005. doi: 10.1158/1055-9965.EPI-12-0867. [DOI] [PubMed] [Google Scholar]
- 102.Chen H., Chen P., Li C. Risk of malignant neoplasm of the pancreas in relation to diabetes: A population-based study in Taiwan. Diabetes Care. 2011;34(5):1177–1179. doi: 10.2337/dc10-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chow W.H., Gridley G., Nyren O., et al. Risk of pancreatic cancer following diabetes mellitus: a nationwide cohort study in Sweden. J. Natl. Cancer Inst. 1995;87(12):930–931. doi: 10.1093/jnci/87.12.930. [DOI] [PubMed] [Google Scholar]
- 104.Gupta S., Vittinghoff E., Bertenthal D., et al. New-onset diabetes and pancreatic cancer. Clin. Gastroenterol. Hepatol. 2006;4(11):1366–1372. doi: 10.1016/j.cgh.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 105.Larsson S.C., Permert J., Hakansson N., Naslund I., Bergkvist L., Wolk A. Overall obesity, abdominal adiposity, diabetes and cigarette smoking in relation to the risk of pancreatic cancer in two Swedish population-based cohorts. Br. J. Cancer. 2005;93(11):1310–1315. doi: 10.1038/sj.bjc.6602868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liao K.F., Lai S.W., Li C.I., Chen W.C. Diabetes mellitus correlates with increased risk of pancreatic cancer: a population-based cohort study in Taiwan. J. Gastroenterol. Hepatol. 2012;27(4):709–713. doi: 10.1111/j.1440-1746.2011.06938.x. [DOI] [PubMed] [Google Scholar]
- 107.Shibata A., Mack T.M., Paganini-Hill A., Ross R.K., Henderson B.E. A prospective study of pancreatic cancer in the elderly. Int. J. Cancer. 1994;58(1):46–49. doi: 10.1002/ijc.2910580109. [DOI] [PubMed] [Google Scholar]
- 108.Stevens R.J., Roddam A.W., Spencer E.A., et al. Factors associated with incident and fatal pancreatic cancer in a cohort of middle-aged women. Int. J. Cancer. 2009;124(10):2400–2405. doi: 10.1002/ijc.24196. [DOI] [PubMed] [Google Scholar]
- 109.Stolzenberg-Solomon R.Z., Pietinen P., Taylor P.R., Virtamo J., Albanes D. A prospective study of medical conditions, anthropometry, physical activity, and pancreatic cancer in male smokers (Finland). Cancer Causes Control. 2002;13(5):417–426. doi: 10.1023/a:1015729615148. [DOI] [PubMed] [Google Scholar]
- 110.Yun J.E., Jo I., Park J., et al. Cigarette smoking, elevated fasting serum glucose, and risk of pancreatic cancer in Korean men. Int. J. Cancer. 2006;119(1):208–212. doi: 10.1002/ijc.21816. [DOI] [PubMed] [Google Scholar]
- 111.Jamal M.M., Yoon E.J., Vega K.J., Hashemzadeh M., Chang K.J. Diabetes mellitus as a risk factor for gastrointestinal cancer among American veterans. World J. Gastroenterol. 2009;15(42):5274–5278. doi: 10.3748/wjg.15.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Giovannucci E., Rimm E.B., Stampfer M.J., Colditz G.A., Willett W.C. Diabetes mellitus and risk of prostate cancer (United States). Cancer Causes Control. 1998;9(1):3–9. doi: 10.1023/a:1008822917449. [DOI] [PubMed] [Google Scholar]
- 113.Leitzmann M.F., Ahn J., Albanes D., et al. Diabetes mellitus and prostate cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Causes Control. 2008;19(10):1267–1276. doi: 10.1007/s10552-008-9198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li Q., Kuriyama S., Kakizaki M., et al. History of diabetes mellitus and the risk of prostate cancer: The Ohsaki Cohort Study. Cancer Causes Control. 2010;21(7):1025–1032. doi: 10.1007/s10552-010-9530-9. [DOI] [PubMed] [Google Scholar]
- 115.Rodriguez C., Patel A.V., Mondul A.M., Jacobs E.J., Thun M.J., Calle E.E. Diabetes and risk of prostate cancer in a prospective cohort of US men. Am. J. Epidemiol. 2005;161(2):147–152. doi: 10.1093/aje/kwh334. [DOI] [PubMed] [Google Scholar]
- 116.Thompson M.M., Garland C., Barrett-Connor E., Khaw K.T., Friedlander N.J., Wingard D.L. Heart disease risk factors, diabetes, and prostatic cancer in an adult community. Am. J. Epidemiol. 1989;129(3):511–517. doi: 10.1093/oxfordjournals.aje.a115162. [DOI] [PubMed] [Google Scholar]
- 117.Velicer C.M., Dublin S., White E. Diabetes and the risk of prostate cancer: the role of diabetes treatment and complications. Prostate Cancer Prostatic Dis. 2007;10(1):46–51. doi: 10.1038/sj.pcan.4500914. [DOI] [PubMed] [Google Scholar]
- 118.Waters K.M., Henderson B.E., Stram D.O., Wan P., Kolonel L.N., Haiman C.A. Association of diabetes with prostate cancer risk in the multiethnic cohort. Am. J. Epidemiol. 2009;169(8):937–945. doi: 10.1093/aje/kwp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Weiderpass E., Ye W., Vainio H., Kaaks R., Adami H.O. Reduced risk of prostate cancer among patients with diabetes mellitus. Int. J. Cancer. 2002;102(3):258–261. doi: 10.1002/ijc.10685. [DOI] [PubMed] [Google Scholar]
- 120.Will J.C., Vinicor F., Calle E.E. Is diabetes mellitus associated with prostate cancer incidence and survival? Epidemiology. 1999;10(3):313–318. [PubMed] [Google Scholar]
- 121.Nicodemus K.K., Sweeney C., Folsom A.R. Evaluation of dietary, medical and lifestyle risk factors for incident kidney cancer in postmenopausal women. Int. J. Cancer. 2004;108(1):115–121. doi: 10.1002/ijc.11532. [DOI] [PubMed] [Google Scholar]
- 122.Adami H.O., McLaughlin J., Ekbom A., et al. Cancer risk in patients with diabetes mellitus. Cancer Causes Control. 1991;2(5):307–314. doi: 10.1007/BF00051670. [DOI] [PubMed] [Google Scholar]
- 123.Atchison E.A., Gridley G., Carreon J.D., Leitzmann M.F., McGlynn K.A. Risk of cancer in a large cohort of U.S. veterans with diabetes. Int. J. Cancer. 2011;128(3):635–643. doi: 10.1002/ijc.25362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Carstensen B., Witte D.R., Friis S. Cancer occurrence in Danish diabetic patients: duration and insulin effects. Diabetologia. 2012;55(4):948–958. doi: 10.1007/s00125-011-2381-4. [DOI] [PubMed] [Google Scholar]
- 125.Chodick G., Heymann A.D., Rosenmann L., et al. Diabetes and risk of incident cancer: a large population-based cohort study in Israel. Cancer Causes Control. 2010;21(6):879–887. doi: 10.1007/s10552-010-9515-8. [DOI] [PubMed] [Google Scholar]
- 126.Dankner R., Chetrit A., Segal P. Glucose tolerance status and 20 year cancer incidence. Isr. Med. Assoc. J. 2007;9(8):592–596. [PubMed] [Google Scholar]
- 127.Folsom A.R., Pankow J.S., Peacock J.M., Bielinski S.J., Heiss G., Boerwinkle E. Variation in TCF7L2 and increased risk of colon cancer: the Atherosclerosis Risk in Communities (ARIC) Study. Diabetes Care. 2008;31(5):905–909. doi: 10.2337/dc07-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hjalgrim H., Frisch M., Ekbom A., Kyvik K.O., Melbye M., Green A. Cancer and diabetes--a follow-up study of two population-based cohorts of diabetic patients. J. Intern. Med. 1997;241(6):471–475. doi: 10.1111/j.1365-2796.1997.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 129.Inoue M., Iwasaki M., Otani T., Sasazuki S., Noda M., Tsugane S. Diabetes mellitus and the risk of cancer: results from a large-scale population-based cohort study in Japan. Arch. Intern. Med. 2006;166(17):1871–1877. doi: 10.1001/archinte.166.17.1871. [DOI] [PubMed] [Google Scholar]
- 130.Jee S.H., Ohrr H., Sull J.W., Yun J.E., Ji M., Samet J.M. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005;293(2):194–202. doi: 10.1001/jama.293.2.194. [DOI] [PubMed] [Google Scholar]
- 131.Johnson J.A., Bowker S.L., Richardson K., Marra C.A. Time-varying incidence of cancer after the onset of type 2 diabetes: evidence of potential detection bias. Diabetologia. 2011;54(9):2263–2271. doi: 10.1007/s00125-011-2242-1. [DOI] [PubMed] [Google Scholar]
- 132.Joshu C.E., Prizment A.E., Dluzniewski P.J., et al. Glycated hemoglobin and cancer incidence and mortality in the Atherosclerosis in Communities (ARIC) Study, 1990-2006. Int. J. Cancer. 2012;131(7):1667–1677. doi: 10.1002/ijc.27394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Khan M., Mori M., Fujino Y., et al. Site-specific cancer risk due to diabetes mellitus history: evidence from the Japan Collaborative Cohort (JACC) Study. Asian Pac. J. Cancer Prev. 2006;7(2):253–259. [PubMed] [Google Scholar]
- 134.Ragozzino M., Melton L.J., Chu C.P., Palumbo P.J. Subsequent cancer risk in the incidence cohort of Rochester, Minnesota, residents with diabetes mellitus. J. Chronic Dis. 1982;35(1):13–19. doi: 10.1016/0021-9681(82)90025-x. [DOI] [PubMed] [Google Scholar]
- 135.Rapp K., Schroeder J., Klenk J., et al. Fasting blood glucose and cancer risk in a cohort of more than 140,000 adults in Austria. Diabetologia. 2006;49(5):945–952. doi: 10.1007/s00125-006-0207-6. [DOI] [PubMed] [Google Scholar]
- 136.Steenland K., Nowlin S., Palu S. Cancer incidence in the National Health and Nutrition Survey I. Follow-up data: diabetes, cholesterol, pulse and physical activity. Cancer Epidemiol. Biomarkers Prev. 1995;4(8):807–811. [PubMed] [Google Scholar]
- 137.Swerdlow A.J., Laing S.P., Qiao Z., et al. Cancer incidence and mortality in patients with insulin-treated diabetes: A UK cohort study. Br. J. Cancer. 2005;92(11):2070–2075. doi: 10.1038/sj.bjc.6602611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wideroff L., Gridley G., Mellemkjaer L., et al. Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J. Natl. Cancer Inst. 1997;89(18):1360–1365. doi: 10.1093/jnci/89.18.1360. [DOI] [PubMed] [Google Scholar]
- 139.Wotton C.J., Yeates D.G., Goldacre M.J. Cancer in patients admitted to hospital with diabetes mellitus aged 30 years and over: record linkage studies. Diabetologia. 2011;54(3):527–534. doi: 10.1007/s00125-010-1987-2. [DOI] [PubMed] [Google Scholar]
- 140.Yeh H., Platz E.A., Wang N., Visvanathan K., Helzlsouer K.J., Brancati F.L. A Prospective Study of the Associations Between Treated Diabetes and Cancer Outcomes. Diabetes Care. 2012;35(1):113–118. doi: 10.2337/dc11-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Aschebrook-Kilfoy B., Sabra M.M., Brenner A., et al. Diabetes and thyroid cancer risk in the National Institutes of Health-AARP Diet and Health Study. Thyroid. 2011;21(9):957–963. doi: 10.1089/thy.2010.0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kitahara C.M., Platz E.A., Beane Freeman L.E., et al. Physical activity, diabetes, and thyroid cancer risk: a pooled analysis of five prospective studies. Cancer Causes Control. 2012 doi: 10.1007/s10552-012-9896-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Meinhold C.L., Ron E., Schonfeld S.J., et al. Nonradiation risk factors for thyroid cancer in the US Radiologic Technologists Study. Am. J. Epidemiol. 2010;171(2):242–252. doi: 10.1093/aje/kwp354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Baur D.M., Klotsche J., Hamnvik O.P., et al. Type 2 diabetes mellitus and medications for type 2 diabetes mellitus are associated with risk for and mortality from cancer in a German primary care cohort 10. Metabolism. 2011;60(10):1363–1371. doi: 10.1016/j.metabol.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 145.Lawlor D.A., Smith G.D., Ebrahim S. Hyperinsulinaemia and increased risk of breast cancer: findings from the British Women's Heart and Health Study. Cancer Causes Control. 2004;15(3):267–275. doi: 10.1023/B:CACO.0000024225.14618.a8. [DOI] [PubMed] [Google Scholar]
- 146.Sandhu M.S., Luben R., Khaw K.T. Self reported non-insulin dependent diabetes, family history, and risk of prevalent colorectal cancer: population based, cross sectional study. J. Epidemiol. Community Health. 2001;55(11):804–805. doi: 10.1136/jech.55.11.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Moreira D.M., Anderson T., Gerber L., et al. The association of diabetes mellitus and high-grade prostate cancer in a multiethnic biopsy series. Cancer Causes Control. 2011;22(7):977–983. doi: 10.1007/s10552-011-9770-3. [DOI] [PubMed] [Google Scholar]
- 148.Moses K.A., Utuama O.A., Goodman M., Issa M.M. The association of diabetes and positive prostate biopsy in a US veteran population. Prostate Cancer Prostatic Dis. 2012;15(1):70–74. doi: 10.1038/pcan.2011.40. [DOI] [PubMed] [Google Scholar]
- 149.Li C., Balluz L.S., Ford E.S., Okoro C.A., Tsai J., Zhao G. Association between diagnosed diabetes and self-reported cancer among U.S. adults: findings from the 2009 Behavioral Risk Factor Surveillance System. Diabetes Care. 2011;34(6):1365–1368. doi: 10.2337/dc11-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Khachatryan L., Scharpf R., Kagan S. Influence of Diabetes Mellitus Type 2 and Prolonged Estrogen Exposure on Risk of Breast Cancer Among Women in Armenia. Health Care Women Int. 2011;32(11):953–971. doi: 10.1080/07399332.2011.569041. [DOI] [PubMed] [Google Scholar]
- 151.Rollison D.E., Giuliano A.R., Sellers T.A., et al. Population-based case-control study of diabetes and breast cancer risk in Hispanic and non-Hispanic White women living in US southwestern states. Am. J. Epidemiol. 2008;167(4):447–456. doi: 10.1093/aje/kwm322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li Q., Li W.W., Yang X., et al. Type 2 diabetes and hepatocellular carcinoma: a case-control study in patients with chronic hepatitis B. Int. J. Cancer. 2012;131(5):1197–1202. doi: 10.1002/ijc.27337. [DOI] [PubMed] [Google Scholar]
- 153.Grainge M.J., West J., Solaymani-Dodaran M., Aithal G.P., Card T.R. The antecedents of biliary cancer: a primary care case-control study in the United Kingdom. Br. J. Cancer. 2009;100(1):178–180. doi: 10.1038/sj.bjc.6604765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Khan Z.R., Neugut A.I., Ahsan H., Chabot J.A. Risk factors for biliary tract cancers. Am. J. Gastroenterol. 1999;94(1):149–152. doi: 10.1111/j.1572-0241.1999.00786.x. [DOI] [PubMed] [Google Scholar]
- 155.Shaib Y.H., El-Serag H.B., Nooka A.K., et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a hospital-based case-control study. Am. J. Gastroenterol. 2007;102(5):1016–1021. doi: 10.1111/j.1572-0241.2007.01104.x. [DOI] [PubMed] [Google Scholar]
- 156.Tao L.Y., He X.D., Qu Q., et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a case-control study in China. Liver Int. 2010;30(2):215–221. doi: 10.1111/j.1478-3231.2009.02149.x. [DOI] [PubMed] [Google Scholar]
- 157.Welzel T.M., Graubard B.I., El-Serag H.B., et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin. Gastroenterol. Hepatol. 2007;5(10):1221–1228. doi: 10.1016/j.cgh.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kantor A.F., Hartge P., Hoover R.N., Narayana A.S., Sullivan J.W., Fraumeni J.F., Jr Urinary tract infection and risk of bladder cancer. Am. J. Epidemiol. 1984;119(4):510–515. doi: 10.1093/oxfordjournals.aje.a113768. [DOI] [PubMed] [Google Scholar]
- 159.Kravchick S., Gal R., Cytron S., et al. Increased incidence of diabetes mellitus in the patients with transitional cell carcinoma of urinary bladder. Pathol. Oncol. Res. 2001;7(1):56–59. doi: 10.1007/BF03032606. [DOI] [PubMed] [Google Scholar]
- 160.MacKenzie T, Zens MS, Ferrara A, Schned A, Karagas MR. Diabetes and risk of bladder cancer. 2011 doi: 10.1002/cncr.25641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ng Y., Husain I., Waterfall N. Diabetes mellitus and bladder cancer--an epidemiological relationship? Pathol. Oncol. Res. 2003;9(1):30–31. doi: 10.1007/BF03033711. [DOI] [PubMed] [Google Scholar]
- 162.Risch H.A., Burch J.D., Miller A.B., Hill G.B., Steele R., Howe G.R. Dietary factors and the incidence of cancer of the urinary bladder. Am. J. Epidemiol. 1988;127(6):1179–1191. doi: 10.1093/oxfordjournals.aje.a114911. [DOI] [PubMed] [Google Scholar]
- 163.Baron J.A., Weiderpass E., Newcomb P.A., et al. Metabolic disorders and breast cancer risk (United States). Cancer Causes Control. 2001;12(10):875–880. doi: 10.1023/a:1013796112348. [DOI] [PubMed] [Google Scholar]
- 164.Beji N.K., Reis N. Risk factors for breast cancer in Turkish women: a hospital-based case-control study. Eur. J. Cancer Care (Engl.) 2007;16(2):178–184. doi: 10.1111/j.1365-2354.2006.00711.x. [DOI] [PubMed] [Google Scholar]
- 165.Cleveland R.J., North K.E., Stevens J., Teitelbaum S.L., Neugut A.I., Gammon M.D. The association of diabetes with breast cancer incidence and mortality in the Long Island Breast Cancer Study Project. Cancer Causes Control. 2012;23(7):1193–1203. doi: 10.1007/s10552-012-9989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Garmendia M.L., Pereira A., Alvarado M.E., Atalah E. Relation between insulin resistance and breast cancer among Chilean women. Ann. Epidemiol. 2007;17(6):403–409. doi: 10.1016/j.annepidem.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 167.Jordan S., Lim L., Vilainerun D., et al. Breast cancer in the Thai Cohort Study: an exploratory case-control analysis. Breast. 2009;18(5):299–303. doi: 10.1016/j.breast.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Weiss H.A., Brinton L.A., Potischman N.A., et al. Breast cancer risk in young women and history of selected medical conditions. Int. J. Epidemiol. 1999;28(5):816–823. doi: 10.1093/ije/28.5.816. [DOI] [PubMed] [Google Scholar]
- 169.Wu A.H., Yu M.C., Tseng C.C., Stanczyk F.Z., Pike M.C. Diabetes and risk of breast cancer in Asian-American women. Carcinogenesis. 2007;28(7):1561–1566. doi: 10.1093/carcin/bgm081. [DOI] [PubMed] [Google Scholar]
- 170.Kune G.A., Kune S., Watson L.F. Colorectal cancer risk, chronic illnesses, operations, and medications: case control results from the Melbourne Colorectal Cancer Study. Cancer Res. 1988;48(15):4399–4404. [PubMed] [Google Scholar]
- 171.Le Marchand L., Wilkens L.R., Kolonel L.N., Hankin J.H., Lyu L.C. Associations of sedentary lifestyle, obesity, smoking, alcohol use, and diabetes with the risk of colorectal cancer. Cancer Res. 1997;57(21):4787–4794. [PubMed] [Google Scholar]
- 172.Rinaldi S., Rohrmann S., Jenab M., et al. Glycosylated hemoglobin and risk of colorectal cancer in men and women, the European prospective investigation into cancer and nutrition. Cancer Epidemiol. Biomarkers Prev. 2008;17(11):3108–3115. doi: 10.1158/1055-9965.EPI-08-0495. [DOI] [PubMed] [Google Scholar]
- 173.Safaee A., Fatemi R., Pourhoseingholi M.A., et al. Positive association between diabetes mellitus and risk of colorectal cancer. Iran. J. Cancer Prev. 2009;2(4):189–193. [Google Scholar]
- 174.Vinikoor L.C., Long M.D., Keku T.O., Martin C.F., Galanko J.A., Sandler R.S. The association between diabetes, insulin use, and colorectal cancer among Whites and African Americans. Cancer Epidemiol. Biomarkers Prev. 2009;18(4):1239–1242. doi: 10.1158/1055-9965.EPI-08-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yang Y.X., Hennessy S., Lewis J.D. Type 2 diabetes mellitus and the risk of colorectal cancer. Clin. Gastroenterol. Hepatol. 2005;3(6):587–594. doi: 10.1016/s1542-3565(05)00152-7. [DOI] [PubMed] [Google Scholar]
- 176.Fortuny J, Sima C, Bayuga S, et al. Risk of endometrial cancer in relation to medical conditions and medication use. 2009. [DOI] [PMC free article] [PubMed]
- 177.Inoue M., Okayama A., Fujita M., Enomoto T., Tanizawa O., Ueshima H. A case-control study on risk factors for uterine endometrial cancer in Japan. Jpn. J. Cancer Res. 1994;85(4):346–350. doi: 10.1111/j.1349-7006.1994.tb02365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Saltzman B.S., Doherty J.A., Hill D.A., et al. Diabetes and endometrial cancer: an evaluation of the modifying effects of other known risk factors. Am. J. Epidemiol. 2008;167(5):607–614. doi: 10.1093/aje/kwm333. [DOI] [PubMed] [Google Scholar]
- 179.Yamazawa K., Matsui H., Seki K., Sekiya S. A case-control study of endometrial cancer after antipsychotics exposure in premenopausal women. Oncology. 2003;64(2):116–123. doi: 10.1159/000067769. [DOI] [PubMed] [Google Scholar]
- 180.Neale R.E., Doecke J.D., Pandeya N., et al. Does type 2 diabetes influence the risk of oesophageal adenocarcinoma? Br. J. Cancer. 2009;100(5):795–798. doi: 10.1038/sj.bjc.6604908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Reavis K.M., Morris C.D., Gopal D.V., Hunter J.G., Jobe B.A. Laryngopharyngeal reflux symptoms better predict the presence of esophageal adenocarcinoma than typical gastroesophageal reflux symptoms. Ann. Surg. 2004;239(6):849–856. doi: 10.1097/01.sla.0000128303.05898.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rubenstein J.H., Davis J., Marrero J.A., Inadomi J.M. Relationship between diabetes mellitus and adenocarcinoma of the oesophagus and gastric cardia. Aliment. Pharmacol. Ther. 2005;22(3):267–271. doi: 10.1111/j.1365-2036.2005.02544.x. [DOI] [PubMed] [Google Scholar]
- 183.Vineis P., Crosignani P., Sacerdote C., et al. Haematopoietic cancer and medical history: a multicentre case control study. J. Epidemiol. Community Health. 2000;54(6):431–436. doi: 10.1136/jech.54.6.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Davila J.A., Morgan R.O., Shaib Y., McGlynn K.A., El-Serag H.B. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54(4):533–539. doi: 10.1136/gut.2004.052167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.El-Serag H.B., Richardson P.A., Everhart J.E. The role of diabetes in hepatocellular carcinoma: a case-control study among United States Veterans. Am. J. Gastroenterol. 2001;96(8):2462–2467. doi: 10.1111/j.1572-0241.2001.04054.x. [DOI] [PubMed] [Google Scholar]
- 186.Hassan M.M., Hwang L.Y., Hatten C.J., et al. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36(5):1206–1213. doi: 10.1053/jhep.2002.36780. [DOI] [PubMed] [Google Scholar]
- 187.Hassan M.M., Curley S.A., Li D., et al. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer. 2010 doi: 10.1002/cncr.24982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Matsuo M. Association between diabetes mellitus and hepatocellular carcinoma: results of a hospital- and community-based case-control study. Kurume Med. J. 2003;50(3-4):91–98. doi: 10.2739/kurumemedj.50.91. [DOI] [PubMed] [Google Scholar]
- 189.Yuan JM, Govindarajan S, Arakawa K, Yu MC. Synergism of alcohol, diabetes, and viral hepatitis on the risk of hepatocellular carcinoma in blacks and whites in the U.S. Cancer. 2004. [DOI] [PubMed]
- 190.Fortuny J., Benavente Y., Bosch R., Garcia-Villanueva M., de Sevilla A.F., de Sanjose S. Type 2 diabetes mellitus, its treatment and risk for lymphoma. Eur. J. Cancer. 2005;41(12):1782–1787. doi: 10.1016/j.ejca.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 191.Cartwright R.A., McKinney P.A., O'Brien C., et al. Non-Hodgkin's lymphoma: case control epidemiological study in Yorkshire. Leuk. Res. 1988;12(1):81–88. doi: 10.1016/s0145-2126(98)80012-x. [DOI] [PubMed] [Google Scholar]
- 192.Cerhan J.R., Bernstein L., Severson R.K., et al. Anthropometrics, physical activity, related medical conditions, and the risk of non-hodgkin lymphoma. Cancer Causes Control. 2005;16(10):1203–1214. doi: 10.1007/s10552-005-0358-7. [DOI] [PubMed] [Google Scholar]
- 193.Lin S.Y., Hsieh M.S., Chen L.S., Chiu Y.H., Yen A.M., Chen T.H. Diabetes mellitus associated with the occurrence and prognosis of non-Hodgkin's lymphoma. Eur. J. Cancer Prev. 2007;16(5):471–478. doi: 10.1097/01.cej.0000236253.93984.8f. [DOI] [PubMed] [Google Scholar]
- 194.Smedby K.E., Hjalgrim H., Askling J., et al. Autoimmune and chronic inflammatory disorders and risk of non-Hodgkin lymphoma by subtype. J. Natl. Cancer Inst. 2006;98(1):51–60. doi: 10.1093/jnci/djj004. [DOI] [PubMed] [Google Scholar]
- 195.Bonelli L., Aste H., Bovo P., et al. Exocrine pancreatic cancer, cigarette smoking, and diabetes mellitus: a case-control study in northern Italy. Pancreas. 2003;27(2):143–149. doi: 10.1097/00006676-200308000-00007. [DOI] [PubMed] [Google Scholar]
- 196.Bueno de Mesquita H.B., Maisonneuve P., Moerman C.J., Walker A.M. Aspects of medical history and exocrine carcinoma of the pancreas: a population-based case-control study in The Netherlands. Int. J. Cancer. 1992;52(1):17–23. doi: 10.1002/ijc.2910520105. [DOI] [PubMed] [Google Scholar]
- 197.Cuzick J., Babiker A.G. Pancreatic cancer, alcohol, diabetes mellitus and gall-bladder disease. Int. J. Cancer. 1989;43(3):415–421. doi: 10.1002/ijc.2910430312. [DOI] [PubMed] [Google Scholar]
- 198.Ekoe J.M., Ghadirian P., Simard A., Baillargeon J., Perret C. Diabetes mellitus and pancreatic cancer: a case-control study in greater Montreal, Quebec, Canada. Rev. Epidemiol. Sante Publique. 1992;40(6):447–453. [PubMed] [Google Scholar]
- 199.Friedman G.D., van den Eeden S.K. Risk factors for pancreatic cancer: an exploratory study. Int. J. Epidemiol. 1993;22(1):30–37. doi: 10.1093/ije/22.1.30. [DOI] [PubMed] [Google Scholar]
- 200.Frye J.N., Inder W.J., Dobbs B.R., Frizelle F.A. Pancreatic cancer and diabetes: is there a relationship? A case-controlled study. Aust. N. Z. J. Surg. 2000;70(10):722–724. doi: 10.1046/j.1440-1622.2000.01940.x. [DOI] [PubMed] [Google Scholar]
- 201.Grote V.A., Rohrmann S., Nieters A., et al. Diabetes mellitus, glycated haemoglobin and C-peptide levels in relation to pancreatic cancer risk: a study within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort 109. Diabetologia. 2011;54:3037–3046. doi: 10.1007/s00125-011-2316-0. [DOI] [PubMed] [Google Scholar]
- 202.Gullo L., Pezzilli R., Morselli-Labate A.M., Italian Pancreatic Cancer Study Group Diabetes and the risk of pancreatic cancer. N. Engl. J. Med. 1994;331(2):81–84. doi: 10.1056/NEJM199407143310203. [DOI] [PubMed] [Google Scholar]
- 203.Hassan M.M., Bondy M.L., Wolff R.A., et al. Risk factors for pancreatic cancer: case-control study. Am. J. Gastroenterol. 2007;102(12):2696–2707. doi: 10.1111/j.1572-0241.2007.01510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hiatt R.A., Klatsky A.L., Armstrong M.A. Pancreatic cancer, blood glucose and beverage consumption. Int. J. Cancer. 1988;41(6):794–797. doi: 10.1002/ijc.2910410603. [DOI] [PubMed] [Google Scholar]
- 205.Jain M., Howe G.R., St Louis P., Miller A.B. Coffee and alcohol as determinants of risk of pancreas cancer: a case-control study from Toronto. Int. J. Cancer. 1991;47(3):384–389. doi: 10.1002/ijc.2910470313. [DOI] [PubMed] [Google Scholar]
- 206.Kalapothaki V., Tzonou A., Hsieh C.C., Toupadaki N., Karakatsani A., Trichopoulos D. Tobacco, ethanol, coffee, pancreatitis, diabetes mellitus, and cholelithiasis as risk factors for pancreatic carcinoma. Cancer Causes Control. 1993;4(4):375–382. doi: 10.1007/BF00051341. [DOI] [PubMed] [Google Scholar]
- 207.Li D., Tang H., Hassan M.M., Holly E.A., Bracci P.M., Silverman D.T. Diabetes and risk of pancreatic cancer: A pooled analysis of three large case-control studies. Cancer Causes Control. 2011;22(2):189–197. doi: 10.1007/s10552-010-9686-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Maisonneuve P., Lowenfels A.B., Bueno-de-Mesquita H.B., et al. Past medical history and pancreatic cancer risk: Results from a multicenter case-control study. Ann. Epidemiol. 2010;20(2):92–98. doi: 10.1016/j.annepidem.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 209.Matsubayashi H., Maeda A., Kanemoto H., et al. Risk factors of familial pancreatic cancer in Japan: current smoking and recent onset of diabetes. Pancreas. 2011;40(6):974–978. doi: 10.1097/MPA.0b013e3182156e1b. [DOI] [PubMed] [Google Scholar]
- 210.Mizuno S., Watanabe S., Nakamura K., et al. A multi-institute case-control study on the risk factors of developing pancreatic cancer. Jpn. J. Clin. Oncol. 1992;22(4):286–291. [PubMed] [Google Scholar]
- 211.Baradaran N., Ahmadi H., Salem S., et al. The protective effect of diabetes mellitus against prostate cancer: role of sex hormones. Prostate. 2009;69(16):1744–1750. doi: 10.1002/pros.21023. [DOI] [PubMed] [Google Scholar]
- 212.Coker A.L., Sanderson M., Zheng W., Fadden M.K. Diabetes mellitus and prostate cancer risk among older men: population-based case-control study. Br. J. Cancer. 2004;90(11):2171–2175. doi: 10.1038/sj.bjc.6601857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gong Z., Neuhouser M.L., Goodman P.J., et al. Obesity, diabetes, and risk of prostate cancer: results from the prostate cancer prevention trial. Cancer Epidemiol. Biomarkers Prev. 2006;15(10):1977–1983. doi: 10.1158/1055-9965.EPI-06-0477. [DOI] [PubMed] [Google Scholar]
- 214.Gonzalez-Perez A., Garcia Rodriguez L.A. Prostate cancer risk among men with diabetes mellitus (Spain). Cancer Causes Control. 2005;16(9):1055–1058. doi: 10.1007/s10552-005-4705-5. [DOI] [PubMed] [Google Scholar]
- 215.Lightfoot N., Conlon M., Kreiger N., Sass-Kortsak A., Purdham J., Darlington G. Medical history, sexual, and maturational factors and prostate cancer risk. Ann. Epidemiol. 2004;14(9):655–662. doi: 10.1016/j.annepidem.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 216.Rosenberg D.J., Neugut A.I., Ahsan H., Shea S. Diabetes mellitus and the risk of prostate cancer. Cancer Invest. 2002;20(2):157–165. doi: 10.1081/cnv-120001141. [DOI] [PubMed] [Google Scholar]
- 217.Tavani A., Gallus S., Bosetti C., et al. Diabetes and the risk of prostate cancer. Eur. J. Cancer Prev. 2002;11(2):125–128. doi: 10.1097/00008469-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 218.Turner E.L., Lane J.A., Donovan J.L., et al. Association of diabetes mellitus with prostate cancer: Nested case-control study (Prostate testing for cancer and Treatment study). Int. J. Cancer. 2011;128(2):440–446. doi: 10.1002/ijc.25360. [DOI] [PubMed] [Google Scholar]
- 219.Zhu K., Lee I.M., Sesso H.D., Buring J.E., Levine R.S., Gaziano J.M. History of diabetes mellitus and risk of prostate cancer in physicians. Am. J. Epidemiol. 2004;159(10):978–982. doi: 10.1093/aje/kwh139. [DOI] [PubMed] [Google Scholar]
- 220.Attner B., Landin-Olsson M., Lithman T., Noreen D., Olsson H. Cancer among patients with diabetes, obesity and abnormal blood lipids: a population-based register study in Sweden. Cancer Causes Control. 2012;23(5):769–777. doi: 10.1007/s10552-012-9946-5. [DOI] [PubMed] [Google Scholar]
- 221.Bosetti C., Rosato V., Polesel J., et al. Diabetes mellitus and cancer risk in a network of case-control studies. Nutr. Cancer. 2012;64(5):643–651. doi: 10.1080/01635581.2012.676141. [DOI] [PubMed] [Google Scholar]
- 222.Jorgensen T.L., Hallas J., Friis S., Herrstedt J. Comorbidity in elderly cancer patients in relation to overall and cancer-specific mortality. Br. J. Cancer. 2012;106(7):1353–1360. doi: 10.1038/bjc.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kuriki K., Hirose K., Tajima K. Diabetes and cancer risk for all and specific sites among Japanese men and women. Eur. J. Cancer Prev. 2007;16(1):83–89. doi: 10.1097/01.cej.0000228404.37858.40. [DOI] [PubMed] [Google Scholar]
- 224.La Vecchia C., Negri E., Franceschi S., D'Avanzo B., Boyle P. A case-control study of diabetes mellitus and cancer risk. Br. J. Cancer. 1994;70(5):950–953. doi: 10.1038/bjc.1994.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Rousseau M., Parent M., Pollak M., Siemiatycki J. Diabetes mellitus and cancer risk in a population-based case-control study among men from Montreal, Canada RID G-9094-2011. Int. J. Cancer. 2006;118(8):2105–2109. doi: 10.1002/ijc.21600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s web site along with the published article.


