Abstract
Salvia miltiorrhiza Bunge, also known as Danshen in Chinese, has been widely used to treat cardiovascular diseases (CVD) in China and other Asia countries. Here, we summarize literatures of the historical traditional Chinese medicine (TCM) interpretation of the action of Salvia miltiorrhiza, its use in current clinical trials, its main phytochemical constituents and its pharmacological findings by consulting Pubmed, China Knowledge Resource Integrated, China Science and Technology Journal, and the Web of Science Databases. Since 2000, 39 clinical trials have been identified that used S. miltiorrhiza in TCM prescriptions alone or with other herbs for the treatment of patients with CVD. More than 200 individual compounds have been isolated and characterized from S. miltiorrhiza, which exhibited various pharmacological activities targeting different pathways for the treatment of CVD in various animal and cell models. The isolated compounds may provide new perspectives in alternative treatment regimes and reveal novel chemical scaffolds for the development of anti-CVD drugs. Meanwhile, there are also some rising concerns of the potential side effects and drug-drug interactions of this plant. The insights gained from this study will help us to better understanding of the actions of this herb for management of cardiovascular disorders. As an herb of red root, S. miltiorrhiza will act as a potential red light to prevent the development of CVD.
Keywords: Salvia miltiorrhiza, traditional chinese medicine (TCM), clinical trials, phytochemistry, pharmacology, cardiovascular disease
1. Introduction
Salvia miltiorrhiza Bunge, also known as red sage or Danshen (Chinese Pinyin name), is a perennial plant (Fig. 1A) in the genus Salvia of the mint family, Lamiaceae [1]. Its roots are highly valued as a “super grade herb (herbs lacking observable toxicity)” in the Shennong’s Herbal Classic of Materia Medica (Shennong Bencao Jing) written during the reign of the Qin and Han dynasties (221 BC to 220 AD), and has been clinically used for more than 2000 years. Following this first record, S. miltiorrhiza was well described in classical traditional Chinese medicine (TCM) works such as in the Compendium of Materia Medica (Bencao Gangmu, Ming dynasty, 1596 AD). In the textbooks of academic TCM, S. miltiorrhiza is characterized as a common drug for promoting blood circulation and removing blood stasis. The herb has been officially recorded in the Chinese pharmacopoeia since 1953. According to the Chinese pharmacopoeia [2], S. miltiorrhiza is the only official source of Salvia Radix & Rhizoma (Danshen) [3]. Phytochemical studies have shown that S. miltiorrhiza contained a large number of lipophilic diterpenoids (such as various tanshinone analogues), hydrophilic phenolic compounds (such as salvianolic acids), flavonoids, and triterpenoids) [4-7]. S. miltiorrhiza has traditionally been used in the treatment of CVD [8], such as atherosclerosis [9], thrombosis [10], and angina pectoris [11, 12]. And data about clinical information of hospitalized patients and outpatients with coronary artery disease and hypertension have demonstrated that S. miltiorrhiza was top-ranked (63.10% and 17.1%, respectively) among all the clinical administered drugs in Beijing, Tianjin [13] and Taiwan [14].
Fig. (1).

The profiles of S. miltiorrhiza. (A) Portion above ground. (B) Roots for pharmaceutical use, (C) Medicinal slices of S. miltiorrhiza root. (Pictures are kindly provided by Zexin Ma from the Museum of Chinese Medicine, Beijing University of Chinese Medicine).
S. miltiorrhiza is widely distributed in the Chinese provinces of Liaoning, Hebei, Beijing, Shandong, Hubei, Hunan, Jiangsu, Jiangxi, Gansu, Henan, and Shanxi. Now, several provinces in China start to domestically plant S. miltiorrhiza Bunge [3]. Suitable growing conditions for this plant are found in locations with sunny, mild and wet environment where average temperature is 17.1°C and the annual average relative humidity is 77% [15]. Currently, S. miltiorrhiza products are available in natural health shops almost all over the world [16].
CVD is a class of disorders that involve the heart and blood vessels, including coronary heart disease, rheumatic heart disease, peripheral vascular disease, heart failure, congenital heart disease and cardiomyopathies [17, 18]. It is becoming a global health burden and is still the number one cause of morbidity and mortality worldwide [19, 20]. Many risk factors could individually or combinedly trigger CVD, such as family history, ethnicity, age, tobacco exposure, high blood pressure, high cholesterol, obesity, physical inactivity, diabetes, unhealthy diets, and harmful use of alcohol. Therefore, multi-targeted therapeutic interventions would likely be required for effective management of CVD.
The current literature review aims to summarize the recent advances of the involvement of S. miltiorrhiza in cardiovascular protective effect in clinical or preclinical studies by consulting Pubmed, China Knowledge Resource Integrated, China Science and Technology Journal, and the Web of Science Databases. We also review its phytochemistry and its explanation in TCM which may help to understand pharmacological activities.
2. The cardiovascular protective activity of Salvia miltiorrhiza in the theory of traditional Chinese medicine
According to the Grand Compendium of Materia Medica [21], “S. miltiorrhiza matches the heart called Chi (Red) Shen”. Thus, S. miltiorrhiza (Danshen) derives its name from its original color of the root (Fig. 1B), which is usually harvested in spring or autumn every year [3]. In TCM, S. miltiorrhiza (Fig. 1C) is recognized as an herb with a bitter-flavor and distribution to the heart. According to Chinese Pharmacopoeia, Salvia Miltiorrhizae Radix & Rhizoma exerts a beneficial action by promoting blood circulation to remove blood stasis and assuage pain, clearing heart heat to relieve restlessness, and cooling blood to remove carbuncle [22]. In TCM perspective, chest pain and heart failure are caused by obstruction of the circulation of Qi and blood. Therefore, S. miltiorrhiza can be used to treat CVD. For details of TCM explanations of this herb, interested readers are encouraged to consult Dr. Guo’s review [23].
3. Salvia miltiorrhiza in clinical trials of CVD
S. miltiorrhiza has been clinically used for management of CVD in China for many years. So far, there are several drugs contained Danshen as its major component which have been developed and marketed in China for the treatment of CVD, including Fufang Danshen tablets, Compound Danshen dripping pills, Danhong injection and Tongxinluo capsule, etc. From 2000 till now, we have identified 39 clinical trials comprised a total of 2431 patients where S. miltiorrhiza was used alone or in combination with other herbs to treat CVD. In the prescriptions of 39 clinical trials, Compound Danshen dripping pills was the most popular one (22 in 39, 56.4%), and offered potential benefits for patients with chronic heart diseases. Among 22 trials with Compound Danshen dripping pills, 9 trials used the Compound Danshen dripping pills alone to treat CVD, and 13 trials used combination treatment. 2 in 39 trials (5.1%) employed the single herb to treat CVD.
The majority of 39 trials targeted the treatment of coronary heart disease. Among them, 6 studies explicitly addressed coronary heart disease, 8 trials studied coronary heart disease combined with angina pectoris, 3 trials observed unstable angina, and 1 trial studied stable angina pectoris. Of the rest, 1 clinical trial treated ischemic cardiovascular disease, 2 trials treated congestive hearts failure, 1 trial treated chronic heart failure, 1 trial treated postmenopausal women with borderline hypercholesterolemia, 1 trial treated hyperlipidemia, 1 trial treated hyperlipidemia and blood hyperviscosity, 2 trials treated hypertension, 2 trials treated diabetes combined with silent myocardial ischemia or chronical heart disease, 1 trial treated cardiovascular neurosis, 1 trial treated cardiovascular complications in peritoneal dialysis patients, 1 trial treated children viral myocarditis, and 7 trials did not specify the disease type. The overall efficacy (with markedly and moderately improved conditions) of trials treating CVD patients was between 63.4% and 99.2% (Table 1). The observations from the clinical trials have demonstrated that S. miltiorrhiza products in China are effective for the management of CVD. However, most of clinical trials are not well-designed. It should be noted that a standardized clinical trial should be randomized, double blind and multi-centered. Obviously, few trials meet the standard.
Table 1.
Clinical cardiovascular diseases trials containing Salvia miltiorrhiza (2000—now).
| Prescription Name | Study Design | Type Of CVD; # of Patient; Duration | Aim and Outcome Measurements | Results (# of Patients) | Refs. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tanshinone IIA + Sulfonic Acid Sodium | Randomized controlled trial | Coronary heart disease 36 15 days |
Significant effect: Markedly improved in clinical symptoms such as chest stuffy, palpitations, chest pain, and short of breath; Electrocardiogram (ECG) returned to normal. Effective: Frequency of clinical symptoms attacks decreased by 50%; ECG ST segment elevated by more than 0.05 mV; Amplitude of lead T wave inversion decreased by more than 25%. Ineffective: No change in ECG. |
Markedly improved (23). Moderately improved (9). Ineffective (4). Overall efficacy: 88.9%. Control treatment: 75.0% using 40ml Shenmai injection + 250ml 5% glucose solution. |
[24] | ||||||||||
| S. miltiorrhiza | Randomized controlled trial | Cardiovascular disease 50 4 weeks |
Clinical efficacy: Significant effect: Clinical symptoms completely improved alleviated by > 90%. Effective: 50% < Clinical symptoms improvement < 90%. Ineffective: Clinical symptoms have no improvement or alleviated by < 50%. ECG efficacy: Significant effect: ECG completely returned to normal. Effective: ST-T wave almost returned to normal. Ineffective: No improvement in ECG. |
Clinical efficacy: Markedly improved (33). Moderately improved (15). Ineffective (2). Overall efficacy: 96%. Control treatment: 86%, using Isosorbide Dinitrate tablet. ECG efficacy: Markedly improved (30). Moderately improved (17). Ineffective (3). Overall efficacy: 94%. Control treatment: 82%, using Isosorbide Dinitrate tablet. |
[25] | ||||||||||
| Danshen and Gegen capsules | Randomized placebo controlled trial (double blinded) | Postmenopausal women with borderline hypercholesterolemia 85 12 months |
Sitting blood pressure, resting ECG, and physical activity scale for the elderly questionnaire; Serum glucose, creatinine, low density lipoprotein (LDL), high density lipoprotein (HDL), triglyceride (TG). |
Primary efficacy: Carotid intima-media thickness decreased. Secondary efficacy: LDL and total cholesterol (TC) decreased. Quality of life: Improvement in general health and blood pressure. |
[26] | ||||||||||
| Compound Danshen dripping pills + Isosorbide Dinitrate sustained released tablets | Randomized controlled trial | Coronary heart disease 49 2 months |
Significant effect: Frequency of angina attacks significantly reduced; Clinical symptoms completely disappeared. Effective: Clinical symptoms improved; Angina attacks decreased. Ineffective: No improvement in clinical symptoms. |
Markedly improved (13). Moderately improved (32). Ineffective (4). Overall efficacy: 93.75%. Control treatment: 75.0%, using Isosorbide Dinitrate tablets |
[27] | ||||||||||
| Compound Danshen dripping pills | Randomized controlled trial | Coronary heart disease 33 10 weeks |
Symptoms of angina pectoris: Significant effect: Angina pectoris disappeared completely or angina pectoris attacks decreased by > 90%. Effective: Angina pectoris attacks decreased by between 50% and 90%. Ineffective: Angina pectoris attacks decreased by < 50%. ECG efficacy: Changes of ST-T wave, including load ECG. Effective: ST-T wave returned to normal or changed from positive to negative. Ineffective: No change in ST-T wave, load ECG test remained positive. |
Symptoms of angina pectoris: Markedly improved (24). Moderately improved (8). Ineffective (1). Overall efficacy: 97.0%. Control treatment: 84.8%, using Isosorbide Dinitrate tablets. |
[28] | ||||||||||
| Prescription Name | Study Design | Type Of CVD; # of Patient; Duration | Aim and Outcome Measurements | Results (# of Patients) | Refs. | ||||||||||
| Compound Danshen dripping pills | Randomized controlled trial | Cardiovascular diseases 39 1 month |
Cure: Clinical symptoms disappeared; Autonomic dysfunction disappeared; ECG and heart rate returned to normal. Significant effect: Significant improvement in clinical symptoms; Heart rate decreased; ECG significantly improved; Autonomic nerve function returned to near normal. Ineffective: No improvement or even worse in clinical symptoms. |
Markedly improved (20). Moderately improved (16). Ineffective (3). Overall efficacy: 92.31%. Control treatment: 71.79% using Isosorbide Dinitrate tablets. |
[29] | ||||||||||
| Compound Danshen dripping pills | Randomized controlled trial | Cardiovascular diseases 39 4 weeks |
Angina pectoris efficacy: Significant effect: Angina pectoris disappeared completely or angina pectoris attacks decreased by > 90%. Effective: Angina pectoris attacks decreased by between 50% and 90%. Ineffective: No obvious improvement in angina pectoris symptoms; Angina attacks decreased by < 50% or even aggravated. ECG efficacy: Significant effect: ST segment returned to normal. Effective: ST segment almost returned to normal. Ineffective: No obvious changes in ST segment. |
Angina pectoris efficacy: Markedly improved (23). Moderately improved (13). Ineffective (3). Overall efficacy: 92.30%. Control treatment: 46.15% using Isosorbide Dinitrate pills and Nifedipine. ECG efficacy: Markedly improved (21). Moderately improved (16). Ineffective (2). Overall efficacy: 94.87%. Control treatment: 76.92% using Isosorbide Dinitrate pills and Nifedipine. |
[30] | ||||||||||
| Compound Danshen dripping pills | Randomized controlled trial | Cardiovascular diseases 25 4 weeks |
Cardiovascular diseases symptoms, serum TC and TG, ECG. | Serum TC and TG levels returned to normal. ECG improved. Control treatment using Isosorbide Dinitrate pills and Nifedipine. |
[31] | ||||||||||
| Compound Danshen dripping pills | Before- and after- control | Coronary heart disease angina pectoris 94 2 months |
Clinical efficacy: Significant effect: (1) Exertional angina: Angina pectoris severity alleviated 2 grade; Grade 1-2 angina pectoris disappeared; Non-exertional angina pectoris: Clinical symptoms disappeared or almost disappeared; Angina attacks decreased by more than 90%; Resting ECG returned to normal; Submaximal exercise test changed from positive to negative or exercise tolerance increased by 2 levels. Effective: (1) Exertional angina: Angina pectoris severity decreased by 1 level; Grade 1 angina pectoris disappeared; (2) Non-exertional angina pectoris: Angina attacks decreased by > 50%; Resting ECG back to normal, or submaximal exercise test ECG ST segment elevated by more than 0.5 mV, but did not return to normal, or amplitude of lead T wave inversion decreased more than 50%, or shape of T wave changed from flat to upright, or exercise tolerance increased by 1 level. Ineffective: No improvement in clinical symptoms, physical signs and ECG; Frequency of angina attacks decreased by < 50%. |
blood viscosity and platelet aggregation rate significantly decreased. Clinical efficacy: Markedly improved (55). Moderately improved (34). Ineffective (5). Overall efficacy: 94%. ECG efficacy: Markedly improved (17). Moderately improved (22). Ineffective (55). Overall efficacy: 75%. |
[32] | ||||||||||
| Prescription Name | Study Design | Type Of CVD; # of Patient; Duration | Aim and Outcome Measurements | Results (# of Patients) | Refs. | ||||||||||
| Compound Danshen dripping pills | Self control | Cardiovascular diseases 439 |
Observe clinical symptoms and treatment efficacy. Psychological status, heart failure, myocardial infarction, arrhythmia, and general health condition scores. | Symptoms markedly improved (203), 46.2%. Partly improved (122), 27.8%. Mildly improved (78), 17.8%. Aggravate (28), 6.4%. Dead (8), 1.8%. |
[33] | ||||||||||
| Compound Danshen dripping pills | Randomized controlled trial | Coronary heart disease angina pectoris 60 |
Angina pectoris efficacy: Significant effect: Clinical symptoms such as chest stuffy, short of breath disappeared; Angina or frequency of angina attacks decreased in response to the same level of exertion; ECG ischemia returned to mormal. Effective: Frequency of angina attacks decreased or chest congestion symptoms alleviated; ECG ischemia ST segment decreased; Shape of T wave changed from flat to upright. Ineffective: No change in angina attacks and ECG. |
Angina pectoris efficacy: Markedly improved (24). Moderately improved (20). Ineffective (8). Overall efficacy: 86.7%. Control treatment 74%. ECG efficacy: Markedly improved (18). Moderately improved (15). Ineffective (13). Overall efficacy: 76.7%. Control treatment using Isosorbide Dinitrate tablets. |
[34] | ||||||||||
| Compound Danshen dripping pills | Randomized controlled trial | Ischemic cardiovascular disease 74 1 month |
Serum TC and TG levels. ECG efficacy: Significant effect: ST segment abnormalities disappeared. Effective: No obvious abnormalities in ST segment. Ineffective: No change in ST segment. |
Serum TC and TG levels decreased. ECG efficacy: Markedly improved (25). Moderately improved (39). Ineffective (10). Overall efficacy: 86.5%. Control treatment: 56.8% using Isosorbide Dinitrate tablets. |
[35] | ||||||||||
| Compound Danshen dripping pills | Randomized controlled trial | Diabetes combined silent myocardial ischemia 45 |
Levels of adiponectin and homocysteine (Hcy). Clinical efficacy: Significant effect: ECG ST segment abnormalities improved or disappeared during the break. Effective: ECG ST segment decreased or elevated by > 0.05 mV during resting or sports; Amplitude of T wave inverted decreased by more than 50%, and shape of T wave appeared upright or inverted. Ineffective: No improvement in ECG, even more serious. |
Markedly improved (30). Moderately improved (14). Ineffective (1). Overall efficacy: 97.8%. Control basic treatment 88.8%. |
[36] | ||||||||||
| Compound Danshen dripping pills + Aspirin | Randomized controlled trial | Coronary heart disease 50 |
Significant effect: Clinical symptoms completely or almost disappeared; ECG completely or almost returned to normal; Blood lipid levels returned to normal. Effective: Clinical symptoms markedly improved; ECG ST segment elevated by more than 0.05mV; Myocardial ischemia attacks decreased by more than 50%; Blood lipid levels improved. Ineffective: No improvement in clinical symptoms, ECG and blood lipid levels. |
LDL decreased, HDL increased. Markedly improved (19). Moderately improved (27). Ineffective (4). Overall efficacy: 92.0%. Control treatment: 76.0% using aspirin. |
[37] | ||||||||||
| Prescription Name | Study Design | Type Of CVD; # of Patient; Duration | Aim and Outcome Measurements | Results (# of Patients) | Refs. | ||||||||||
| Compound Danshen dripping pills + Isosorbide Mononitrate sustained release tablets | Randomized controlled trial | Elderly patients with unstable angina 40 4 weeks |
Observe the clinical therapeutic effects. | Recovery (18) Markedly improved (12). Moderately improved (7). Ineffective (3). Overall efficacy: 92.5%. Control treatment: 75.0% using Simvastatin. |
[38] | ||||||||||
| Compound Danshen dripping pills + low molecular weight Heparin | Randomized controlled trial | Coronary heart disease angina pectoris 80 1 month |
Significant effect: Angina pectoris almost disappeared or angina attacks decreased by more than 80%; ECG returned to normal or near normal. Effective: Angina attacks decreased by between 50% and 80% or duration shorten; ECG ST segment elevated by more than 0.05 mV, but did not return to normal; Amplitude of T wave inversion decreased, or shape of T wave changed from flat to upright, or elevated ST segment decreased by more than 50%. Ineffective: No improvement in angina pectoris; Elevated ST segment decreased by less than 50%, or even worse. |
Frequency and duration of angina pectoris attacks decreased. Markedly improved (42). Moderately improved (29). Ineffective (9). Overall efficacy: 88.75%. Control treatment: 76.25% using aspirin and nitroglycerin. |
[39] | ||||||||||
| Compound Danshen injection + Lulutong injection | Randomized controlled trial | Cardiovascular diseases 40 3 weeks |
Significant effect: Clinical symptoms disappeared; Cardiac function increased by more than 2 levels. Effective: Clinical symptoms decreased; Cardiac function increased by more than 1 level. Ineffective: No changes in symptoms and cardiac function. |
Markedly improved (23). Moderately improved (15). Ineffective (2). Overall efficacy: 95.0%. Control treatment: 75.0% using basic treatment. |
[40] | ||||||||||
| Compound Danshen dripping pills + Valsartan | Randomized controlled trial | Senile primary hypertension 34 6 months |
Significant effect: Diastolic blood pressure decreased by ≥ 10 mmHg and returned to normal, or diastolic blood pressure decreased by ≥ 20 mmHg. Effective: Diastolic blood pressure decreased by < 10 mmHg, but did not return to normal, or diastolic blood pressure decreased by between 10 and 19 mmHg, or systolic pressure decreased by more than 30 mmHg. Ineffective: No improvement in blood pressure. |
Markedly improved (24). Moderately improved (7). Ineffective (3). Overall efficacy: 91.18%. Control treatment: 67.65% using Valsartan. |
[41] | ||||||||||
| Compound Danshen dripping pills + Carvedilol | Randomized controlled trial | Congestive hearts failure 47 90 days |
Significant effect: Clinical symptoms of heart failure and physical signs disappeared; Cardiac function improved by 2 levels. Effective: Clinical symptoms of heart failure and physical signs significantly improved; Cardiac function improved by 1 level. Ineffective: No improvement in clinical symptoms of heart failure and physical signs, or cardiac function aggregated by 1 level. |
Markedly improved (15). Moderately improved (24). Ineffective (8). Overall efficacy: 83.0%. Control treatment: 73.3% using conventional treatment include angiotensin converting-enzyme inhibition, Diuretics and Digoxin. |
[42] | ||||||||||
| Compound Danshen dripping pill + Simvastatin | Randomized controlled trial | Hyperlipidemia 75 12 weeks |
Significant effect: TC level decreased by ≥ 20% or TG level decreased by ≥ 40%. Effective: 10% ≤ TC level decrease ≤20%; 20% ≤ TG level decrease ≤ 40%. Ineffective: No obvious improvement in blood lipids. |
Basically control (28). Markedly improved (20). Moderately improved (19). Ineffective (8). Overall efficacy: 89.33%. Control treatment: 70.83% using Simvastatin. |
[43] | ||||||||||
| Prescription Name | Study Design | Type Of CVD; # of Patient; Duration | Aim and Outcome Measurements | Results (# of Patients) | Refs. | ||||||||||
| Compound Danshen dripping Pills | Self control | Hyperlipidemia and blood hyperviscosity 40 2 weeks |
TG, TG, HDL, hematocrit, whole blood viscosity, plasma viscosity. | TG, TC, HDL decreased; Plasma viscosity, whole blood viscosity and hematocrit decreased. | [44] | ||||||||||
| Shenfu Injection | Randomized controlled trial | Chronic heart failure 129 4 weeks |
Cure: Cardiac function returned to 1 level; Clinical symptoms almost disappeared; No abnormality in physical examination. Significant effect: Cardiac function improved by 2 levels, but did not reach 1 level; Significant improvement in clinical symptoms and physical examination. Effective: Cardiac function improved by 1 level; Clinical symptoms and physical examination improved. Ineffective: No improvement in clinical symptoms and physical examination, or aggravated or dead. |
Cure (60). Markedly improved (43). Moderately improved (17). Ineffective (9). Overall efficacy: 93.0%. Control treatment: 69.0% using Salvia injection |
[45] | ||||||||||
| Compound Danshen dripping pills + Isosorbide Dinitrate tablet | Randomized controlled trial | Coronary heart disease angina pectoris 98 2 weeks |
Angina pectoris efficacy: Significant effect: The same level of exertion cannot induce angina, or frequency of angina attacks decreased by 80%, or nitroglycerin consumption decreased by 50%. Effective: Angina attacks or nitroglycerin consumption decreased by between 50% and 80%. Ineffective: Angina attacks or nitroglycerin consumption decreased by less than 50%. Emphasis: Angina attacks or nitroglycerin consumption increased. ECG efficacy: Significant effect: Rest ischemic ST segment back to normal. Effective: Ischemic ST segment improved, but did not return to normal. Ineffective: No change in ischemic ST segment. |
Angina pectoris efficacy: Markedly improved (26). Moderately improved (62). Ineffective (10). Overall efficacy: 89.8%. Control treatment 63.2%. ECG efficacy: Markedly improved (32). Moderately improved (49). Ineffective (17). Overall efficacy: 82.7%. Control treatment: 54. 7% using Isosorbide Dinitrate tablet. |
[46] | ||||||||||
| Compound Danshen dripping pills + Betaloc tablet | Randomized controlled trial | Stable angina pectoris 63 Unstable angina pectoris 33 |
Significant effect: Angina pectoris completely disappeared; Heart function improved from IV level to above III level, or from III level to above II level; ST segment returned to normal. Effective: Angina pectoris improved; Pain alleviated; Frequency and duration attacks decreased; ST segment almost returned to normal. Ineffective: No improvement in Angina or angina aggravated; Cardiac function worsen and hard-to-heal; Left ventricular failure; ECG depressed or elevated; even myocardial infarction. |
Stable angina pectoris: Markedly improved (51). Moderately improved (11). Ineffective (1). Overall efficacy: 81%. Control treatment 63.2%. Unstable angina pectoris: Markedly improved (26). Moderately improved (4). Ineffective (3). Overall efficacy: 79%. Control treatment: 54. 7% using basic treatment. |
[47] | ||||||||||
| Prescription Name |
Study Design |
Type Of CVD; # of Patient; Duration | Aim and Outcome Measurements | Results (# of Patients) | Refs. | ||||||||||
| Compound Danshen dripping pills | Randomized controlled trial | Coronary heart disease 30 |
Angina pectoris efficacy: Significant effect: Same level of exertion cannot induce angina, or frequency of angina attacks decreased by more than 80%. Effective: Angina attacks or nitroglycerin consumption decreased by between 50% and 80%. Ineffective: Angina attacks or nitroglycerin consumption decreased by < 50%. Aggravate: Frequency, duration and degree of angina attacks and nitroglycerin consumption increased. ECG efficacy: Significant effect: Resting ECG returned to normal or near normal. Effective: ECG ST segment elevated by more than 0.05 mV; Amplitude of lead T wave inversion decreased by more than 25%, or shape of T wave changed from flat to upright. Ineffective: No improvement in resting ECG. |
Angina pectoris efficacy: Markedly improved (15). Moderately improved (10). Ineffective (4). Aggravate: (1). Overall efficacy: 83.3%. Control treatment 50%. ECG efficacy: Markedly improved (10). Moderately improved (17). Ineffective (3). Aggravate: (0). Overall efficacy: 90%, Control treatment: 66.7% using Astragalus. |
[48] | ||||||||||
| Compound Danshen injection + Shenmai injection | No control | Congestive heart failure 15 25 days |
Ineffective: Symptoms of heart failure and physical signs markedly improved; Cardiac function improved by more than 2 levels. Effective: Symptoms of heart failure and physical signs improved; Cardiac function improved by more than 1 level. Significant effect: No improvement in symptoms of heart failure. |
Markedly improved (8). Moderately improved (6). Ineffective (1). Overall efficacy: 93%. No control treatment. |
[49] | ||||||||||
| Danhong Injection | Randomized controlled trial | Unstable angina pectoris 80 14 days |
Blood routine examination, liver or renal functions, platelet aggregation, levels of fibrinogen and D-dimmer. | Plasma levels of fibrinogen and D-dimmer decreased; Platelet aggregation ratio decreased. Control treatment using Alprostadil lipid emulsion injection. |
[50] | ||||||||||
| Gehong decoction | No control | Cardiovascular neurosis 23 |
Significant effect: Clinical symptoms completely disappeared. Effective: Clinical symptoms obviously improved. Ineffective: No change in clinical symptoms. |
Markedly improved (21). Moderately improved (2). Ineffective (0). Overall efficacy: 100%. No Control treatment. |
[51] | ||||||||||
| Tongxinluo capsule | Randomized controlled trial | Angina pectoris with coronary heart disease 48 4 weeks |
Clinical efficacy: Significant effect: Same level of exertion cannot induce angina pectoris, or angina pectoris attacks decreased by more than 80%. Effective: Angina attacks decreased by between 50% and 80%. Ineffective: Angina attacks decreased by < 50%. ECG efficacy: Significant effect: Resting ECG returned to normal or/and submaximal exercise test changed from positive to negative, or exercise tolerance increased by more than 2 levels. Effective: Resting ECG returned to normal or/and submaximal exercise test ECG ischemic ST segment elevated by more than 0.05mm, but did not return to normal; Amplitude of T wave inversion decreased by more than 50%, or T wave changed from flat to upright; Exercise tolerance improved by 1 level. Ineffective: No change in ECG. |
Clinical efficacy: Markedly improved (32). Moderately improved (12). Ineffective (4). Overall efficacy: 91.78%. Control treatment 57.14%. ECG efficacy: Markedly improved (12). Moderately improved (19). Ineffective (17). Overall efficacy: 64.37%. Control treatment: 35.72% using Fufang Danshen tablets |
[52] | ||||||||||
| Prescription name |
Study Design |
Type Of CVD; # of patient; Duration | Aim and Outcome measurements | Results (# of patients) | Refs. | ||||||||||
| Yindan Xinnaotong Capsule +Aspirin + β –receptor blocker + Calcium Antagonist + Nitrates | Randomized controlled trial (grouped by visiting order) | Coronary heart disease angina pectoris 50 |
Angina pectoris efficacy: Significant effect: Frequency and duration of angina pectoris attacks decreased by more than 80%. Effective: Frequency and duration of angina pectoris attacks decreased by more than 50%. Ineffective: Frequency and duration of angina pectoris attacks decreased by less than 50%. ECG efficacy: Significant effect: ECG returned to normal. Effective: ST segment elevated by more than 0.05 mV, but did not return to normal; Amplitude of lead T wave inversion decreased by more than 25%, or shape of T wave changed from flat to upright; Atrio- and intra- ventricular block improved. Ineffective: No change in ECG. Blood lipid efficacy: Significant effect: TC decrease ≥ 20%; TG decrease ≥ 40%; HDL increase ≥ 0.26 mM; (TC-HDL)/HDL decrease ≥ 20%. Effective: 10% ≤ TC decrease < 20%; 20% ≤ TG decrease < 40%; 0.104 mM ≤ HDL increase < 0.26 mM; 10% ≤ (TC-HDL)/HDL decrease < 20%. Ineffective: No change in blood lipid. |
Angina pectoris efficacy: Markedly improved (27). Moderately improved (19). Ineffective (4). Overall efficacy: 92%. Control treatment 74%. ECG efficacy: Markedly improved (20). Moderately improved (23). Ineffective (7). Overall efficacy: 86%. Control treatment 70%. Blood lipid efficacy: Markedly improved (18). Moderately improved (22). Ineffective (20). Overall efficacy: 80%. Control treatment: 62% using aspirin, β–antagonist, calcium antagonists, nitrates. |
[53] | ||||||||||
| Compound Danshen dripping pills + Perindopril. | Randomized controlled trial | Hypertension complicated with diabetes 30 3 months |
Significant effect: Diastolic blood pressure decreased by more than 10 mmHg and returned to normal, or decreased by ≥ 20 mmHg. Effective: Diastolic blood pressure returned to normal or decreased by between 10 and 19 mmHg, or systolic pressure decreased by 30 mmHg. Ineffective: No improvement in blood pressure. |
Markedly improved (24). Moderately improved (5). Ineffective (1). Overall efficacy: 96.7%. Control treatment: 76.7% using Perindopril. |
[54] | ||||||||||
| Yixinshu Capsule | Self control | Cardiovascular diseases 135 13 weeks |
Significant effect: ECG returned to normal or near normal. Effective: ST segment elevated by more than 0.05 mV, but did not return to normal; Amplitude of lead T wave inversion decreased by more than 25%, or shape of T wave changed from flat to upright. Ineffective: No change in ECG. |
Markedly improved (22). Moderately improved (112). Ineffective (1). Overall efficacy: 99.2%. |
[55] | ||||||||||
| Guanxin Danshen dripping pills | Randomized controlled trial | Coronary heart disease angina pectoris 20 6 weeks |
Serum C-reactive protein (CRP) and matrix metalloproteinases (MMP)-9. Significant effect: Angina attacks decrease > 80%; Nitroglycerin consumption decrease > 80%, or same level of exertion cannot induce angina pectoris ; Resting ECG returned to normal. Effective: 50%<angina attacks and nitroglycerin consumption decrease <50%; Resting ECG ischemic ST elevation ≥ 0.1 mV; Amplitude of T wave inversion decreased by more than 25% or shape of T wave changed from flat to upright. Ineffective: Angina attacks and nitroglycerin consumption decrease < 50%. No improvement in resting ECG. |
Serum CRP, MMP-9 markedly decreased Markedly improved (12). Moderately improved (7). Ineffective (1). Overall efficacy: 95.0%. Control treatment 70.0%. |
[56] | ||||||||||
| Yiqi Huoxue granules | Randomized controlled trial | Cardiovascular complications with peritoneal dialysis patients 18 6 months |
Left ventricular hypertrophy (LVH), cardiovascular calcification (CVC), congestive heart failure (CHF), the ratio of the early (E) to late (A) ventricular filling velocities (E/A), left ventricular ejection fraction (LVEF) evaluated by color B-ultrasound. Serum cystatin C (CysC), Hcy. |
Serum levels of Hcy, CysC and tumor necrosis factor-α (TNF-α) decreased; LVEF and E/A increased; Incidence of LVH, CHF decreased; CVC increased. | [57] | ||||||||||
| Prescription name | Study design | Type Of CVD; # of patient; duration | Aim and Outcome measurements | Results (# of patients) | Refs. | ||||||||||
| Compound Danshen dripping pills | Randomized controlled trial | Coronary heart disease 46 1 month |
Clinical efficacy: Significant effect: No angina pectoris attacks or frequency of angina attacks decreased by more than 80%; No significant chest pain during angina pectoris attacks. Effective: Frequency of angina attacks decreased by between 50% and 80%; Chest pain obviously alleviated during angina pectoris attack. Ineffective: No obvious improvement in frequency of angina attacks and chest pain, or chest pain developed into acute myocardial infarction or sudden cardiac death. ECG efficacy: Significant effect: Resting ECG almost returned to normal. Effective: Amplitude of ST segment increased by 0.1 mV; Amplitude of T wave inversion decreased by more than 50%, or shape of T wave changed from flat to upright. Ineffective: No obvious improvement in ECG, or even appeared myocardial infarction during the attack. |
Clinical efficacy: Markedly improved (22). Moderately improved (19). Ineffective (5). Overall efficacy: 89.1%. Control treatment 69.6%. ECG efficacy: Markedly improved (25). Moderately improved (19). Ineffective (2). Overall efficacy: 95.7%. Control treatment: 80.4% using Isosorbide Dinitrate Tablets |
[58] | ||||||||||
| Danshen Chuanxiongqin injection + Huangqi injection | Randomized controlled trial | Viral myocarditis in children 42 30 days |
Significant effect: Clinical symptoms completely disappeared; ECG returned to normal; Myocardial enzymes returned to normal. Effective: Clinical symptoms improved; ECG improved, but did not return to normal. Ineffective: No improvement in clinical symptoms, ECG and myocardial enzymes. |
Clinical efficacy Markedly improved (26). Moderately improved (13). Ineffective (3). Overall efficacy: 93%. Control treatment: 72% using conventional treatment. |
[59] | ||||||||||
| Danshen Chuanxiongqin injection | Randomized controlled trial | Coronary heart disease angina pectoris 35 14 days |
Significant effect: Frequency of angina attacks decreased by≥ 80%; Resting ECG returned to normal. Effective: Frequency of angina attacks decreased by between 50% and 80%; Resting ECG improved. Ineffective: No improvement in clinical symptoms or even worse. |
Frequency and duration of angina pectoris attacks, and heart rate improved. Markedly improved (26). Moderately improved (6). Ineffective (3). Overall efficacy: 93.43%. Control treatment: 77.14% using composite salvia miltiorrhiza injection. |
[60] | ||||||||||
| 5 g hydrophilic extracts of S. miltiorrhiza | Randomized controlled trial | Diabetes with chronic heart disease 62 60 days |
Serum malondialdehyde (MDA), glutathione (GSH), superoxide dismutase (SOD), paraoxonase (PONase) and glutathione reductase (GSSG-R). Soluble vascular cell adhesion molecule-1 (sVCAM-1), von Willebrand factor (vWF) and oxidative low density lipoprotein (oxLDL) |
Serum MDA level reduced; Serum GSH level increased; Serum SOD, PONase and GR activities increased; Serum levels of sVCAM-1, vWF and oxLDL decreased. |
[61, 62] | ||||||||||
Note: The ingredients of TCM prescriptions.
(1) Compound Danshen dripping pills: Borneolum Synthcticum, Notoginseng Radix, Salvia Miltiorrhizae Radix et Rhizoma.
(2) Danhong injection: Salvia Miltiorrhizae Radix et Rhizoma, Carthami Flos.
(3) Gehong decoction: Puerariae Lobatae Radix 30g, Carthami Flos 10g, Cinnamomi Ramulus 10g, Notopterygii Rhizoma et Radix 10g, Angelicae Sinensis Radix 12g, Chuanxiong Rhizoma 12g, Salvia Miltiorrhizae Radix et Rhizoma 20g.
(4) Tongxinluo capsule: Ginseng Radix, Hirudo, Scolopendra, Cicadae Periostracum,Paeoniae Rubra Radix, Borneolum Synthcticum, Salvia Miltiorrhizae Radix et Rhizoma.
(5) Yindan Xinnaotong soft capsule: Ginkgo Folium, Salvia Miltiorrhizae Radix et Rhizoma, Asari Radix et Rhizoma, Notoginseng Radix, Crataegi Pinnatifidae Fructus, Gynostemmatis Pentaphylli Herba seu Radix, Allium sativum L. (Garlic), Borneolum Synthcticum.
(6) Yixinshu Capsule: Ginseng Radix, Ophiopogonis Radix, Schisandrae Chinensis Fructus, Salvia Miltiorrhizae Radix et Rhizoma, Astragali Mongolici Radix, Chuanxiong Rhizoma, Crataegi Pinnatifidae Fructus.
(7) Guanxin Danshen dripping pills: Salvia Miltiorrhizae Radix et Rhizoma, Notoginseng Radix, Dalbergiae Odoriferae Lignum.
(8) Yiqi Huoxue granule: Astragali Mongolici Radix 20 g, Notoginseng Radix 3 g, Salvia Miltiorrhizae Radix et Rhizoma 10 g, Allii Macrostemi Bulbus 10 g.
The main outcome measures were introduced to evaluate the efficacy of the TCM prescription as follows: (1) Clinical symptoms and physical signs, such as frequency of angina attacks, cardiac function, and blood pressure; (2) Biomarkers, such as antioxidant indexes, inflammation markers and blood lipids levels; (3) Improvement of electrocardiogram (ECG), such as ST segment and T wave inversion; (4) Adverse effects during the treatment. Of 39 clinical trials, 31 trials (79.5%) observed the improvement of clinical symptoms and physical signs, 20 (51.3%) trials described the improvement of ECG, 11 (28.2%) trials examined the biomarkers of CVD, 12 (30.8%) trials reported the adverse effects treatment with the prescription, in which abdominal complaints, nausea, and dyspepsia were most often appeared. And the adverse effects completely alleviated after cessation of treatment.
4. Phytochemistry of Salvia miltiorrhiza
The study on the chemical components of S. miltiorrhiza was initiated as early as in 1930s. Following that, a lot of researchers have focused on isolating and characterizing ingredients from this plant. Currently, more than 200 compounds have been identified from S. miltiorrhiza according to the Chinese Academy of Sciences Chemistry Database (www.organchem.csdb.cn) and Chinese Herbal Drug Database [63]. They can be classified into two major groups: water-soluble (hydrophilic) phenolic compounds and nonpolar (lipid-soluble) diterpenoid compounds. Salvianolic acids and diterpenoid tanshinones are two representative hydrophilic components and lipophilic components in this plant (Fig. 2) [23, 64-66].
Fig. (2).
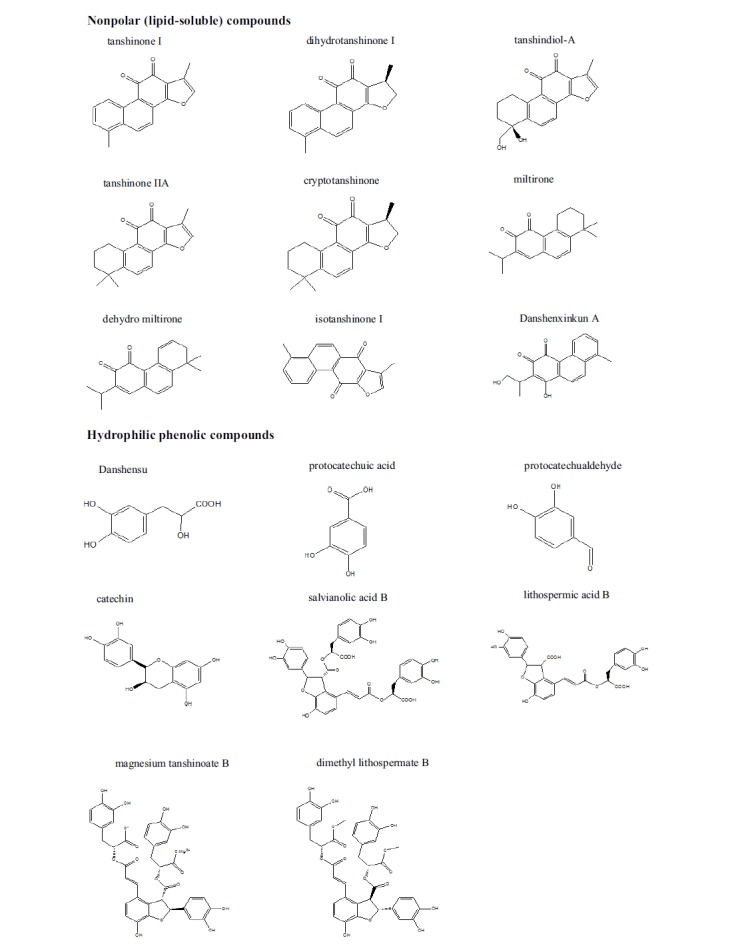
Chemical structure and names of compounds isolated from S. miltiorrhiza.
The basic chemical structure of the water soluble phenolic acids of S. miltiorrhiza is composed of C6C3 units. The total phenolic acids are usually isolated from this plant with water, methanol, ethanol, or aqueous actone. Most of the salvianolic acids are colorless or tan amorphous powders. As a result of the presence of α-3,4-dihydroxyphenyl in the structure, the phenolic acids are very sensitive to light and heat, and easliy oxidized in air.
Diterpenoid tanshinones are usually classified into three types, including diterpenoid tanshinone, tricyclic diterpenoid tanshinone, and royleanone tanshinone. The basic mother nucleus of diterpenoid tanshinones contain 1,2-o-naphthoquinone with furan or dihyrofuran rings, such as tanshinone І, dihydrotanshinone І, tanshindiol-A. The general structure of tricyclic diterpenoid tanshinone is featured as the ligation with an isopropyl group instead of a furan or dihydrofuran ring, such as miltirone, dehydro miltirone. The group of royleanone tanshinone is featured as 1,4-p-quinone, such as isotanshinone, Danshenxinkun A. Most compounds of the diterpenoid tanshinones are red color and stable in their solid state.
The biological actions of these compounds isolated from S. miltiorrhiza have been clarified over the last few years, and several mechanisms have been proposed for the cardiovascular protection effects [67] of this plant, including anti-inflammation [1], antioxidation [65, 68] anti-thrombosis [69], anti-proliferation of vascular smooth muscle cells, inhibition of the expression of adhesion molecules in vascular endothelium and leukocytes [64, 69-71], improvement of acute myocardial ischemia [72], and so on [73].
5. Effects of S. miltiorrhiza and its isolated compounds on the development of CVD in animal experiments
The preclinical pharmacological effects of S. miltiorrhiza and its isolated compounds for the treatment of CVD have been extensively studied over the past few decades. The results have shown that S. miltiorrhiza offered therapeutic benefits on hypertension, atherosclerosis, myocardial ischemia and reperfusion injury in the animal experiments. The recent progress of S. miltiorrhiza in CVD animal disease models are summarized in Table 2. In the following paragraphs, we review the recent advances of S. miltiorrhiza regarding cardiac protective effects in vivo and in vitro studies.
Table 2.
The efficacy of S. miltiorrhiza in CVD animal models.
| Animal Model | Drug and Dose | Duration | Main Findings | Refs. | |
|---|---|---|---|---|---|
| Spontaneously hypertensive Rats | Danshensu; 10 mg/kg/d. | 6 weeks | Decrease in heart weight to body weight index and blood pressure; Decrease in ventricular tachycardia and ventricular fibrillation; Increase in serum nitric oxide content and inducible nitric oxide synthase activity. | [74] | |
| Spontaneously hypertensive Rats | S. miltiorrhiza; 1 g/kg/d. | 12 weeks | Decrease in left ventricular mass index, cardiomyocyte size, diameter, collagen volume fraction, perivascular circumferential area, and tumor necrosis factor-α (TNF-α) expression. | [75] | |
| Hypertension rats | Tanshinone II-A; 70, 35 mg/kg/d. | 6 weeks | Attenuatation of the attendant interstitial fibrosis; Inhition of matrix metalloproteinase (MMP)-9 and tissue inhibitors of MMP-1 expressions. | [76] | |
| Myocardial ischemia/reperfusion injury rat | Cryptotanshinone; 125 or 250 µg/kg. | 10 minitues before ischemia |
Inhibition of the translocation of nuclear transcription factor-kappa B and suppressed expression of inflammatory cytokines (TNF-α, interleukin-1 β), and decreased the myeloperoxidase activity. | [77] | |
| Porcine closed-chest model | Salvianolate; 10 mg/kg/day. |
7 days | Improvement in myocardial perfusion; Increase in capillary density and decrease in infarct sizes; Elevatation of superoxide dismutase (SOD) activity, thioredoxin activity and glutathione concentration; Decrease in malondialdehyde concentration. | [78] | |
| Myocardial ischemia rats | Salvianolic acid B; 20 mg/kg/d. |
7 days | Improvement in the abnormal metabolites. | [79] | |
| Cholesterol-fed Rabbits | 5% water-soluble extract of S. miltiorrhiza. | 12 weeks | Improvement in endothelial damage, atherosclerotic area in the abdominal aorta, and cholesterol deposition in the thoracic aorta. | [80] | |
| LAD ligation induced MI rats | Tanshinone II A; 60 mg/kg/day. | 1 week | Decrease in infarct sizes and collagen deposition; Improvement in heart recovery. | [81] | |
| High fatty diet induced rabbit atherosclerosis | Tanshinone II A; 6.25, 15.00 and 37.50 mg/kg/day. | 2 months | Increase in SOD activity; Decrease in malondialdehyde level; Decrease in cluster of differentiation (CD) 40 expression and MMP-2 activity. | [1] | |
| Atherosclerosis rats | Salvianolate; 60, 120 or 240 mg/kg. | 12 weeks | Decrease in total cholesterol, low density lipoprotein (LDL), interleukin-6 and C-reactive protein; Increase in the numbers of CD4+CD25+Foxp3+ cells. | [82] | |
| Atherosclerosis rabbits | Salvianolic acid B; 8 mg/kg/d. TanshinoneIIA; 24 mg/kg/d. |
8 weeks | Decrease in triglyceride level; Increase in nitric oxide level. | [9] | |
| Hypoxia or monocrotaline rats | Tanshinone IIA; 10 mg/kg. | 4 weeks or 21 days | Decrease in pulmonary artery wall remodeling (thickened small pulmonary artery vessel walls and deflated vessel lumina) and right ventricular systolic pressure. | [83, 84] | |
| Isoproterenol induced myocardial hypertrophy rats | Danshensu; 3 and 10 mg/kg/. | from day 4 to day 7 during isoproterenol treatment | Decrease in heart/body weight index and arrhythmia scores; Improvement in left ventricle systolic pressure and left ventricle end diastolic pressure and electrocardiogram parameters; Increase in SOD and connexin 43 expression. | [85] | |
| Phenylephrine induced male SD rats | Magnesium tanshinoate B; 0.7-175 mg/kg. | infusion of the same agent via the left femoral vein every 15 mins | Decrease in blood pressure. | [86] | |
| Endothelin-1 induced portal hypertension mice |
S. miltiorrhiza; 0.125 g/mouse. Salvianolic acid B; 0.5 mg/mouse. |
3 days | Decrease in the average blood flow velocity in liver. | [87] | |
| Isolated rat hearts of ischemia reperfusion (I/R) | Danshensu; 1 and 10 µM. The extract of S. miltiorrhiza; 29.76 or 59.52 mg/kg. |
5 days | Increase in CF, heart rate, left ventricular developed pressure, and the recovery of ± dp/dtmax; Decrease in infarct sizes; Inhibition of oxidative stress through regualting Akt/extracellular signal-regulated kinase 1/2/nuclear factor erythroid-2-related factor 2 signaling pathway. | [88, 89] | |
| Apolipoprotein E-deficient mice | Cryptotanshinone; 15 and 45 mg/kg/day. | 22 weeks | Significant reduction in atherosclerotic plaque formation and enhanced plaque stability; Decrease in lectin-like oxidized LDL receptor-1 (LOX-1) and matrix metalloproteinase (MMP)-9. | [90] | |
| Animal Model | Drug and Dose | Duration | Main Findings | Refs. | |
| Carotid artery balloon injury rats | Magnesium lithospermate B; 10 mg/kg/day. | 28 days | Inhibition of neointimal formation. | [68] | |
| LAD induced M/I mice | Danshen; 3 and 6 g/kg/day. | 4 weeks | Increase in hypoxia-inducible factor 1α and vascular endothelial growth factor A expression. | [91] | |
| LAD induced M/I rats | S. miltiorrhiza polysaccharides pretreatment with 400 and 800 mg/kg | one week | Decrease in the infarct sizes; Improvement of Na+–K+-ATPase and Ca2+-Mg2+-ATPase activities; Alleviatation of oxidative stress; Inhibition of myocardial apoptosis | [92] | |
5.1. Effects of Salvia miltiorrhiza in the Development of Hypertension
Hypertension is a chronical medical condition in which the blood pressure in the arteries is persistently higher than it should be. Increased peripheral resistance, high cardiac output, an elevated heart rate, a reduction in the number or density of capillaries, increased active arteriolar vasoconstriction and decreased peripheral venous compliance account for the high pressure. Long term high blood pressure can cause coronary artery disease, stroke, heart failure and peripheral vascular disease [93].
It is well established that Ca2+ dependent and independent K+ effluxes limit membrane depolarization and contraction and further buffer vascular resistance in arterial reactivity [94, 95]. Tanshinone IIA treatment (10 mg/kg for 4 weeks [83] or 21 days [84], i.p.) attenuated chronic intermittent hypoxia or sustained hypoxia or monocrotaline (subcutaneous injection of 50 mg/kg [84]) induced pulmonary artery wall remodeling (thickened small pulmonary artery vessel walls and deflated vessel lumina) and increased right ventricular systolic pressure (RVSP) in rats. The underlying mechanisms for these improvements may be attributed to modulate the expressions of KV2.1 and KV1.5 [83], and to inhibit basal intracellular Ca2+ concentration and store-operated Ca2+ entry (SOCE) via attenuating the expressions of canonical transient receptor potential (TRPC) 1 and TRPC 6 [84]. However, Tanshinone IIA did not affect rats RVSP and the mean carotid arterial pressure under normoxia exposure.
Connexins (Cxs) and endothelial nitric oxide synthase (eNOS) harmonize the adaptation of smooth muscle cells and endothelial cells. Using spontaneously hypertensive rats, Tang et al. found that administration of Danshensu (10 mg/kg/d for 6 weeks) decreased blood pressure and inhibited arrhythmias via increasing serum nitric oxide (NO) content and nitric oxide synthase activity [74]. Using isoproterenol (2.5 mg/kg/d for 7 days, s.c.) induced myocardial hypertrophy rats, Danshensu treatment (3 and 10 mg/kg/d from day 4 to 7 of isoproterenol treatment, i.p.) reduced heart/body weight index and arrhythmia scores as well as improved left ventricle systolic pressure and left ventricle end diastolic pressure and electrocardiogram parameters by regulating antioxidant enzymes and promoting left ventricular Cx-43 expression [85].
Matrix metalloproteinases and tissue inhibitors of metalloproteinases (MMPs/TIMPs) impact vascular relaxation/contraction via regulating ion channels in the endothelium and vascular smooth muscle during vascular remodeling [96, 97]. Fang et al. demonstrated that supplyment with tanshinone IIA (70 mg/kg/d for 6 weeks) prevented cardiac fibrosis and left ventricular hypertrophy as well as improved cardiac relaxation in 2-kidney-2-clip induced renovascular hypertension rats [76]. Tanshinone IIA conferred its beneficial effects by decreasing collagen volume content via decreasing mRNA levels of MMP-9, TIMP-1 and TIMP-2 and increasing the mRNA ratio of MMP-2 to TIMP-2 [76].
Angiotensin-converting enzyme (ACE) controls the fluid-electrolyte balance and blood pressure through converting the hormone angiotensin (Ang) I to the active vasoconstrictor Ang II and subsequently causing blood vessels to constrict [98, 99]. Addition of lithospermic acid B (10-1000 μg/ml) dose-dependently inhibited ACE plasma activities. Lithospermic acid B (100-400 μg/ml) treatment also attenuated Ang I induced contraction in the endothelium intact aortic rings [100].
Hypertension is associated with elevated myocardial tumor necrosis factor-α (TNF-α) production [101]. S. miltiorrhiza (1 g/kg for 12 weeks, i.p.) treatment decreased left ventricular mass index, cardiomyocytes sizes, collagen volume fraction, perivascular circumferential area through regulating TNF-α expression in spontaneously hypertensive rats [75]. Interestingly, the investigator claimed that the cardio protective effect of this herb was independent of blood pressure.
Further, magnesium tanshinoate B (MTB, 0.7–175 mg/kg via the jugular vein) was evidenced to exhibit hypotensive effect on phenylephrine induced elevated blood pressure in male SD rats [86]. Tian et al found that S. miltiorrhiza (0.125 g/mouse) and salvianolic acid B (0.5 mg/mouse) decreased the average blood flow velocity in liver in endothelin (ET)-1 induced portal hypertension mice evaluated by laser-Doppler flow instrument [87]. But the mechanisms remain unclear.
Taken together, Salvia miltiorrhiza and its ingredients, including lithospermic acid B, tanshinone IIA and Danshensu, offer therapeutic promise on hypertension through regulating expressions of TRPC/Ca2+, Cx/eNOS/ET-1, MMP/TIMPs, ACE and TNF-α. However, the underlying mechanisms for their pharmacological actions of salvianolic acid B and tanshinoate B still remain unknown. Besides spontaneously hypertensive rats, a lot of measures are employed to evaluate anti-hypertensive effects of the herbs, such as hypoxia, monocrotaline, 2-kidney-2-clip, Ang I, phenylephrine and ET-1. These efforts may afford experimental evidences for clinical use of this herb.
5.2. Effects of Salvia miltiorrhiza in the Development of Artherosclerosis
Atherosclerosis is a condition where the arteries become narrowed and lost of elasticity of the artey walls due to an excessive build up of sticky plaques within the arterial intima [102, 103]. S. miltiorrhiza exihibits beneficial effects for the treatment of artherosclerosis through the following manners.
First, S. miltiorrhiza has the ability of inhibiting oxidative stress. Oxidative stress leads to aggravation of endothelial cells and disruption of vascular wall microenvironment as well as overexpression of adhesion molecules such as intracellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), which induced cardiac dysfunction [104]. By preventing low density lipoprotein (LDL) from oxidation, S. miltiorrhiza (5% water-soluble extract of S. miltiorrhiza for 12 weeks) reduced the atherosclerotic area in the abdominal aorta by 56% and cholesterol deposition in the thoracic aorta by 50% in cholesterol-fed rabbits [80]. In vitro, both S. miltiorrhiza and salvianolic acid B exhibited free radical scavenging activity in DPPH assay, and were also effective in preventing Cu2+ induced LDL oxidation [80]. Further, tanshinone IIA treatment (15 and 37.5 mg/kg for 2 months by gavage) reduced malondialdehyde (MDA) level and increased superoxide dismutase (SOD) activity in high fatty diet (HFD) induced atherosclerosis rabbits [1].
Zhiping Liu et al. showed that treatment with cryptotanshinone (15 and 45 mg/kg/day) resulted in a significant reduction in atherosclerotic plaque formation and enhanced plaque stability in HFD insulted apolipoprotein E deficient mice by reducing the expressions of lectin-like oxidized LDL receptor-1 (LOX-1) and MMP-9 through inhibiting NADPH oxidase 4 (NOX4)-mediated ROS generation and consequent nuclear transcription factor-kappa B (NF-κB) activation [90]. Treatment with salvianolic acid B (8 m/kg/d) and tanshinone IIA (24 mg/kg/d) for 8 weeks were demonstrated to inhibit atherogenesis of HFD-fed rabbits by increasing NO release [9].
Second, S. miltiorrhiza is effective in inhibiting inflammation in the development of CVD. Inflammatory mediators increase local susceptibility to plaque formation which contributes to the development of atherosclerosis [105]. Using atherosclerosis rats challegened by HFD, salvianolate treatment dose-dependently (60, 120 and 240 mg/kg for 12 weeks, i.p.) alleviated the atherosclerotic process via decreasing the levels of proinflammatory cytokines (plasma interleukin-6 (IL-6) and C reactive protein (CRP)) and increasing the number of regulatory T cells (Tregs) (cluster of differentiation (CD)4+CD25+Foxp3+) [82]. Tregs is demonstrated to prevent the development of atherosclerosis by reducing IL-6 expression [106] and CRP production [107]. Interestingly, although salvianolate treatment did cause a decrease in the levels of total cholesterol and LDL, it had no effect on that of triglyceride and HDL.
Platelet CD40 stimulates activations of leukocyte and endothelial cells and further promotes atherosclerosis [108]. Meanwhile, increased MMP-2 activity potentiates inflammation insults and plaque instability in the injured vascular wall [109]. Tanshinone IIA was demonstrated to downregulate CD40 expression and decrease MMP-2 activity in atherosclerosis rabbits [1].
In a rat carotid artery balloon injury model, magnesium lithospermate B treatment (10 mg/kg/day for 28 days, i.p) prevented neointimal formation [68]. Wan et al studied the effect of the lipophilic fraction (tanshinone IIA and cryptotanshinone) of S. miltiorrhiza roots on isolated porcine coronary arteries induced by U46619 (a thromboxane A analogue). The results demonstrated that the lipophilic fraction at a concentration of 0.1 mg/mL induced complete relaxation in porcine coronary artery [110]. However, the mechanisms responsible for these effects still warrant further investigation.
In short, S. miltiorrhiza and its ingredients may serve as anti-oxidant and anti-inflammation agents to prevent the development of atherosclerosis. S. miltiorrhiza, salvianolic acid B, tanshinone IIA, salvianolate and cryptotanshinone are well studied in this respect. Meanwhile, HFD induced atherosclerotic rabbit and rat models are employed to evaluate the effect of this herb on atherosclerosis.
5.3. Effects of S. miltiorrhiza in the Improvement of Myocardial Ischemia (MI)
Myocardial ischemia (MI, also known as angina) is a heart condition caused by a lack of blood supplement to the heart, which is the result of a partial or complete blockage of coronary arteries and may consequently accelerate the deterioration of cardiac function [17]. Reperfusion therapy is the most effective therapeutic strategy for improving the prognosis of patients with acute myocardial infarction but accompanied with the potential risk of worsening tissue damage after ischemia [17, 111, 112]. MI exerts multiple insults in myocardium, frequently accompanied by reactive oxygen species (ROS) generation, intracellular calcium overload, myocardial apoptosis, endothelial dysfunction, enhanced adhesion of leukocytes and mast cell degranulation [71, 113, 114].
Using real-time myocardial contrast echocardiography and coloured microspheres techniques, Han et al. demonstrated that adminstration with salvianolate (10 mg/kg/day for 7 days, i.v.) improved myocardial microvascular reflow and increased capillary density as well as decreased infarct sizes in a porcine closed-chest model [78]. The beneficial effect of salvianolate was probobaly related to its ability of decreasing oxidative stress and apoptosis, which was supported by elevated SOD activity, thioredoxin activity and glutathione concentration, and reduced MDA concentration, as well as decreased terminal deoxynucleotide transferase-mediated dUTP nick end labelling-positive cells and increased ratio of B-cell lymphoma 2 to Bax expression [78].
Monocyte chemotactic protein (MCP)-1 is upregulated in the development of MI both in clincal trial [115] and animal model [116], which facilliates infiltration, activation and cytokine secretion of inflammatory cells. MCP-1 null mice exhibited decreased macrophage recruitment in the infarcted heart, delayed phagocytosis of dead cardiomyocytes, diminished fibroblast infiltration and attenuated left ventricular remodeling [117]. Meanwhile, Ang II and TNF-α stimulate MCP-1 production [118] and amplify the pro-inflammatory response through NF-κB and mitogen activated protein kinase (MAPK) p38 signaling pathway [119, 120]. Tanshinone IIA (60 mg/kg/day for 7 days) treatment decreased infarct sizes and collagen deposition and improved heart recovery in permanent left anterior descending coronary artery (LAD) ligation induced MI rats [81]. The cardioprotective effect of tanshinone IIA could be attributed to its abilty of inhibiting inflammatory responses by reducing expressions of MCP-1, transforming growth factor (TGF)-β1, TNF-α and NF-κB [81]. In addition, tanshinone IIA (2-8 μM) treatment also reduced MCP-1 and TGF-β1 secretion in TNF-α stimulated cardiac fibroblasts [81].
In addition, Danshensu (1 and 10 µM) [88] and the extract of S. miltiorrhiza (29.76 or 59.52 mg/kg for 5 days by gavage) [89] treatment contributed to the recovery of cardiac function after MI, and the tolerance of heart against MI injury by improving coronary flow, heart rate and left ventricular developed pressure, and the recovery of ± dp/dtmax as well as reducing infarct sizes via inhibiting oxidative stress through regualting Akt/extracellular signal-regulated kinase (ERK) 1/2/nuclear factor erythroid-2-related factor 2 (Nrf2) signaling pathways in isolated rat hearts MI model.
Jin et al. observed the effect of cryptotanshinone treatment in LAD induced MI rats. They oberved the alterations of hemodynamic parameters (left ventricular end diastolic pressure and ± dp/dtmax) and the infarct area as well as area at risk. The results demonstrated that cryptotanshinone (250 μg/kg) reversed the MI induced alterations by modualting inflammatory pathway evidenced via inhibiting NF-κB translocation and reducing expressions of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) as well as suppressing neutrophil infiltration and myeloperoxidase activity through regualting p38 mitogen-activated protein kinase (MAPK)/ c-Jun N-terminal kinase (JNK)/ERK in ischemic myocardial tissues [77].
Activation of the cardiac angiogenesis program is a promising therapeutic strategy to match the increasing demands of oxygen and nutrients in the stressed heart [121]. The hypoxia-inducible factor 1α (HIF1α) and vascular endothelial growth factor A (VEGFA) contribute to cardiac angiogenesis [122]. Employing LAD induced MI mice, Danshen (3 and 6 g/kg/day for 4 weeks, i.p.) was proved to increase HIF1α and VEGFA expression which futher contributed to improving cardiac function and cardiac angiogenesis [91].
Song et al oberved the effect of S. miltiorrhiza polysaccharides on LAD induced MI rats. They found that pretreatment with S. miltiorrhiza polysaccharides (400 and 800 mg/kg for 1 week) reduced the infarct sizes and improved Na+-K+-ATPase and Ca2+-Mg2+-ATPase activities by alleviating oxidative stress and inhibiting myocardial apoptosis [92]. It is known that myocardial Na+-K+-ATPase and Ca2+-Mg2+-ATPase activities are associated with myocardial damage such as ischemic cardiac dysfunction and arrhythmias [123].
Using LAD induced MI rats, Lu et al. [79] found that salvianolic acid B (20 mg/kg/d for 7 days) treatment was effective in resolving MI. They demonstrated that salvianolic acid B achieved its therapeutic effect by improving some of biomarkers, including 15(S)-HETE, 2',3'-cyclic adenosine monophosphate (cAMP), dihydrosphingosine, phytosphingosine, L-isoleucyl-L-proline, 2',3'-cyclic guanosine monophosphate, 1-phenylethylamine, thromboxane B2, hypoxanthine, L-homoserine, carnosine, allantoin, L-valine, L-phenylalanine, dihydrobiopterin, 2-oxoisocaproic acid, L-isoleucine, L-tryptophan and glyceraldehydes. However, the authors did not conduct further experiments to verify these alterations.
In conclusion, Danshen, the extract of S. miltiorrhiza, salvianolate, tanshinone IIA, Danshensu, cryptotanshinone, S. miltiorrhiza polysaccharides and salvianolic acid B are demonstrated to exhibit protective effect on MI through scavenging oxidative stress, reducing inflammation and improving angiogenesis via regualting p38MAPK/JNK/ERK and Akt/ERK1/2/Nrf2 pathways. Among these, S. miltiorrhiza polysaccharides also showed its beneficial role against MI. Given lack of effective measures to determine its contributors, there is still a long way to go for exploiting contributions of polysaccharides for the treatment of CVD.
5.4. Effects of Salvia miltiorrhiza on Endothelial Cells, Smooth Muscle Cells and Myocardial Cells
5.4.1. The Protective Effects of Salvia miltiorrhiza on Endothelial Cells
The vascular endothelium, positioned at the interface between blood and tissue, plays a pivotal role in maintaining the integrity of the vessel wall. The death or injury of endothelial cells may contribute to the initial endothelial pathophysiological processes, including angiogenesis, atherosclerosis, and thrombosis.
NO plays the cardioprotective role through regualting blood pressure and vascular tone, and inhibiting platelet aggregation and leukocyte adhesion as well as preventing smooth muscle cell proliferation. An impairment of NO production may result in hypertension or atherosclerosis [124]. Further, ET-1 is a vasoconstricting peptide produced primarily in the endothelium which contributes to constrict blood vessels and raise blood pressure. Activating transcription factor (ATF)-3 mediated inflammatory and apoptotic responses as well as oxidantvie stress in the endothelium [125]. NO promotes ATF-3 expression and further results in a repression of MMP-2 promoter activity [126]. Tanshinone IIA (3 and 10 μM [127]; 0-100 μM [128]) treatment inhibited ET-1 release and stimulated NO production via inducing eNOS activation and ATF-3 expression in human umbilical vein endothelial cells (HUVECs). Further, tanshinone IIA (3-10 μM) exhibited resistance to H2O2 induced apoptosis via decreasing CD40 expression [129] and inducing ATF-3 expression [130] in HUVECs.
Cryptotanshinone also exhibits mitigating effects against oxidative stress and inflammation insults. Using HUVECs exposed to H2O2, TNF-α and oxidized LDL insults [77, 90], cryptotanshinone treatment was shown to inhibit the resultant NF-κB activity and LOX-1 expression. Further, cryptotanshinone inhibited LOX-1-mediated adhesion of THP-1 monocytes to HUVECs by reducing the expression of VCAM-1, ICAM-1 and E-selectin via inhibiting NOX4/ROS/NF-κB signaling pathway in HUVECs [90, 131]. It is accepted that increased expression and activation of ICAM-1 and VCAM-1 as well as E-selectin contribute to the attachment of circulating monocytes/leukocytes to the surface of endothelial cells, which initials atherosclerosis [132].
Vascular endothelial growth factor (VEGF) regulates the endothelial hyperpermeability which resulted in inflammation and subsequent ischemic reperfusion injury or atherosclerosis. The extracts of S. miltiorrhiza and its active components (Danshensu and salvianolic acid B) inhibited TNF-α induced hyper-permeability by inhibiting VEGF expression [133] in HUVECs. In addition, salvianolic acid B and Danshensu (1-20 μg/ml) also protected HUVECS against hydrogen peroxide damage [65].
Danshen aqueous extract and its pure compounds (Danshensu, protocatechuic acid, catechin and protocatechualdehyde at dose of 10 μg/ml) protected HUVEC against homocysteine-induced endothelial dysfunction by tube formation assay. The efficacy was demonstrated as the following descending order: Danshen aqueous extract, Danshensu, protocatechuic acid, catechin and protocatechualdehyde [134].
5.4.2. The Protective Effects of Salvia miltiorrhiza on Smooth Muscle Cells
Vascular smooth muscle cells (VSMCs) are one of the major constituents of blood vessel walls and deeply involved in the development of CVD. Wang et al. demonstrated that tanshinones (a mixture of tanshinone I, tanshinone II, and cryptotanshinone at the dosages of 0.4, 2, 10 and 50 μg/ml) inhibited VSMCs proliferation by decreasing ERK1/2 signaling as well as decreasing the cyclin D expression through increasing the p21waf1/cip1 expression, which are essential for cell cycle progression from G0/G1 phase to S Phase [70]. Tanshinone IIA (12.5 μM) treatment also inhibited hypoxia (4% O2, 60 h) induced pulmonary artery smooth muscle cells (PASMCs) proliferation and migration, and suppressed prolonged hypoxia induced TRPC expression in PASMCs from normoxic rats [84].
TRPC proteins may act as molecualr components of store-operated Ca2+ channels which mediate Ca2+ influx [135]. Elevation of intracellular Ca2+ concentration ([Ca2+]i) contributes to cell growth and contraction [136]. Administration of sodium tanshinone IIA sulfonate (10 mg/kg) reduced SOCE and inhibited elevation of basal [Ca2+]i through supressing TRPC1 and TRPC6 expression in distal PASMCs from chronical hypoxic rats [84].
Acute hypoxia inhibits KV channel function and triggers contraction in PASMCs [137]. Tanshinone IIA (25 μg/ml) partly reversed acute hypoxia (pO2 at about 15 mmHg for 30 min) induced downregulated IKV currents in PASMCs [83].
Using primary mesenteric vascular smooth muscle cells isolated from spontaneously hypertensive rats, Danshensu treatment enhanced the K+ and Ca2+ activated K+ channel current density when the test potential was set at +60 mV [74]. Ca2+-activated K+ (KCa) channels along with K+ (KV) channels play an important role in regulating vascular tone and blood pressure [138].
Both phosphatidylinositide 3 kinase (PI3K)/Akt and MAPK/ERK pathways are involved in regulating smooth muscle cells [139, 140]. Tanshinone IIA was able to inhibit human aortic smooth muscle cell migration by inhibiting phosphorylation of Akt and ERK and c-jun. Meanwhile, tanshinone IIA also has the ability of reducing MMP-9 activity and NF-κB activation [141].
Following endothelial injury and excessive oxidative stress, VSMCs proliferate and migrate to the intima, leading to intima hyperplasia and development of CVD [10, 142, 143]. In an experiment performed by Hur et al. demonstrated that magnesium lithospermate B (10 μM) attenuated platelet-derived growth factor-BB induced VSMCs proliferation and migration via inhibiting phosphorylation of PI3K/Akt and MAPK/ERK pathways by scavenging reactive oxygen species [68].
In addition, dimethyl lithospermate B was effective in eliminating the arrhythmogenic substrate which was responsible for the Brugada syndrome by slowing INa inactivation via increased inward current during the early phase of the action potential in canine [144]. It maybe use as a pharmacological adjunct to implantable cardioverter/defibrillator usage.
5.4.3. The Protective Effects of Salvia miltiorrhiza on Myocardial Cells
The hypertrophy of myocardial cells impairs myocardial contractility and leads to LV hypertrophy [145]. Tanshinone VI (10 μM) attenuated ET-1, phenylephrine, or insulin like growth factor-1 (IGF-1) induced increases in protein synthesis in neonatal rat cardiac myocytes. Tanshinone VI (10 μM) treatment attenuated fetal bovine serum (5%) or IGF-1 (0.01 μM) induced hypertrophy via decreasing collagen synthesis in cardiac fibroblasts [146]. Thus, tanshinone VI may improve the development of cardiac remodeling under pathophysiological conditions.
In the development of MI, cAMP activates protein kinase A (PKA) which results in phosphorylation of L-type Ca2+ channel and consequent Ca2+ influx, leading to stronger muscle contraction. Salvianolic acid B treatment (0.001, 0.01 and 0.1 mg/mL for 2 h) decreased Ca2+ and cAMP, and inhibited PKA in H9C2 cell (a myogenic cell line derived from embryonic rat heart ventricle) [79].
In addition, S. miltiorrhiza is reported to be an effective inhibitor of Ang II action. However, the different fractions of the roots of S. miltiorrhiza exhibited different effects on Ang II treated neonatal rat cardiac cells (cardiomyocytes and non-cardiomyocytes). The ethyl acetate insoluble fraction from the methanol extract attenuated hypertrophy of cardiomyocytes. The methanol eluate fraction of the water extract treatment inhibited hyperplasia of non-cardiomyocyte [147].
To summarized in vitro experiments, S. miltiorrhiza and its active ingredients (Danshensu, salvianolic acid B, protocatechuic acid, catechin, protocatechualdehyde, tanshinone, tanshinone IIA, lithospermate B, tanshinone VI and cryptotanshinone) exhibit protective effects in CVD disease models of endothelial cell and smooth muscle cells as well as myocardial cells through mutiple targets.
Furthermore, S. miltiorrhiza is also synergistically cooperated with other herbs (Radix Puerariae Lobatae [148-150], Compound Danshen tablet [151], Fufang Xueshuantong capsule [152]), Danhong injection [153], or western drug (Atorvastatin [154]) to treatment of CVD animals. However, it is reported that tanshinones and S. miltiorrhiza root extracts contained potent human carboxylesterase (CE) inhibitors, while CEs hydrolyze clinically used drugs [155]. For drug interactions of this herb, interested readers are encouraged to consult Su et al’s review [16]. Therefore, remedies containing tanshinones should be alerted for potential drug-drug interactions when patients are taking additional western drugs.
Currently, there is a rising concern about the safety of S. miltiorrhiza products. And a few investigators reported potential side effects, such as abdominal discomfort [5, 156], decreased appetite, convulsions, dystonia syndromes [157], and allergy [158]. However, these side effects are relieved when patients stop taking the medications [16]. Meanwhile, these observations are not well designed. Thus, the clinical physicians still need strong and scientific evidences to make sure whether Salvia miltiorrhiza is suitable for long term consuming.
In summary, S. miltiorrhiza and its ingredients (Danshensu, salvianolic acid B, protocatechuic acid, catechin and protocatechualdehyde, tanshinone, tanshinone IIA, tanshinone VI, lithospermate B, cryptotanshinone and polysaccharides) are extensively studied in animal and cell disease models of CVD. The results reveal that S. miltiorrhiza plays beneficial roles in improving CVD, such as atherosclerosis, hypertension and myocardial ischemia. These pharmacological activities are associated with scavengering oxidative stress and resolving inflammation as well as improving angiogenesis via regulating TRPC/Ca2+, Cx/eNOS/ET-1, MMP/ TIMPs, p38MAPK/JNK/ERK, Akt/ERK/Nrf2 and Nox4/ROS/NF-κB pathways. The researchers and clinical physicians should pay attention to the potential side effects and drug-drug interactions.
Conclusion and outlook
S. miltiorrhiza is one of the most frequently used herbs in formulations prescribed for the clinical treatment of CVD in China. Since 2000, there are 39 clinical trials used S. miltiorrhiza to treat CVD. Although most studies reported promising efficacy in improving clinical symptoms, their significance is still need to be improved through well designed clinical trials in order to provide sufficient evidence to fully demonstrate the effects of S. miltiorrhiza products. The investigators are also needed to pay more attention to potential adverse effects and drug-drug interactions of this herb. Currently, there have been more than 200 compounds have been identified from S. miltiorrhiza Bunge. Most of these compounds exhibit multipotent pharmacological activities. Various in vitro and in vivo preclinical experiments showed S. miltiorrhiza and its ingredients could lower hypertension, improve atherosclerosis and myocardial ischemia reperfusion. The identified active compounds, including Danshensu, salvianolic acid B, protocatechuic acid, catechin, protocatechualdehyde, tanshinone, tanshinone IIA, tanshinone VI, lithospermate B, cryptotanshinone and polysaccharides, are considered to be responsible for its cardioprotective effects through different cell signaling pathways. Fig. (3) briefly illustrates the different pharmacological activities of S. miltiorrhiza and its ingredients. The results strongly support the notion that S. miltiorrhiza has beneficial therapeutic properties and has a potential of being an effective alternative remedy for the management of CVD.
Fig. (3).
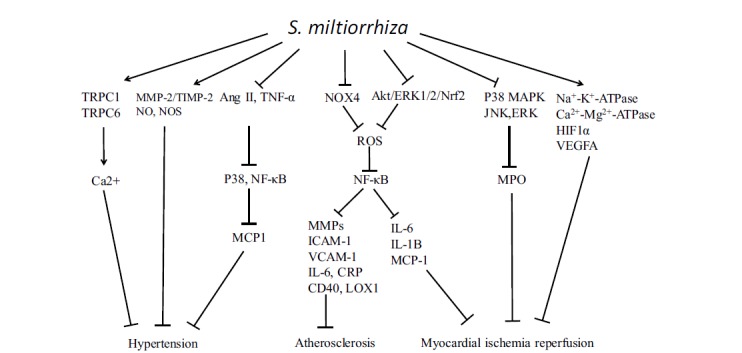
The revealed pathways targeted by S. miltiorrhiza. S. miltiorrhiza could downregulate the levels of Nox4, ROS, NF-κB, MMPs, ICAM-1, VCAM-1, IL-6, CRP, CD40, and LOX1, which contributes to inhibition of atherosclerosis. S. miltiorrhiza suppresses P38MAPK/JNK/ERK pathway, and activates Na+-K+-ATPase, Ca2+-Mg2+-ATPase, HIF1α and VEGFA, which facilitates improvement of CVD. Further, S. miltiorrhiza upregulates TRPC1/TRPC6/Ca2+, MMP-2/TIMP-2, NO and NOS expression, and inhibits Ang II, TNF-α/P38/NF-κB/MCP1 pathways, which contribute to the inhibition of hypertension.
Taditional clinical Chinese medicine illuminates the possible indications of S. miltiorrhiza for patients with CVD. However, the potential pharmacological effects are still required to be studied by more strong scientific evidences. Furthermore, the holistic and dialectical approach of TCM theory suggests the scientists to probe the possible synergistic actions among multiple constituents of this herb and other co-prescribed herbs. More new drugs are expected to source from this plant with better therapeutic effects and lower side effects by mining the ingredients. The existing ingredients in this plant are useful for developing potent compounds drugs, which may offer synergistic actions and exhibit better anti-CVD effects compared to the individual compound. These approaches are also useful for guiding our research to employ an integrative therapeutic approach to treat complex diseases such as CVD which could be superior to the conventional single target – single drug approach. This will shed the light on TCM researchers and patients with CVD around the world, and will also benefit for the development of TCM herbs such as S. miltiorrhiza in the near future.
ACKNOWLEDGEMENTS
The authors thank Dr. Shuiping Zhou (Tasly Institute; Tianjin, China) for his kind contribution.
This work was supported by grants from National Natural Science Foundation of China (NSFC81273995, NSFC81274041), international cooperation projects of MOE (2011DFA30920) and key drug development program of MOST (20122X09103201) as well as the 111 project of MOE (B07007). These funding agencies have no roles in the study design; in data collection, analysis and interpretation; in the writing of the report; and in the decision to submit the article for publication.
List of ABBREVIATIONS
- ACE
Angiotensin-converting enzyme
- Ang
Angiotensin
- ATF-3
Activating transcription factor 3
- cAMP
Cyclic adenosine monophosphate
- CD
Cluster of differentiation
- CE
Carboxylesterase
- CHF
Congestive heart failure
- CRP
C-reactive protein
- CVC
Cardiovascular calcification
- CVD
Cardiovascular disease
- Cx
Connexin
- CysC
Cystatin C
- E/A
The ratio of the early (E) to late (A) ventricular filling velocities
- ECG
Electrocardiogram
- eNOS
Endothelial NO synthase
- ERK
Extracellular signal-regulated kinase
- ET-1
Endothelin-1
- GSH
Glutathione
- GR
Glutathione reductase
- Hcy
Homocysteine
- HDL
High density lipoprotein
- HFD
High fat diet
- HIF1α
Hypoxia-inducible factor 1α
- HUVEC
Human umbilical vein endothelial cell
- ICAM-1
Intracellular adhesion molecule-1
- IGF-1
Insulin-like growth factor-1
- IL-1β
Interleukin-1β
- IL-6
Interleukin-6
- I/R
Ischemia reperfusion
- JNK
c-Jun N-terminal kinase
- LAD
Left anterior descending coronary artery
- LDL
Low density lipoprotein
- LOX-1
Oxidized low-density lipoprotein receptor-1
- LVDP
Left ventricular developed pressure
- LVEDP
Left ventricle end diastolic pressure
- LVEF
Left ventricular ejection fraction
- LVH
Left ventricular hypertrophy
- MAPK
Mitogen-activated protein kinase
- MCP-1
Monocyte chemoattractant protein
- MDA
Malondialdehyde
- MI
Myocardial infarction
- MMP
Matrix metalloproteinase
- NF-κB
Nuclear transcription factor-kappa B
- NO
Nitric oxide
- NOX4
NADPH oxidase 4
- Nrf2
Nuclear factor erythroid-2-related factor 2
- oxLDL
Oxidized low-density lipoprotein
- PASMCs
Pulmonary artery smooth muscle cells
- PI3K
Phosphoinositide-3 kinase
- PKA
Protein kinase A
- PONase
Paraoxonase
- RVSP
Right ventricular systolic pressure
- ROS
Reactive oxygen species
- SOCE
Store-operated Ca2+ entry
- SOD
Superoxide dismutase
- sVCAM-1
Soluble vascular cell adhesion molecule-1
- TC
Total cholesterol
- TCM
Traditional Chinese medicine
- TG
Triglyceride
- TGF
Transforming growth factor
- TIMP
Tissue inhibitors of metalloproteinase
- TNF-α
Tumor necrosis factor-α
- Tregs
Regulatory T cells
- TRPC
Canonical transient receptor potential
- VCAM-1
Vascular cell adhesion molecule-1
- VEGF
Vascular endothelial growth factor
- VEGFA
Vascular endothelial growth factor A
- VSMCs
Vascular smooth muscle cells
- vWF
von Willebrand factor
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
References
- 1.Fang Z.Y., Lin R., Yuan B.X., Yang G.D., Liu Y., Zhang H. Tanshinone IIA downregulates the CD40 expression and decreases MMP-2 activity on atherosclerosis induced by high fatty diet in rabbit. J. Ethnopharmacol. 2008;115:217–222. doi: 10.1016/j.jep.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 2.PPRC-2010 . Pharmacopeia of the People's Republic of China 2010. Beijing: China Medical Science Press; 2010. [Google Scholar]
- 3.Li M.H., Chen J.M., Peng Y., Wu Q., Xiao P.G. Investigation of Danshen and related medicinal plants in China. J. Ethnopharmacol. 2008;120:419–426. doi: 10.1016/j.jep.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Li Y.G., Song L., Liu M., Hu Z.B., Wang Z.T. Advancement in analysis of Salviae miltiorrhizae Radix et Rhizoma (Danshen). J. Chromatogr. A. 2009;1216:1941–1953. doi: 10.1016/j.chroma.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 5.Zhou L., Zuo Z., Chow M.S. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J. Clin. Pharmacol. 2005;45:1345–1359. doi: 10.1177/0091270005282630. [DOI] [PubMed] [Google Scholar]
- 6.Wang X., Morris-Natschke S.L., Lee K.H. New developments in the chemistry and biology of the bioactive constituents of Tanshen. Med. Res. Rev. 2007;27:133–148. doi: 10.1002/med.20077. [DOI] [PubMed] [Google Scholar]
- 7.Lu Y., Foo L.Y. Polyphenolics of Salvia--a review. Phytochemistry. 2002;59:117–140. doi: 10.1016/s0031-9422(01)00415-0. [DOI] [PubMed] [Google Scholar]
- 8.Luo J., Song W., Yang G., Xu H., Chen K. Compound Danshen (Salvia miltiorrhiza) dripping pill for coronary heart disease: an overview of systematic reviews. Am. J. Chin. Med. 2015;43:25–43. doi: 10.1142/S0192415X15500020. [DOI] [PubMed] [Google Scholar]
- 9.lv B, Fan Y, Sun L. The influence of salvianolic acid B and tanshinone IIA on the nitric oxide and triglyceride in sera of atherosclerosis rabbit. J Tianjin Univ Trad Chinese Med. 2006;25:32–34. [Google Scholar]
- 10.Wu W.Y., Wang Y.P. Pharmacological actions and therapeutic applications of Salvia miltiorrhiza depside salt and its active components. Acta Pharmacol. Sin. 2012;33:1119–1130. doi: 10.1038/aps.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang M., Liu A., Guan S., Sun J., Xu M., Guo D. Characterization of tanshinones in the roots of Salvia miltiorrhiza (Dan-shen) by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2006;20:1266–1280. doi: 10.1002/rcm.2447. [DOI] [PubMed] [Google Scholar]
- 12.Guan X., Dei-Anane G., Bruns H., et al. Danshen protects kidney grafts from ischemia/reperfusion injury after experimental transplantation. Transpl. Int. 2009;22:232–241. doi: 10.1111/j.1432-2277.2008.00770.x. [DOI] [PubMed] [Google Scholar]
- 13.Gao Z.Y., Xu H., Shi D.Z., Wen C., Liu B.Y. Analysis on outcome of 5284 patients with coronary artery disease: the role of integrative medicine. J. Ethnopharmacol. 2012;141:578–583. doi: 10.1016/j.jep.2011.08.071. [DOI] [PubMed] [Google Scholar]
- 14.Yang P.R., Shih W.T., Chu Y.H., Chen P.C., Wu C.Y. Frequency and co-prescription pattern of Chinese herbal products for hypertension in Taiwan: a Cohort study. BMC Complement. Altern. Med. 2015;15:163. doi: 10.1186/s12906-015-0690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang L. Cyclopedia of Danshen. Beijing, China: People's Medical Publishing House; 2013. [Google Scholar]
- 16.Su C.Y., Ming Q.L., Rahman K., Han T., Qin L.P. Salvia miltiorrhiza: Traditional medicinal uses, chemistry, and pharmacology. Chin. J. Nat. Med. 2015;13:163–182. doi: 10.1016/S1875-5364(15)30002-9. [DOI] [PubMed] [Google Scholar]
- 17.Buja L.M., Vander Heide R.S. Pathobiology of cardiovascular diseases: past, present, and future perspectives. Cardiovasc. Pathol. 2016;25:214–220. doi: 10.1016/j.carpath.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Buja L.M., Vander Heide R.S. Pathobiology of Ischemic Heart Disease: Past, Present and Future. Cardiovasc. Pathol. 2016;25:214–220. doi: 10.1016/j.carpath.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Dyakova M., Shantikumar S., Colquitt J.L., et al. Systematic versus opportunistic risk assessment for the primary prevention of cardiovascular disease. Cochrane Libr. 2016 doi: 10.1002/14651858.CD010411.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aragona CO, Imbalzano E, Mamone F, et al. Endothelial Progenitor Cells for Diagnosis and Prognosis in Cardiovascular Disease. 2016. [DOI] [PMC free article] [PubMed]
- 21.Li S. Ben Cao Gang Mu. Beijing, China: People's Medical Publishing House; 1982. [Google Scholar]
- 22. Pharmacopoeia of the People's Republic of China, Vol. 1. Beijing Chemical Industry Press: Beijing, China.
- 23.Guo Y., Li Y., Xue L., et al. Salvia miltiorrhiza: an ancient Chinese herbal medicine as a source for anti-osteoporotic drugs. J. Ethnopharmacol. 2014;155:1401–1416. doi: 10.1016/j.jep.2014.07.058. [DOI] [PubMed] [Google Scholar]
- 24.Bi Z. Effect curative observation of tanshinone II A sulfonate treatment of coronary heart disease. Chinese J Clin Rational Drug Use. 2014;7:152–153. [Google Scholar]
- 25.Ma M. Clinical observation of Salvia miltiorrhiza in the treatment of cardiovascular diseases. Chinese Med Mod Distance Educ China. 2011;9:62. [Google Scholar]
- 26.Kwok T., Leung P.C., Lam C., et al. A randomized placebo controlled trial of an innovative herbal formula in the prevention of atherosclerosis in postmenopausal women with borderline hypercholesterolemia. Complement. Ther. Med. 2014;22:473–480. doi: 10.1016/j.ctim.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z. Clinical diagnosis and therapeutic effect observation of coronary heart disease 96 cases. Cardiovasc Dis Prevent Control Knowl; 2014. pp. 63–65. [Google Scholar]
- 28.Lin D., Lin X., Yuan X. Clinical analysis of compound danshen dropping pill in the treatment of coronary heart disease. J Clin Exp Med. 2013;26:82–83. [Google Scholar]
- 29.Ding L. Clinical curative effect of compound danshen dropping pill on cardiovascular disease. Cardiovasc Dis Prevent Knowl; 2015. pp. 102–103. [Google Scholar]
- 30.Liu C. Observation of Clinical effect of compound Danshen dropping pill in the treatment of cardiovascular diseases. Henan Med Res. 2015;24:116. [Google Scholar]
- 31.Wang J., Huang M., Ma C. Effect of compound danshen dropping pill in the treatment of cardiovascular diseases. Guide China Med. 2012;10:263–264. [Google Scholar]
- 32.Wang C. Clinical analysis of Compound danshen dropping pill for treatment of coronary heart disease angina pectoris 94 cases. Jilin Med J. 2012;33:6111–6112. [Google Scholar]
- 33.Zheng C. Clinical observation of 439 patients with heart medicine by salvia miltiorrhiza preparation and combine traditional Chinese and western medicine nursing. LiShiZhen Med Mater Med Res. 2013;24:893–894. [Google Scholar]
- 34.Zhang L. Compound danshen dropping pill treatment of coronary heart disease angina pectoris 60 cases. Chinese Med Modern Distance Educ China. 2010;8:16. [Google Scholar]
- 35.Dai X. Clinical study on compound danshen dropping pill treatment of ischemic cardiovascular disease Asia-Pacific Tradit Med. 2014.
- 36.Guo H. Efficacy observation of compound danshen dropping pill on treating diabetic patients with asymptomatic myocardial ischemia. Chinese J Clin Rational Drug Use. 2015;8:123–124. [Google Scholar]
- 37.Li X. Combine traditional Chinese and western medicine treatment of cardiovascular disease 100 cases. Health. 2012;6:83–84. [Google Scholar]
- 38.Sheng Y., Liu Y. Clinical curative effect observation of Isosorbide mononitrate zyban joint compound danshen dropping pill treatment of old age not stable type angina pectoris. China's Rural Health; 2015. pp. 1–10. [Google Scholar]
- 39.Yan D. Clinical observation of combining traditional Chinese and western medicine treatment of coronary heart disease angina pectoris 80 cases. Chinese J Integr Med Cardio-. Cerebrovasc. Dis. 2013;11:928–929. [Google Scholar]
- 40.Sun Q. Emergency treatment for cardiovascular diseases and observation of curative effect. Cardiovas Dis J Integr Tradit Chinese Western Med. 2015;3:77–78. [Google Scholar]
- 41.Qiu P. Curative effect observation of Valsartan combined with compound Danshen dripping pills in the treatment of Senile primary hypertension. Modern Med Health. 2010;26:57–58. [Google Scholar]
- 42.Xiang W., Yang Q. Curative effect observation of Carvedilol combined with compound Danshen dripping pills in the treatment of congestive hearts failure. Zhejiang J Integr Tradit Chinese Western Med. 2011;21:314–315. [Google Scholar]
- 43.Gong X. Clinical study on compound danshen dropping pill combined with simvastatin treatment of hyperlipidemia. China J Chinese Med. 2013;28:1575–1576. [Google Scholar]
- 44.Ren C., Wang H., Cui J. Clinical analysis and health guidance of Danshen dripping pills in the treatment of hyperlipemia and blood hyperviscosity in 40 cases. China Pract Med. 2011;6:231–232. [Google Scholar]
- 45.Yang X., Wang Z. The observation on the therapeutic effect of Shenfu Injection in the treatment of 129 cases of chronic heart failure. China Modern Doctor. 2009;47:80. [Google Scholar]
- 46.Wang T., Tang R., Li W. Compound danshen dropping pill with isosorbide dinitrate treat coronary heart disease angina pectoris. J Jilin Med Coll. 2009;30:277–278. [Google Scholar]
- 47.Zhang T. Clinical study on compound danshen dropping pill combined with betaloc piecefor the treatment of coronary heart disease. Guide China Med. 2010;8:147–148. [Google Scholar]
- 48.Zhao X., Bai S. Curative effect comparison of astragalus and compound salvia miltiorrhiza on the treatment of coronary heart disease angina pectoris. Hei Long Jiang Med Pharm. 2002;25:41. [Google Scholar]
- 49.Huang Z., He G. Clinical observation of Shenmai and compound salvia miltiorrhiza injection for the treatment of congestive heart failure. Chinese Commun Doctors. 2007;23:45. [Google Scholar]
- 50.Li C.L., Si Q.J., Cai L.L. Antithrombotic study of Danhong Injection in the treatment of old patient with unstable angina pectoris. Chinese J Clin Healthcare. 2012;15:584–585. [Google Scholar]
- 51.Hu X., Chen G. Clinical efficacy observation of GeHong tang on treatment of cardiovascular neurosis 23 cases. J Clin Exp Med. 2006;5:231. [Google Scholar]
- 52.Liu Y. Clinical observation of the effective of Tongxinluo capsule on angina pectoris in patients with coronary heart disease. J Liaoning Univ TCM. 2008;10:98–99. [Google Scholar]
- 53.Luo F., Zhang W., Li X. Observation of yindan xinnaotong capsule for treatment of coronary heart disease angina pectoris 50 cases. Chinese J Integr Med Cardio-.Cerebrovasc. Dis. 2011;9:366–367. [Google Scholar]
- 54.Fei B. Clinical curative effect observation of perindopril combined with compound Danshen dripping pills in the treatment of hypertension complicated with diabetes in 60 cases. Chinese Commun Doctors. 2015;35:90–92. [Google Scholar]
- 55.Yang S. Curative effect observation of Yixinshu capsule alleviate symptoms of cardiovascular disease. Chinese J Integr Med Cardio-. Cerebrovasc. Dis. 2011;9:880–881. [Google Scholar]
- 56.Zhang l Di C, Chen F. The effect of guanxindanshen droping pills on serum c-reactive protein and matrix metalloproteinases 9 in patients with coronary heart disease angina pectoris. J Clin Med Pract. 2013;17:64–65. [Google Scholar]
- 57.Ai Q., Sheng M., Jiang Y., Xu L., Xu L., Yin L. The clinical observation of yiqihuoxue granule treating cardiovascular complications in peritoneal dialysis patients. Chinese J Integr Tradit Western Nephrol. 2015;16:968–972. [Google Scholar]
- 58.Li X. Efficacy analysis of compound Danshen dropping pill treatment of coronary heart disease 46 cases. China's Naturopathy. 2015;23:50–51. [Google Scholar]
- 59.Niu M., Yang J., Li J. Curative effect analysis of astragalus injection with Danshen Chuanxiongqin injection therapy infantile viral myocarditis. Chinese Remedies Clin. 2012;12:1470–1471. [Google Scholar]
- 60.Zheng Z. Curative effect observation of Danshen Chuanxiongqin injection treatment for coronary heart disease angina pectoris. Cardiovas Disease J Integr Tradit Chinese Western Med. 2015;3:120–121. [Google Scholar]
- 61.Qian Q., Qian S., Fan P., Huo D., Wang S. Effect of Salvia miltiorrhiza hydrophilic extract on antioxidant enzymes in diabetic patients with chronic heart disease: a randomized controlled trial. Phytother. Res. 2012;26:60–66. doi: 10.1002/ptr.3513. [DOI] [PubMed] [Google Scholar]
- 62.Qian S., Wang S., Fan P., Huo D., Dai L., Qian Q. Effect of Salvia miltiorrhiza hydrophilic extract on the endothelial biomarkers in diabetic patients with chronic artery disease. Phytother. Res. 2012;26:1575–1578. doi: 10.1002/ptr.4611. [DOI] [PubMed] [Google Scholar]
- 63.Wang L., Li Y., Guo Y., et al. Herba epimedii: An ancient chinese herbal medicine in the prevention and treatment of osteoporosis. Curr. Pharm. Des. 2016;22:328–349. doi: 10.2174/1381612822666151112145907. [DOI] [PubMed] [Google Scholar]
- 64.Ho J.H., Hong C.Y. Salvianolic acids: small compounds with multiple mechanisms for cardiovascular protection. J. Biomed. Sci. 2011;18:30. doi: 10.1186/1423-0127-18-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao G.R., Zhang H.M., Ye T.X., et al. Characterization of the radical scavenging and antioxidant activities of danshensu and salvianolic acid B. Food Chem. Toxicol. 2008;46:73–81. doi: 10.1016/j.fct.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 66.Fan G.W., Gao X.M., Wang H., et al. The anti-inflammatory activities of Tanshinone IIA, an active component of TCM, are mediated by estrogen receptor activation and inhibition of iNOS. J. Steroid Biochem. Mol. Biol. 2009;113:275–280. doi: 10.1016/j.jsbmb.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 67.Zhang X., Ma Z., Liang Q., et al. Tanshinone IIA exerts protective effects in a LCA-induced cholestatic liver model associated with participation of pregnane X receptor. J. Ethnopharmacol. 2015;164:357–367. doi: 10.1016/j.jep.2015.01.047. [DOI] [PubMed] [Google Scholar]
- 68.Hur K.Y., Seo H.J., Kang E.S., et al. Therapeutic effect of magnesium lithospermate B on neointimal formation after balloon-induced vascular injury. Eur. J. Pharmacol. 2008;586:226–233. doi: 10.1016/j.ejphar.2008.02.072. [DOI] [PubMed] [Google Scholar]
- 69.Lu J., Song H.P., Li P., Zhou P., Dong X., Chen J. Screening of direct thrombin inhibitors from Radix Salviae Miltiorrhizae by a peak fractionation approach. J. Pharm. Biomed. Anal. 2015;109:85–90. doi: 10.1016/j.jpba.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 70.Wang H., Gao X., Zhang B. Tanshinone: an inhibitor of proliferation of vascular smooth muscle cells. J. Ethnopharmacol. 2005;99:93–98. doi: 10.1016/j.jep.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 71.Han J.Y., Fan J.Y., Horie Y., et al. Ameliorating effects of compounds derived from Salvia miltiorrhiza root extract on microcirculatory disturbance and target organ injury by ischemia and reperfusion. Pharmacol. Ther. 2008;117:280–295. doi: 10.1016/j.pharmthera.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 72.Xia H., Sun L., Lou H., Rahman M.M. Conversion of salvianolic acid B into salvianolic acid A in tissues of Radix Salviae Miltiorrhizae using high temperature, high pressure and high humidity. Phytomedicine. 2014;21:906–911. doi: 10.1016/j.phymed.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 73.Bi H.C., Zuo Z., Chen X., et al. Preclinical factors affecting the pharmacokinetic behaviour of tanshinone IIA, an investigational new drug isolated from Salvia miltiorrhiza for the treatment of ischaemic heart diseases. Xenobiotica. 2008;38:185–222. doi: 10.1080/00498250701767675. [DOI] [PubMed] [Google Scholar]
- 74.Tang Y., Wang M., Chen C., Le X., Sun S., Yin Y. Cardiovascular protection with danshensu in spontaneously hypertensive rats. Biol. Pharm. Bull. 2011;34:1596–1601. doi: 10.1248/bpb.34.1596. [DOI] [PubMed] [Google Scholar]
- 75.Sun L., Zheng Z. Effect of salvia miltiorrhiza Bge on left ventricular hypertrophy and the expression of tumor necrosis factor-alpha in spontaneously hypertensive rats. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2007;27:245–247. doi: 10.1007/s11596-007-0307-7. [DOI] [PubMed] [Google Scholar]
- 76.Fang J., Xu S.W., Wang P., et al. Tanshinone II-A attenuates cardiac fibrosis and modulates collagen metabolism in rats with renovascular hypertension. Phytomedicine. 2010;18:58–64. doi: 10.1016/j.phymed.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 77.Jin Y.C., Kim C.W., Kim Y.M., et al. Cryptotanshinone, a lipophilic compound of Salvia miltiorrriza root, inhibits TNF-alpha-induced expression of adhesion molecules in HUVEC and attenuates rat myocardial ischemia/reperfusion injury in vivo. Eur. J. Pharmacol. 2009;614:91–97. doi: 10.1016/j.ejphar.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 78.Han B., Zhang X., Zhang Q., et al. Protective effects of salvianolate on microvascular flow in a porcine model of myocardial ischaemia and reperfusion. Arch. Cardiovasc. Dis. 2011;104:313–324. doi: 10.1016/j.acvd.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 79.Lu Y., Zheng Y., Liu X., et al. Metabolomic profiles of myocardial ischemia under treatment with salvianolic acid B. Chin. Med. 2012;7:6. doi: 10.1186/1749-8546-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu Y.J., Hong C.Y., Lin S.J., Wu P., Shiao M.S. Increase of vitamin E content in LDL and reduction of atherosclerosis in cholesterol-fed rabbits by a water-soluble antioxidant-rich fraction of Salvia miltiorrhiza. Arterioscler. Thromb. Vasc. Biol. 1998;18:481–486. doi: 10.1161/01.atv.18.3.481. [DOI] [PubMed] [Google Scholar]
- 81.Ren Z.H., Tong Y.H., Xu W., Ma J., Chen Y. Tanshinone II A attenuates inflammatory responses of rats with myocardial infarction by reducing MCP-1 expression. Phytomedicine. 2010;17:212–218. doi: 10.1016/j.phymed.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 82.Meng C., Zhuo X.Q., Xu G.H., Liu J.L. Protection of salvianolate against atherosclerosis via regulating the inflammation in rats. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2014;34:646–651. doi: 10.1007/s11596-014-1331-z. [DOI] [PubMed] [Google Scholar]
- 83.Zheng L., Liu M., Wei M., et al. Tanshinone IIA attenuates hypoxic pulmonary hypertension via modulating KV currents. Respir. Physiol. Neurobiol. 2015;205:120–128. doi: 10.1016/j.resp.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 84.Wang J., Jiang Q., Wan L., et al. Sodium tanshinone IIA sulfonate inhibits canonical transient receptor potential expression in pulmonary arterial smooth muscle from pulmonary hypertensive rats. Am. J. Respir. Cell Mol. Biol. 2013;48:125–134. doi: 10.1165/rcmb.2012-0071OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tang Y., Wang M., Le X., et al. Antioxidant and cardioprotective effects of Danshensu (3-(3, 4-dihydroxyphenyl)-2-hydroxy-propanoic acid from Salvia miltiorrhiza) on isoproterenol-induced myocardial hypertrophy in rats. Phytomedicine. 2011;18:1024–1030. doi: 10.1016/j.phymed.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 86.Leung S.W., Zhu D.Y., Man R.Y. Effects of the aqueous extract of Salvia Miltiorrhiza (danshen) and its magnesium tanshinoate B-enriched form on blood pressure. Phytother. Res. 2010;24:769–774. doi: 10.1002/ptr.3047. [DOI] [PubMed] [Google Scholar]
- 87.Tian T., Xu L.M. Effects of Salviae miltiorrhizae and salvianolic acid B on microcirculation of liver in mice with portal hypertension. Zhong Xi Yi Jie He Xue Bao. 2009;7:151–156. doi: 10.3736/jcim20090211. [DOI] [PubMed] [Google Scholar]
- 88.Yu J., Wang L., Akinyi M., et al. Danshensu protects isolated heart against ischemia reperfusion injury through activation of Akt/ERK1/2/Nrf2 signaling. Int. J. Clin. Exp. Med. 2015;8:14793–14804. [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou R., He L.F., Li Y.J., Shen Y., Chao R.B., Du J.R. Cardioprotective effect of water and ethanol extract of Salvia miltiorrhiza in an experimental model of myocardial infarction. J. Ethnopharmacol. 2012;139:440–446. doi: 10.1016/j.jep.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 90.Liu Z., Xu S., Huang X., et al. Cryptotanshinone, an orally bioactive herbal compound from Danshen, attenuates atherosclerosis in apolipoprotein E-deficient mice: role of lectin-like oxidized LDL receptor-1 (LOX-1). Br. J. Pharmacol. 2015;172:5661–5675. doi: 10.1111/bph.13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ai F., Chen M., Li W., et al. Danshen improves damaged cardiac angiogenesis and cardiac function induced by myocardial infarction by modulating HIF1alpha/VEGFA signaling pathway. Int. J. Clin. Exp. Med. 2015;8:18311–18318. [PMC free article] [PubMed] [Google Scholar]
- 92.Song M., Huang L., Zhao G., Song Y. Beneficial effects of a polysaccharide from Salvia miltiorrhiza on myocardial ischemia-reperfusion injury in rats. Carbohydr. Polym. 2013;98:1631–1636. doi: 10.1016/j.carbpol.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 93.Lackland D.T., Weber M.A. Global burden of cardiovascular disease and stroke: hypertension at the core. Can. J. Cardiol. 2015;31:569–571. doi: 10.1016/j.cjca.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 94.Dai Z.K., Cheng Y.J., Chung H.H., Wu J.R., Chen I.J., Wu B.N. KMUP-1 ameliorates monocrotaline-induced pulmonary arterial hypertension through the modulation of Ca2+ sensitization and K+-channel. Life Sci. 2010;86:747–755. doi: 10.1016/j.lfs.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 95.Ko E.A., Park W.S., Firth A.L., Kim N., Yuan J.X., Han J. Pathophysiology of voltage-gated K+ channels in vascular smooth muscle cells: modulation by protein kinases. Prog. Biophys. Mol. Biol. 2010;103:95–101. doi: 10.1016/j.pbiomolbio.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 96.Pushpakumar S.B., Kundu S., Metreveli N., Tyagi S.C., Sen U. Matrix metalloproteinase inhibition mitigates renovascular remodeling in salt-sensitive hypertension. Physiol. Rep. 2013;1:e00063. doi: 10.1002/phy2.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Raffetto J.D., Khalil R.A. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem. Pharmacol. 2008;75:346–359. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bernstein K.E., Giani J.F., Shen X.Z., Gonzalez-Villalobos R.A. Renal angiotensin-converting enzyme and blood pressure control. Curr. Opin. Nephrol. Hypertens. 2014;23:106–112. doi: 10.1097/01.mnh.0000441047.13912.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carey R.M. The intrarenal renin-angiotensin system in hypertension. Adv. Chronic Kidney Dis. 2015;22:204–210. doi: 10.1053/j.ackd.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 100.Kang D.G., Oh H., Chung H.T., Lee H.S. Inhibition of angiotensin converting enzyme by lithospermic acid B isolated from Radix Salviae miltiorrhiza Bunge. Phytother. Res. 2003;17:917–920. doi: 10.1002/ptr.1250. [DOI] [PubMed] [Google Scholar]
- 101.Bergman M.R., Kao R.H., McCune S.A., Holycross B.J. Myocardial tumor necrosis factor-alpha secretion in hypertensive and heart failure-prone rats. Am. J. Physiol. 1999;277:H543–H550. doi: 10.1152/ajpheart.1999.277.2.H543. [DOI] [PubMed] [Google Scholar]
- 102.Aluganti Narasimhulu C., Fernandez-Ruiz I., Selvarajan K., et al. Atherosclerosis - do we know enough already to prevent it? Curr. Opin. Pharmacol. 2016;27:92–102. doi: 10.1016/j.coph.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 103.Linton M.F., Yancey P.G., Davies S.S., Jerome W.G., Linton E.F., Vickers K.C. 2000. The role of lipids and lipoproteins in atherosclerosis. [Google Scholar]
- 104.Corte V.D., Tuttolomondo A., Pecoraro R., Pinto A. Inflammation, endothelial dysfunction and arterial stiffness as therapeutic targets in cardiovascular medicine. Curr. Pharm. Des. 2016;22(30):4658–4668. doi: 10.2174/1381612822666160510124801. [DOI] [PubMed] [Google Scholar]
- 105.Thompson P.L., Nidorf S.M., Eikelboom J. Targeting the unstable plaque in acute coronary syndromes. Clin. Ther. 2013;35:1099–1107. doi: 10.1016/j.clinthera.2013.07.332. [DOI] [PubMed] [Google Scholar]
- 106.He S., Li M., Ma X., Lin J., Li D. CD4+CD25+Foxp3+ regulatory T cells protect the proinflammatory activation of human umbilical vein endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2010;30:2621–2630. doi: 10.1161/ATVBAHA.110.210492. [DOI] [PubMed] [Google Scholar]
- 107.Poledne R., Lorenzova A., Stavek P., et al. Proinflammatory status, genetics and atherosclerosis. Physiol. Res. 2009;58(Suppl. 2):S111–S118. doi: 10.33549/physiolres.931915. [DOI] [PubMed] [Google Scholar]
- 108.Gerdes N., Seijkens T., Lievens D., et al. Platelet CD40 exacerbates atherosclerosis by transcellular activation of endothelial cells and leukocytes. Arterioscler. Thromb. Vasc. Biol. 2016;36:482–490. doi: 10.1161/ATVBAHA.115.307074. [DOI] [PubMed] [Google Scholar]
- 109.Galis Z.S., Khatri J.J. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ. Res. 2002;90:251–262. [PubMed] [Google Scholar]
- 110.Wan A.K., Leung S.W., Zhu D.Y., Man R.Y. Vascular effects of different lipophilic components of “Danshen”, a traditional Chinese medicine, in the isolated porcine coronary artery. J. Nat. Prod. 2008;71:1825–1828. doi: 10.1021/np800119k. [DOI] [PubMed] [Google Scholar]
- 111.Ferdinandy P., Schulz R., Baxter G.F. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol. Rev. 2007;59:418–458. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- 112.Minamino T. Cardioprotection from ischemia/reperfusion injury. Circ. J. 2012;76:1074–1082. doi: 10.1253/circj.cj-12-0132. [DOI] [PubMed] [Google Scholar]
- 113.Mahmood S. Mozaffari, Jun Yao Liu, Worku Abebe, Baban B. Mechanisms of load dependency of myocardial ischemia reperfusion injury. Am. J. Cardiovasc. Dis. 2013;4:180–196. [PMC free article] [PubMed] [Google Scholar]
- 114.Turer A.T., Hill J.A. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am. J. Cardiol. 2010;106:360–368. doi: 10.1016/j.amjcard.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kobusiak-Prokopowicz M., Orzeszko J., Mazur G., et al. Chemokines and left ventricular function in patients with acute myocardial infarction. Eur. J. Intern. Med. 2007;18:288–294. doi: 10.1016/j.ejim.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 116.Kohno T., Anzai T., Naito K., et al. Angiotensin-receptor blockade reduces border zone myocardial monocyte chemoattractant protein-1 expression and macrophage infiltration in post-infarction ventricular remodeling. Circ. J. 2008;72:1685–1692. doi: 10.1253/circj.cj-08-0115. [DOI] [PubMed] [Google Scholar]
- 117.Xia Y., Frangogiannis N.G. MCP-1/CCL2 as a therapeutic target in myocardial infarction and ischemic cardiomyopathy. Inflamm. Allergy Drug Targets. 2007;6:101–107. doi: 10.2174/187152807780832265. [DOI] [PubMed] [Google Scholar]
- 118.Lam S.Y., Tipoe G.L., Liong E.C., Fung M.L. Chronic hypoxia upregulates the expression and function of proinflammatory cytokines in the rat carotid body. Histochem. Cell Biol. 2008;130:549–559. doi: 10.1007/s00418-008-0437-4. [DOI] [PubMed] [Google Scholar]
- 119.Ho A.W., Wong C.K., Lam C.W. Tumor necrosis factor-alpha up-regulates the expression of CCL2 and adhesion molecules of human proximal tubular epithelial cells through MAPK signaling pathways. Immunobiology. 2008;213:533–544. doi: 10.1016/j.imbio.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 120.Takahashi M., Suzuki E., Takeda R., et al. Angiotensin II and tumor necrosis factor-alpha synergistically promote monocyte chemoattractant protein-1 expression: roles of NF-kappaB, p38, and reactive oxygen species. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H2879–H2888. doi: 10.1152/ajpheart.91406.2007. [DOI] [PubMed] [Google Scholar]
- 121.Mozid A.M., Holstensson M., Choudhury T., et al. Clinical feasibility study to detect angiogenesis following bone marrow stem cell transplantation in chronic ischaemic heart failure. Nucl. Med. Commun. 2014;35:839–848. doi: 10.1097/MNM.0000000000000130. [DOI] [PubMed] [Google Scholar]
- 122.Giatromanolaki A., Bai M., Margaritis D., et al. Hypoxia and activated VEGF/receptor pathway in multiple myeloma. Anticancer Res. 2010;30:2831–2836. [PubMed] [Google Scholar]
- 123.Ke Y.S., Wang D.G., Wang H.G., Yang S.Y. Endoxin antagonist lessens myocardial ischemia reperfusion injury. Cardiovasc. Drugs Ther. 2004;18:289–293. doi: 10.1023/B:CARD.0000041248.20065.47. [DOI] [PubMed] [Google Scholar]
- 124.Zhao Y., Vanhoutte P.M., Leung S.W. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015;129:83–94. doi: 10.1016/j.jphs.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 125.Aung H.H., Altman R., Nyunt T., et al. Induction of lipotoxic brain microvascular injury is mediated by activating transcription factor 3-dependent inflammatory and oxidative stress pathways. J. Lipid Res. 2016 doi: 10.1194/jlr.M061853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen H.H., Wang D.L. Nitric oxide inhibits matrix metalloproteinase-2 expression via the induction of activating transcription factor 3 in endothelial cells. Mol. Pharmacol. 2004;65:1130–1140. doi: 10.1124/mol.65.5.1130. [DOI] [PubMed] [Google Scholar]
- 127.Hong H.J., Hsu F.L., Tsai S.C., et al. Tanshinone IIA attenuates cyclic strain-induced endothelin-1 expression in human umbilical vein endothelial cells. Clin. Exp. Pharmacol. Physiol. 2012;39:63–68. doi: 10.1111/j.1440-1681.2011.05637.x. [DOI] [PubMed] [Google Scholar]
- 128.Huang K.J., Wang H., Xie W.Z., Zhang H.S. Investigation of the effect of tanshinone IIA on nitric oxide production in human vascular endothelial cells by fluorescence imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2007;68:1180–1186. doi: 10.1016/j.saa.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 129.Lin R., Wang W.R., Liu J.T., Yang G.D., Han C.J. Protective effect of tanshinone IIA on human umbilical vein endothelial cell injured by hydrogen peroxide and its mechanism. J. Ethnopharmacol. 2006;108:217–222. doi: 10.1016/j.jep.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 130.Chan P., Chen Y.C., Lin L.J., et al. Tanshinone IIA attenuates H(2)O(2) -induced injury in human umbilical vein endothelial cells. Am. J. Chin. Med. 2012;40:1307–1319. doi: 10.1142/S0192415X12500966. [DOI] [PubMed] [Google Scholar]
- 131.Zhao W, Wu C, Chen X. Cryptotanshinone inhibits oxidized LDLinduced adhesion molecule expression via ROS dependent NFkappaB pathways. . Cell Adhes Migrat . 2016. [DOI] [PMC free article] [PubMed]
- 132.Ling S., Nheu L., Komesaroff P.A. Cell adhesion molecules as pharmaceutical target in atherosclerosis. Mini Rev. Med. Chem. 2012;12:175–183. doi: 10.2174/138955712798995057. [DOI] [PubMed] [Google Scholar]
- 133.Ding M., Ye T.X., Zhao G.R., Yuan Y.J., Guo Z.X. Aqueous extract of Salvia miltiorrhiza attenuates increased endothelial permeability induced by tumor necrosis factor-alpha. Int. Immunopharmacol. 2005;5:1641–1651. doi: 10.1016/j.intimp.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 134.Chan K., Chui S.H., Wong D.Y., Ha W.Y., Chan C.L., Wong R.N. Protective effects of Danshensu from the aqueous extract of Salvia miltiorrhiza (Danshen) against homocysteine-induced endothelial dysfunction. Life Sci. 2004;75:3157–3171. doi: 10.1016/j.lfs.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 135.Cheng K.T., Ong H.L., Liu X., Ambudkar I.S. Contribution and regulation of TRPC channels in store-operated Ca2+ entry. Curr. Top. Membr. 2013;71:149–179. doi: 10.1016/B978-0-12-407870-3.00007-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang J., Fu X., Yang K., et al. Hypoxia inducible factor-1-dependent up-regulation of BMP4 mediates hypoxia-induced increase of TRPC expression in PASMCs. Cardiovasc. Res. 2015;107:108–118. doi: 10.1093/cvr/cvv122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang J., Weigand L., Wang W., Sylvester J.T., Shimoda L.A. Chronic hypoxia inhibits Kv channel gene expression in rat distal pulmonary artery. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;288:L1049–L1058. doi: 10.1152/ajplung.00379.2004. [DOI] [PubMed] [Google Scholar]
- 138.Toth P., Csiszar A., Tucsek Z., et al. Role of 20-HETE, TRPC channels, and BKCa in dysregulation of pressure-induced Ca2+ signaling and myogenic constriction of cerebral arteries in aged hypertensive mice. Am. J. Physiol. Heart Circ. Physiol. 2013;305:H1698–H1708. doi: 10.1152/ajpheart.00377.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Roffe S., Hagai Y., Pines M., Halevy O. Halofuginone inhibits Smad3 phosphorylation via the PI3K/Akt and MAPK/ERK pathways in muscle cells: effect on myotube fusion. Exp. Cell Res. 2010;316:1061–1069. doi: 10.1016/j.yexcr.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 140.Zhou J., Du T., Li B., Rong Y., Verkhratsky A., Peng L. Crosstalk between MAPK/ERK and PI3K/AKT signal pathways during brain ischemia/reperfusion. ASN Neuro. 2015:7. doi: 10.1177/1759091415602463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jin U.H., Suh S.J., Chang H.W., et al. Tanshinone IIA from Salvia miltiorrhiza BUNGE inhibits human aortic smooth muscle cell migration and MMP-9 activity through AKT signaling pathway. J. Cell. Biochem. 2008;104:15–26. doi: 10.1002/jcb.21599. [DOI] [PubMed] [Google Scholar]
- 142.Ross R. The pathogenesis of atherosclerosis a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 143.Perrins C.J., Bobryshev Y.V. Current advances in understanding of immunopathology of atherosclerosis. Virchows Arch. 2011;458:117–123. doi: 10.1007/s00428-010-1006-5. [DOI] [PubMed] [Google Scholar]
- 144.Fish J.M., Welchons D.R., Kim Y.S., Lee S.H., Ho W.K., Antzelevitch C. Dimethyl lithospermate B, an extract of Danshen, suppresses arrhythmogenesis associated with the Brugada syndrome. Circulation. 2006;113:1393–1400. doi: 10.1161/CIRCULATIONAHA.105.601690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Voulgari C., Papadogiannis D., Tentolouris N. Diabetic cardiomyopathy: from the pathophysiology of the cardiac myocytes to current diagnosis and management strategies. Vasc. Health Risk Manag. 2010;6:883–903. doi: 10.2147/VHRM.S11681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Maki T., Kawahara Y., Tanonaka K., Yagi A., Takeo S. Effects of tanshinone VI on the hypertrophy of cardiac myocytes and fibrosis of cardiac fibroblasts of neonatal rats. Planta Med. 2002;68:1103–1107. doi: 10.1055/s-2002-36337. [DOI] [PubMed] [Google Scholar]
- 147.Ouyang X., Takahashi K., Komatsu K., et al. Protective effect of Salvia miltiorrhiza on angiotensin II-induced hypertrophic responses in neonatal rat cardiac cells. Jpn. J. Pharmacol. 2001;87:289–296. doi: 10.1254/jjp.87.289. [DOI] [PubMed] [Google Scholar]
- 148.Wong S.M., Chiu P.Y., Leung H.Y., et al. Myocardial post-conditioning with Danshen-Gegen decoction protects against isoproterenol-induced myocardial injury via a PKCepsilon/mKATP-mediated pathway in rats. Chin. Med. 2011;6:7. doi: 10.1186/1749-8546-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chiu P.Y., Wong S.M., Leung H.Y., et al. Acute treatment with Danshen-Gegen decoction protects the myocardium against ischemia/reperfusion injury via the redox-sensitive PKCvarepsilon/mK(ATP) pathway in rats. Phytomedicine. 2011;18:916–925. doi: 10.1016/j.phymed.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 150.Hu F., Koon C.M., Chan J.Y., Lau K.M., Kwan Y.W., Fung K.P. Involvements of calcium channel and potassium channel in Danshen and Gegen decoction induced vasodilation in porcine coronary LAD artery. Phytomedicine. 2012;19:1051–1058. doi: 10.1016/j.phymed.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 151.Ren-an Q., Juan L., Chuyuan L., et al. Study of the protective mechanisms of Compound Danshen Tablet (Fufang Danshen Pian) against myocardial ischemia/reperfusion injury via the Akt-eNOS signaling pathway in rats. J. Ethnopharmacol. 2014;156:190–198. doi: 10.1016/j.jep.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 152.Sheng S., Wang Y., Long C., Su W., Rong X. Chinese medicinal formula Fufang Xueshuantong capsule could inhibit the activity of angiotensin converting enzyme. Biotechnol Biotechnol Equip. 2014;28:322–326. doi: 10.1080/13102818.2014.911611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chen J., Deng J., Zhang Y., et al. Lipid-lowering effects of Danhong injection on hyperlipidemia rats. J. Ethnopharmacol. 2014;154:437–442. doi: 10.1016/j.jep.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 154.Xu S., Liu Z., Huang Y., et al. Effectiveness of combination therapy of atorvastatin and non lipid-modifying tanshinone IIA from Danshen in a mouse model of atherosclerosis. Int. J. Cardiol. 2014;174:878–880. doi: 10.1016/j.ijcard.2014.04.226. [DOI] [PubMed] [Google Scholar]
- 155.Hatfield M.J., Tsurkan L.G., Hyatt J.L., et al. Modulation of esterified drug metabolism by tanshinones from Salvia miltiorrhiza (“Danshen”). J. Nat. Prod. 2013;76:36–44. doi: 10.1021/np300628a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang G., Wang L., Xiong Z.Y., Mao B., Li T.Q. Compound salvia pellet, a traditional Chinese medicine, for the treatment of chronic stable angina pectoris compared with nitrates: a meta-analysis. Med. Sci. Monit. 2006;12:SR1–SR7. [PubMed] [Google Scholar]
- 157.Cheng T.O. Cardiovascular effects of Danshen. Int. J. Cardiol. 2007;121:9–22. doi: 10.1016/j.ijcard.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 158.Liu L. The clinical application and side effects of Danshen injection. J Mod Med Health. 2012;28:1689–1691. [Google Scholar]


