Abstract
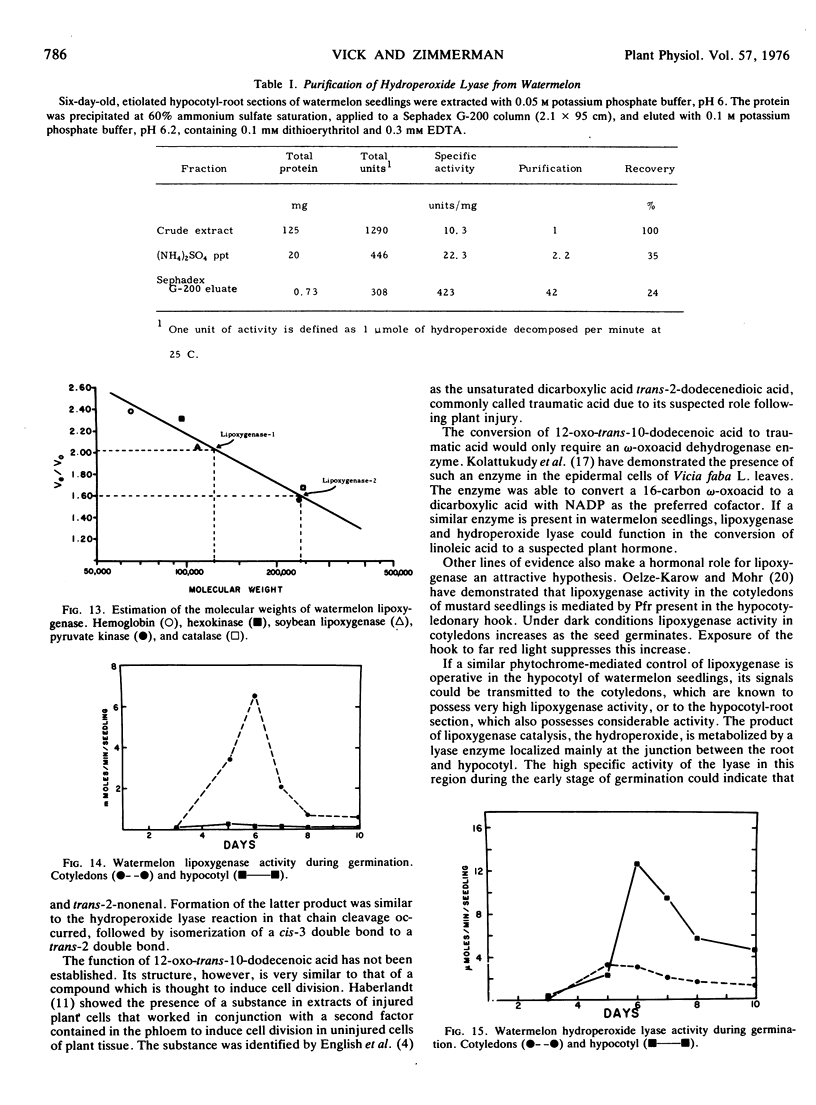
Lipoxygenase (EC 1.13.1.13) was found in seedlings of Citrullus lanatus (Thunb.) Matsum. and Nakai (watermelon). The enzyme has pH optima of 4.4 and 5.5 and is inhibited by 0.2 mM nordihydroguaiaretic acid. It is present in two functional units with estimated molecular weights of 120,000 and 240,000, respectively.
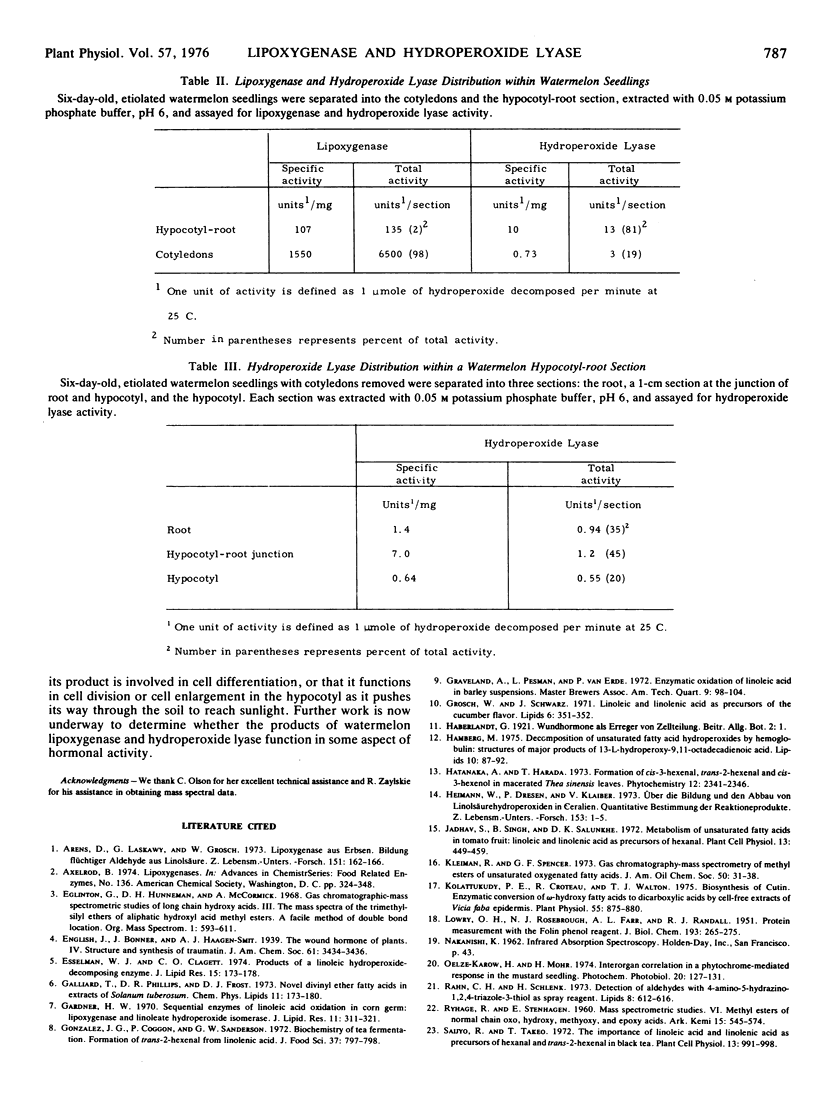
A new enzyme, tentatively termed hydroperoxide lyase, has been partially purified from watermelon seedlings. The enzyme, located principally in the region of the hypocotyl-root junction, catalyzes the conversion of 13-l-hydroperoxy-cis-9-trans-11-octadecadienoic acid to 12-oxo-trans-10-dodecenoic acid and hexanal. The hydroperoxide lyase enzyme from watermelon has a molecular weight in excess of 250,000, a pH optimum in the range of 6 to 6.5, and is inhibited by p-chloromercuribenzoic acid. Its presence has also been demonstrated in other cucurbits.
The maximum activity of both enzymes occurs on the 6th day of germination. The identification of the products of the hydroperoxide lyase reaction suggests that lipoxygenase and hydroperoxide lyase may be involved in the conversion of certain polyunsaturated fatty acids to traumatic acid (trans-2-dodecenedioic acid).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Esselman W. J., Clagett C. O. Products of linoleic hydroperoxide-decomposing enzyme of alfalfa seed. J Lipid Res. 1974 Mar;15(2):173–178. [PubMed] [Google Scholar]
- Gardner W. H. Sequential enzymes of linoleic acid oxidation in corn germ: lipoxygenase and linoleate hydroperoxide isomerase. J Lipid Res. 1970 Jul;11(4):311–321. [PubMed] [Google Scholar]
- Hamberg M. Decomposition of unsaturated fatty acid hydroperoxides by hemoglobin: Structures of major products of 13L-hydroperoxy-9,11-octadecadienoic acid. Lipids. 1975 Feb;10(2):87–92. doi: 10.1007/BF02532161. [DOI] [PubMed] [Google Scholar]
- Kolattukudy P. E., Croteau R., Walton T. J. Biosynthesis of Cutin: Enzymatic Conversion of omega-Hydroxy Fatty Acids to Dicarboxylic Acids by Cell-free Extracts of Vicia Faba Epidermis. Plant Physiol. 1975 May;55(5):875–880. doi: 10.1104/pp.55.5.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Rahn C. H., Schlenk H. Detection of aldehydes with 4-amino-5-hydrazino-1,2,4-triazole-3-thiol as spray reagent. Lipids. 1973 Nov;8(11):612–616. doi: 10.1007/BF02533143. [DOI] [PubMed] [Google Scholar]
- VIOQUE E., HOLMAN R. T. Characterization of the ketodienes formed in the oxidation of linoleate by lipoxidase. Arch Biochem Biophys. 1962 Dec;99:522–528. doi: 10.1016/0003-9861(62)90301-6. [DOI] [PubMed] [Google Scholar]
- Zimmerman D. C. A new product of linoleic acid oxidation by a flaxseed enzyme. Biochem Biophys Res Commun. 1966 May 25;23(4):398–402. doi: 10.1016/0006-291x(66)90740-6. [DOI] [PubMed] [Google Scholar]
- Zimmerman D. C., Vick B. A. Hydroperoxide isomerase: a new enzyme of lipid metabolism. Plant Physiol. 1970 Sep;46(3):445–453. doi: 10.1104/pp.46.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]


