Abstract
Background:
Gestational diabetes mellitus (GDM) is associated with both short- and long-term adverse health consequences for both the mother and her offspring. The aim was to study the prevalence and risk factors for GDM in Beijing.
Methods:
The study population consisted of 15,194 pregnant women attending prenatal care in 15 hospitals in Beijing, who delivered between June 20, 2013, and November 30, 2013, after 28 weeks of gestation. The participants were selected by cluster sampling from the 15 hospitals identified through random systematic sampling based on the number of deliveries in 2012. A questionnaire was designed to collect information.
Results:
A total of 2987 (19.7%) women were diagnosed with GDM and 208 (1.4%) had diabetes in pregnancy (DIP). Age (OR: 1.053, 95% CI: 1.033–1.074, P < 0.01), family history of diabetes mellitus (OR: 1.481, 95% CI: 1.254–1.748, P < 0.01), prepregnancy body mass index (BMI) (OR: 1.481, 95% CI: 1.254–1.748, P < 0.01), BMI gain before 24 weeks (OR: 1.126, 95% CI: 1.075–1.800, P < 0.01), maternal birth weight (P < 0.01), and fasting plasma glucose at the first prenatal visit (P < 0.01) were identified as risk factors for GDM. In women with birth weight <3000 g, GDM rate was significantly higher.
Conclusions:
One out of every five pregnant women in Beijing either had GDM or DIP and this constitutes a huge health burden for health services. Prepregnancy BMI and weight gain before 24th week are important modifiable risk factors for GDM. Ensuring birth weight above 3000 g may help reduce risk for future GDM among female offsprings.
Keywords: Gestational Diabetes Mellitus, Maternal Low Birth Weight, Risk Factors
Introduction
Gestational diabetes mellitus (GDM) is described as diabetes diagnosed during pregnancy which is not overt diabetes.[1] It is associated with both short- and long-term adverse health consequences for both the mother and her offspring.[2,3,4,5] GDM with its potential to increase future rates of diabetes is arousing international attention of late. In China with the highest number of people with diabetes, the epidemiology of GDM has not been extensively studied. Till now, lack of uniform policy and diagnostic protocol, coupled with lack of awareness about GDM and its consequences, has meant that the health-care practitioners (HCPs) have not given adequate attention to screening for and treating GDM. The Ministry of Health (MOH) of China published the diagnostic criteria for GDM in July 2011[6] which was amended in August 2014.[7] In addition, a World Diabetes Foundation-funded project (WDF 10-517) has helped build capacity through high-quality training of HCPs in China. These developments have ensured that all large hospitals providing maternal health services comply with the testing protocol according to the new MOH guidelines. In China, especially in cities, nearly all pregnant women deliver in hospitals and this gave us the opportunity to investigate the prevalence of and the risk factors for GDM among pregnant women attending maternal health services in the public hospitals in Beijing.
Methods
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee of the institute. Informed written consent was obtained from all patients prior to their enrollment in this study.
Study population and sampling method
All public hospitals that offer delivery services in Beijing constituted the sampling frame for our study. All pregnant women attending a particular hospital were taken as a cluster. The survey adopted a systemic cluster sampling method. Random seed and sampling intervals were decided and sorted by the number of deliveries in 2012, 15 hospitals in Beijing were chosen by cluster sampling (listed in the acknowledgment). The public hospitals cater to over 90% of all deliveries in Beijing and therefore the study population is representative of pregnant women delivering in Beijing. The survey included pregnant women who delivered after at least 28 weeks of pregnancy between June 20, 2013, and November 30, 2013. The survey and data collection were started and completed at the same time in all the selected hospitals.
Sample size
With an estimated incidence of GDM between 10% and 30% based on previous reports and considering 95% confidence interval (95% CI) width within 3%, the sample size was estimated to be between 3650 and 1603.
Taking the higher number and rounding it off gives the estimated number to be 3650. Considering refusal to participate and defaulter rate of around 30%, a sample size of 5214 was considered to be adequate. We collected data on 15,194 pregnant women, adequately powered for calculating the prevalence of GDM, as well as to analyze the risk factors and build a sizeable cohort for a postpartum follow-up study later on.
Investigation method and quality control
The study developed a questionnaire to collect and record the data. The questionnaire was evaluated by a team of professionals and pilot tested before finalization. Two training workshops were held to train the investigators from the selected hospitals. A quality control center was set up at the Beijing Maternity Hospital to monitor and supervise the quality of data collection and provide assistance and feedback.
The questionnaire consisted of three parts. The first part relates to basic information about demography, socioeconomic conditions and lifestyle, family history of diabetes mellitus (DM), and general medical history. This part, took 5–10 min to complete, was filled during face-to-face interviews when the women were hospitalized for delivery. The second part relates to information about the index pregnancy such as findings of physical examinations and laboratory tests, history of complications during pregnancy and childbirth, and maternal and fetal outcome. This part was filled by the investigators based on the patients’ medical record and completed within 1 day after the patients’ discharge from the hospital. The third part relates to the postpartum period. According to the protocol, all women were required to come back for routine checkup including a basic physical examination at 6–12 weeks postpartum. For women with GDM, a 75 g OGTT test is given at that time to assess their glycemic status. The information for the third part is being collected by the investigators in the outpatient department and from the hospital information system and will be used for the follow-up study.
Being an observational study, part two of the questionnaire was filled using medical records and thus our study has elements of retrospective analysis. In some cases, women did not report for routine prenatal visits until the second or third trimester, while in some other cases, women delivered at a particular hospital but had attended prenatal care at some other place. Thus, incomplete information of routine prenatal examination was unavoidable in some cases.
Data from the questionnaires were transferred to computer (double input with Epidata software, EpiData Association, Denmark), and logical consistency was checked. The responder and delivery list was cross-checked to make a supplementary investigation for missing information.
Diagnostic criteria for gestational diabetes mellitus and diabetes mellitus
All hospitals included in the study implemented the MOH China guideline.
Routine fasting plasma glucose (FPG) test to rule out previously undiagnosed diabetes was done at the time of booking or in the first trimester. A reading ≥7.0 mmol/L was considered diagnostic of DM.
Between 24 and 28 weeks of gestation, a diagnostic 2 h 75 g OGTT test was done on all pregnant women. GDM was diagnosed by the 2014 MOH China criteria when one of the following plasma glucose values was met or exceeded: 0 h, 5.1 mmol/L; 1 h, 10.0 mmol/L; and 2 h, 8.5 mmol/L. Even if the test was done after 28 weeks, it was considered valid. Diabetes in pregnancy (DIP) was diagnosed when FPG was ≥7.0 mmol/L and/or the 2 h value was ≥11.1 mmol/L.
At 6–12 weeks postpartum, all women with GDM underwent a 75 g 2 h OGTT test. The results of FPG and 2 h values are recorded. The diagnosis of DM was made if the FPG value was ≥7.0 mmol/L and/or the 2 h value was ≥11.1 mmol/L; impaired glucose tolerance was diagnosed if the 2 h value was between 7.8 and 11.0 mmol/L; and impaired fasting glucose was diagnosed if fasting value was between 6.1 and 6.9 mmol/L.
Statistical analysis
Data analysis was done using the Predictive Analysis Software (PASW) PASW Statistics 18.0 (SPSS Inc., IBM, USA). The quantitative parameters followed the normal distribution, expressed as mean ± standard deviation (SD), and tested by independent t-tests. Pearson's Chi-square test and Fisher's exact test were applied to examine differences between the groups for categorical parameters. Association of GDM diagnosis with other factors was examined by multivariable binary logistic regression. Sixteen variables were included in the logistic regression model using conditional forward method. All reported P values were two tailed, and P < 0.05 was established as the level of significance.
Results
Prevalence of gestational diabetes mellitus
Data of 15,194 pregnant women who delivered at the selected maternity hospitals in Beijing between June 20, 2013, and November 30, 2013 after at least 28 weeks of gestation were collected. Their mean age ± SD was 28.3 ± 4.3 years. The mean pregestational body mass index (BMI) ± SD was 21.6 ± 3.3. Two-thirds (9675) of the women were urban residents.
A total of 2987 (19.7%) pregnant women were diagnosed as GDM and 208 (1.4%) as DIP. Of the women with DIP, 141 (67.8%) were first diagnosed with DM during the index pregnancy. The basic information comparing GDM and non-GDM population is shown in Table 1 (Table 1 did not cover the information of 208 DIP women.).
Table 1.
Demographic data of the samples in nongestational diabetes mellitus and gestational diabetes mellitus groups
| Characteristics | Non-GDM (n = 11,999) | GDM (n = 2987) | P | ||
|---|---|---|---|---|---|
| n | Values | n | Values | ||
| Age (years) | 11,999 | 28.0 ± 4.2 | 2987 | 29.4 ± 4.7 | <0.01 |
| Prepregnancy BMI (kg/m2) | 11,908 | 21.3 ± 3.1 | 2964 | 22.7 ± 3.6 | <0.01 |
| BMI gain before 24 weeks (kg/m2) | 10,705 | 3.4 ± 1.6 | 2754 | 3.5 ± 1.7 | <0.01 |
| MBW (g) | 4239 | 3210.0 ± 520.5 | 1143 | 3156 ± 541.5 | <0.01 |
| First trimester Hb (g/L) | 9398 | 128.8 ± 11.7 | 2448 | 130.5 ± 9.9 | <0.01 |
| First trimester FPG (mmol/L) | 10,802 | 4.65 ± 0.42 | 2764 | 4.98 ± 1.35 | <0.01 |
| DM family history | 11,999 | 1424 (11.9) | 2987 | 594 (19.9) | <0.01 |
| Urban | 11,846 | 7493 (63.3) | 2924 | 2028 (69.4) | <0.01 |
| Singleton | 11,999 | 11,814 (98.5) | 2987 | 2927 (98.0) | 0.07 |
| HBsAg (+) | 11,916 | 199 (1.7) | 2976 | 38 (1.3) | 0.13 |
| Vaginal infection positive | 11,463 | 654 (5.7) | 2813 | 147 (5.2) | 0.32 |
Data were shown as mean ± standard deviation or n (%). GDM: Gestational diabetes mellitus; BMI: Body mass index; MBW: Maternal birth weight; FPG: Fasting plasma glucose; SD: Standard deviation; HBsAg: Hepatitis B surface antigen; Hb: Hemoglobin.
Risk factors for gestational diabetes mellitus
After excluding women with DIP, data of the remaining 14,986 pregnant women were used to analyze the risk factors for GDM. The study used “GDM diagnosis” (0 normal, 1 GDM) as dependent variable to carry out logistic regression analysis. Sixteen independent variables as described below were selected by conditional forward method. About 4703 cases without missing information were included in analysis.
There were five continuous variables: age, prepregnancy BMI, BMI gain before 24 weeks, gravida, and parity. Eleven categorical variables included were as follows: education level, average monthly income, family history of DM, place of residence (urban or rural), maternal birth weight (MBW), hepatitis B antigen status, vaginal infection, FPG level in the first trimester, hemoglobin level in the first trimester, and the number and the gender of fetus.
The grouping of categorical variables used in logistic regression analysis is shown in Table 2. Using conditional forward stepwise method, six variables [Table 3] were preserved in the equation and ten were removed. The variables that were removed were education level, income level, gravidity, parity, location, hepatitis B antigen status, vaginal infection, hemoglobin level in the first trimester, the number and the gender of fetus as they showed no statistical correlation with GDM diagnosis. Age, prepregnancy BMI, BMI gain before 24 weeks, family history of DM, MBW, and FPG level in the first trimester showed statistical differences and these six variables were considered risk factors for GDM.
Table 2.
Categorical variables included in the logistic regression
| Variables | Categories |
|---|---|
| First trimester FPG (mmol/L) | <4.10 |
| 4.10–4.59 | |
| 4.60–5.09 | |
| 5.10–5.09 | |
| 5.60–6.09 | |
| 6.10–6.99 | |
| MBW (g) | <2000 |
| 2000–2499 | |
| 2500–2999 | |
| 3000–3499 | |
| 3500–3999 | |
| ≥4000 | |
| Education | Graduate and above |
| College | |
| High school | |
| Junior school or lower | |
| Average monthly income (RMB) | <1000 |
| 1000–2999 | |
| 3000–4999 | |
| ≥5000 | |
| Anemia (Hb g/L) | No (≥120) |
| Mild (90–119) | |
| Moderate (60–89) | |
| Severe (30–59) | |
| Extremely severe (<30) | |
| Family history of DM | Positive |
| Negative | |
| Place of residence | Urban |
| Rural | |
| Number of fetus | Single |
| Multiple | |
| Gender of fetus | Male |
| Female | |
| Hepatitis B antigen | Positive |
| Negative | |
| Vaginal infection | Present |
| Absent |
MBW: Maternal birth weight; FPG: Fasting plasma glucose; DM: Diabetes mellitus; Hb: Hemoglobin.
Table 3.
Variables in the equation in logistic regression
| Variables | β | SE | P | OR | 95% CI |
|---|---|---|---|---|---|
| Age | 0.052 | 0.010 | <0.01* | 1.053 | 1.033–1.074 |
| Prepregnancy BMI | 0.103 | 0.011 | <0.01* | 1.109 | 1.084–1.134 |
| BMI gain before 24 weeks | 0.119 | 0.024 | <0.01* | 1.126 | 1.075–1.180 |
| Family DM history | 0.392 | 0.085 | <0.01* | 1.481 | 1.254–1.748 |
| Maternal birth weight (g) | <0.01* | ||||
| <2000 | 1 | ||||
| 2000–2499 | –0.421 | 0.400 | 0.29 | 0.656 | 0.300–1.436 |
| 2500–2999 | –0.654 | 0.371 | 0.08 | 0.520 | 0.251–1.075 |
| 3000–3499 | –0.832 | 0.366 | 0.02† | 0.435 | 0.213–0.891 |
| 3500–3999 | –0.802 | 0.370 | 0.03† | 0.448 | 0.217–0.927 |
| >4000 | –1.090 | 0.386 | <0.01* | 0.336 | 0.158–0.336 |
| FPG level (mmol/L) | <0.01* | ||||
| <4.10 | 1 | ||||
| 4.10–4.59 | 0.065 | 0241 | 0.79 | 1.067 | 0.665–1.711 |
| 4.60–5.09 | 0.577 | 0.231 | 0.01† | 1.781 | 1.132–2.804 |
| 5.10–5.59 | 1.459 | 0.235 | <0.01* | 4.303 | 2.715–6.822 |
| 5.60–6.09 | 2.270 | 0.283 | <0.01* | 9.680 | 5.558–16.860 |
| 6.10–6.99 | 2.334 | 0.441 | <0.01* | 10.318 | 4.345–24.499 |
| Constant | –5.718 | 0.576 | <0.01* | 0.003 |
*P<0.01; †P<0.05. BMI: Body mass index; FPG: Fasting plasma glucose; OR: Odds ratio; CI: Confidence interval; DM: Diabetes mellitus; SE: Standard error.
As shown in Table 3, the risk for GDM increased with increasing age. With each 1 year increase in age (between the ages of 17 and 46 years), the risk for GDM increased by 5.3% (1.053-fold risk). Women with a family history of DM had 1.481-fold increased risk for GDM compared to women with no family history of DM. Prepregnancy BMI and BMI gain before 24 weeks were both significant risk factors; 1 unit increase in BMI above 14 increased the risk by 11% (1.109-fold risk), while 1 unit increase in BMI gain before 24 weeks increased the risk by 13% (1.126-fold risk). MBW is a high risk of GDM, especially when MBW <3000 g. However, with the increase of MBW, the risk association of GDM was decreasing. FPG level in the first trimester had the highest risk association with GDM. Women with FPG values of 5.60 mmol/L had 9-fold higher risk of GDM compared to women with FPG value below 4.1 mmol/L.
Association between gestational diabetes mellitus and maternal birth weight
A subset of 5382 pregnant women in our study were able to recall their own birth weight. Of these, 1143 (21.2%) had GDM which was slightly higher than the whole study population (19.7%). MBW below 3000 g increased the risk of GDM whereas MBW above 3000 g appeared protective. Table 4 and Figure 1 show that increasing MBW from below 2000 g up to 3000 g was associated with a decreasing GDM prevalence. At MBW of 3000 g, the GDM prevalence (19.9%) approximated the GDM rate for the whole study population (19.7%) and remained steady even with MBW in the 4000 g range. However, the number of women with MBW above 4000 g is rather small to draw any conclusion with regard to risk associated with higher MBW.
Table 4.
Association between gestational diabetes mellitus and maternal birth weight
| Maternal birth weight (g) | n | GDM | Rate (%) |
|---|---|---|---|
| <2000 | 46 | 16 | 34.8 |
| 2000~ | 224 | 63 | 28.1 |
| 2500~ | 1056 | 246 | 23.3 |
| 3000~ | 2542 | 505 | 19.9 |
| 3500~ | 1073 | 230 | 21.4 |
| 4000~ | 441 | 83 | 18.8 |
| Total | 5382 | 1143 | 21.2 |
GDM: Gestational diabetes mellitus.
Figure 1.
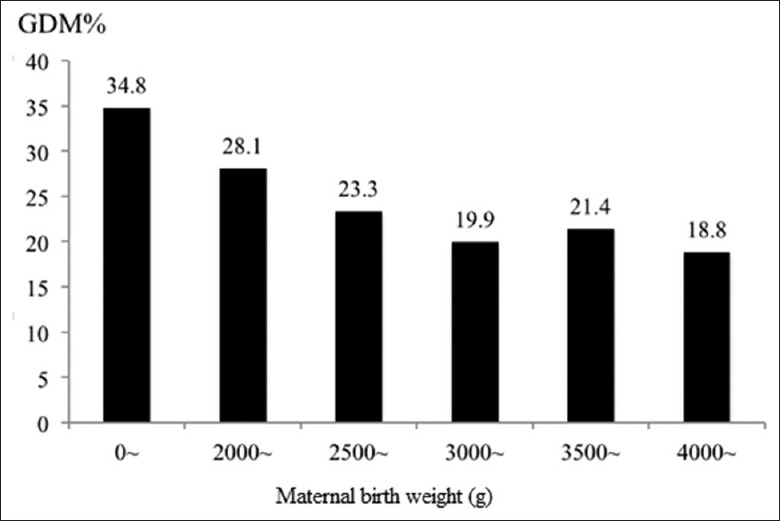
Association between gestational diabetes mellitus and maternal birth weight. GDM: Gestational diabetes mellitus.
Discussion
This is one of the largest studies on GDM in China using the random sampling approach. The study is adequately powered not only to estimate the prevalence of GDM, but also to study the risk factors associated with GDM. Our present study confirms our earlier observation about the high rate of GDM in China. A prevalence rate of 19.7% exceeded the 17.5% prevalence reported in our earlier study.[8] In addition, the rate for newly diagnosed DIP was almost 1%.
Maternal age, prepregnancy BMI, excessive weight gain before 24 weeks of gestation, and family history of diabetes were identified as common risk factors for GDM as also noted by previous studies.[9,10,11,12,13] Our study confirmed that these risk factors play a role also among pregnant Chinese women who develop GDM. Maternal age is an important risk factor. In our study, the mean maternal age was 28.3 years, and each 1 year increase in maternal age above 17 years increased the risk by 5%. As more women join the workforce, especially in the urban areas, delayed marriage and pregnancy is the new norm that is unlikely to change. Family history of diabetes with an odds ratio of 1.48 was an important risk factor noted in our study. Given that over half of the women with diabetes in China are unaware of their condition,[14] the chances that, in our study, even more women may have had a positive family member of diabetes cannot be ruled out. Animal studies[15] have shown that prenatal exposure to a diabetic intrauterine milieu in the female offspring leads to an increased risk of developing gestational diabetes. Several clinical studies[16,17,18] have also shown an excess of maternal history of diabetes among women with GDM. We have not yet analyzed our data to see if this is also true in the Chinese setting. Higher prepregnancy BMI is a known risk factor for GDM. In our study, each one unit increase in prepregnancy BMI was associated with an 11% increase in risk for GDM. As noted by others as well, excessive weight gain in early pregnancy was an important risk factor noted in our study. Ensuring optimal weight before conception and preventing excessive weight gain in early part of pregnancy are two important actions that may help reduce GDM rates in the future. Thus, health education of girls and reproductive age women before pregnancy is important. In our future research, we hope to study optimal prepregnancy BMI and weight gain in early pregnancy to lower the risk of GDM among Chinese women.
The first trimester FPG is an important marker of subsequent GDM as reported by us earlier.[8] In the present study also, we noted that FPG level in the first trimester had the highest risk association with GDM. Women with FPG values of 5.60 mmol/L had a 9-fold higher risk of GDM compared to women with FPG values below 4.1 mmol/L.
We did not find any association between GDM and multifetal pregnancy unlike the higher association with twin pregnancies reported by Whitelaw and Gayle.[13] Egeland and Irgens[19] did not find an elevated risk of GDM among 9271 multifetal pregnancies after controlling for other risk factors such as advanced age, parity, maternal history of diabetes, and women's own birth weight. The higher association of GDM with twin pregnancy may be related to higher weight gain and not necessarily to the number of fetus. Xiao et al.[20] reported that female fetus is associated with greater maternal insulin resistance during pregnancy and thus is at a greater risk of GDM. In our study, we found no relationship between the gender of the fetus and the rate of GDM.
Multigravida and multiparous women are more likely to develop GDM compared to primigravida and nulliparous women; however, after adjusting for age, prepregnancy BMI, and other confounders, the excess risk disappears.[21] We did not find any association between gravidity and parity and higher rate of GDM. Given the single child policy in China, our study was anyway inadequately powered (too few multigravidas) to demonstrate any difference.
Lower socioeconomic background including low level of education has been reported to be a risk factor for GDM, mostly from studies[22,23] reported from the developed countries. These factors may play a role through their ability to impact lifestyle. In our study, most social conditions including education, income, and place of residence showed no correlation with GDM diagnosis. However, it is hard to draw any conclusion based on our study from Beijing. The economic transition in the big cities in China is a recent phenomenon, and the disparity within the local population is limited and has not existed long enough to make a significant impact. When large economic, social, and nutritional variances occur, the social factors might play a role.
While seemingly not affecting GDM rate directly in our study, these socioeconomic factors do exert their influence through MBW. While low birth weight[24] as a consequence of maternal starvation[25] has been shown to increase the risk of type 2 diabetes in China, few previous studies from China have demonstrated the association between low MBW and incidence of GDM. MBW has been reported to be a risk factor for GDM, and studies have shown a U-shaped relationship between MBW and GDM[26,27,28,29,30] with both low and high MBW increasing the risk of GDM.
In our study, women with low birth weight had a high prevalence of GDM. Increasing MBW from below 2000 g up to 3000 g was associated with decreasing GDM prevalence. At MBW of 3000 g, the GDM prevalence (19.9%) approximated the GDM rate for the whole study population (19.7%) and remained steady even with MBW in the 4000 g range. Studies from Italy, Poland, and the USA[31,32,33] report a similar trend. However, given that the number of women with MBW above 4000 g was rather small in our study, it cannot be concluded that higher MBW will not adversely affect GDM rates in China. The mothers in our study were born around late 1980s when the nutrition condition in China overall was not good.
Because of the large sample size and systemic cluster sampling, our study population is representative of pregnant women giving birth in Beijing hospitals. The study is also adequately powered to study the prevalence of GDM and its risk factors. Despite this, the research has some limitations. First of all, part of the information was collected through patient interviews, and being dependent on patients’ memory, it is subject to bias, and there is some degree of inaccuracy, particularly with regard to MBW and family history of DM. Second, the research was an observational study, thus not all information was available in all cases. Despite these lacunae, we believe our study provides useful information with regard to GDM in China and creates a platform for further research to fill the knowledge gaps.
Financial support and sponsorship
The study was supported by the World Diabetes Foundation (WDF10-517 and WDF14-908).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The 15 hospitals which were the study sites are: Tongzhou Maternal and Child Health Hospital of Beijing; Peking University First Hospital; Peking University Third Hospital; Aviation General Hospital; Chinese people's Liberation Army General Hospital of Beijing Military Region; Navy General Hospital; Beijing Hospital of Chinese Traditional and Western Medicine; Pinggu District Maternal and Child Health Hospital of Beijing; Beijing Daxing District Hongxing Hospital; Beijing Chuiyangliu Hospital; Miyunxian Hospital; Peking University Shougang Hospital; Changping District Hospital of Chinese Traditional Medicine; Beijing Jingmei Group General Hospital, Beijing No. 6 Hospital, Beijing, China. The authors thank all the project collaborators in the 15 hospitals: Wen-Ying Meng, Nan Li, Yong-Qing Wang, Zhen-Yu Cai, Li-Xin Shang, Ying Sun, Xue-Ying Zhang, Li-Ping Ji, Jia-Xiu Liu, Li Wei, Yun-Feng Wang, Yu-Feng Sun, Hai-xia, Yu Li-Jun Chen, and Tian-Xia Luo.
Footnotes
Edited by: Yi Cui
References
- 1.American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2012;35(Suppl 1):s11–63. doi: 10.2337/dc12-s011. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Getahun D, Fassett MJ, Jacobsen SJ. Gestational diabetes: Risk of recurrence in subsequent pregnancies. Am J Obstet Gynecol. 2010;203:467.e1–6. doi: 10.1016/j.ajog.2010.05.032. doi: 10.1016/j.ajog.2010.05.032. [DOI] [PubMed] [Google Scholar]
- 3.O’Sullivan JB. Body weight and subsequent diabetes mellitus. JAMA. 1982;248:949–52. doi: 10.1001/jama.1982.03330080031024. [PubMed] [Google Scholar]
- 4.Chu C, Gui YH, Ren YY, Shi LY. The impacts of maternal gestational diabetes mellitus (GDM) on fetal hearts. Biomed Environ Sci. 2012;25:15–22. doi: 10.3967/0895-3988.2012.01.003. doi: 10.3967/0895-3988.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Chang Y, Chen X, Zhang ZK. Intrauterine exposure to maternal diabetes is associated with adiposity in children at 6 years of age in China. Biomed Environ Sci. 2015;28:140–2. doi: 10.3967/bes2015.017. doi: 10.3967/bes2015.017. [DOI] [PubMed] [Google Scholar]
- 6.Yang HX. Diagnostic criteria for gestational diabetes mellitus (WS 331-2011) Chin Med J. 2012;125:1212–3. [PubMed] [Google Scholar]
- 7.Chinese Society of Gynecology and Obstetrics, Society of Perinatal Medicine. Diagnosis and therapy guideline of pregnancy with diabetes mellitus (in Chinese) Chin J Obstet Gynecol. 2014;49:561–9. [PubMed] [Google Scholar]
- 8.Zhu WW, Yang HX, Wei YM, Yan J, Wang ZL, Li XL, et al. Evaluation of the value of fasting plasma glucose in the first prenatal visit to diagnose gestational diabetes mellitus in china. Diabetes Care. 2013;36:586–90. doi: 10.2337/dc12-1157. doi: 10.2337/dc12-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Cianni G, Volpe L, Lencioni C, Miccoli R, Cuccuru I, Ghio A, et al. Prevalence and risk factors for gestational diabetes assessed by universal screening. Diabetes Res Clin Pract. 2003;60:131–7. doi: 10.1016/j.diabres.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Savona-Ventura C, Vassallo J, Marre M, Karamanos BG MGSD-GDM Study Group. A composite risk assessment model to screen for gestational diabetes mellitus among Mediterranean women. Int J Gynaecol Obstet. 2013;120:240–4. doi: 10.1016/j.ijgo.2012.10.016. doi: 10.1016/j.ijgo.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Ogura T, Hamada H, Yoshikawa H. The influence of maternal pre-pregnancy BMI on prenatal risks and treatment effects for patients newly diagnosed with GDM based on the revision of the diagnostic criteria. Int J Gynecol Obstet. 2012;119(Suppl 3):S775. [Google Scholar]
- 12.Burke AE, Bennett WL, Jamshidi RM, Gilson MM, Clark JM, Segal JB, et al. Reduced incidence of gestational diabetes with bariatric surgery. J Am Coll Surg. 2010;211:169–75. doi: 10.1016/j.jamcollsurg.2010.03.029. doi: 10.1016/j.jamcollsurg.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 13.Whitelaw B, Gayle C. Gestational diabetes. Obstet Gynaecol Reprod Med. 2011;21:41–6. doi: 10.1016/j.ogrm.2010.11.001. [Google Scholar]
- 14.Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–101. doi: 10.1056/NEJMoa0908292. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 15.Aerts L, Van Assche FA. Is gestational diabetes an acquired condition? J Dev Physiol. 1979;1:219–25. [PubMed] [Google Scholar]
- 16.Tabák AG, Tamás G, Péterfalvi A, Bosnyák Z, Madarász E, Rákóczi I, et al. The effect of paternal and maternal history of diabetes mellitus on the development of gestational diabetes mellitus? J Endocrinol Invest. 2009;32:606–10. doi: 10.1007/BF03346517. doi: 10.1007/BF03346517. [DOI] [PubMed] [Google Scholar]
- 17.Harder T, Franke K, Kohlhoff R, Plagemann A. Maternal and paternal family history of diabetes in women with gestational diabetes or insulin-dependent diabetes mellitus type I. Gynecol Obstet Invest. 2001;51:160–4. doi: 10.1159/000052916. doi: 10.1159/000052916. [DOI] [PubMed] [Google Scholar]
- 18.McLean M, Chipps D, Cheung NW. Mother to child transmission of diabetes mellitus: Does gestational diabetes program Type 2 diabetes in the next generation? Diabet Med. 2006;23:1213–5. doi: 10.1111/j.1464-5491.2006.01979.x. doi: 10.1111/j.1464-5491.2006.01979.x. [DOI] [PubMed] [Google Scholar]
- 19.Egeland GM, Irgens LM. Is a multiple birth pregnancy a risk factor for gestational diabetes? Am J Obstet Gynecol. 2001;185:1275–6. doi: 10.1067/mob.2001.118853. doi: 10.1067/mob.2001.118853. [DOI] [PubMed] [Google Scholar]
- 20.Xiao L, Zhao JP, Nuyt AM, Fraser WD, Luo ZC. Female fetus is associated with greater maternal insulin resistance in pregnancy. Diabet Med. 2014;31:1696–701. doi: 10.1111/dme.12562. doi: 10.1111/dme.12562. [DOI] [PubMed] [Google Scholar]
- 21.Al-Rowaily MA, Abolfotouh MA. Predictors of gestational diabetes mellitus in a high-parity community in Saudi Arabia. East Mediterr Health J. 2010;16:636–41. [PubMed] [Google Scholar]
- 22.Cullinan J, Gillespie P, Owens L, Avalos G, Dunne FP ATLANTIC DIP Collaborators. Is there a socioeconomic gradient in the prevalence of gestational diabetes mellitus. Ir Med J. 2012;105(5 Suppl):21–3. [PubMed] [Google Scholar]
- 23.Anna V, van der Ploeg HP, Cheung NW, Huxley RR, Bauman AE. Sociodemographic correlates of the increasing trend in prevalence of gestational diabetes mellitus in a large population of women between 1995 and 2005. Diabetes Care. 2008;31:2288–93. doi: 10.2337/dc08-1038. doi: 10.2337/dc08-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao X, Zhang ZX, Cohen HJ, Wang H, Li W, Wang T, et al. Evidence of a relationship between infant birth weight and later diabetes and impaired glucose regulation in a Chinese population. Diabetes Care. 2008;31:483–7. doi: 10.2337/dc07-1130. doi: 10.2337/dc07-1130. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, He Y, Qi L, Jaddoe VW, Feskens EJ, Yang X, et al. Exposure to the Chinese famine in early life and the risk of hyperglycemia and type 2 diabetes in adulthood. Diabetes. 2010;59:2400–6. doi: 10.2337/db10-0385. doi: 10.2337/db10-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Claesson R, Aberg A, Marsál K. Abnormal fetal growth is associated with gestational diabetes mellitus later in life: Population-based register study. Acta Obstet Gynecol Scand. 2007;86:652–6. doi: 10.1080/00016340701207682. doi: 10.1080/00016340701207682. [DOI] [PubMed] [Google Scholar]
- 27.Innes KE, Byers TE, Marshall JA, Barón A, Orleans M, Hamman RF. Association of a woman's own birth weight with subsequent risk for gestational diabetes. JAMA. 2002;287:2534–41. doi: 10.1001/jama.287.19.2534. [DOI] [PubMed] [Google Scholar]
- 28.Pettitt DJ, Knowler WC. Long-term effects of the intrauterine environment, birth weight, and breast-feeding in Pima Indians. Diabetes Care. 1998;21(Suppl 2):B138–41. [PubMed] [Google Scholar]
- 29.Savona-Ventura C, Chircop M. Birth weight influence on the subsequent development of gestational diabetes mellitus. Acta Diabetol. 2003;40:101–4. doi: 10.1007/s005920300013. doi: 10.1007/s005920300013. [DOI] [PubMed] [Google Scholar]
- 30.Gale CR, Martyn CN, Kellingray S, Eastell R, Cooper C. Intrauterine programming of adult body composition. J Clin Endocrinol Metab. 2001;86:267–72. doi: 10.1210/jcem.86.1.7155. doi: 10.1210/jcem.86.1.7155. [DOI] [PubMed] [Google Scholar]
- 31.Williams MA, Emanuel I, Kimpo C, Leisenring WM, Hale CB. A population-based cohort study of relation between maternal birth weight and risk of gestational diabetes mellitus in four racial/ethnic groups. Paediatr Perinat Epidemiol. 1999;13:452–65. doi: 10.1046/j.1365-3016.1999.00219.x. doi: 10.1046/j.1365-3016.1999.00219.x. [DOI] [PubMed] [Google Scholar]
- 32.Yeung EH, Hu FB, Solomon CG, Chen L, Louis GM, Schisterman E, et al. Life-course weight characteristics and the risk of gestational diabetes. Diabetologia. 2010;53:668–78. doi: 10.1007/s00125-009-1634-y. doi: 10.1007/s00125-009-1634-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogonowski J, Miazgowski T, Engel K, Celewicz Z. Birth weight predicts the risk of gestational diabetes mellitus and pregravid obesity. Nutrition. 2014;30:39–43. doi: 10.1016/j.nut.2013.05.021. doi: 10.1016/j.nut.2013.05.021. [DOI] [PubMed] [Google Scholar]


