Abstract
Background:
Excessive reactive oxygen species (ROS) may lead to a number of reproductive diseases such as polycystic ovary syndrome. This study aimed to establish an animal model of ovarian oxidative stress and to assess the protective effect of curcumin against oxidative injury.
Methods:
Ovarian oxidative stress was induced in female Kunming mice (n = 40) with intraperitoneal injection of 8 mg/kg sodium arsenite (As) once every other day for 16 days; meanwhile, they were, respectively, treated by intragastric administration of 0, 100, 150, or 200 mg/kg (n = 10/group) curcumin once per day for 21 days. Ten normal mice were used as control. Then, the mice were injected intraperitoneally with BrdU and sacrificed; the right ovaries were collected for hematoxylin and eosin (HE) staining and BrdU immunohistochemistry, and the left ovaries for enzyme-linked immunosorbent assay (ELISA) and Western blotting analyses.
Results:
The ELISA results showed that ROS (11.74 ± 0.65 IU/mg in 8 mg/kg AS + 0 mg/kg curcumin group vs. 10.71 ± 0.91 IU/mg in control group, P = 0.021) and malondialdehyde (MDA) (0.32 ± 0.02 nmol/g in 8 mg/kg AS + 0 mg/kg curcumin group vs. 0.27 ± 0.02 nmol/g in control group, P = 0.048) increased while superoxide dismutase (SOD) (3.96 ± 0.36 U/mg in 8 mg/kg AS + 0 mg/kg curcumin group vs. 4.51 ± 0.70 U/mg in control group, P = 0.012) and glutathione peroxidase (17.36 ± 1.63 U/g in 8 mg/kg AS + 0 mg/kg curcumin group vs. 18.92 ± 1.80 U/g in control group, P = 0.045) decreased in the ovary after injection of As, indicating successful modeling of oxidative stress. Curcumin treatment could considerably increase SOD (4.57 ± 0.68, 4.49 ± 0.27, and 4.56 ± 0.25 U/mg in 100 mg/kg, 150 mg/kg, and 200 mg/kg curcumin group, respectively, all P < 0.05) while significantly reduce ROS (10.64 ± 1.38, 10.73 ± 0.71, and 10.67 ± 1.38 IU/mg in 100 mg/kg, 150 mg/kg, and 200 mg/kg curcumin group, respectively, all P < 0.05) and MDA (0.28 ± 0.02, 0.25 ± 0.03, and 0.27 ± 0.04 nmol/g in 100 mg/kg, 150 mg/kg, and 200 mg/kg curcumin group, respectively; both P < 0.05) in the ovary. HE staining and BrdU immunohistochemistry of the ovarian tissues indicated the increased amount of atretic follicles (5.67 ± 0.81, 5.84 ± 0.98, and 5.72 ± 0.84 in 100 mg/kg, 150 mg/kg, and 200 mg/kg curcumin group, respectively, all P < 0.05), and the inhibited proliferation of granular cells under oxidative stress would be reversed by curcumin. Furthermore, the Western blotting of ovarian tissues showed that the p66Shc expression upregulated under oxidative stress would be lowered by curcumin.
Conclusion:
Curcumin could alleviate arsenic-induced ovarian oxidative injury to a certain extent.
Keywords: Antioxidant, Curcumin, Mouse, Ovary, Oxidative Stress, p66Shc
Introduction
Oxidative stress is the imbalance between antioxidants and reactive oxygen species (ROS) (such as superoxide anion, hydrogen peroxide, and hydroxyl radicals), because of increased production and/or decreased detoxification.[1] Oxidative stress is present in all organs and cells. The ovary is a metabolically active organ in which ROS are generated during normal physiological functioning.[2] Excessive ROS production may overpower the body's natural antioxidant defense system, creating an environment unsuitable for normal female physiology.[1] Indeed, oxidative stress has been recently reported to play an important role in the normal functioning of the female reproductive system and in the pathogenesis of reproductive diseases such as endometriosis, polycystic ovary syndrome, and unexplained infertility.[3]
The expression of various biomarkers of oxidative stress has been demonstrated in normal human ovarian cycles.[3] Excess ROS in the follicle promotes apoptosis[3] and may damage oocytes and granulosa cells in the follicle.[4] There is a delicate balance between ROS and antioxidant enzymes in ovarian tissues. The antioxidant enzymes neutralize ROS and protect the oocyte and embryo.[5] Enzymatic antioxidants bear a metal ion core, which gives them the ability to take on different valences as they transfer electrons to other molecules for detoxification process.[1] Thus, they can neutralize excess ROS and prevent damage to cells. Endogenous antioxidant enzymes include superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx), and glutathione oxidase.[1] p66Shc is a 66-kDa Src collagen homolog (Shc) adaptor protein that is one of the three main isoforms encoded by the SHC1 gene (p46Shc, p52Shc, and p66Shc).[6] The p66Shc isoform is also involved in signaling pathways that regulate the cellular response to oxidative stress and cell lifespan.[7] Granulosa cells closely interact and provide support to the developing and maturing oocyte, share the oocyte's microenvironment, and minimize damage caused by ROS. Granulosa cells can produce antioxidants, which potentially protect the oocyte from ROS-induced damage.[8]
Arsenic is a drug that has double effects. It can exert toxic effects on the female reproductive system and can be used in anticancer therapy.[9] The mechanisms by which arsenic exerts its toxic effects are still unclear, although chromosomal abnormalities, oxidative stress, altered DNA repair, altered DNA methylation patterns, and altered cell proliferation have been proposed.[10] Of these, oxidative stress is one of the most acknowledged mechanisms. In this experiment, arsenic was used to induce oxidative stress in the mouse ovary.
Curcumin is a yellow-orange dye extracted from the Indian spice turmeric. Its activity as an antioxidant and free radical scavenger has been demonstrated by several in vitro studies.[11] This activity comes from either the hydroxyl group or the methylene group of the β-diketone (heptadiene-dione) moiety.[12] The median lethal dose of curcumin is 2 g/kg. Clinical trials have shown that the continuous use of curcumin for 4 months had no obvious side effects.[13] The aim of this study was to establish whether curcumin can protect the mouse ovary against oxidative injury and to explore the underlying cellular and biochemical mechanisms.
Methods
Animals
Female Kunming mouse (n = 50) (mean age of 49 ± 5 days, mean body weight of 30 ± 5 g) were provided by the Hubei Medical Experimental Animal Center (China). The animals were housed separately under a standard cycle of 12-h light/darkness and provided with water and food chow ad libitum. Before the experiment, all rats were monitored for several days to observe their health condition. All animal experiments were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Drugs and reagents
Curcumin (molecular weight of 368.4 Da, 94% purity) was purchased from Sigma (St. Louis, MO, USA). Carboxymethyl cellulose is a nontoxic, nonimmunogenic solvent with a good absorbance; 0.5% carboxymethyl cellulose solution was used as a solvent to make curcumin into a suspension for absorption. Sodium carboxymethyl cellulose solution (0.5%) was donated by the Pharmacy of the Affiliated People's Hospital of Hubei Medical College, China. The mouse ROS ELISA kit, MDA ELISA kit, SOD ELISA kit, and GPx ELISA kit were purchased from R&D Systems (Minneapolis, MN, USA). The rabbit anti-mouse P-Shc-R polyclonal antibody was purchased from Biosynthesis Biotechnology Co., Ltd. (China). Rabbit anti-goat IgG and Horse anti-mouse antibodies were purchased from Jinqiao Co., Ltd. (China). An electrochemiluminescence color kit was purchased from Pierce Chemical (Dallas, TX, USA). Prestained protein marker was purchased from Fermentas (Thermo Fisher Scientific, Waltham, MA, USA).
Experimental design
Fifty Kunming mice with two regular estrus cycles without coitus were weighed. Matured female Kunming mice, as required by the methods of vaginal smear, were randomly divided into five groups: control group, 0 mg/kg curcumin group, 100 mg/kg curcumin-treated group, 150 mg/kg curcumin-treated group, and 200 mg/kg curcumin-treated group (n = 10/group). Sodium arsenite (As) (dissolved in distilled water) was used to induce ovarian oxidative stress in mice of the latter four groups (n = 40), using intraperitoneal injection of 8 mg/kg sodium As once every other day for 16 days.[14] These mice were meanwhile treated by intragastric administration of 0, 100, 150, or 200 mg/kg (n = 10/group) curcumin (dissolved in 0.5% sodium carboxymethyl cellulose solution) once per day for 21 days. Ten remaining mice were used as control (injected with distilled water as vehicle for sodium As and received 0.5% sodium carboxymethyl cellulose solution as vehicle for curcumin).
Sample collection
After 21 days, all the mice were injected intraperitoneally with 10 μmol/L of BrdU at a dose of 20 μl/g and sacrificed. Layer by layer, the abdominal cavity of each group was opened to obtain the ovaries. After being weighed, the right ovary was collected for hematoxylin and eosin (HE) staining and immunohistochemistry. The left ovary was frozen at −70°C for enzyme-linked immunosorbent assay (ELISA) and Western blotting analyses. The rate of the weight gain percent (the percentage of weight gain relative to the initial weight) and the index of ovaries (percentage weight of the ovary) were recorded.
Counting follicles after hematoxylin and eosin staining
The right ovaries were embedded in paraffin after a 12-h fixation in 4% paraformaldehyde. They were serially sectioned (6 μm), mounted on glass slides, and stained with HE. Ovarian follicles were counted according to a previous study.[2] In brief, every fifth ovary section was scanned under a dot slide-digital virtual microscope. To avoid repeated counts of the same follicle, only those with a visible oocyte nucleus were included. Since oocyte nuclei measure between 20 and 30 μm in diameter, counting every fifth section of the ovary ensured a distance of 30 μm between sections and thus minimizes the chance of multiple counts of the same ovarian follicle. The number of primordial follicles, primary follicles, secondary follicles, and atretic follicles in all the serial sections of an ovary were counted. The following follicle classification[15,16] was utilized: Type 1, primordial follicle, one layer of flattened granulosa cells surrounding the oocyte; Type 2: primary follicle, one to fewer than two complete layers of cuboidal granulosa cells; Type 3: secondary follicle, an oocyte surrounded by greater than one layer of cuboidal granulosa cells, with no visible antrum; Type 4: atretic follicle, the morphology of follicle is irregular, granulosa cells became less or disappeared.
BrdU immunohistochemistry
The sections were dewaxed with gradient alcohol. They were washed once with phosphate-buffered saline (PBS) for 5 min and subjected to antigen retrieval in 0.01 mol/L sodium citrate buffer, pH 6.0, for 20 min in a microwave. They were allowed to cool to room temperature and washed once with PBS for 5 min. The DNA was nicked in 2 N HCl for 30 min at 37°C. Endogenous HRP was blocked with 3% H2O2 in methanol for 10 min, and then washed with water and 1 × PBS for 5 min, separately. Nonspecific staining was blocked with 10% horse serum in 1 × PBS at 37°C for 1 h. Excess blocking reagent was removed and replaced with mouse anti-BrdU (1:400) diluted with blocking reagent, overnight at 4°C. The slides were each washed three times with PBS for 5 min to remove excess PBS buffer. The sections were incubated at 25°C with biotinylated horse anti-rabbit IgG (1:200) antibody diluted with 1% BSA in 1 × PBS for 30 min. Sections were washed three times with 1 × PBS buffer for 5 min. The excess 1 × PBS buffer was removed and sections were incubated with AB Complex/HRP for 30 min at 25°C and then washed three times with 1 × PBS buffer for 5 min. Sections were developed with 3,3’-diaminobenzidinetetrahydrochloride (DAB, 0.03–0.05% DAB in 0.05 mol/L Tris-HCl pH 7.6/0.01–0.03% H2O2) for 5–10 min. The slides were rinsed with water, counterstained with hematoxylin, dehydrated, and mounted. The integrated optical density of staining was measured by the Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
Enzyme-linked immunosorbent assay for reactive oxygen species, malondialdehyde, superoxide dismutase, and glutathione peroxidase
The ovarian tissues were homogenized with 19-fold volume of ice-cold 1 × PBS using a glass homogenizer at 4°C. After 10 min of centrifugation at 3000 r/min, the supernatant was obtained. The ROS, malondialdehyde (MDA), SOD, and GPx in the supernatant were determined with ELISA kits for mouse ROS, MDA, SOD, and GPx according to the manufacturer's protocols. The optical density was detected at 450 nm with a microplate reader (Anthos Labtec Instruments, Salzburg, Austria).
Western blotting for p66Shc
To determine the effect of different concentrations of curcumin on the p66Shc protein in ovarian tissues, the expression of p66Shc was measured by Western blotting. The ovary was fully homogenized with RIPA buffer (Sigma, St. Louis, MO, USA) at 4°C, and then centrifuged for 5 min at 10,000 r/min. Total proteins in the homogenates were quantified using the bicinchoninic acid protein assay kit (Fermentas, Thermo Fisher Scientific, Waltham, MA, USA). Total proteins in each sample were separated by SDS-PAGE on 12% gels and transferred onto nitrocellulose membranes (Pall Life Sciences, Ann Arbor, MI, USA). The membranes were blocked for 1 h at room temperature with 5% nonfat dry milk in TBS-T (50 mmol/L Tris-HCl, 150 mM NaCl, and 0.05% Tween-20). After washing three times with TBS-T, the membranes were incubated overnight at 4°C with the primary antibodies (P-Shc and α-tubulin (Biosynthesis Biotechnology Co., Ltd., China; diluted at 1:1000 with TBS-T). The membranes were washed three times with TBS-T. HRP-conjugated rabbit anti-goat antibody diluted at 1:5000 with TBS-T was added and incubated for 1 h at room temperature. Chemiluminescence and imaging were performed, and a snapshot was captured. The bands were scanned and quantified using the Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
Statistical analysis was carried out using SPSS version 17.0 (IBM, Armonk, NY, USA). Normally distributed measurement data with homogeneity of variance were expressed as mean ± standard deviation (SD). Comparisons among multiple subgroups were performed using one-way analysis of variance (ANOVA) with Scheffe's test for post hoc analysis. Statistical significance was determined as P < 0.05.
Results
Oxidative stress model in mouse and effects of curcumin on oxidation markers
During the experiment, the weight of the mice in the five groups all increased with no obvious difference among them [Table 1]. There was no significant difference in the ovarian indexes among the groups either [Table 1].
Table 1.
Comparison of the rate of weight gain and index of ovaries in the different groups (n = 10 per group)
| Items | Control group | Curcumin group | F | P | |||
|---|---|---|---|---|---|---|---|
| 0 mg/kg | 100 mg/kg | 150 mg/kg | 200 mg/kg | ||||
| Rate of weight gain (%) | 16.52 ± 9.62 | 11.75 ± 5.88 | 16.29 ± 4.86 | 15.10 ± 5.67 | 13.02 ± 4.19 | 9.62 | 0.614 |
| Index of ovaries (%) | 0.86 ± 0.19 | 0.85 ± 0.15 | 0.96 ± 0.22 | 0.95 ± 0.2 | 1.0 ± 0.24 | 7.12 | 0.521 |
All data were shown as mean ± SD. Comparisons among multiple subgroups were performed using one-way ANOVA. ANOVA: Analysis of variance; SD: Standard deviation.
As measured by ELISA [Table 2], after intraperitoneal injection of sodium As, the levels of ROS (P = 0.021) and MDA (P = 0.048) significantly increased while that of the SOD (P = 0.012) and GPx (P = 0.045) significantly reduced in the ovary, indicating successful modeling of ovarian oxidative stress. In comparison with the 0 mg/kg curcumin group, ROS and MDA in the ovary were significantly lower in the 100 mg/kg (P = 0.038, P = 0.019), 150 mg/kg (P = 0.031, P = 0.021), and 200 mg/kg (P = 0.035, P = 0.001) curcumin groups (all P < 0.05); and ovarian SOD levels were significantly higher in the 100 mg/kg (P = 0.024), 150 mg/kg (P = 0.015), and 200 mg/kg (P = 0.008) curcumin groups (all P < 0.05). However, there were no significant differences concerning the level of GPx when compared with the 0 mg/kg curcumin group. In general, the results suggested that curcumin could alleviate ovarian oxidative stress induced by arsenic.
Table 2.
Comparison of ovarian ROS, MDA, SOD, and GPx levels in different groups (n = 10 per group)
| Items | Control group | Curcumin group | F | P | |||
|---|---|---|---|---|---|---|---|
| 0 mg/kg | 100 mg/kg | 150 mg/kg | 200 mg/kg | ||||
| ROS (IU/mg) | 10.71 ± 0.91 | 11.74 ± 0.65* | 10.64 ± 1.38† | 10.73 ± 0.71† | 10.67 ± 1.38† | 2.655 | 0.045 |
| MDA (nmol/g) | 0.27 ± 0.02 | 0.32 ± 0.02* | 0.28 ± 0.02† | 0.25 ± 0.03† | 0.27 ± 0.04† | 1.254 | 0.032 |
| SOD (U/mg) | 4.51 ± 0.70 | 3.96 ± 0.36* | 4.57 ± 0.68† | 4.49 ± 0.27† | 4.56 ± 0.25† | 2.008 | 0.011 |
| GPx (U/g) | 18.92 ± 1.80 | 17.36 ± 1.63* | 17.98 ± 1.86 | 18.01 ± 1.20 | 18.45 ± 1.65 | 7.968 | 0.041 |
All data were shown as mean ± SD. Comparisons among multiple subgroups were performed using one-way ANOVA. *P<0.05 versus the control group; †P<0.05 versus the 0 mg/kg curcumin treatment group. ROS: Reactive oxygen species; MDA: Malondialdehyde; SOD: Superoxide dismutase; GPx: Glutathione peroxidase; ANOVA: Analysis of variance.
Effects of curcumin on ovary cell proliferation under oxidative stress
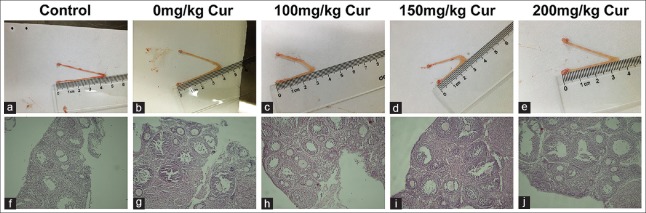
According to HE staining of slices of the right ovary, it was found that the ovarian tissue was destroyed and there were few structurally complete follicles and abundant atretic follicles in the 0 mg/kg curcumin group compared to the normal control [Figure 1; Table 3]. The number of atretic follicles was markedly reduced by curcumin treatment [all P < 0.05, Table 3].
Figure 1.
Representative images of H and E staining on slices of the right ovary in the control group and 0, 100, 150, or 200 mg/kg curcumin groups (n = 10/group) groups (original magnification ×10). a-e: Ovarian tissue to the naked eye in the five groups. f-j: Representative images of H and E staining on slices of the right ovary in the five groups. Cur: curcumin.
Table 3.
Comparison of the number of follicles per ovary in different groups (n = 10 per group)
| Items | Control group | Curcumin group | F | P | |||
|---|---|---|---|---|---|---|---|
| 0 mg/kg | 100 mg/kg | 150 mg/kg | 200 mg/kg | ||||
| Primordial follicles | 5.96 ± 0.97 | 6.11 ± 0.58 | 6.24 ± 0.23 | 6.57 ± 1.10 | 5.85 ± 0.55 | 9.67 | 0.842 |
| Primary follicles | 3.56 ± 0.35 | 3.78 ± 0.25 | 4.05 ± 0.38 | 3.25 ± 0.58 | 3.09 ± 0.37 | 8.32 | 0.912 |
| Secondary follicles | 4.71 ± 0.85 | 4.25 ± 0.88 | 3.99 ± 0.94 | 4.92 ± 0.94 | 4.23 ± 0.75 | 16.41 | 1.000 |
| Atretic follicles | 5.12 ± 0.84 | 6.34 ± 0.75* | 5.67 ± 0.81† | 5.84 ± 0.98† | 5.72 ± 0.84† | 12.31 | 0.026 |
All data were shown as mean ± SD. Comparisons among multiple subgroups were performed using one-way ANOVA. *P<0.05 versus the control group; †P<0.05 versus the 0 mg/kg curcumin treatment group. ANOVA: Analysis of variance; SD: Standard deviation.
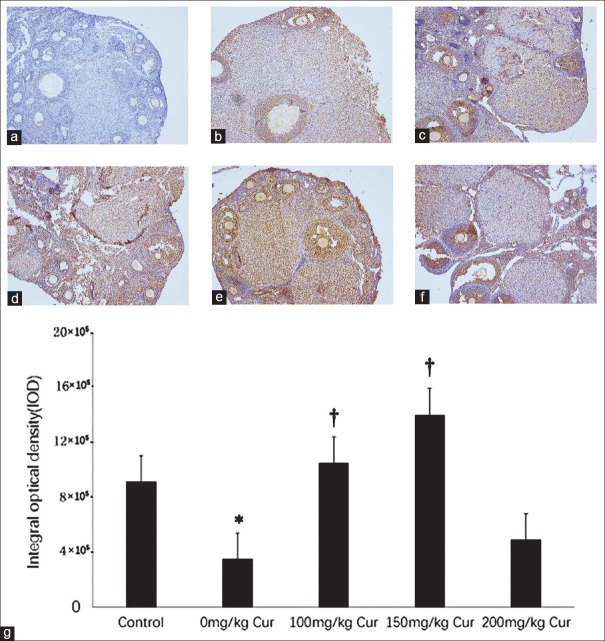
According to BrdU staining [Figure 2], the proliferation of granular cells in the 0 mg/kg curcumin group (3.51 ± 1.97 × 105) was obviously inhibited compared with normal control (9.16 ± 2.66 × 105) (P = 0.032). However, the number of granular cells in the 100 mg/kg (10.53 ± 3.52 × 105, P = 0.010) and 150 mg/kg (14.04 ± 3.55 × 105, P = 0.000) curcumin groups were significantly higher than the 0 mg/kg curcumin group.
Figure 2.
BrdU immunohistochemistry of cell proliferation (a-f) and the integrated optical density (g) in the five groups. The brown granules represent the proliferating granular cells. (a) Negative control (control group without anti-BrdU antibody); (b) control group; (c) 0 mg/kg curcumin; (d) 100 mg/kg curcumin; (e) 150 mg/kg curcumin; (f) 200 mg/kg curcumin (a-f, original magnification ×200). (g): integrated optical density in the five groups. n = 10 for each group; *P < 0.05 versus the control group; †P < 0.05 versus the 0 mg/kg curcumin treatment group.
Taken together, the results suggested that the increased amount of atretic follicles and the inhibited proliferation of granular cells under arsenic-induced oxidative stress would be reversed by curcumin.
Effects of curcumin on the expression of p66Shc in the ovary under oxidative stress
To elucidate the mechanism of curcumin on lipid peroxidation, the expression of p66Shc in ovaries was examined by Western blotting [Figure 3]. The expression of the p66Shc protein was obviously high in the 0 mg/kg group (0.28 ± 0.004) compared with the control group (0.23 ± 0.002) (P = 0.021), but lower in the 100 mg/kg (0.18 ± 0.001) (P = 0.017), 150 mg/kg (0.24 ± 0.007) (P = 0.049), and 200 mg/kg (0.20 ± 0.003) (P = 0.035) curcumin groups. There were no obvious differences between the curcumin groups and the control group (all P > 0.05). The results suggested that the p66Shc expression upregulated under oxidative stress would be lowered by curcumin.
Figure 3.
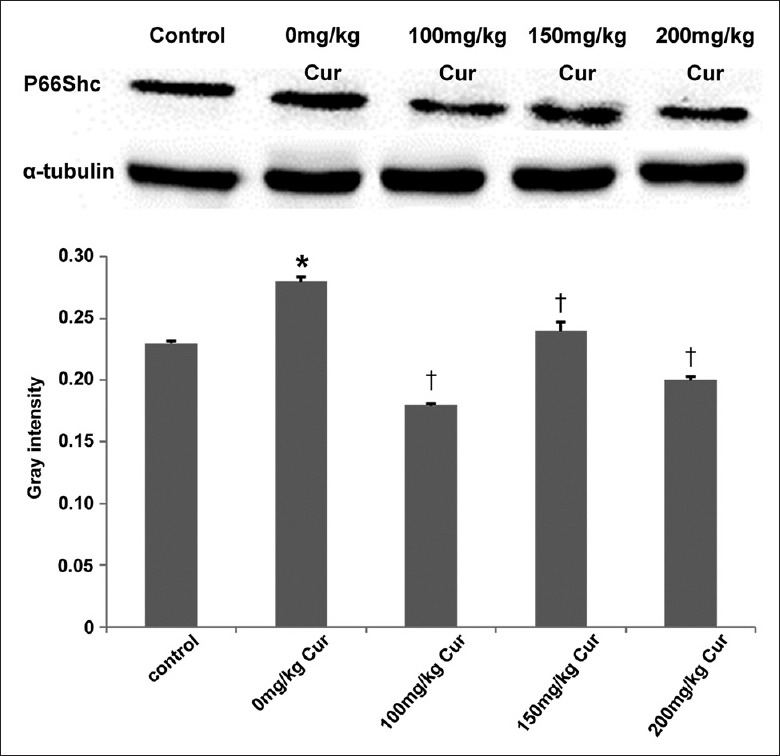
p66Shc expression in the five groups. n = 10 for each group; *P < 0.05 versus the control group; †P < 0.05 versus the 0 mg/kg curcumin treatment group. Cur: Curcumin.
Discussion
Oxidative stress causes damage to cellular components including proteins, lipids, and DNA.[17] In this study, one of the most commonly used chemicals, arsenic sodium, caused oxidative stress in the ovary by elevating the levels of ROS and MDA. Thus, exposure to arsenic sodium could lead to infertility and other adverse reproductive outcomes.
Nowadays, many drugs such as Vitamin C,[18] Vitamin E,[18] and NAC[19] have been found to be useful against oxidative stress-related diseases. However, there is no specific therapy for oxidative damage in the ovary, hence finding one that assures the safety of antioxidant active substance is of great importance. Curcumin, a plant polyphenolic compound widely used in the traditional Chinese medicine, shows great potential as a therapeutic agent. Recently, several pharmacological effects of curcumin including anti-carcinogen,[20] anti-angiogenesis,[21] and antioxidant effects[22] have been reported. In the present study, we selected curcumin as a therapeutic intervention for ovarian oxidative stress. After treatment with curcumin, we observed that all mice showed no significant differences in food consumption and normal daily life activities, which agree with the findings of a previous study.[23]
Many studies have demonstrated that oxidative stress is directly associated with the aging of ovarian follicles, endometriosis, unexplained female infertility, and low success rates in assisted reproductive techniques.[3] The biomarkers of oxidative stress damage are determined by the antioxidant system and the oxidative stress products. ROS play important roles in the folliculogenesis, oocyte maturation, luteal regression, and fertilization.[24] Lipid peroxide decomposes to produce a variety of substances such as MDA. As a result, MDA was selected as a biomarker of oxidative stress in addition to ROS in this study. Results showed that curcumin could reduce the concentration of ROS and MDA in the ovary. Cells have defense systems that prevent injury by ROS. These include the production of antioxidant enzymes such as SOD and GPx. SOD participates in the dismutation of superoxide to H2O2 while GPx converts H2O2 into H2O and O2. It has been reported that curcumin plays a key role in promoting the antioxidant effect in tissues such as liver, heart, and kidney.[11] In the present study, it was also found that curcumin could increase the levels of SOD and GPx in mouse ovaries. This indicates that curcumin could protect the ovaries from oxidative injuries by enhancing the concentration of antioxidant enzymes. In addition, from the pathology biopsy results, it was shown that curcumin could maintain the integrity of the cell morphology as evidenced in the curcumin-treated group compared with the 0 mg/kg curcumin group, in agreement with the results of the oxidation markers.
p66Shc, the 66Kd Shc protein, is an age-related adaptor protein that has a substantial impact on mitochondrial metabolism through regulation of cellular response to oxidative stress.[25] It was reported that high-level As induces severe redox imbalance by decreasing the levels of glutathione and increasing the levels of ROS through the oxidative stress adaptor p66Shc, which induces apoptosis by activating the cytochrome c-caspase.[26] In the present study, it was shown that arsenic promoted the expression of p66Shc, which decreased after treatment with curcumin. p66Shc is a ROS-generated enzyme. It can clear mitochondrial membrane, react with the cytochrome C to produce H2O2, and open mitochondrial permeability transition pore to release ROS to the cytoplasm. When the expression of p66Shc is upregulated, the generation of ROS increases and the oxidative damage to cells becomes severe,[7] meaning that the concentration of ROS correlates positively with the expression of p66Shc. We postulated that arsenic plays its offensive role through the p66Shc/ROS pathway, and curcumin could influence this pathway to play a protective role. However, this requires further verification.
In addition, we also examined the influence of curcumin on the proliferation of ovarian cells and found that the proliferating cells were mainly granular cells. These cells provide nutrition and support for ovarian follicles’ growth and development.[8] We found that the number of proliferating granular cells in the curcumin-treated groups was significantly higher than in the 0 mg/kg curcumin group. Cell proliferation is one of the most important indicators that can reflect the severity of cell damage, as well as offer protection to oxidative injuries. The high proliferating granular cells in the curcumin-treated group in this study show that curcumin can protect the ovaries from oxidative injuries. This protective role is played by direct reduction of the oxidative product and elevation of the antioxidant enzyme, which reduces damage to ovarian structure and function and promotes the proliferation of granular cells.
Of course, the present study is not without limitations. Animal models are never perfect. Arsenic is a harmful element that very probably induces systemic effects other than ovarian oxidative stress, and these systemic effects could participate in metabolic imbalances that could bias our results. In addition, only a few proteins were examined, and the mRNA expression levels were not assessed. Additional studies using a wider panel of markers should provide more insights into the mechanisms responsible for arsenic toxicity to the ovaries as well as the mechanisms for the protective effects of curcumin.
In conclusion, curcumin can scavenge free radicals and improve the antioxidant state in mouse ovary. Then, it plays a key role in the generation of ROS through the regulation of p66Shc. Moreover, curcumin could promote the proliferation of granular cells in mouse ovaries. Thus, curcumin, a well-established, safe, and nontoxic traditional Chinese medicine, might have a potential application in the treatment of ovarian oxidative stress.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Min Chen
References
- 1.Al-Gubory KH, Fowler PA, Garrel C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol. 2010;42:1634–50. doi: 10.1016/j.biocel.2010.06.001. doi: 10.1016/j.biocel.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Bernal AB, Vickers MH, Hampton MB, Poynton RA, Sloboda DM. Maternal undernutrition significantly impacts ovarian follicle number and increases ovarian oxidative stress in adult rat offspring. PLoS One. 2010;5:e15558. doi: 10.1371/journal.pone.0015558. doi: 10.1371/journal.pone.0015558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: A review. Reprod Biol Endocrinol. 2012;10:49. doi: 10.1186/1477-7827-10-49. doi: 10.1186/1477-7827-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamura H, Takasaki A, Taketani T, Tanabe M, Kizuka F, Lee L, et al. Melatonin as a free radical scavenger in the ovarian follicle. Endocr J. 2013;60:1–13. doi: 10.1507/endocrj.ej12-0263. doi: 10.1507/endocrj.EJ12-0263. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;3:28. doi: 10.1186/1477-7827-3-28. doi: 10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Migliaccio E, Mele S, Salcini AE, Pelicci G, Lai KM, Superti-Furga G, et al. Opposite effects of the p52shc/p46shc and p66shc splicing isoforms on the EGF receptor-MAP kinase-fos signalling pathway. EMBO J. 1997;16:706–16. doi: 10.1093/emboj/16.4.706. doi: 10.1093/emboj/16.4.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Betts DH, Bain NT, Madan P. The p66(Shc) adaptor protein controls oxidative stress response in early bovine embryos. PLoS One. 2014;9:e86978. doi: 10.1371/journal.pone.0086978. doi: 10.1371/journal.pone.0086978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Z, Wells D. The human oocyte and cumulus cells relationship: New insights from the cumulus cell transcriptome. Mol Hum Reprod. 2010;16:715–25. doi: 10.1093/molehr/gaq031. doi: 10.1093/molehr/gaq031. [DOI] [PubMed] [Google Scholar]
- 9.Chattopadhyay S, Ghosh S, Debnath J, Ghosh D. Protection of sodium arsenite-induced ovarian toxicity by coadministration of L-ascorbate (Vitamin C) in mature Wistar strain rat. Arch Environ Contam Toxicol. 2001;41:83–9. doi: 10.1007/s002440010223. doi: 10.1007/s002440010223. [DOI] [PubMed] [Google Scholar]
- 10.Reichard JF, Puga A. Effects of arsenic exposure on DNA methylation and epigenetic gene regulation. Epigenomics. 2010;2:87–104. doi: 10.2217/epi.09.45. doi: 10.2217/epi.09.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta SC, Patchva S, Koh W, Aggarwal BB. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol. 2012;39:283–99. doi: 10.1111/j.1440-1681.2011.05648.x. doi: 10.1111/j.1440-1681.2011.05648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem Pharmacol. 2008;76:1590–611. doi: 10.1016/j.bcp.2008.08.008. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10:6847–54. doi: 10.1158/1078-0432.CCR-04-0744. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Zhang C, Zhang Y, Ru X, Gong Q. Establishment of a mouse model of ovarian oxidative stress (in Chinese) J South Med Univ. 2012;32:1643–5. doi: 10.3969/j.issn.1673-4254.2012.11.027. [PubMed] [Google Scholar]
- 15.Wang XN, Roy SK, Greenwald GS. In vitro DNA synthesis by isolated preantral to preovulatory follicles from the cyclic mouse. Biol Reprod. 1991;44:857–63. doi: 10.1095/biolreprod44.5.857. doi: 10.1095/biolreprod44.5.857. [DOI] [PubMed] [Google Scholar]
- 16.Borgeest C, Symonds D, Mayer LP, Hoyer PB, Flaws JA. Methoxychlor may cause ovarian follicular atresia and proliferation of the ovarian epithelium in the mouse. Toxicol Sci. 2002;68:473–8. doi: 10.1093/toxsci/68.2.473. doi: 10.1093/toxsci/68.2.47. [DOI] [PubMed] [Google Scholar]
- 17.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Jhala DD, Chinoy NJ, Rao MV. Mitigating effects of some antidotes on fluoride and arsenic induced free radical toxicity in mice ovary. Food Chem Toxicol. 2008;46:1138–42. doi: 10.1016/j.fct.2007.11.009. doi: 10.1016/j.fct.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Wang W, Craig ZR, Basavarajappa MS, Hafner KS, Flaws JA. Mono-(2-ethylhexyl) phthalate induces oxidative stress and inhibits growth of mouse ovarian antral follicles. Biol Reprod. 2012;87:152. doi: 10.1095/biolreprod.112.102467. doi: 10.1095/biolreprod.112.102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh M, Singh N. Curcumin counteracts the proliferative effect of estradiol and induces apoptosis in cervical cancer cells. Mol Cell Biochem. 2011;347:1–11. doi: 10.1007/s11010-010-0606-3. doi: 10.1007/s11010-010-0606-3. [DOI] [PubMed] [Google Scholar]
- 21.Yoysungnoen P, Wirachwong P, Changtam C, Suksamrarn A, Patumraj S. Anti-cancer and anti-angiogenic effects of curcumin and tetrahydrocurcumin on implanted hepatocellular carcinoma in nude mice. World J Gastroenterol. 2008;14:2003–9. doi: 10.3748/wjg.14.2003. doi: 10.3748/wjg.14.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang D, Li T, Liu Z, Arbez N, Yan J, Moran TH, et al. LRRK2 kinase activity mediates toxic interactions between genetic mutation and oxidative stress in a Drosophila model: Suppression by curcumin. Neurobiol Dis. 2012;47:385–92. doi: 10.1016/j.nbd.2012.05.020. doi: 10.1016/j.nbd.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 23.Carroll RE, Benya RV, Turgeon DK, Vareed S, Neuman M, Rodriguez L, et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev Res (Phila) 2011;4:354–64. doi: 10.1158/1940-6207.CAPR-10-0098. doi: 10.1158/1940-6207.capr-10-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agarwal A, Gupta S, Sekhon L, Shah R. Redox considerations in female reproductive function and assisted reproduction: From molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10:1375–403. doi: 10.1089/ars.2007.1964. doi: 10.1089/ars.2007.1964. [DOI] [PubMed] [Google Scholar]
- 25.Wu L, Sun Y, Hu YJ, Yang Y, Yao LL, Zhou XX, et al. Increased p66Shc in the inner ear of D-galactose-induced aging mice with accumulation of mitochondrial DNA 3873-bp deletion: p66Shc and mtDNA damage in the inner ear during aging. PLoS One. 2012;7:e50483. doi: 10.1371/journal.pone.0050483. doi: 10.1371/journal.pone.0050483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang C, Liu C, Li D, Yao N, Yuan X, Yu A, et al. Intracellular redox imbalance and extracellular amino acid metabolic abnormality contribute to arsenic-induced developmental retardation in mouse preimplantation embryos. J Cell Physiol. 2010;222:444–55. doi: 10.1002/jcp.21966. doi: 10.1002/jcp.21966. [DOI] [PubMed] [Google Scholar]