Abstract
Background:
X-linked Charcot-Marie-Tooth type 1 (CMT1X) disease is one of the most common forms of inherited neuropathy caused by mutations in the gap junction beta-1 protein (GJB1) gene (also known as connexin 32). This study presented the clinical and genetic features of a series of Chinese patients with GJB1 gene mutations.
Methods:
A total of 22 patients from unrelated families, who were referred to Department of Neurology, Peking University First Hospital from January 2005 to January 2016, were identified with GJB1 mutations. Their clinical records and laboratory findings were retrospectively collected and reviewed. Mutations in the GJB1 gene were analyzed by targeted next-generation sequencing (NGS). Nucleotide alternations were confirmed with Sanger sequencing.
Results:
The CMT1X patients predominantly showed distal muscle weakness of lower limbs with mild sensory disturbance. The mean age of onset was 15.6 ± 8.7 years (ranging from 1 year to 42 years). The sudden onset of cerebral symptoms appeared in four patients (18.2%); two were initial symptoms. One case had constant central nervous system (CNS) signs. There were 19 different heterozygous mutations, including 15 known mutations and four novel mutations (c.115G>T, c.380T>A, c.263C>A, and c.818_819insGGGCT). Among the 22 Chinese patients with CMT1X, the frequency of the GJB1 mutation was 4.5% in transmembrane domain 1 (TM1), 4.5% in TM2, 22.7% in TM3, 9.1% in TM4, 4.5% in extracellular 1 (EC1), 27.3% in EC2, 9.1% in intracellular loop, 13.6% in the N-terminal domain, and 4.5% in the C-terminal domain. CMT1X with CNS impairment appeared in five (22.7%) of these patients.
Conclusions:
This study indicated that CNS impairment was not rare in Chinese CMT1X patients. Mutations in the EC2 domain of the GJB1 gene were hotspot in Chinese CMT1X patients.
Keywords: Connexin 32, Gap Junction Beta-1 Protein, Neuropathy, X-linked Charcot-Marie-Tooth Type 1
Introduction
X-linked Charcot-Marie-Tooth type 1 (CMT1X) disease is a hereditary chronic progressive disease, which is caused by mutations in the gap junction beta-1 protein (GJB1) gene encoding the gap junction protein connexin 32 (Cx32). Clinically, the disease is characterized by the chronic progressive wasting and weakness of distal muscles in the lower limbs with sensory disturbance usually from the second decade. Some patients experience sensorineural hearing loss and central nervous system (CNS) involvement.[1,2,3,4] Nerve conduction velocity test shows a moderate neuropathy, and sural biopsy usually shows axonal or mixed axonal-demyelinating neuropathy pathology.[5,6] CMT1X has been described in Chinese patients since 2001.[3,7,8,9,10,11,12,13,14,15,16,17,18,19] Most of these were case reports,[8,14,17,19,20] summarized electrophysiological findings,[2,10,12,13,14,18] or pathological features in a few cases.[18,19] Here, we reported the clinical features and genetic mutations of GJB1 gene in a large Chinese patient cohort.
Methods
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki and was approved by the local Ethics Committee of Peking University First Hospital. Informed written consent was obtained from all patients prior to their enrollment in this study.
Patients
Ninety-two patients with CMT from unrelated families were diagnosed by next-generation sequencing (NGS) at the Department of Neurology, Peking University First Hospital from January 2005 to January 2016. Among them, a total of 22 patients with GJB1 mutations were recruited in this study, which comprised 23.9% of the total CMT patients. As for the other CMT genes, the mutation frequency was 35.9% in peripheral myelin protein 22 gene, 17.4% in mitofusin 2 gene, 2.2% in myelin protein zero gene, 5.4% in inverted formin, FH2 and WH2 domain containing (INF2) gene, 4.3% in ganglioside-induced differentiation associated protein 1 gene, 2.2% in neurofilament light gene, 2.2% in periaxin gene, 1.1% in SH3 domain and tetratricopeptide repeats 2 gene, 1.1% in early growth response 2 gene, 1.1% in glycyl-tRNA synthetase gene, 1.1% in leucine rich repeat and sterile alpha motif containing 1 gene, 1.1% in FIG4 phosphoinositide 5-phosphatase (FIG4) gene, 1.1% in alanyl-tRNA synthetase gene, and 1.1% in dehydrogenase E1 and transketolase domain containing 1 gene.
The clinical records and electrophysiological characteristics of these 22 CMT1X patients with GJB1 mutations were retrospectively collected and reviewed. Muscle weakness was evaluated using the Medical Research Council score. All patients were interviewed and examined by two neurologists.
Mutation analysis
Genomic DNA was extracted from the peripheral blood samples of all patients. Mutations in the GJB1 gene were analyzed by targeted NGS. NGS panel covered all of the exons and their flanking sequences of genes known to be associated with hereditary neuropathies (gene list available on request). The exons and their flanking splice sites were captured and subsequently sequenced on an Illumina HiSeq 2500 Sequencer (Illumina, San Diego, CA, USA). The sequencing files were mapped to reference sequences with Burrows-Wheeler Aligner and Picard tools and then called with control samples with the GATK 3.0 HaplotypeCaller (Broad Institute, USA). Nucleotide alternations were confirmed with Sanger sequencing. The segregation analyses of the mutations were confirmed in the parents and the affected family members. For the novel mutations, 1000 healthy controls of Chinese origin were screened. The biological relevance of the novel amino acid changes was studied using both PolyPhen-2 (http://www.genetics.bwh.harvard.edu/pph2/) and Mutation Taster (http://www.mutationtaster.org/) programs.
Results
Patients’ clinical and electrophysiological characteristics
These 22 patients came from unrelated families, including 20 males and 2 females. The clinical and electrophysiological characteristics of these CMT1X patients are shown in Table 1. The mean age of onset was 15.6 ± 8.7 years (ranging from 1 year to 42 years). Fifteen (68.2%) patients exhibited first symptoms in their second decade. Five (22.7%) of them presented symptoms after 20 years old. Two patients (9.1%) had an earlier onset before 10 years old, including one from infancy. The mean duration from onset to diagnostic time was 8.2 ± 4.5 years (ranging from 2 years to 18 years).
Table 1.
The clinical and electrophysiological characteristics of 22 Chinese CMT1X patients in this study
| Patient number | Age (years) | Gender | Onset age (years) | Muscle strength in distal UL | Muscle strength in distal LL | Sensory loss | CNS lesions | CMAP in median nerves (mV) | MCV in median nerves (m/s) | SNAP in median nerves (mV) | SCV in median nerves (m/s) | CMAP in ulnar nerves (mV) | MCV in ulnar nerves (m/s) | SNAP in ulnar nerves (mV) | SCV in ulnar nerves (m/s) | CMAP in tibial nerves (mV) | MCV in tibial nerves (m/s) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 34 | Male | 21 | IV | II | − | − | 2.7 | 35.5 | NE | NE | NA | NA | NA | NA | NA | NA |
| 2 | 14 | Male | 11 | IV | IV | − | + | 2.5 | 36.0 | 12.1 | 41.0 | 1.8 | 31 | 1.8 | 34.0 | NA | NA |
| 3 | 23 | Male | 8 | V– | I–II | − | + | NE | NE | NE | NE | 4.7 | 33.1 | NE | NE | NE | NE |
| 4 | 13 | Male | 10 | III | I–II | − | + | 0.8 | 28.9 | NE | NE | 10.3 | 27.7 | NE | NE | 3.5 | 38.4 |
| 5 | 20 | Female | 15 | IV | 0–II | − | − | 2.1 | 38.0 | NA | NA | 0.7 | 46.7 | NA | NA | 0.3 | 38.4 |
| 6 | 24 | Male | 14 | V | V– | − | − | 2.7 | 43.9 | 8.7 | 43.0 | 4.8 | 33.1 | 4.6 | 37.6 | 1.0 | NA |
| 7 | 17 | Male | 14 | V | IV | − | − | 5.2 | 34.5 | 3.6 | 40.6 | NA | NA | 2.9 | 35.3 | 0.2 | 32.4 |
| 8 | 18 | Female | 12 | V– | IV | − | − | 3.0 | 35.0 | 1.87 | 38.6 | 4.4 | 32 | −1.47 | 35.8 | 0.1 | 27.7 |
| 9 | 37 | Male | 24 | V– | I–II | − | + | 0.3 | 27.3 | 2.3 | 32.1 | 1.9 | 30.9 | 2.0 | 30.3 | 0.1 | 29.2 |
| 10 | 19 | Male | 10 | V– | IV | UL, LL | − | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 11 | 50 | Male | 32 | IV | II | UL, LL | − | 2.3 | 38.9 | NE | NE | NA | NA | NE | NE | NE | NE |
| 12 | 34 | Male | 24 | V– | IV– | LL | − | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 13 | 15 | Male | 10 | V | 0–V– | − | + | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 14 | 24 | Male | 14 | V | 0–V– | LL | − | 4.7 | 38.0 | 8.7 | 66.0 | 1.5 | 32 | 9.6 | 71.0 | 0.6 | 32.0 |
| 15 | 13 | Male | 11 | V | IV | UL, LL | − | NA | 28.0 | NA | NA | NA | NA | NA | NA | 4.8 | 32.4 |
| 16 | 44 | Male | 42 | V | IV | − | − | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 17 | 26 | Male | 18 | V | I | LL | − | 3.4 | 39.8 | NE | NE | 3.2 | 41.7 | 5.7 | 37.6 | NE | NE |
| 18 | 27 | Male | 15 | IV | II | UL, LL | − | 0.2 | NA | 1.4 | 42.0 | 0.3 | NA | 1.4 | 41.7 | 0.2 | NA |
| 19 | 19 | Male | 12 | IV | I–II | UL, LL | − | 0.8 | 28.9 | NE | NE | 9.1 | 27.7 | NE | NE | NA | NA |
| 20 | 21 | Male | 11 | V | I–IV | − | − | 1.8 | 33.9 | 1.4 | 36.4 | 1.8 | 34 | NE | NE | 0.0 | 23.0 |
| 21 | 19 | Male | 14 | V | 0–IV | − | − | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 22 | 12 | Male | 1 | IV | IV | − | − | NA | 37.9 | NA | 38.2 | NA | NA | NA | NA | NA | 39.2 |
CMT1X: X-linked Charcot-Marie-Tooth type 1; LL: Low limbs; UL: Upper limbs; +: Positive; −: Negative; CNS: Central nervous system; MCV: Motor conduction velocity; CMAP: Compound motor action potential; SCV: Sensory conduction velocity; SNAP, Sensory nerve action potential; NE: Not elicited; NA: Not available.
All patients showed distal muscle wasting and weakness of the lower limbs, with the involvement of the upper limbs in 13 cases. Muscle strength ranged from III to V in distal upper limbs and from 0 to V in distal lower limbs. Sensory loss in distal limbs appeared in eight cases. Pes cavus occurred in 16 cases. CNS involvement appeared in five patients (22.7%), including four cases with transient CNS symptoms and one case with constant CNS signs. Four cases (18.2%) with transient symptoms presented with sudden onset of cerebral symptoms including aphasia, dysphagia, quadriplegia, or paralysis induced by fever or infection. The symptoms usually lasted from hours to days, and the patients completely recovered without special treatment. CNS symptoms in two patients appeared initially before the development of peripheral neuropathy. One case with constant CNS involvement additionally presented with nystagmus and ataxia on physical examination.
In median nerves, the mean motor nerve conduction velocity (MCV) was 35.0 ± 4.8 m/s (range: 27.3–43.9 m/s), the mean amplitude of compound muscle action potential (CAMP) was 2.3 ± 1.5 mV (range: 0.2–5.2 mV), the mean sensory nerve conduction velocity (SCV) was 42.0 ± 9.6 m/s (range: 32.1–66.0 m/s), and the mean sensory nerve action potential (SNAP) amplitude was 5.0 ± 4.2 mV (range: 1.4–12.1 mV). In ulnar nerves, the mean MCV was 33.6 ± 5.7 m/s (range: 27.7–46.7 m/s), the mean CAMP amplitude was 3.7 ± 3.2 mV (range: 0.3–10.3 mV), the mean SCV was 40.4 ± 12.8 m/s (range: 30.3–71.0 m/s), and the SNAP mean amplitude was 3.3 ± 3.3 mV (ranging from −1.47 mV to 9.6 mV). In tibial nerves, the mean MCV was 32.5 ± 5.5 m/s (range: 23.0–39.2 m/s) and the mean CAMP amplitude was 1.1 ± 1.7 mV (range: 0.0–4.8 mV).
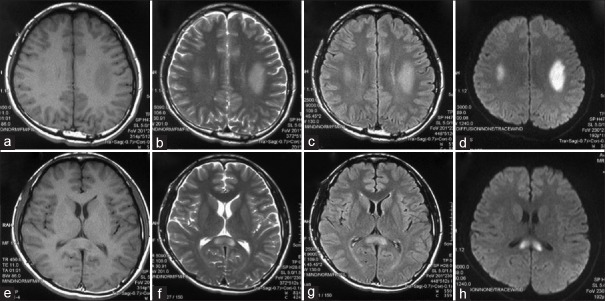
The cranial magnetic resonance imagings (MRIs) of patient 2 revealed abnormal signals in the splenium and genu of the corpus callosum in the first episode and in the bilateral posterior limbs of the internal capsule and splenium of the corpus callosum in the second episode. The cranial MRIs of patient 3 revealed abnormal signals in the centrum semiovale bilaterally in the first episode, the signals enlarged at the same location in the second episode, and widened at the third episode; the abnormal signals were resolved at intervals. The cranial MRIs of patient 4 revealed abnormal signals in the posterior limb of the internal capsule and the periventricular area bilaterally. The MRIs of patient 13 revealed abnormal signals in the bilateral centrum semiovale and splenium of the corpus callosum [Figure 1].
Figure 1.
Brain magnetic resonance imagings of a male patient with X-linked Charcot-Marie-Tooth type 1 (patient 13) revealed abnormal signals in the bilateral centrum semiovale (a–d) and splenium of the corpus callosum (e–h). (a and e): T1 weighted; (b and f): T2 weighted; (c and d): Fluid attenuated inversion recovery; and (d and h): Diffusion-weighted magnetic resonance imaging.
Gap junction beta-1 protein mutations
This study identified 19 different heterozygous mutations in these 22 CMT1X patients [Table 2]. The c.44G>T, c.59T>G, c.62G>A, c.194A>G, c.379A>T, C.403_404insT, c.424C>T, c.425G>A, c.490C>T, c.533A>G, c.548G>A, c.547C>T, c.556G>A, c.590C>T, and c.614A>G mutations were reported previously. Four (c.115G>T, c.263C>A, c.380T>A, and c.818_819insGGGCT) were novel mutations. Patient 22 with a c.818_819insGGGCT mutation had an X-linked family history. Five family members had peripheral neuropathy. Patients with c.115G>T, c.380T>A, and c.263C>A mutations were sporadic, and the mutation was not found in their parents. All novel mutations were not found in 1000 healthy controls and also not found in NCBI SNP database, indicating that they were not benign polymorphisms. The novel missense mutations (c.115G>T, c.380T>A, and c.263C>A) were highly conserved in the Cx32 proteins across all mammalian species. The pathogenicities of the novel missense mutations were predicted to be possibly damaging (c.380T>A) and probably damaging (c.115G>T and c.263C>A) by Polyphen-2 and disease causing by Mutation Taster software. Patient 12 with pure peripheral neuropathy and patient 13 with CNS impairment had the same c.425G>A mutations.
Table 2.
GJB1 mutations in 22 CMT1X patients from unrelated families in this study
| Patient number | Nucleotide changes | Amino acid changes | Domain | Novel mutation |
|---|---|---|---|---|
| 1 | c.44G>T | R15L | N-terminal domain | No |
| 2 | c.59T>G | I20T | N-terminal domain | No |
| 3 | c.62G>A | G21D | N-terminal domain | No |
| 4 | c.115G>T | A39S | TM1 | Yes |
| 5 | c.194A>G | Y65C | EC1 | No |
| 6 | c.263C>A | A88D | TM2 | Yes |
| 7 | c.379A>T | I127F | IC | Yes |
| 8 | c.380T>A | I127N | IC | No |
| 9 | C.403_404insT | Y135fsX146 | TM3 | No |
| 10 | c.424C>T | R142W | TM3 | No |
| 11 | c.424C>T | R142W | TM3 | No |
| 12 | c.425G>A | R142Q | TM3 | No |
| 13 | c.425G>A | R142Q | TM3 | No |
| 14 | c.490C>T | R164W | EC2 | No |
| 15 | c.533A>G | D178G | EC2 | No |
| 16 | c.548G>A | R183H | EC2 | No |
| 17 | c.548G>A | R183H | EC2 | No |
| 18 | c.547C>T | R183C | EC2 | No |
| 19 | c.556G>A | E186K | EC2 | No |
| 20 | c.590C>T | A197V | TM4 | No |
| 21 | c.614A>G | N205S | TM4 | No |
| 22 | c.818_819insGGGCT | L273fs | C-terminal domain | Yes |
CMT1X: X-linked Charcot-Marie-Tooth type 1; EC: Extracellular domain; TM: Transmembrane domain; IC: Intracellular loop; GJB1: Gap junction beta-1 protein.
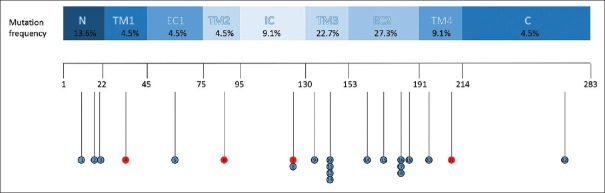
Among 22 patients, the frequency of the GJB1 mutations was 4.5% in transmembrane domain 1 (TM1), 4.5% in TM2, 22.7% in TM3, 9.1% in TM4, 4.5% in extracellular 1 (EC1), 27.3% in EC2, 9.1% in intracellular loop, 13.6% in the N-terminal domain, and 4.5% in the C-terminal domain [Figure 2].
Figure 2.
Distribution of the respective amino acid changes on the connexin 32 protein structure identified in 22 patients with X-linked Charcot-Marie-Tooth type 1. Different domains are indicated by rectangles with different colors. The positions of novel mutations are indicated in red.
Discussion
This study confirmed that CMT1X was a common form of inherited neuropathy in Chinese patients with CMT because 23.9% of all 92 CMT families were CMT1X. This study observed that the proportion of CMT1X in Chinese population was greater than other reports. The proportion of CMT1X patients for all CMTs was 10.7% in Europe (n = 997),[21] 15.2% in the USA (n = 527),[22] 12.0% in Australia (n = 224),[23] and 10.9% in Japan (n = 128).[24] In a study of multi-ethnic Malaysian patients with CMTs (n = 25), CMT1X patients were all Chinese and accounted for 24% of the total CMT patients.[25]
In this study, all CMT1X patients showed distal muscle weakness predominantly in the lower limbs with a mean onset age of 15.6 ± 8.7 years, similar to other reports.[22] Most patients began presenting their symptoms in their second or third decade. Patients with an earlier onset before 10 years old or in infancy were rare in the present study and other reports.[22,26,27] Electrophysiological findings of our patients further confirmed that CMT1X is an intermediate neuropathy.
Several types of hereditary neuropathies were associated with other organ involvement, such as distal motor neuropathy with optic atrophy,[28] hereditary transthyretin amyloidosis with cardiomyopathy,[29] and dominant inherited intermediate CMT with focal segmental glomerulosclerosis.[30] This study confirmed that CNS involvement was common in Chinese CMT1X. Dysarthria and hemiparesis were the main symptoms of our patients, and other similar symptoms have been occasionally reported in other countries.[31,32,33,34,35,36] CNS symptoms developed after peripheral neuropathy symptoms in some patients in the present study, which has also been reported in previous studies.[32,36] Brain MRIs showed abnormal transient lesions involving bilateral white matter in all patients. However, the involvement of the corpus callosum which was frequently reported did not appear in all cases in the present study.[34,37] The isolated involvement of the centrum semiovale, internal capsule, and the periventricular area appeared in the present study as well as in other reports.[38,39]
We found that the EC2 domain of Cx32 protein was more affected in our patients. The EC2 domain was a hotspot mutation domain and was affected in 44% of Korean patients,[40] which was more frequent compared to our patients. Mutations in the EC1 and EC2 domains of Cx32 were in 65% of the patients with Spanish or Portuguese descent.[41] However, EC1 and EC2 were not hotspot mutation domains in Japanese[24] and Malaysian[25] patients. We found no relationship between the position of mutations and CNS involvement in CMT1X. In the present series, mutations of five patients with CNS involvement were located in the N-terminal, TM1, and TM3 domains of GJB1 gene. The c.425G>A mutation was found in patients with pure peripheral neuropathy as well as in patients with additional CNS involvement. Four novel mutations in GJB1 gene were found in our cohort, which expands the spectrum of mutations in CMT1X.
In summary, CNS impairment was not rare in Chinese CMT1X patients. The CMT1X diagnosis should be considered in children with transient CNS impairment with or without any signs of peripheral neuropathy. Mutations in the EC2 domain of the GJB1 gene were hotspot in Chinese CMT1X patients.
Financial support and sponsorship
This study was supported by a grant from the National Science Foundation of China (No. 81471185).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank the participating patients and their families for their cooperation. We also thank Ms. Jing Liu, Ms. Yue-Huan Zuo and Ms. Qiu-Rong Zhang for their technical assistance in the preparation of nerve biopsies.
Footnotes
Edited by: Xin Chen
References
- 1.Shu XM, Tian MQ, Li J, Peng LY, Yu XH. X-linked hereditary motor sensory neuropathy type 1 (CMTX1) in a three-generation Gelao Chinese family. Neuropediatrics. 2015;46:424–7. doi: 10.1055/s-0035-1564619. doi: 10.1055/s-0035-1564619. [DOI] [PubMed] [Google Scholar]
- 2.Luan XH, Chen B, Zheng RL, Zhang W, Wang ZX, Yuan Y. Transient white matter lesions in X-linked Charcot-Marie-Tooth disease type 1 with novel I20T mutation of gap junction protein beta 1 gene (in Chinese) Chin J Neurol. 2009;42:241–4. [Google Scholar]
- 3.Xie C, Zhou X, Zhu D, Liu W, Wang X, Yang H, et al. CNS involvement in CMTX1 caused by a novel connexin 32 mutation: A 6-year follow-up in neuroimaging and nerve conduction. Neurol Sci. 2016;37:1063–70. doi: 10.1007/s10072-016-2537-6. doi: 10.1007/s10072-016-2537-6. [DOI] [PubMed] [Google Scholar]
- 4.Sargiannidou I, Kim GH, Kyriakoudi S, Eun BL, Kleopa KA. A start codon CMT1X mutation associated with transient encephalomyelitis causes complete loss of Cx32. Neurogenetics. 2015;16:193–200. doi: 10.1007/s10048-015-0442-4. doi: 10.1007/s10048-015-0442-4. [DOI] [PubMed] [Google Scholar]
- 5.Hattori N, Yamamoto M, Yoshihara T, Koike H, Nakagawa M, Yoshikawa H, et al. Demyelinating and axonal features of Charcot-Marie-Tooth disease with mutations of myelin-related proteins (PMP22, MPZ and Cx32): A clinicopathological study of 205 Japanese patients. Brain. 2003;126:134–51. doi: 10.1093/brain/awg012. [DOI] [PubMed] [Google Scholar]
- 6.Hahn AF, Ainsworth PJ, Bolton CF, Bilbao JM, Vallat JM. Pathological findings in the X-linked form of Charcot-Marie-Tooth disease: A morphometric and ultrastructural analysis. Acta Neuropathol. 2001;101:129–39. doi: 10.1007/s004010000275. [DOI] [PubMed] [Google Scholar]
- 7.Luo W, Tang B, Xiao J. X-linked recessive Charcot-Marie-Tooth disease and Cx32 gene mutation (in Chinese) Chin J Inter Med. 2001;40:543–5. [PubMed] [Google Scholar]
- 8.Da YW, Jia JP, Yang JF, Don XM. Clinical, electrophysiological and connexin 32 gene mutation analysis with X-linked dominant Charcot-Marie Tooth disease. Chin J Nerv Ment Dis. 2005;31:435–7. [Google Scholar]
- 9.Song SJ, Song SJ, Yan M, Wang XZ, Zhang YZ, Zou JH, et al. The same mutation Glu208Lys in the GJB1 gene was detected in 2 families with X-linked Charcot-Marie-Tooth disease (in Chinese) Hereditas. 2007;29:800–4. doi: 10.1360/yc-007-0800. doi: 10.1360/yc-007-0800. [DOI] [PubMed] [Google Scholar]
- 10.Lin P, Mao F, Liu Q, Yang W, Shao C, Yan C, et al. A novel deletion mutation in GJB1 causes X-linked Charcot-Marie-Tooth disease in a Han Chinese family. Muscle Nerve. 2010;42:922–6. doi: 10.1002/mus.21790. doi: 10.1002/mus.21790. [DOI] [PubMed] [Google Scholar]
- 11.Li Q, Chen M, Liu K, Lin X, Chui D. Vocal cord paresis and probable X-linked Charcot-Marie-Tooth disease with novel GJB1 mutation. Int J Neurosci. 2010;120:731–4. doi: 10.3109/00207454.2010.513461. doi: 10.3109/00207454.2010.513461. [DOI] [PubMed] [Google Scholar]
- 12.Feng Y, Li L, Li X, Wang G, Li J, Liu Q. Clinical and genetic analysis of a Chinese family affected with X-linked Charcot-Marie-Tooth disease (in Chinese) Chin J Med Genet. 2013;30:659–61. doi: 10.3760/cma.j.issn.1003-9406.2013.06.005. doi: 10.3760/cma.j.issn.1003-9406.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Xiao F, Tan JZ, Zhang X, Wang XF. A novel mutation in GJB1 (c.212T>G) in a Chinese family with X-linked Charcot-Marie-Tooth disease. J Clin Neurosci. 2015;22:513–8. doi: 10.1016/j.jocn.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 14.Wang R, He J, Li JJ, Ni W, Wu ZY, Chen WJ, et al. Clinical and genetic spectra in a series of Chinese patients with Charcot-Marie-Tooth disease. Clin Chim Acta. 2015;451:263–70. doi: 10.1016/j.cca.2015.10.007. doi: 10.1016/j.jocn.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 15.Li LX, Zhao SY, Liu ZJ, Ni W, Li HF, Xiao BG, et al. Improving molecular diagnosis of Chinese patients with Charcot-Marie-Tooth by targeted next-generation sequencing and functional analysis. Oncotarget. 2016;7:27655–64. doi: 10.18632/oncotarget.8377. doi: 10.18632/oncotarget.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun B, Chen ZH, Ling L, Li YF, Liu LZ, Yang F, et al. Mutation analysis of gap junction protein beta 1 and genotype-phenotype correlation in X-linked Charcot-Marie-Tooth disease in Chinese patients. Chin Med J. 2016;129:1011–6. doi: 10.4103/0366-6999.180511. doi: 10.4103/0366-6999.180511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang RX, Tang BS, Zi XH, Zhao GH, Zhang FF, Luo W, et al. Mutation of Cx32 gene, clinical and electrophysiological features in patients with Charcot-Marie-Tooth disease. J Clin Neurol. 2005;18:327–9. [Google Scholar]
- 18.Chen SD, Li ZX, Guan YT, Zhou XJ, Jiang JM, Hao Y. A novel mutation of gap junction protein β 1 gene in X-linked Charcot-Marie-Tooth disease. Muscle Nerve. 2011;43:887–92. doi: 10.1002/mus.21992. doi: 10.1002/mus.21992. [DOI] [PubMed] [Google Scholar]
- 19.Li M, Cheng TS, Ho PW, Chan KH, Mak W, Cheung RT, et al. -459C>T point mutation in 5’ non-coding region of human GJB1 gene is linked to X-linked Charcot-Marie-Tooth neuropathy. J Peripher Nerv Syst. 2009;14:14–21. doi: 10.1111/j.1529-8027.2009.00201.x. doi: 10.1111/j.1529-8027.2009.00201.x. [DOI] [PubMed] [Google Scholar]
- 20.Luan XH, Qiao XH, Lv H, Wang ZX, Li YX, Yuan Y. Pathologic and genetic features in 6 Chinese X-linked Charcot-Marie-Tooth disease type 1 families (in Chinese) Chin J Neurol. 2012;45:6–10. [Google Scholar]
- 21.Fridman V, Bundy B, Reilly MM, Pareyson D, Bacon C, Burns J, et al. CMT subtypes and disease burden in patients enrolled in the Inherited Neuropathies Consortium natural history study: A cross-sectional analysis. J Neurol Neurosurg Psychiatry. 2015;86:873–8. doi: 10.1136/jnnp-2014-308826. doi: 10.1136/jnnp-2014-308826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saporta AS, Sottile SL, Miller LJ, Feely SM, Siskind CE, Shy ME. Charcot-Marie-Tooth disease subtypes and genetic testing strategies. Ann Neurol. 2011;69:22–33. doi: 10.1002/ana.22166. doi: 10.1002/ana.22166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicholson GA. Mutation testing in Charcot-Marie-Tooth neuropathy. Ann N Y Acad Sci. 1999;883:383–8. doi: 10.1111/j.1749-6632.1999.tb08599.x. [PubMed] [Google Scholar]
- 24.Numakura C, Lin C, Ikegami T, Guldberg P, Hayasaka K. Molecular analysis in Japanese patients with Charcot-Marie-Tooth disease: DGGE analysis for PMP22, MPZ, and Cx32/GJB1 mutations. Hum Mutat. 2002;20:392–8. doi: 10.1002/humu.10134. doi: 10.1002/humu.10134. [DOI] [PubMed] [Google Scholar]
- 25.Shahrizaila N, Samulong S, Tey S, Suan LC, Meng LK, Goh KJ, et al. X-linked Charcot-Marie-Tooth disease predominates in a cohort of multi-ethnic Malaysian patients. Muscle Nerve. 2013;49:198–201. doi: 10.1002/mus.23892. doi: 10.1002/mus.23892. [DOI] [PubMed] [Google Scholar]
- 26.Yiu EM, Geevasinga N, Nicholson GA, Fagan ER, Ryan MM, Ouvrier RA. A retrospective review of X-linked Charcot-Marie-Tooth disease in childhood. Neurology. 2011;76:461–6. doi: 10.1212/WNL.0b013e31820a0ceb. doi: 10.1212/WNL.0b013e31820a0ceb. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Yin F. A Review of X-linked Charcot-Marie-Tooth disease. J Child Neurol. 2016;31:761–72. doi: 10.1177/0883073815604227. doi: 10.1177/0883073815604227. [DOI] [PubMed] [Google Scholar]
- 28.Fang XJ, Zhang W, Lyu H, Wang ZX, Wang WW, Yuan Y. Compound heterozygote mutation of C12orf65 causes distal motor neuropathy and optic atrophy. Chin Med J. 2017;130:242–4. doi: 10.4103/0366-6999.198019. doi: 10.4103/0366-6999.198019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meng LC, Lyu H, Zhang W, Liu J, Wang ZX, Yuan Y. Hereditary transthyretin amyloidosis in eight Chinese families. Chin Med J. 2015;128:2902–5. doi: 10.4103/0366-6999.168048. doi: 10.4103/0366-6999.168048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin S, Wang W, Wang R, Lv H, Zhang W, Wang Z, et al. INF2 mutations associated with dominant inherited intermediate Charcot-Marie-Tooth neuropathy with focal segmental glomerulosclerosis in two Chinese patients. Clin Neuropathol. 2015;34:275–81. doi: 10.5414/NP300835. doi: 10.4103/0366-6999.168048. [DOI] [PubMed] [Google Scholar]
- 31.Taylor RA, Simon EM, Marks HG, Scherer SS. The CNS phenotype of X-linked Charcot-Marie-Tooth disease: More than a peripheral problem. Neurology. 2003;61:1475–8. doi: 10.1212/01.wnl.0000095960.48964.25. doi: 10.1212/01.WNL.0000095960.48964.25. [DOI] [PubMed] [Google Scholar]
- 32.Anand G, Maheshwari N, Roberts D, Padeniya A, Hamilton-Ayers M, van der Knaap M, et al. X-linked hereditary motor sensory neuropathy (type 1) presenting with a stroke-like episode. Dev Med Child Neurol. 2010;52:677–9. doi: 10.1111/j.1469-8749.2010.03674.x. doi: 10.1111/j.1469-8749.2010.03674.x. [DOI] [PubMed] [Google Scholar]
- 33.Fusco C, Frattini D, Pisani F, Spaggiari F, Ferlini A, Della Giustina E. Coexistent central and peripheral nervous system involvement in a Charcot-Marie-Tooth syndrome X-linked patient. J Child Neurol. 2010;25:759–63. doi: 10.1177/0883073809344119. doi: 10.1177/0883073809344119. [DOI] [PubMed] [Google Scholar]
- 34.Karadima G, Koutsis G, Raftopoulou M, Floroskufi P, Karletidi KM, Panas M. Four novel connexin 32 mutations in X-linked Charcot-Marie-Tooth disease. Phenotypic variability and central nervous system involvement. J Neurol Sci. 2014;341:158–61. doi: 10.1016/j.jns.2014.04.007. doi: 10.1016/j.jns.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Sato K, Kubo S, Fujii H, Okamoto M, Takahashi K, Takamatsu K, et al. Diffusion tensor imaging and magnetic resonance spectroscopy of transient cerebral white matter lesions in X-linked Charcot-Marie-Tooth disease. J Neurol Sci. 2012;316:178–80. doi: 10.1016/j.jns.2012.01.017. doi: 10.1016/j.jns.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 36.Kim GH, Kim KM, Suh SI, Ki CS, Eun BL. Charcot-Marie-Tooth disease masquerading as acute demyelinating encephalomyelitis-like illness. Pediatrics. 2014;134:e270–3. doi: 10.1542/peds.2012-3243. doi: 10.1542/peds.2012-3243. [DOI] [PubMed] [Google Scholar]
- 37.Appu M, Mar S. Novel familial pathogenic mutation in gap junction protein, beta-1 gene (GJB1) associated with transient neurological deficits in a patient with X-linked Charcot-Marie-Tooth disease. Muscle Nerve. 2014;50:1023–4. doi: 10.1002/mus.24343. doi: 10.1002/mus.24343. [DOI] [PubMed] [Google Scholar]
- 38.Okada K, Fujiwara H, Tsuji S. X-linked Charcot-Marie-Tooth disease with transient splenium lesion on MRI. Intern Med. 2006;45:33–4. doi: 10.2169/internalmedicine.45.1477. [DOI] [PubMed] [Google Scholar]
- 39.Zhao Y, Xie Y, Zhu X, Wang H, Li Y, Li J. Transient, recurrent, white matter lesions in X-linked Charcot-Marie-tooth disease with novel mutation of gap junction protein beta 1 gene in China: A case report. BMC Neurol. 2014;14:156. doi: 10.1186/s12883-014-0156-5. doi: 10.1186/s12883-014-0156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim Y, Choi KG, Park KD, Lee KS, Chung KW, Choi BO. X-linked dominant Charcot-Marie-Tooth disease with connexin 32 (Cx32) mutations in Koreans. Clin Genet. 2012;81:142–9. doi: 10.1111/j.1399-0004.2011.01642.x. doi: 10.1111/j.1399-0004.2011.01642.x. [DOI] [PubMed] [Google Scholar]
- 41.Casasnovas C, Banchs I, Corral J, Martínez-Matos JA, Volpini V. Clinical and molecular analysis of X-linked Charcot-Marie-Tooth disease type 1 in Spanish population. Clin Genet. 2006;70:516–23. doi: 10.1111/j.1399-0004.2006.00724.x. doi: 10.1111/j.1399-0004.2006.00724.x. [DOI] [PubMed] [Google Scholar]