Abstract
Background:
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are life-threatening diseases with high mortality rates. This study was designed to analyze the pathogenic factors, clinical manifestations, complications, treatment, and prognosis of SJS/TEN and to explore the differences between surviving and deceased patients.
Methods:
SJS/TEN patients admitted to Beijing Friendship Hospital from January 2006 to December 2015 were included in the study. Patients’ data were retrospectively analyzed. Comparative studies were performed on the survival group and the deceased group, and Fisher's exact probability test was used for statistical analysis.
Results:
Among the 88 patients included, 40 (45.5%) were male with a mean age of 45 ± 18 years. Forty-eight (54.5%) had SJS, 34 (38.6%) had SJS/TEN, and 6 (6.8%) had TEN. Fifty-three (60.2%) cases were caused by medications, mainly antibiotics (n = 24) followed by traditional Chinese medicines (n = 7). Forty-two cases (47.7%) developed visceral damage. Eighty-two patients improved or recovered and were discharged from hospital, and six patients died. Comparative studies on the survival group and the deceased group showed that the presence of malignant tumor (χ2 = 27.969, P < 0.001), connective tissue diseases (χ2 = 9.187, P = 0.002), previous abnormal liver/kidney functions (χ2 = 6.006, P = 0.014), heart rate >100 times/min (χ2 = 6.347, P = 0.012), detached skin area >20% (χ2 = 5.594, P = 0.018), concurrent mucosal involvement at the mouth, eyes, and external genitals (χ2 = 4.945, P = 0.026), subsequent accompanying liver/kidney damage (χ2 = 11.839, P = 0.001, and χ2 = 36.302, P < 0.001, respectively), and SCORTEN score >2 (χ2 = 37.148, P < 0.001) increased the risk of death.
Conclusions:
SJS/TEN is mainly caused by medications, and nearly half of patients develop visceral damage. Multiple factors increase the mortality risk.
Keywords: Mortality, Relative Risk, Stevens-Johnson Syndrome, Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis Overlap, Toxic Epidermal Necrolysis
Introduction
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are life-threatening diseases characterized by widespread red rash, blisters, and shedding of dead skin, with mucosal involvement. They are also associated with visceral damage.[1] SJS and TEN are generally considered as different phases of the disease, and the diagnosis is based on clinical manifestations. Prodromal symptoms include fever, cough, sore throat, and other discomforts. The acute phase typically occurs during the first 8–12 days with a rapid spreading of mucous membrane death and a positive result for Nicolsky's sign.[2] Bastuji-Garin et al.[3] proposed that disease classification should be based on the percentage of the total body surface area (BSA) of the involved skin. Epidermal detachment <10% of the BSA is classed as SJS, detachment above 30% as TEN, and detachment between 10% and 30% as intermediate (SJS/TEN overlap).[3]
SJS/TEN is usually caused by medications, a variety of which can cause allergic reactions including antibiotics, antituberculosis drugs, anticonvulsants, nonsteroidal anti-inflammatory drugs, and allopurinol.[2]
SJS/TEN are systematic diseases. In addition to the damage to the skin, gastrointestinal tract, and respiratory tract mucosa, they can also result in visceral involvement (e.g., liver, kidneys, lungs, and hematopoietic system), leading to organ dysfunction or even failure. The reported mortality rates of SJS and TEN are 10% and 34%, respectively.[4,5] Bastuji-Garin et al.[3] also established the TEN-specific severity-of-illness score (SCORTEN) for predicting the mortality risk. The following seven factors were considered high-risk, with each equivalent to one point: age >40 years, presence of a malignancy, heart rate >120 times/min, serum urea level >10 mmol/L (>27 mg/dl), percentage of epidermal detachment >10% of the BSA, serum glucose level >14 mmol/L (>250 mg/dl), and serum bicarbonate level <20 mEq/L. A higher score means a higher risk of mortality: the risk is 3% for 1 point and 90% for 5 or more points.
By summarizing the clinical and laboratory findings of SJS/TEN patients who were treated in a Chinese tertiary hospital over the past 10 years, this study sought to analyze the pathogenic factors, types of causative drugs, clinical manifestations, organ damage, treatment, and prognosis of SJS/TEN and to explore the differences in these features between surviving and deceased patients.
Methods
Ethical aprroval
As a retrospective study and data analysis was performed anonymously, this study was exempt from the ethical approval and informed consent from patients.
Patient selection
SJS/TEN patients admitted to Beijing Friendship Hospital from January 2006 to December 2015 were included.
Definition of disease
Diagnostic criteria were based on those proposed by Bastuji-Garin et al.[3] WHO-Uppsala Monitoring Centre causality categories were used to determine whether a drug caused allergies.[6] “Incubation period” refers to the period from the first usage of the drug to the clinical onset allergy. If a drug was continuously used for more than 3 months, withdrawn for more than 14 days, or had an incubation period shorter than 3 days, it was not considered to have caused allergy.[7] Disease severity and mortality were assessed using the SCORTEN standard system.[3,8]
Statistical analysis
Statistical analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). Fisher's exact probability test was used for statistical analysis and a value of P < 0.05 was considered statistically significant.
Results
Demographic data
Data from 95 patients were included in the study, 88 of whom met the diagnostic criteria for SJS/TEN. The clinical manifestations, including raised atypical targets or skin pathology, confirmed the presence of bullous erythema multiforme (BEM) in the remaining seven cases.
Among the 88 patients diagnosed with SJS/TEN, 48 (54.5%) had SJS, 34 (38.6%) had SJS/TEN overlap, and 6 (6.8%) had TEN. There were 40 (45.5%) male patients and 48 (54.5%) females, with a male-to-female ratio of 1:1.2. The mean age of patients was 45 ± 18 years (range: 7–83 years) [Table 1]. Age distribution showed that the 31–40 age group had the highest number of patients (n = 20), followed by the 21–30 age group (n = 17). Groups of age ≤10 years or 81–90 years had the lowest number of patients: one and two cases, respectively [Figure 1]. The length of hospital stay was 3–69 days (median: 10 days; interquartile range: 7–15 days). Six (6.8%) patients had a history of drug allergy: three to sulfonamides, one to penicillin, one to amoxicillin, and one to levofloxacin. There were 29 cases with underlying disease: 10 cases of Type II diabetes, 7 cases of high blood pressure, 3 cases of coronary heart disease, 2 cases of liver disorder, 2 cases of kidney disorder, 2 cases of epilepsy, 2 cases of low white blood cell count, and 1 case each of the following diseases: nephrotic syndrome, rheumatoid arthritis, system lupus erythematosus, Sjögren's syndrome, lung cancer, hemophagocytic syndrome (primary disease for T cell lymphoma), benign cerebellar tumor, hyperthyroidism, primary biliary cirrhosis, gout, atrophic gastritis, chronic urticaria, renal transplantation, and depression. Seven patients had two types of underlying disease, and three had three types of underlying disease.
Table 1.
Demographic information of SJS/TEN patients
| Variables | Total (n = 88) | SJS (n = 48) | SJS/TEN overlap (n = 34) | TEN (n = 6) |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Male | 40 (45.5) | 20 (41.7) | 19 (55.9) | 1 (16.7) |
| Female | 48 (54.5) | 28 (58.3) | 15 (44.1) | 5 (83.3) |
| Mean age (years), mean ± SD | 45 ± 18 | 48 ± 19 | 39 ± 15 | 49 ± 13 |
| Median hospital stay (days), median (inter quartile range) | 10 (7–15) | 10 (8–14) | 9 (7–14) | 37 (20–55) |
| History of drug allergy, n (%) | 6 (6.8) | 4 (8.3) | 2 (5.9) | 0 |
| Underlying disease, n (%) | 29 (33.0) | 16 (33.3) | 10 (29.4) | 3 (50.0) |
SJS: Stevens-Johnson syndrome; TEN: Toxic epidermal necrolysis; SD: Standard deviation.
Figure 1.
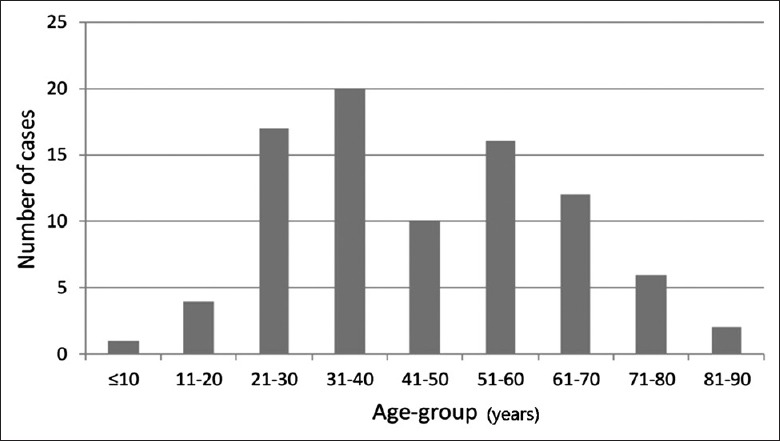
Age distribution among patients with Stevens-Johnson syndrome/toxic epidermal necrolysis (n = 88).
Causes of Stevens-Johnson syndrome/toxic epidermal necrolysis and toxic epidermal necrolysis
Among the 88 patients, SJS/TEN was caused by infection in 13 (14.8%) patients (including upper respiratory tract infections in 11 patients, 1 case of skin infection, and 1 case of mammary gland infection) and by medications in 53 patients (60.2%). The etiologies were unclear in the remaining 22 (25.0%) patients [Figure 2].
Figure 2.
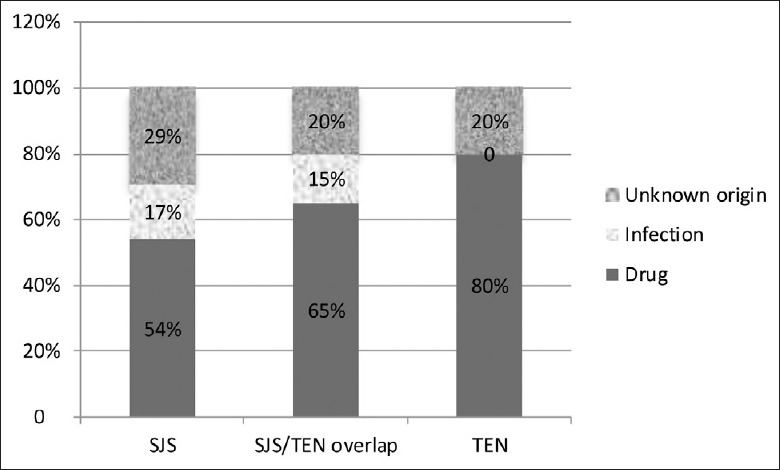
Pathogenic factors of Stevens-Johnson syndrome/toxic epidermal necrolysis patients (n = 88).
Among the 53 cases caused by medications, 4 had used multiple drugs before the rash occurred and therefore could not identify the specific causative agent. The remaining 49 cases had mostly used antibiotics (n = 24) or traditional Chinese medicines (TCMs, n = 7). The allergies were caused by a wide variety of medications: levofloxacin was the most common (n = 3) followed by amoxicillin, azithromycin, clindamycin, cefdinir, cefradine, carbamazepine, lamotrigine, lysine acetylsalicylate, and acetaminophen, each of which had caused 2 cases. The compound medications pseudoephedrine hydrochloride and dextromethorphan hydrobromide, and Xiaojinwan, a kind of TCM, also caused allergies in two cases each. Each of the other listed drugs caused allergy in a single case [Table 2].
Table 2.
Drugs that caused allergies in SJS/TEN patients
| Responsible drug(s) | Total (n = 49) | SJS (n = 18) | SJS/TEN overlap (n = 26) | TEN (n = 5) |
|---|---|---|---|---|
| Antibiotics | 24 (49.0) | 10 (55.6) | 12 (46.2) | 2 (40.0) |
| Levofloxacin | 3 (6.1) | 2 (11.1) | 1 (3.8) | 0 |
| Amoxicillin | 2 (4.1) | 0 | 1 (3.8) | 1 (20.0) |
| Azithromycin | 2 (4.1) | 1 (5.6) | 1 (3.8) | 0 |
| Clindamycin | 2 (4.1) | 1 (5.6) | 1 (3.8) | 0 |
| Cefdinir | 2 (4.1) | 2 (11.1) | 0 | 0 |
| Cefradine | 2 (4.1) | 0 | 2 (7.6) | 0 |
| Amoxicillin and clavulanate potassium | 1 (2.0) | 0 | 1 (3.8) | 0 |
| Cefoperazone sodium and sulbactam sodium | 1 (2.0) | 0 | 1 (3.8) | 0 |
| Cefuroxime axetil | 1 (2.0) | 0 | 1 (3.8) | 0 |
| Ceftizoxime | 1 (2.0) | 1 (5.6) | 0 | 0 |
| Latamoxef | 1 (2.0) | 1 (5.6) | 0 | 0 |
| Meropenem | 1 (2.0) | 0 | 1 (3.8) | 0 |
| Roxithromycin | 1 (2.0) | 0 | 1 (3.8) | 0 |
| Moxifloxacin hydrochloride | 1 (2.0) | 1 (5.6) | 0 | 0 |
| Etimicin sulfate | 1 (2.0) | 1 (5.6) | 0 | 0 |
| Mesylate pefloxacin | 1 (2.0) | 0 | 1 (3.8) | 0 |
| Norvancomycin | 1 (2.0) | 0 | 0 | 1 (20.0) |
| Anticonvulsant | 5 (10.2) | 0 | 4 (15.4) | 1 (20.0) |
| Carbamazepine | 2 (4.1) | 0 | 1 (3.8) | 1 (20.0) |
| Lamotrigine | 2 (4.1) | 0 | 2 (7.6) | 0 |
| Valproate | 1 (2.0) | 0 | 1 (3.8) | 0 |
| NSAID | 4 (8.2) | 1 (5.6) | 2 (7.6) | 1 (20.0) |
| Aspisol | 2 (4.1) | 1 (5.6) | 1 (3.8) | 0 |
| Acetaminophen | 2 (4.1) | 0 | 1 (3.8) | 1 (20.0) |
| Allopurinol | 1 (2.0) | 0 | 1 (3.8) | 0 |
| Omeprazole | 1 (2.0) | 1 (5.6) | 0 | 0 |
| Iohexol | 1 (2.0) | 1 (5.6) | 0 | 0 |
| Interferon | 1 (2.0) | 0 | 1 (3.8) | 0 |
| Compound medicine | 5 (10.2) | 2 (11.1) | 2 (7.6) | 1 (20.0) |
| Pseudoephedrine hydrochloride | 2 (4.1) | 1 (5.6) | 1 (3.8) | 0 |
| Dextromethorphan hydrobromide | 2 (4.1) | 1 (5.6) | 0 | 1 (20.0) |
| Anjiahuangmin | 1 (2.0) | 0 | 1 (3.8) | 0 |
| Traditional Chinese Medicine | 7 (14.3) | 3 (16.7) | 4 (15.4) | 0 |
| Xiaojinwan | 2 (4.1) | 0 | 2 (7.6) | 0 |
| Wei C Yinqiao Pian | 1 (2.0) | 1 (5.6) | 0 | 0 |
| Sanhuang Pian | 1 (2.0) | 1 (5.6) | 0 | 0 |
| Zhengqingfengtongning | 1 (2.0) | 1 (5.6) | 0 | 0 |
| Herbal medicine | 2 (4.1) | 0 | 2 (7.6) | 0 |
The data was presented by n (%). SJS: Stevens-Johnson syndrome; TEN: Toxic epidermal necrolysis; NSAID: Nonsteroidal anti-inflammatory drug.
Laboratory findings
Laboratory tests showed that 42 cases (47.7%) developed visceral damage. Liver disorders were the most common, as demonstrated by the increased levels of liver enzymes among 32 patients (36.4%). The second most common was gastrointestinal tract damage (n = 11, 12.5%) and the symptoms included diarrhea, gastrointestinal tract hemorrhage, and appetite loss. Ten cases (11.4%) developed lung damage, as manifested by dyspnea and patchy spot-like shadows on X-ray. There were eight (9.1%) cases of kidney damage, seven (8.0%) cases of disseminated intravascular coagulation (DIC), five (5.7%) cases of myocardial damage (as manifested by increased levels of troponin T or creatine kinase-MB, possibly accompanied by ST-T changes in electrocardiography), and two cases of encephalopathy (as manifested by consciousness disorder and delirium) [Table 3].
Table 3.
Clinical manifestations, visceral damage, and mortalities among SJS/TEN patients
| Variables | Total (n = 88) | SJS (n = 48) | SJS/TEN overlap (n = 34) | TEN (n = 6) |
|---|---|---|---|---|
| Fever, n (%) | 62 (70.5) | 30 (62.5) | 26 (76.5) | 6 (100.0) |
| Leukocytosis, n (%) | 35 (39.8) | 16 (33.3) | 16 (47.1) | 3 (50.0) |
| % BSA, mean ± SD | 12.5 ± 12.5 | 5.2 ± 2.2 | 16.6 ± 6.6 | 50.0 ± 10.0 |
| Organ involvement, n (%) | ||||
| Hepatitis | 32 (36.4) | 17 (35.4) | 12 (35.3) | 3 (50.0) |
| Renal dysfunction | 8 (9.1) | 3 (6.3) | 3 (8.8) | 2 (33.3) |
| Gastrointestinal | 11 (12.5) | 3 (6.3) | 4 (11.8) | 4 (66.7) |
| Respiratory | 10 (11.4) | 3 (6.3) | 3 (8.8) | 4 (66.7) |
| Encephalopathy | 2 (2.3) | 0 | 1 (2.9) | 1 (16.7) |
| Myocarditis | 5 (5.7) | 1 (2.1) | 3 (8.8) | 1 (16.7) |
| DIC | 7 (8.0) | 3 (6.3) | 2 (5.9) | 2 (33.3) |
| Mortality, n (%) | 6 (6.8) | 2 (4.2) | 2 (5.9) | 2 (33.3) |
SJS: Stevens-Johnson syndrome; TEN: Toxic epidermal necrolysis; BSA: Body surface area; SD: Standard deviation; DIC: Disseminated intravenous coagulation.
Treatment and clinical course
Among the 88 patients, 41 (46.6%) received systematic glucocorticoid treatment. Specifically, 15 of the 48 SJS patients, 20 of the 34 SJS/TEN overlap patients, and all 6 TEN patients received systematic glucocorticoid treatment. In addition, the combined application of intravenous immunoglobulin (IVIG) was administered to two SJS/TEN overlap patients and four TEN patients.
Among the 88 patients, 82 improved or recovered completely and were discharged from the hospital, and 6 died, including 2 from each of the following groups: SJS, SJS/TEN overlap, and TEN. Comparative studies on patient health history, clinical manifestations, test results, and treatment protocols were performed between the survival group and the deceased group. The results showed that death was closely correlated with the status of the systemic disease, area of detached skin, and severity of liver/kidney damage [Table 4]. Causes of death included infection, organ failure, and pulmonary embolism resulting from detached vein thrombosis in the lower limbs [Table 5].
Table 4.
Univariate analysis of the clinical characteristics of the survival and deceased groups (n = 88)
| Variables | Survived group (%) | Deceased group (%) | χ2 | P |
|---|---|---|---|---|
| Age >70 years | 8.5 | 16.7 | 0.447 | 0.504 |
| Male gender | 46.3 | 33.3 | 0.382 | 0.537 |
| Recent pathological history | ||||
| Cancer | 0.0 | 33.3 | 27.969 | <0.001 |
| Renal disease | 1.2 | 16.7 | 6.006 | 0.014 |
| Liver disease | 1.2 | 16.7 | 6.006 | 0.014 |
| Prior infections | 41.5 | 50.0 | 0.167 | 0.683 |
| Autoimmune diseases | 3.7 | 33.3 | 9.187 | 0.002 |
| Diabetes | 9.8 | 33.3 | 3.086 | 0.079 |
| Eye involvement | 32.9 | 66.7 | 2.789 | 0.095 |
| Oral mucosa involvement | 36.6 | 66.7 | 2.134 | 0.144 |
| Genital involvement | 22.0 | 50.0 | 2.421 | 0.120 |
| Involvement of three mucosal areas | 14.6 | 50.0 | 4.945 | 0.026 |
| Heart rate >100 beats/min | 12.2 | 50.0 | 6.347 | 0.012 |
| WBC >10.0×109/L | 41.5 | 50.0 | 0.167 | 0.683 |
| BSA involvement >20% | 13.4 | 50.0 | 5.594 | 0.018 |
| Liver involvement | 30.5 | 100.0 | 11.839 | 0.001 |
| BUN >10 mg/dl | 2.4 | 66.7 | 36.302 | <0.001 |
| Serum bicarbonate <20 mEq/L | 2.4 | 50.0 | 23.599 | <0.001 |
| Steroid treatment | 42.7 | 100.0 | 7.381 | 0.007 |
| Antibiotics use | 46.3 | 83.3 | 3.062 | 0.080 |
| Infection | 46.3 | 83.3 | 3.062 | 0.080 |
| SCORTEN >2 | 8.5 | 83.3 | 37.148 | <0.001 |
WBC: White blood cell; BSA: Body surface area; BUN: Blood urea nitrogen; SCORTEN: Toxic epidermal necrolysis specific severity of illness score.
Table 5.
Analysis of the six deceased cases among patients with SJS/TEN
| Case number | Disease | Age | Sex | Underlying disease | Causative drugs | Indication for drug therapy | Maximum skin detachment (%) | Clinical course of the skin lesion | Severe complications and cause of death | Maximum doses of corticosteroids and other therapies | SCORTEN | Time to death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SJS | 62 | Female | Hemophagocytic syndrome, T cell lymphoma | NA | NA | 8 | Improved | Lung infection, liver/kidney failure | mPSL 40 mg/d | 4 | 14 |
| 2 | SJS | 62 | Female | Systemic lupus erythematosus | Imipenem, vancomycin | Lung infection | 5 | No change | Lung infection, respiratory failure | DXM 10 mg/d | 2 | 6 |
| 3 | SJS/TEN overlap | 83 | Male | Lung cancer | Meropenem | Lung infection | 22 | Improved | Lung infection, respiratory failure, DIC | mPSL 80 mg/d, IVIG 20 g/day | 5 | 12 |
| 4 | SJS/TEN overlap | 66 | Male | Mesenteric venous thrombosis with intestinal necrosis | Cefoperazone sodium and sulbactam sodium | Peritonitis | 18 | Healed | Peritonitis, bacterial sepsis, fungal sepsis | mPSL 80 mg/d, IVIG 20 g/day | 3 | 30 |
| 5 | TEN | 45 | Female | Sjögren’s disease, primary biliary cirrhosis | Norvancomycin | Lung infection | 65 | Improved | Lung infection, respiratory failure | mPSL 120 mg/d, IVIG 20 g/day | 4 | 10 |
| 6 | TEN | 67 | Female | Type II diabetes | Amoxicillin | Upper respiratory tract infection | 70 | Healed | Pulmonary embolism resulting from the detached vein thrombosis in lower limbs | mPSL 160 mg/d, IVIG 20 g/day | 3 | 46 |
Time to death: Time between the onset of eruption and death. SJS: Stevens–Johnson syndrome; mPSL: Methylprednisolone; DXM: Dexamethasone; IVIG: Intravenous immunoglobulin; DIC: Disseminated intravenous coagulation; TEN: Toxic epidermal necrolysis; SCORTEN: Toxic epidermal necrolysis specific severity of illness score; NA: Not available.
Discussion
In this study, 88 of the 95 patients met the diagnostic criteria of SJS/TEN, and 7 of them were re-diagnosed with BEM. Normally, BEM and SJS/TEN were distinguished by clinical manifestations and pathological examinations. BEM is characterized by the epidermal detachment of <10% BSA, coupled with localized typical target lesions or raised atypical targets and is typically caused by infection, of which herpes simplex virus is the most common. Skin biopsies were characterized histologically by lichenoid infiltrate.[9]
SJS/TEN was mostly caused by medication use. However, vaccination, chemical exposure, and certain virus/mycoplasma infections might have also been responsible for the induction of disease. Among the 88 cases in the present study, disease severity increased along the spectrum of SJS, SJS/TEN overlap, and TEN, and cases with drug-induced diseases increased from 54% to 80%. In contrast, infection-associated cases decreased from 29% to 20%. These results suggest that TEN is more likely to be caused by drug use, which was consistent with previously reported studies.[9,10]
Among the seven patients who were allergic to TCMs, five were allergic to Chinese patented medicines (two cases of Xiaojin tablets; one case of Vitamin C Yinqiao tablets; one case of Sanhuang tablets; and one case of Zhengqing Fengtongning). Two patients were allergic to Chinese herbal medicines. We searched the China Knowledge Resource Integrated Database and found that allergies to the above four Chinese patent medicines had already been reported in the literature. A further literature search on each of the 23 medicinal materials contained in these 4 Chinese patented medicines showed that 14 had been described as allergy-inducing. Therefore, allergies to TCMs are not uncommon, probably because most Chinese patented medicines contain multiple medicinal materials, which contain multiple ingredients, and allergy to any of these ingredients can lead to allergic reactions. Interestingly, while some articles had reported allergic reactions to Zhengqing Fengtongning, this drug contains only one ingredient, sinomenine hydrochloride. However, most relevant studies on sinomenine hydrochloride have focused on its purification methods and therapeutic efficacies but not on the induction of clinical allergy or its underlying mechanisms. In total, there are more than 12,000 known TCMs, only 1% of which have been shown to cause an adverse reaction.[11] However, since TCMs are widely used in China and their components are relatively complex, clinicians must be particularly cautious when prescribing TCMs, especially when studies to date on the ingredients of TCMs and their pharmacological mechanisms remain inadequate.[11,12,13,14]
Mortalities were determined according to the SCORTEN grading system: 0–1 point, 3%; 2 points, 12%; 3 points, 35%; 4 points, 58%; and 5 points and above, >90%.[3,8] Among the six cases of death, one had 5 points, two had 4 points, two had 3 points, and one had 2 points. Analysis of the causes of death and statistical analyses showed that gender, age (>70 years), infection status, and diabetes status were not significantly different between patients who survived and those who did not. However, the presence or absence of malignant tumor, connective tissue disease, or liver/kidney disorder was significantly different between the survival and the deceased groups, with the presence of these diseases correlating with death. Clinically, mucosal damage to the mouth, eyes, or genitals was not individually correlated with death; however, concurrent mucosal damage to all three areas was a factor associated with death. Heart rate >100/min or skin detachment area >20% was also correlated with death. Laboratory results showed no statistically significant differences in increased levels of white blood cells, accompanying infection, and antibiotic use between the survival and deceased groups. However, liver damage, blood urea nitrogen >10 mg/dl, bicarbonate level <20 mEq/L, and SCORTEN >2 increased the risk of death. Glucocorticoid usage was also significantly different between the survival and deceased groups: the proportion of glucocorticoid usage was 100% in the deceased group but only 43% in the survival group. However, we cannot conclude that glucocorticoid usage contributed to death because patients in the deceased group had higher SCORTEN scores and more severe underlying diseases, which led to a higher rate of glucocorticoid usage. Therefore, the more likely cause of death was the diseases themselves, and the use of glucocorticoids failed to save the patients’ lives.
All six cases of death had underlying diseases, including two with the connective tissue diseases systemic lupus erythematosus, and Sjögren's disease, accompanied by primary biliary cirrhosis; two diabetes cases, with one previously admitted to hospital because of mesenteric venous thrombosis with intestinal necrosis; and two cases with malignant tumors, one of lung cancer and the other of primary T cell lymphoma with hemophagocytic syndromes. In the six deceased patients, four cases were directly caused by infection, one by liver failure, and one by pulmonary embolism caused by a detached vein thrombosis in the lower limbs. Patients who developed liver failure also had accompanying hemophagocytic syndromes, which resulted in lung infection and rapid liver/kidney deterioration in addition to their original liver/kidney damage. Five patients had accompanying infections, four of which were lung infections. Among these four cases, three developed respiratory failure and one had accompanying DIC. The infections were caused by bacteria and fungi, including Pseudomonas aeruginosa, methicillin-resistant Staphylococcus aureus, Stenotrophomonas maltophilia, Klebsiella pneumoniae, Acinetobacter baumannii, Leclercia adecarboxylata, Candida albicans, and Candida parapsilosis. Most of these pathogens are opportunistic. The infections were mainly acquired in hospital, which might correlate with the low immunity and long hospital stay of patients. Four of the five patients developed definite infection before corticoid usage, and subsequent infection control was insufficient, which directly led to death. These results indicate that infection, particularly lung infection, requires special attention when corticosteroids are used in the clinic to treat SJS/TEN.
As a retrospective study carried out in a tertiary-care setting in China, our current study was limited by its sample size, which might have caused bias in the analysis (e.g., in the types and proportions of allergy-inducing drugs). Six of the 88 patients died, yielding a mortality rate of 6.8%, which was lower than that reported in the most literature.[4,5] We analyzed the causes of death in terms of underlying diseases, clinical manifestations after disease onset, and laboratory findings; however, the small number of reported deaths might also have introduced bias.
In conclusions, SJS/TEN is mostly caused by medications, most commonly antibiotics, although TCMs are also considered causative factors in allergy induction. Accompanying visceral damage was common, particularly liver damage that accounted for 36% of the total cases. The following factors increased the risk of death: presence of malignant tumor, connective tissue disease, previous abnormal liver/kidney function, heart rate >100/min, detached skin area >20%, concurrent mucosal involvement in the mouth, eyes, and external genitals, consequent accompanying liver/kidney damage, and SCORTEN >2. Glucocorticoid application combined with IVIG was effective for the treatment of severe cases. During corticoid application, opportunistic, hospital-acquired infections should be prevented. Death was mostly caused by infection, followed by visceral damage.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Ning-Ning Wang
References
- 1.Yamane Y, Matsukura S, Watanabe Y, Yamaguchi Y, Nakamura K, Kambara T, et al. Retrospective analysis of Stevens-Johnson syndrome and toxic epidermal necrolysis in 87 Japanese patients – Treatment and outcome. Allergol Int. 2016;65:74–81. doi: 10.1016/j.alit.2015.09.001. doi: 10.1016/j.alit. 2015.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Law EH, Leung M. Corticosteroids in Stevens-Johnson syndrome/toxic epidermal necrolysis: Current evidence and implications for future research. Ann Pharmacother. 2015;49:335–42. doi: 10.1177/1060028014560012. doi: 10.1177/1060028014560012. [DOI] [PubMed] [Google Scholar]
- 3.Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129:92–6. [PubMed] [Google Scholar]
- 4.Kim HI, Kim SW, Park GY, Kwon EG, Kim HH, Jeong JY, et al. Causes and treatment outcomes of Stevens-Johnson syndrome and toxic epidermal necrolysis in 82 adult patients. Korean J Intern Med. 2012;27:203–10. doi: 10.3904/kjim.2012.27.2.203. doi: 10.3904/kjim.2012.27.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee HY, Tey HL, Pang SM, Thirumoorthy T. Systemic lupus erythematosus presenting as Stevens-Johnson syndrome and toxic epidermal necrolysis: A report of three cases. Lupus. 2011;20:647–52. doi: 10.1177/0961203310385162. doi: 10.1177/0961203310385162. [DOI] [PubMed] [Google Scholar]
- 6.The Use of the WHO-UMC System for Standardised Case Causality Assessment. [Last accessed on 2017 Mar 29]. Available from: https://www.who-umc.org/media/2768/standardised-case-causality-assessment.pdf .
- 7.Kardaun SH, Sekula P, Valeyrie-Allanore L, Liss Y, Chu CY, Creamer D, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): An original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071–80. doi: 10.1111/bjd.12501. doi: 10.1111/bjd.12501. [DOI] [PubMed] [Google Scholar]
- 8.Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P. SCORTEN: A severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149–53. doi: 10.1046/j.1523-1747.2000.00061.x. doi: 10.1046/j.1523-1747.2000.00061.x. [DOI] [PubMed] [Google Scholar]
- 9.Kohanim S, Palioura S, Saeed HN, Akpek EK, Amescua G, Basu S, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis – A comprehensive review and guide to therapy. I. Systemic disease. Ocul Surf. 2016;14:2–19. doi: 10.1016/j.jtos.2015.10.002. doi: 10.1016/j.jtos.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Chantaphakul H, Sanon T, Klaewsongkram J. Clinical characteristics and treatment outcome of Stevens-Johnson syndrome and toxic epidermal necrolysis. Exp Ther Med. 2015;10:519–24. doi: 10.3892/etm.2015.2549. doi: 10.3892/etm.2015.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Liu Z. Prevention and treatment of viral respiratory infections by traditional Chinese herbs. Chin Med J. 2014;127:1344–50. doi: 10.3760/cma.j.issn.0366-6999.20132029. [PubMed] [Google Scholar]
- 12.Han L, Guo S, Wang Y, Yang L, Liu S. Experimental drugs for treatment of autoimmune myocarditis. Chin Med J. 2014;127:2850–9. doi: 10.3760/cma.j.issn.0366-6999.20140748. [PubMed] [Google Scholar]
- 13.Shi YL, Liu WJ, Zhang XF, Su WJ, Chen NN, Lu SH, et al. Effect of Chinese herbal medicine Jinlida granule in treatment of patients with impaired glucose tolerance. Chin Med J. 2016;129:2281–6. doi: 10.4103/0366-6999.190676. doi: 10.4103/0366-6999.190676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hua W, Gao RL, Zhao BC, Wang J, Chen XH, Cai C, et al. The efficacy and safety of Wenxin Keli in patients with frequent premature ventricular contractions: A randomized, double-blind, placebo-controlled, parallel-group, multicenter trial. Chin Med J. 2015;128:2557–64. doi: 10.4103/0366-6999.166026. doi: 10.4103/0366-6999.166026. [DOI] [PMC free article] [PubMed] [Google Scholar]


