Abstract
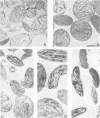
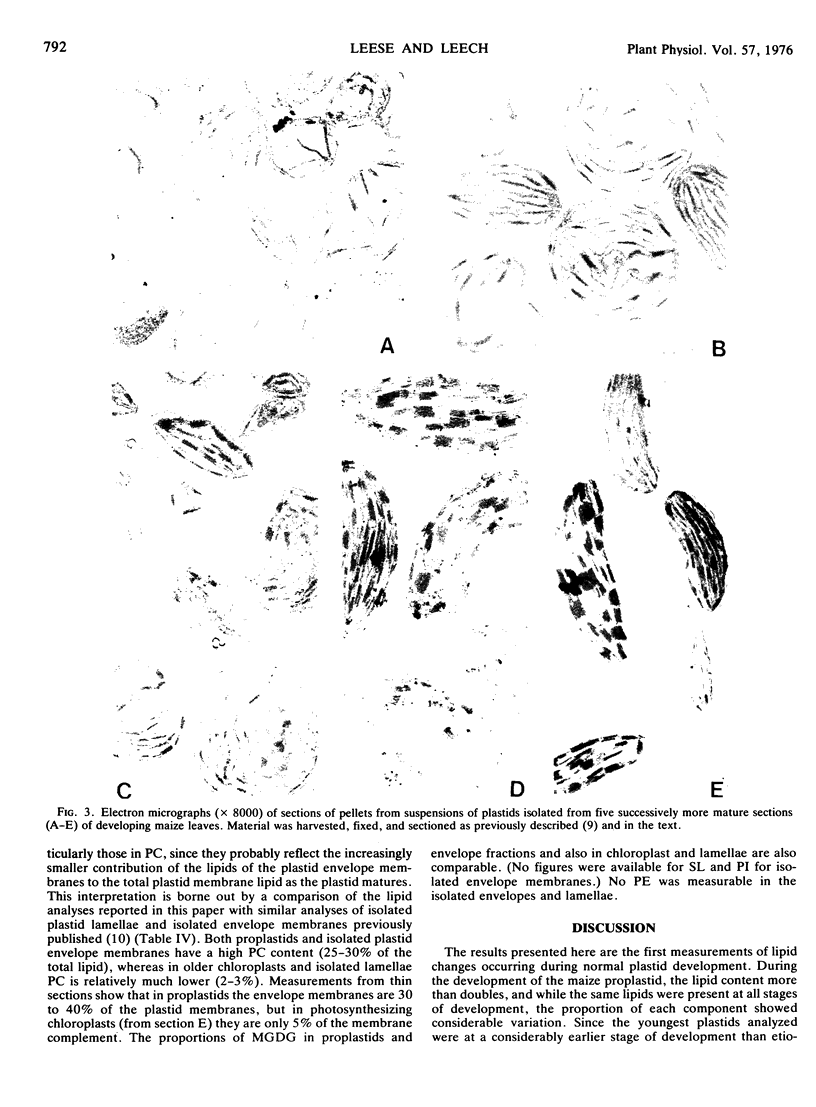
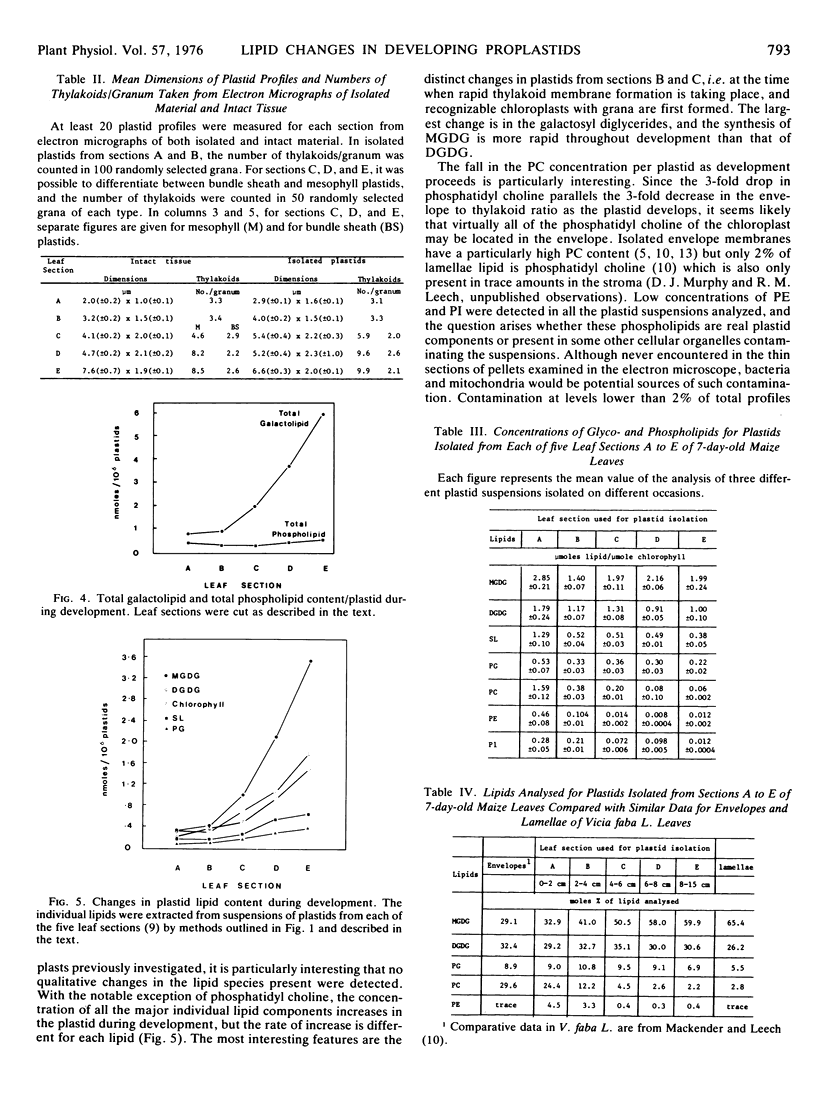
Changes in lipid composition were followed as a proplastid develops into a chloroplast. Methods were devised for the isolation of developing proplastids from sections of five different ages from the same 7-day-old maize (Zea mays var. Kelvedon Glory) leaf. Electron micrographs illustrate the homogeneity of the five types of plastid suspension, minimal contamination with other cytoplasmic membranes, and the presence of morphologically intact plastids in the proportions 85% (youngest), 85%, 80%, 70% and 60% (oldest), respectively. Both bundle sheath and mesophyll plastids are well preserved in isolation. Plastid numbers were determined from calibration curves of the chlorophyll content of each type of suspension, and lipid values then expressed as nmoles/106 plastids. Monogalactosyl diglyceride (MGDG), digalactosyl diglyceride (DGDG), sulfoquinovosyl diglyceride, and phosphatidyl glycerol (PG) all increase during plastid development but the rate of increase is different for each lipid. The largest changes are in MGDG (6-fold) and DGDG (4-fold). Phosphatidyl choline shows a continuous decline during plastid development. Phosphatidyl inositol and phosphatidyl ethanolamine were found in all the suspensions in low concentrations (0.4-4.0% of total lipid): calculations showed their presence could not be accounted for by bacterial or mitochondrial contamination. The increase in PG parallels the chlorophyll changes during development and at maturity 1 molecule of PG is present per 3 molecules of chlorophyll. The results are discussed in the context of the molecular structure of the photosynthetic thylakoid membranes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M. The molecular organization of chloroplast thylakoids. Biochim Biophys Acta. 1975 Aug 15;416(2):191–235. doi: 10.1016/0304-4173(75)90007-5. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Douce R., Holtz R. B., Benson A. A. Isolation and properties of the envelope of spinach chloroplasts. J Biol Chem. 1973 Oct 25;248(20):7215–7222. [PubMed] [Google Scholar]
- Klein S., Schiff J. A. The Correlated Appearance of Prolamellar Bodies, Protochlorophyll(ide) Species, and the Shibata Shift during Development of Bean Etioplasts in the Dark. Plant Physiol. 1972 Apr;49(4):619–626. doi: 10.1104/pp.49.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R. M., Rumsby M. G., Thomson W. W. Plastid differentiation, acyl lipid, and Fatty Acid changes in developing green maize leaves. Plant Physiol. 1973 Sep;52(3):240–245. doi: 10.1104/pp.52.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackender R. O., Leech R. M. The Galactolipid, Phospholipid, and Fatty Acid Composition of the Chloroplast Envelope Membranes of Vicia faba. L. Plant Physiol. 1974 Mar;53(3):496–502. doi: 10.1104/pp.53.3.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poincelot R. P. Differences in lipid composition between intact and membrane-stripped spinach chlorplasts. Biochim Biophys Acta. 1971 Jun 8;239(1):57–60. doi: 10.1016/0005-2760(71)90192-5. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D., Laetsch W. M. Structure and function of developing barley plastids. Plant Physiol. 1974 Aug;54(2):148–159. doi: 10.1104/pp.54.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Batt R. D. Quantitative analysis of sulfolipid (sulfoquinovosyl diglyceride) and galactolipids (monogalactosyl and digalactosyl diglycerides) in plant tissues. Anal Biochem. 1968 Jan;22(1):74–88. doi: 10.1016/0003-2697(68)90261-3. [DOI] [PubMed] [Google Scholar]
- Roughan P. G., Boardman N. K. Lipid Composition of Pea and Bean Leaves during Chloroplast Development. Plant Physiol. 1972 Jul;50(1):31–34. doi: 10.1104/pp.50.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Trémolières A., Lepage M. Changes in Lipid Composition during Greening of Etiolated Pea Seedlings. Plant Physiol. 1971 Feb;47(2):329–334. doi: 10.1104/pp.47.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]