Abstract
Background:
Parkinson's disease (PD) patients with long-term levodopa (L-DOPA) treatment are suffering from severe circadian dysfunction. However, it is hard to distinguish that the circadian disturbance in patients is due to the disease progression itself, or is affected by L-DOPA replacement therapy. This study was to investigate the role of L-DOPA on the circadian dysfunction in a rat model of PD.
Methods:
The rat model of PD was constructed by a bilateral striatal injection with 6-hydroxydopamine (6-OHDA), followed by administration of saline or 25 mg/kg L-DOPA for 21 consecutive days. Rotarod test, footprint test, and open-field test were carried out to evaluate the motor function. Striatum, suprachiasmatic nucleus (SCN), liver, and plasma were collected at 6:00, 12:00, 18:00, and 24:00. Quantitative real-time polymerase chain reaction was used to examine the expression of clock genes. Enzyme-linked immunosorbent assay was used to determine the secretion level of cortisol and melatonin. High-performance liquid chromatography was used to measure the neurotransmitters. Analysis of variance was used for data analysis.
Results:
L-DOPA alleviated the motor deficits induced by 6-OHDA lesions in the footprint and open-field test (P < 0.01, P < 0.001, respectively). After L-DOPA treatment, Bmal1 decreased in the SCN compared with 6-OHDA group at 12:00 (P < 0.01) and 24:00 (P < 0.001). In the striatum, the expression of Bmal1, Rorα was lower than that in the 6-OHDA group at 18:00 (P < 0.05) and L-DOPA seemed to delay the peak of Per2 to 24:00. In liver, L-DOPA did not affect the rhythmicity and expression of these clock genes (P > 0.05). In addition, the cortisol secretion was increased (P > 0.05), but melatonin was further inhibited after L-DOPA treatment at 6:00 (P < 0.01).
Conclusions:
In the circadian system of advanced PD rat models, circadian dysfunction is not only contributed by the degeneration of the disease itself but also long-term L-DOPA therapy may further aggravate it.
Keywords: Circadian Rhythm, Levodopa, Oxidopamine, Parkinson Disease
Introduction
Parkinson's disease (PD) is a common movement disorder, affecting about 1.7% in the aged population over 65 years.[1] The main pathology of PD is featured by the loss of dopaminergic neurons and the formation of Lewy bodies. Interestingly, the patients often have more complaints about their movement dysfunction in the late day than in the morning.[2] Moreover, sleep disturbance, visual deficits, and autonomic dysfunction have also been found to fluctuate in the whole day.[3,4,5] The secretion patterns of several hormones involved in the rhythm also differ from that of the healthy controls.[6] Therefore, all these manifestations imply that the circadian rhythm of PD patients may be disrupted.
In mammals, circadian rhythm is controlled by hypothalamic suprachiasmatic nucleus (SCN), and regulated by a transcriptional feedback loop with clock genes, such as circadian locomotor cycle kaput (Clock), Brain and muscle Arnt-like protein-1 (Bmal1), Period (Per), Cryptochrome (Cry), Reverse erythroblastosis virus (Rev-erb), Retinoic acid receptor-related orphan receptor (Rorα) genes, and their corresponding proteins.[7] Clock and Bmal1 are two critical transcriptional activators, which stimulate the expression of Per and Cry at the beginning of the day. Per and Cry heterodimers translocate into the nucleus and inhibit the transcription of Bmal1 and Clock. Eventually, the Per and Cry proteins accumulate in the day and then degrade at night, followed by the beginning of Bmal1 and Clock transcription, which initiates the cycle over again.[8] In addition, Rev-erb and Rorα play a role in maintaining the circle stable.[9]
So far, L-DOPA serves as the most effective therapy in PD. L-DOPA obviously alleviates the motor symptoms, especially in the early stage. However, long-term L-DOPA therapy has been reported to be associated with several detrimental motor and nonmotor symptoms.[10] Furthermore, PD patients in the advanced stage often suffer from the worsening of circadian rhythmic disturbance. Nevertheless, it remains to be clarified whether the disrupted circadian rhythm in advanced PD patients is related to L-DOPA treatment or disease progression itself. The current study hypothesized that long-term L-DOPA treatment may aggravate the circadian rhythms of PD patients. Using the rat dyskinesia model to mimic the late stage PD, we investigated the effect of long-term L-DOPA treatment on the circadian rhythm in a rat model of PD.
Methods
Animals
Male Sprague-Dawley rats (200–250 g) were purchased from Shanghai Laboratory Animal Center (SLAC, Shanghai, China). The experimental protocols were approved by the Institutional Animal Care and Use Committee of Soochow University. Rats were maintained under a 12/12 h light/dark cycle (light on at 8:00, light off at 20:00) at 22°C with free access to food and tap water.
Experimental protocol
The overall experimental procedure is shown in Figure 1a. Briefly, sham or lesioned rats were made by a bilateral striatal injection with saline (4 μl for each side) or 6-hydroxydopamine (6-OHDA) (8 μg/4 μl for each side, 2-μg 6-OHDA in 1-μl saline containing 0.02% of ascorbic acid, Sigma, St. Louis, MO, USA) on a Stoelting stereotaxic apparatus (Stoelting Co., Kiel, WI, USA) following the coordinates: AP +1.0, ML ±3.5, DV −4.5 mm from bregma at the rate of 0.5 μl/min.[11] The syringe needle (Hamilton, USA) was left in place for 5 min to assure the solution diffusion. A total number of 42 rats were used in this experiment, 21 days after the surgery, animals were randomly divided into three groups (n = 14 each group): (a) sham group (sham-operated, treated with saline); (b) 6-OHDA group (6-OHDA-lesioned, also treated with saline); and (c) L-DOPA group (6-OHDA-lesioned, treated with L-DOPA). Animals received the intraperitoneal administration with saline or 25 mg/kg of levodopa methyl ester mixed with 6.25 mg/kg of benserazide tablets (Roche Diagnostics, Penzberg, Germany) dissolved in sterile saline once daily at 15:00 for another 21 consecutive days. The animals underwent behavioral tests and were then sacrificed at 24:00, 6:00, 12:00, and 18:00. The plasma collected for enzyme-linked immunosorbent assay (ELISA) was from the left ventricle and centrifuged at 800 ×g for 3 min. The brain and liver were immediately harvested (n = 3 for each time point, equals to n = 12 in each group, used for quantitative polymerase chain reaction [QPCR] and high performance liquid chromatography [HPLC]), and the caudate putamen (striatum) and SCN were carefully dissected out, the left side of the brain was later used for QPCR, the right side was used for HPLC. The rest rats (n = 2 for each group) were used for immunofluorescence.
Figure 1.
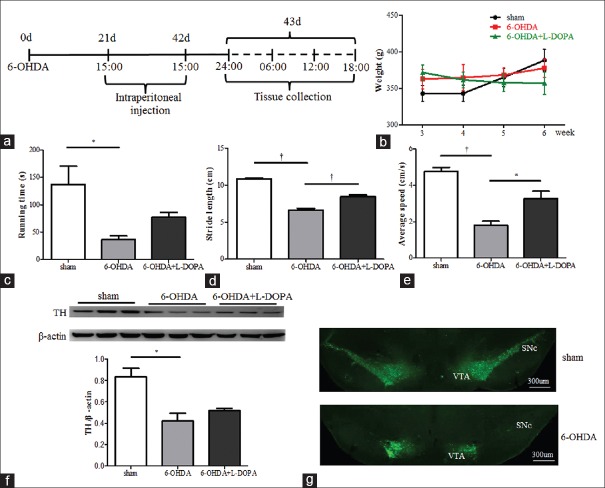
Motor deficiency in 6-OHDA rats and L-DOPA improved it. (a) A simple illustration of the experimental procedure. (b) The body weight measurement (n = 14 for each group). (c) Rotarod test (n = 14 for each group, *P < 0.01). (d) Footprint test (n = 14 for each group, †P < 0.001) and (e) Open field test (n = 14 for each group, *P < 0.01, †P < 0.001). (f and g) Loss of TH neurons in the substantia nigra of 6-OHDA lesioned rats, as revealed by western blotting (n = 3 for each group, *P < 0.01) and immunofluorescence (n = 2 for each group). 6-OHDA: 6-hydroxydopamine.
Behavioral and body weight measurements
Body weight measurement
During L-DOPA treatment, body weights were recorded weekly till the end of the experiment for all the groups.
Rotarod performance
Rotarod test was carried out by the Rotarod system (ZH-300, Zhenghua, Anhui Province, China) to evaluate the motor function. Each rat was trained for 3 times/day with 3 consecutive days before the real test. The training speed is from 4 to 20 r/min at the rate of 0.5 r/s. After completing the training, rats were placed onto a rotating rod with steady acceleration to 20 r/min. The duration time of staying on the rotating rod was recorded. Each rat was given three trials in 30-min intervals and the average time was recorded as the final result.
Footprint analysis
Footprint analysis was used to assess limb coordination and the distance between the centers of ipsilateral adjacent footprints. The hindpaws of each rat were dipped in yellow dye and footprints were recorded on the white paper. The average distance of five sequential steps was then measured and recorded.
Open field test
To evaluate 6-OHDA-induced motor deficits, the rats were tested in an open field (50 cm × 50 cm × 30 cm) of an automated Flexfield/Open Field Activity System (DigBehv-LR4 System, JiLiang Software Technology Co. Ltd., Shanghai, China). It was constructed by plywood planks and divided into 4 quadrants of equal size. The rat was initially placed in the center, and the traveling distance and average speed of the freely moving rats were recorded during 10-min session and analyzed using JLDigBehv-LR4 System software (Jiliang Software Technology Co. Ltd., Shanghai, China).
Immunofluorescence
Rats were randomly chosen from sham and 6-OHDA groups (n = 2 for each group). They were anesthetized with 4% chloral hydrate (400 mg/kg, i.p.) and transcardially perfused with 4% paraformaldehyde (PFA). Brains were harvested and postfixed in PFA followed by dehydration in a solution of 25% sucrose. Coronal sections of the midbrain in 20-μm thickness were cut on a cryostat microtome (Leica, Wetzlar, Germany), and incubated by mouse monoclonal anti-tyrosine hydroxylase (TH) (1:1000, Sigma, St. Louis, USA) at 4°C overnight. Then, slides were incubated with anti-mouse Alexa488 secondary antibody for 1 h at room temperature. Finally, coverslips were mounted with DAPI (Vector Laboratories, USA) and photographed under a positive Zeiss microscope (Axio Scope A1, Zeiss Corp., Goettingen, Germany).
Western blotting
Tissues from left striatum (n = 3 for each group) were homogenized in lysis buffer (150 mmol/L NaCl, 25 mmol/L Tris, 5 mmol/L EDTA, 1% Nonidet; pH 7.5) with protease inhibitor cocktail tablets (Roche Diagnostics, Penzberg, Germany). Protein lysates were separated by 10% sodium dodecyl sulfate-polyacrylamide gels and transferred onto polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA). Then, the membranes were blocked with 5% milk in 0.1% Tris-buffered saline/Tween 20 for 1 h and incubated with primary mouse anti-TH antibody (1:5000, Sigma T1299) and mouse anti-β-actin antibody (1:8000, Sigma) at 4°C overnight. Membranes were briefly washed and incubated with secondary antibodies for another 1 h. Finally, membranes were visualized by a chemiluminescence kit (Bio-Rad, 170–5061). The densitometric analysis was performed using ImageJ software (National Institute of Health, Bethesda, MD, USA).
Melatonin and cortisol measurement
Plasma was collected and subjected to ELISA (R&D Systems, USA) according to the manufacturer's instructions. Absorbance was measured at 450 nm using a microplate reader (Tecan M200, Grodig, Austria).
Neurotransmitter measurement
The right side of the striatal content of DA, DOPAC and 5-HIAA were determined by HPLC with an electrochemical detector (Antec, Zoeterwoude, The Netherlands). Homogenates were prepared in 4% perchloric acid and centrifuged at 4°C (14,000 ×g, 20 min). The supernatants were filtered with 0.22-μm syringe filters and applied to the HPLC system. The standard samples of the monoamines and their metabolites were similarly processed as the brain homogenates. Then, the monoamine level was quantified with the standard curves and the results were expressed as ng per mg wet tissue.
Quantitative real-time polymerase chain reaction
The left side of striatal and SCN content, liver were used for QPCR analysis. Total RNA was extracted using Trizol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA), 1 μg RNA was reverse transcribed into cDNA using a cDNA synthesis kit (Roche Diagnostics, Germany). Real-time PCR was conducted on the 7500 real-time PCR system (Applied Biosystems, Foster City, CA, USA) with the following primers: 18S (forward: 5’-TCAACACGGGAAACCTCAC-3’, reverse: 5’-CGCTCCACCAACTAAGAAC-3’), Bmal1 (forward: 5’-CTTGTCTGTAAAACTTGCCTGTGAC-3’, reverse: 5’-GTAGATCAGAGGGCGACGGCTA-3’), Clock (forward: 5’-GTAGGTTTCCAGTCCTGTCG-3’, reverse: 5’-TGGGGTCTATGCTTCCTGGT-3’); Per2, (forward: 5’-AGGATCCAAGAACGGCACAG-3’, reverse: 5’-CGGACCTGGCTTCAGTTCAT-3’); Rorα (forward: 5’-CCAAACTTGACAGCATCTCGA-3’, reverse: 5’-GAAGGCTGCAAGGGCTTTTTCAGGA-3’). The results were normalized to 18S RNA.
Statistical analysis
Data were analyzed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA). Data were presented as mean ± standard error. The significance of multiple groups and time-dependent variation in each group were analyzed using one-way analysis of variance (ANOVA), followed by Tukey's post hoc test. Two-way ANOVA was applied for the significance of differences of circadian phases among groups, and Tukey's post hoc test for multiple comparisons. Differences of values at the same time points were performed using one-way ANOVA, followed by Dunnett's post hoc test. P < 0.05 was considered statistically significant.
Results
Improved motor ability after L-DOPA treatment
There was a slight increase in the body weight in both sham and 6-OHDA group rats (n = 14 for each group) during the treatment period. In contrast, we observed the trend of weight loss in 6-OHDA-lesioned rats receiving L-DOPA treatment [Figure 1b]. When compared to sham group, 6-OHDA group showed less running time in the rotarod test (n = 14 for each group, P < 0.01, ANOVA), and L-DOPA tended to improve it [Figure 1c]. In the footprint and open field test, L-DOPA alleviated the motor deficits induced by 6-OHDA lesions (n = 14 for each group, P < 0.01, P < 0.001 respectively, ANOVA) [Figure 1d and 1e]. As expected, Western blotting showed that the striatal TH protein level decreased by 50% in 6-OHDA-lesioned rats (n = 3 for each group, P < 0.01, ANOVA). However, there was no significant difference between 6-OHDA and L-DOPA group [Figure 1f]. Consistently, the immunostaining demonstrated that the number of TH-positive cells was decreased in the substantia nigra pars compacta in 6-OHDA group [Figure 1g].
Modified expression of clock genes in suprachiasmatic nucleus and striatum but not in liver after L-DOPA treatment
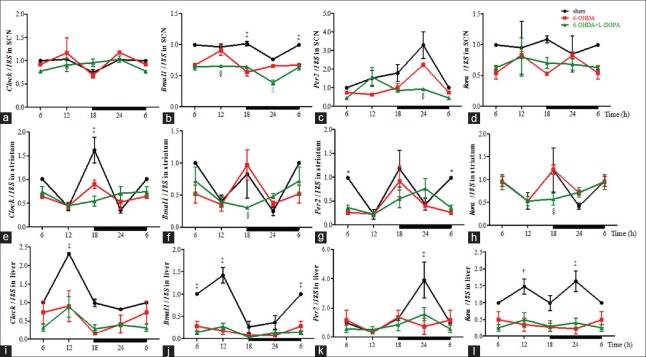
In the circadian pacemaker SCN, the QPCR analysis (n = 3 for each time point, equals to n = 12 in each group) showed an obvious effect of time on the expression profile of Clock, Bmal1, Per2 in sham group [P < 0.01, P < 0.01, P < 0.05, ANOVA, Figure 2a–2c]. Also, Rorα showed the similar pattern with Bmal1 in sham group [Figure 2d]. Two-way ANOVA showed that there was difference in the mRNA levels of Bmal1, Per2 among three groups (treatment, F2,40 = 78.72, P < 0.0001; F2,40 = 11.68, P < 0.001). Clock did not show a significant difference between sham and 6-OHDA group while the daily rhythm of Bmal1 in the 6-OHDA-lesioned rat was markedly decreased at 18:00 and 6:00 (P < 0.001). When compared to the 6-OHDA group, the levels of Bmal1 expression were even lower at 12:00 (P < 0.05) and 24:00 (P < 0.01) in L-DOPA treatment group. Similarly, Per2 showed a significant decrease at 24:00 (P < 0.05), and its peak value exhibited an approximately 12-h phase advance.
Figure 2.
QPCR analysis of the mRNA level of clock genes in the SCN (a-d), striatum (e-h) and liver (i-l). The left side of the brain was used for QPCR analysis, n = 3 for each time point, equals to n = 12 in each group. *P < 0.05, †P < 0.01, ‡P < 0.001 for 6-OHDA group versus sham group; §P < 0.05, ||P < 0.01 for 6-OHDA group versus L-DOPA group. 6-OHDA: 6-hydroxydopamine; SCN: Suprachiasmatic nucleus; QPCR: Quantitative polymerase chain reaction.
Next, in the striatum, the mRNA expression of Clock and Per2 [Figure 2e and 2g] displayed time-dependent variations in the sham group (P < 0.001, P < 0.05, ANOVA). However, these rhythms were abolished after administration of 6-OHDA, and L-DOPA did not improve it. Two-way ANOVA showed there was a significant difference in the mRNA levels of Clock among three groups (treatment, F2,40 = 5.00, P < 0.05). A significant decrease of Clock was shown in 6-OHDA group at 18:00 (P < 0.001), as well as Per2 at 6:00 (P < 0.05). Interestingly, when compared to the 6-OHDA group, the phase of the four clock genes was delayed in L-DOPA-treated rats. Specifically, Bmal1 and Rorα [Figure 2f and 2h] reduced about 60% at 18:00 (P < 0.05). In addition, L-DOPA seemed to delay the peak of Per2 to 24:00. Furthermore, the expression pattern of Rorα was altered in L-DOPA group, with the peak shifting from 18:00 to 6:00.
Meanwhile, the peripheral organ, liver, showed that the 24-h rhythm of Clock, Bmal1 and Per2 [Figure 2i–2k] was observed in the sham group (P < 0.001, P < 0.0001, P < 0.05, ANOVA). However, the rhythmicity of Clock and Bmal1 was blunted in 6-OHDA ± L-DOPA group, and two-way ANOVA showed that there was a significant difference among three groups (treatment, F2,40 = 57.87, P < 0.0001; F2,40 = 21.43, P < 0.0001). When compared to the sham group, the expression of Clock and Bmal1 in the 6-OHDA ± L-DOPA group was generally decreased throughout the whole day, especially in the daytime such as 12:00 (P < 0.001, P < 0.0001). Similarly, the peak of Per2 was decreased at 24:00 (P < 0.001). In addition, Rorα mRNA levels [Figure 2l] also declined both in the daytime (P < 0.01) and the nighttime (P < 0.001). However, L-DOPA did not affect the changes of the expression profiles of clock genes induced by 6-OHDA.
Changed secretion levels of cortisol and melatonin after L-DOPA treatment
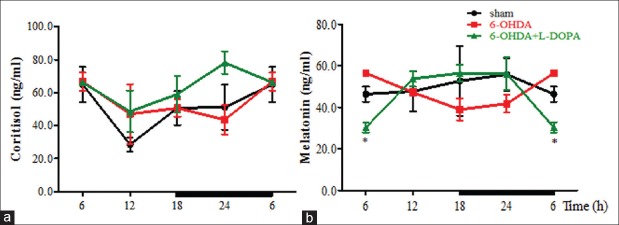
Cortisol and melatonin served as two main biomarkers of the circadian system. In our study, the secretion pattern of these two hormones showed no obvious time-dependent variation in three groups (P > 0.05, ANOVA). However, the area under curve (AUC) analysis showed the cortisol secretion level maintained in 6-OHDA + L-DOPA group, and L-DOPA treatment further elevated the cortisol secretion compared to 6-OHDA group [Figure 3a, sham: 150 ± 20 ng/ml×min; 6-OHDA: 150 ± 40 ng/ml×min; L-DOPA: 190 ± 20 ng/ml×min; P > 0.05, ANOVA]. Besides, sham and 6-OHDA group showed the maximal cortisol secretion in the early morning at 6:00, whereas L-DOPA treatment put this peak forward about 6 h. AUC analysis of melatonin in the plasma indicated a decrease in 6-OHDA group [Figure 3b, sham: 160 ± 20 ng/ml×min; 6-OHDA: 130 ± 20 ng/ml×min; L-DOPA: 150 ± 10 ng/ml×min, P > 0.05, ANOVA]. Its peak reached at 24:00 in the sham group, while it shifted to 6:00 in 6-OHDA group. Notably, L-DOPA treatment seemed to reverse the shift phase to the normal. However, at 6:00, the melatonin level was much lower in L-DOPA group than that in 6-OHDA group (P < 0.01).
Figure 3.
The concentrations of cortisol (a) and melatonin (b) in plasma were detected by ELISA method and expressed as ng/ml. n = 3 for each time point, equals to n = 12 in each group. *P < 0.01 for 6-OHDA group versus L-DOPA group. 6-OHDA: 6-hydroxydopamine; ELISA: Enzyme-linked immunosorbent assay.
Altered rhythm of dopamine and serotonin metabolism in striatum
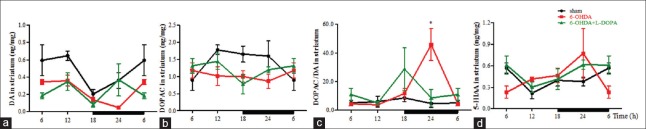
We measured the amount of DA, 5-HT and their metabolites in the right side of striatum [Figure 4a–4d] (n = 3 for each time point, equals to n = 12 in each group). A time-dependent rhythm of DA was observed (P < 0.01, ANOVA), which was abolished in 6-OHDA ± L-DOPA group (treatment, F2,40 = 16.96, P < 0.0001). Moreover, the AUC analysis indicated that the average amount of DA in unilateral striatum declined in 6-OHDA group, which was partially elevated by L-DOPA treatment (sham: 1.19 ± 0.20 ng/mg×min; 6-OHDA: 0.49 ± 0.03 ng/mg×min; L-DOPA: 0.71 ± 0.22 ng/mg×min). The ratio of DOPAC/DA was elevated in 6-OHDA group (P < 0.05) and the peak of DA metabolism shifted from 24:00 to 18:00. 5-HIAA reached its peak at about 6:00 in the sham group, whereas its peak was advanced to 24:00 in 6-OHDA ± L-DOPA group.
Figure 4.
Neurotransmitters content of DA, DOPAC, 5-HIAA were analyzed in the striatum (a-d) with HPLC, and the results were expressed as ng/mg wet tissue. The right side of the brain was used for HPLC analysis, n = 3 for each time point, equals to n = 12 in each group. *P < 0.05 for 6-OHDA group versus sham group. 6-OHDA: 6-hydroxydopamine; HPLC: High performance liquid chromatography.
Discussion
In clinical practice, the long course of PD was always accompanied with long-term and high-dose levodopa treatment. Thus, it was difficult to distinguish whether the disease itself or the drug therapy contributed to the rhythm dysregulation. Unfortunately, few basic studies focused on the effect of chronic L-DOPA therapy on the circadian rhythm in advanced PD models. Therefore, 6-OHDA-induced PD rats with 21 days of high-dose L-DOPA administration was used to probe into the issue. Based on the expression profile of clock genes, we found dysfunctions of circadian rhythm were more severe after long-term L-DOPA treatment in 6-OHDA-lesioned rats.
Recently, several studies reported the circadian dysfunctions in PD patients.[12,13] Moreover, such circadian dysfunctions are more common in advanced patients. Our previous research showed the higher incidence rate of rapid eye movement sleep behavior disorder was associated with the longer PD duration.[14] Other studies also showed that patients with lower insomnia scores and more sleep fragmentations had higher unified Parkinson's disease rating scale (UPDRS) scores.[15,16] Heart rate oscillation was also related to the disease severity.[17] Meanwhile, many clinical studies also proposed that chronic anti-parkinsonian medication, such as levodopa, had a close relationship with the severe motor or nonmotor symptoms.[6] Hence, we hypothesized that the severe circadian dysfunction in PD patients may also be related to the long-term L-DOPA treatment.
SCN was the central controller of circadian rhythm, destruction of the SCN could dysregulate a series of behavioral and physiological rhythms.[18] The postmortem study showed α-synuclein accumulation and the formation of Lewy bodies in SCN. Researchers also observed the decline of clock genes expression in rotenone-induced PD rodents.[19] Consistently, we observed that the clock gene expression was also downregulated in 6-OHDA group. Moreover, L-DOPA treatment further suppressed their transcription. The reduction in Bmal1 transcription in SCN was particularly apparent after L-DOPA treatment. Decreased Bmal1 may result in less formation of CLOCK-BMAL1 heterodimers, which regulated the clock-controlled gene expression through E-Box. Similarly, the antioxidant activity, which was under the control of circadian rhythm, may also be suppressed, and thus accelerated the progression of PD.[20,21]
The reduction of clock genes expression was also observed in the striatum. The decrease was more evident probably due to the high susceptibility of this brain region. Administration of L-DOPA for 21 days did not restore the arrhythm of these clock genes in 6-OHDA-lesioned rats. Instead, the amplitude was much lower than that in 6-OHDA group. Dopamine could affect the clock genes expression through dopamine receptors. The expression of D2R in the striatum was decreased in 6-OHDA group, whereas the remaining receptor was in highly sensitive state.[22] D2R played a negative role in clock genes. Activation of D2R could lead to the lower level of intracellular cyclic adenosine monophosphate (cAMP) due to the inhibition of the adenylyl cyclase enzyme and then resulted in the less expression of clock genes through the cAMP-responsive element (CRE). In addition, D2R was reported to enhance the transcriptional capacity of the CLOCK-BMAL1 complex through a CREB-dependent mechanism, and then inhibited Per2 transcription by E-Box element.[23] Thus, on L-DOPA administration, D2R may be activated and then exerted an inhibitory effect on the expression of clock genes in the striatum.
Liver, an important metabolic organ, showed robust circadian rhythm according to the environment.[24,25] Surprisingly, the circadian oscillation in the liver was totally abolished after midbrain dopamine depletion by 6-OHDA lesion, which could not be alleviated by L-DOPA replacement therapy. Perhaps, the liver was out of the control by the impaired SCN oscillator and could not be self-sustained. What's more, the hepatic rhythm of clock genes could be entrained by feeding.[26] Less food taken in our PD model might reduce the circadian inputs to the liver. In addition, the levodopa we applied was accompanied with the decarboxylase inhibitor, which prohibited the L-DOPA metabolizing into its biologically active form in the peripheral system. Therefore, L-DOPA might exert no effect on the liver.
To get more evidence of L-DOPA on the circadian rhythm, we further investigated the hormones and transmitters. Clinical research reported that the melatonin secretion patterns in PD patient showed a decreased amplitude and phase advance in the dopaminergic-treated group.[27] The altered nychthemeral pattern of melatonin was observed in our 6-OHDA model. The phase was advanced to daytime and the amplitude showed a decrease which might reflect the desynchronization in PD patients. However, we did not find any rhythm of the plasma cortisol among three groups, but the AUC was slightly larger in the 6-OHDA group than that in the sham group, which was also evidenced by 24-h blood sample in PD patients.[28] Surprisingly, L-DOPA treatment could elevate the serum cortisol level by 30%. Similarly, results were reported in PD patients with stable levodopa compliance.[29] This phenomenon may be clarified by the fact that 6-OHDA lesions not only cause degeneration of dopaminergic neurons but also neurons in the hippocampus, decreasing the expression of hippocampal mineralocorticoid receptors.[30] Meanwhile, this higher secretion of glucocorticoids may be related to the cognitive impairment in advanced PD patients.[31] Normally, plasma dopamine level reached its peak in the morning,[32] but this rhythm of dopamine secretion was lost and flattened due to the loss of dopaminergic neuron after 6-OHDA lesions. In addition, the expression pattern of 5-HIAA was altered with the shift of peak. L-DOPA treatment partially reversed this changed pattern and might have a positive effect on depression, in consistence with the clinical observations that L-DOPA therapy could ameliorate the depression in PD patients.[33]
However, several limitations should be taken into consideration. Although dopamine is thought to play an important role in regulating the circadian rhythm, if combined with the samples from advanced PD patients who take large doses of levodopa regularly, we could verify the results in our model and provide stronger evidence. What else, further researches on the mutual regulation of dopamine and clock genes are needed to give an explanation to the phenomenon we have found.
In conclusion, long-term L-DOPA treatment exacerbated the disruptions of circadian rhythm in 6-OHDA-lesioned rats, that might provide some guidance for PD treatment. For instance, the advanced PD patients can hardly control the motor symptoms without levodopa; it is hard to say what will happen to the circadian system if levodopa is reduced in these patients. Fortunately, several interventions, such as physical activity, melatonin therapy, and bright light therapy, which have been proved to be potential approaches for restoring the circadian arhythmicity, can be applied to the advanced PD patients. Besides, deep brain stimulation has beneficial effects on nonmotor symptoms, such as sleep, cardiovascular, and sensory dysfunctions,[34] perhaps it can also confer some advantages to the circadian system.
Financial support and sponsorship
This work was supported by grants from the National Natural Science Foundation of China (No. 91649114), Jiangsu Provincial Special Program of Medical Science (No. BL2014042), the Plans for Graduate Research and Innovation in Colleges and Universities of Jiangsu Province (No. KYZZ15_0334), and Suzhou Clinical Research Center of Neurological Disease (No. Szzx201503). This was also partly supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions and Jiangsu Provincial Medical Key Discipline Project.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
References
- 1.Zhang ZX, Roman GC, Hong Z, Wu CB, Qu QM, Huang JB, et al. Parkinson's disease in China: Prevalence in Beijing, Xian, and Shanghai. Lancet. 2005;365:595–7. doi: 10.1016/S0140-6736(05)17909-4. doi: 10.1016/S0140-6736(05)17909-4. [DOI] [PubMed] [Google Scholar]
- 2.Bonuccelli U, Del Dotto P, Lucetti C, Petrozzi L, Bernardini S, Gambaccini G, et al. Diurnal motor variations to repeated doses of levodopa in Parkinson's disease. Clin Neuropharmacol. 2000;23:28–33. doi: 10.1097/00002826-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Faludi B, Janszky J, Komoly S, Kovács N. Sleep disturbances in Parkinson's disease: Characteristics, evaluation and therapeutic approaches. Orv Hetil. 2015;156:1091–9. doi: 10.1556/650.2015.30191. doi: 10.1556/650.2015.30191. [DOI] [PubMed] [Google Scholar]
- 4.Struck LK, Rodnitzky RL, Dobson JK. Circadian fluctuations of contrast sensitivity in Parkinson's disease. Neurology. 1990;40(3 Pt 1):467–70. doi: 10.1212/wnl.40.3_part_1.467. [DOI] [PubMed] [Google Scholar]
- 5.Oh YS, Kim JS, Lee KS. Orthostatic and supine blood pressures are associated with white matter hyperintensities in Parkinson disease. J Mov Disord. 2013;6:23–7. doi: 10.14802/jmd.13006. doi: 10.14802/jmd.13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Videnovic A, Noble C, Reid KJ, Peng J, Turek FW, Marconi A, et al. Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson disease. JAMA Neurol. 2014;71:463–9. doi: 10.1001/jamaneurol.2013.6239. doi: 10.1001/jamaneurol.2013.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozekmekçi S, Apaydin H, Kiliç E. Clinical features of 35 patients with Parkinson's disease displaying REM behavior disorder. Clin Neurol Neurosurg. 2005;107:306–9. doi: 10.1016/j.clineuro.2004.09.021. doi: 10.1016/j.clineuro.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 8.Videnovic A, Lazar AS, Barker RA, Overeem S. ‘The clocks that time us’ – Circadian rhythms in neurodegenerative disorders. Nat Rev Neurol. 2014;10:683–93. doi: 10.1038/nrneurol.2014.206. doi: 10.1038/nrneurol.2014.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowrey PL, Takahashi JS. Genetics of circadian rhythms in Mammalian model organisms. Adv Genet. 2011;74:175–230. doi: 10.1016/B978-0-12-387690-4.00006-4. doi: 10.1016/B978-0-12-387690-4.00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu B, Xiao ZY, Li JZ, Yuan J, Liu YM. Study of an integrated non-motor symptoms questionnaire for Parkinson's disease. Chin Med J. 2010;123:1436–40. [PubMed] [Google Scholar]
- 11.Hu LF, Lu M, Tiong CX, Dawe GS, Hu G, Bian JS. Neuroprotective effects of hydrogen sulfide on Parkinson's disease rat models. Aging Cell. 2010;9:135–46. doi: 10.1111/j.1474-9726.2009.00543.x. doi: 10.1111/j.1474-9726.2009.00543.x. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Wang Y, Wang F, Hu LF, Liu CF. A new perspective for Parkinson's disease: Circadian rhythm. Neurosci Bull. 2017;33:62–72. doi: 10.1007/s12264-016-0089-7. doi: 10.1007/s12264-016-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Videnovic A, Golombek D. Circadian and sleep disorders in Parkinson's disease. Exp Neurol. 2013;243:45–56. doi: 10.1016/j.expneurol.2012.08.018. doi: 10.1016/j.expneurol.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gong Y, Xiong KP, Mao CJ, Shen Y, Hu WD, Huang JY, et al. Clinical manifestations of Parkinson disease and the onset of rapid eye movement sleep behavior disorder. Sleep Med. 2014;15:647–53. doi: 10.1016/j.sleep.2013.12.021. doi: 10.1016/j.sleep.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 15.Norlinah MI, Afidah KN, Noradina AT, Shamsul AS, Hamidon BB, Sahathevan R, et al. Sleep disturbances in Malaysian patients with Parkinson's disease using polysomnography and PDSS. Parkinsonism Relat Disord. 2009;15:670–4. doi: 10.1016/j.parkreldis.2009.02.012. doi: 10.1016/j.parkreldis.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Lee JE, Kim KS, Shin HW, Sohn YH. Factors related to clinically probable REM sleep behavior disorder in Parkinson disease. Parkinsonism Relat Disord. 2010;16:105–8. doi: 10.1016/j.parkreldis.2009.08.005. doi: 10.1016/j.parkreldis.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Harnod D, Wen SH, Chen SY, Harnod T. The association of heart rate variability with parkinsonian motor symptom duration. Yonsei Med J. 2014;55:1297–302. doi: 10.3349/ymj.2014.55.5.1297. doi: 10.3349/ymj.2014.55.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inouye ST, Shibata S. Neurochemical organization of circadian rhythm in the suprachiasmatic nucleus. Neurosci Res. 1994;20:109–30. doi: 10.1016/0168-0102(94)90029-9. doi: 10.1016/0168-0102(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 19.Mattam U, Jagota A. Daily rhythms of serotonin metabolism and the expression of clock genes in suprachiasmatic nucleus of rotenone-induced Parkinson's disease male Wistar rat model and effect of melatonin administration. Biogerontology. 2015;16:109–23. doi: 10.1007/s10522-014-9541-0. doi: 10.1007/s10522-014-9541-0. [DOI] [PubMed] [Google Scholar]
- 20.Belden WJ, Dunlap JC. SIRT1 is a circadian deacetylase for core clock components. Cell. 2008;134:212–4. doi: 10.1016/j.cell.2008.07.010. doi: 10.1016/j.cell.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren JP, Zhao YW, Sun XJ. Toxic influence of chronic oral administration of paraquat on nigrostriatal dopaminergic neurons in C57BL/6 mice. Chin Med J. 2009;122:2366–71. [PubMed] [Google Scholar]
- 22.Hood S, Cassidy P, Cossette MP, Weigl Y, Verwey M, Robinson B, et al. Endogenous dopamine regulates the rhythm of expression of the clock protein PER2 in the rat dorsal striatum via daily activation of D2 dopamine receptors. J Neurosci. 2010;30:14046–58. doi: 10.1523/JNEUROSCI.2128-10.2010. doi: 10.1523/JNEUROSCI.2128-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yujnovsky I, Hirayama J, Doi M, Borrelli E, Sassone-Corsi P. Signaling mediated by the dopamine D2 receptor potentiates circadian regulation by CLOCK: BMAL1. Proc Natl Acad Sci U S A. 2006;103:6386–91. doi: 10.1073/pnas.0510691103. doi: 10.1073/pnas.0510691103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–61. doi: 10.1101/gad.183500. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peirson SN, Butler JN, Duffield GE, Takher S, Sharma P, Foster RG. Comparison of clock gene expression in SCN, retina, heart, and liver of mice. Biochem Biophys Res Commun. 2006;351:800–7. doi: 10.1016/j.bbrc.2006.10.118. doi: 10.1016/j.bbrc.2006.10.118. [DOI] [PubMed] [Google Scholar]
- 26.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–3. doi: 10.1126/science.291.5503.490. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 27.Bordet R, Devos D, Brique S, Touitou Y, Guieu JD, Libersa C, et al. Study of circadian melatonin secretion pattern at different stages of Parkinson's disease. Clin Neuropharmacol. 2003;26:65–72. doi: 10.1097/00002826-200303000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Breen DP, Vuono R, Nawarathna U, Fisher K, Shneerson JM, Reddy AB, et al. Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol. 2014;71:589–95. doi: 10.1001/jamaneurol.2014.65. doi: 10.1001/jamaneurol.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartmann A, Veldhuis JD, Deuschle M, Standhardt H, Heuser I. Twenty-four hour cortisol release profiles in patients with Alzheimer's and Parkinson's disease compared to normal controls: Ultradian secretory pulsatility and diurnal variation. Neurobiol Aging. 1997;18:285–9. doi: 10.1016/s0197-4580(97)80309-0. [DOI] [PubMed] [Google Scholar]
- 30.Seckl JR, Dickson KL, Yates C, Fink G. Distribution of glucocorticoid and mineralocorticoid receptor messenger RNA expression in human postmortem hippocampus. Brain Res. 1991;561:332–7. doi: 10.1016/0006-8993(91)91612-5. doi: 10.1016/0006-8993(91)91612-5. [DOI] [PubMed] [Google Scholar]
- 31.Wolkowitz OM. Prospective controlled studies of the behavioral and biological effects of exogenous corticosteroids. Psychoneuroendocrinology. 1994;19:233–55. doi: 10.1016/0306-4530(94)90064-7. doi: 10.1016/0306-4530(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 32.Sowers JR, Vlachakis N. Circadian variation in plasma dopamine levels in man. J Endocrinol Invest. 1984;7:341–5. doi: 10.1007/BF03351014. doi: 10.1007/BF03351014. [DOI] [PubMed] [Google Scholar]
- 33.Bellante F, Dethy S, Zegers de Beyl D. Depression, anxiety and non-motor symptoms on initiation of intrajejunal levodopa/carbidopa therapy. Acta Neurol Belg. 2016;116:39–41. doi: 10.1007/s13760-015-0497-x. doi: 10.1007/s13760-015-0497-x. [DOI] [PubMed] [Google Scholar]
- 34.Wang XH, Zhang L, Sperry L, Olichney J, Farias ST, Shahlaie K, et al. Target selection recommendations based on impact of deep brain stimulation surgeries on nonmotor symptoms of Parkinson's disease. Chin Med J. 2015;128:3371–80. doi: 10.4103/0366-6999.171464. doi: 10.4103/0366-6999.171464. [DOI] [PMC free article] [PubMed] [Google Scholar]