Abstract
Background:
Various types of lumbar dural punctures may contribute to neurological injury. The etiologies of dural injury include; inadvertent dural punctures due to epidurals placed for labor anesthesia, epidural steroid injections (ESI/transforaminal TESI; approximately 9 million ESI performed in the US per year), deliberate placement of intradural pain devices, and spontaneous cerebrospinal fluid (CSF) fistulas. Resulting neurological complications may include; spinal headaches/intracranial hypotension, subdural hematomas, and 6th nerve cranial palsies. Furthermore, uniquely in the cervical spine, inadvertent cervical dural punctures attributed to cervcial ESI (CESI) may lead to intramedullary spinal cord injuries (e.g. resulting in monoparesis to quadriplegia) or spinal cord strokes due to intravascular/vertebral artery injections.
Methods/Results:
In 8 studies, inadvertent lumbar dural punctures contributed to intracranial hypotension, subdural hematomas, and double vision/6th cranial nerve palsies. In 5 of the 6 studies, inadvertent dural punctures occurring during CESI were responsible for intramedullary spinal cord injuries, or direct intravascular/vertebral injections resulting in monoplegia/quadriplegia.
Conclusions:
Inadvertent lumbar dural punctures led to multiple neurological complications including intracranial hypotension, subdural hematomas, and double vision/6th cranial nerve palsies. Uniquely, inadvertent cervical dural punctures solely due to CESI directly resulted in intramedullary spinal cord injuries or cord stroked and monoplegia/quadriplegia attributed to intravascular/vertebral artery injections. The potential neurological risks/complications/adverse events attributed to lumbar and cervical ESI must be taken into account before spine surgeons and others order these procedures.
Keywords: Cervical, complications, epidural steroid injection, lumbar, monoplegia, new deficit, quadriplegia, risks, sixth cranial nerve palsy
INTRODUCTION
Various types of lumbar and cervical dural punctures result in significant neurological injury. In the lumbar spine, lumbar dural punctures may be attributed to; inadvertent epidural injections for labor/anesthesia, deliberate epidural steroid injections (ESI) [(lumbar LESI)/transforaminal TFESI)], direct placement of intradural/intrathecal pain devices, deliberately when placing intradural pain devices, or in rare instances, spontaneously. Neurological complications attributed to these lumbar punctures include; spinal headaches/intracranial hypotension, subdural hematomas, and 6th cranial nerve palsies/double vision. Cervical dural punctures solely attributed to cervical ESI (CESI), uniquely risked monoparesis/quadrliplegia due to intramedullary spinal cord injections/injury, or stroke due to intravascular vertebral artery injections. The neurological complications/adverse events (AE)/risks of lumbar and cervical ESI must be carefully considered when spine surgeons and other specialists order these procedures often for minimal spinal complaints.
LUMBAR SPINAL EPIDURAL INJECTIONS IN US
Look at how often non FDA (Food and Drug Administration) approved ESI are being perfomed in the U.S. Manchikanti et al. estimated that approximately 9 million epidural steroid injections are performed in the US per year.[15] They examined the frequency of LESI performed in the US Medicare population between 2000 and 2014, and found it increase by 99%; 36.2% were lumbar interlaminar or caudal epidural injections. In addition, over this period, there was a 609% increase in lumbar transforaminal ESI. Bhatia et al. assessed the data regarding the efficacy of LESI based on 8 randomized controlled trials; 366 patients received TFESI with steroids, whereas 405 had TFESI with a local anesthetic alone without steroids.[2] Although the TFESI steroids afforded “moderate” analgesia at 3 months, they had no impact on “physical disability or incidence of surgery”. In short, they had no long-term documented efficacy. Furthermore, there were marked variations in the quality of meta-analysis data, and they recommended better “well designed, large, randomized studies” be performed in the future. An additional notation by Yang et al. was that lumbar ESI performed within 3 months of one-level spine surgery correlated with an increased risk of wound infection (e.g. 0.8–1.7% of patients).[21] They, therefore, recommended that more than 3 months elapse between LESI and single level spine procedures. Another concern is that recent ESI (e.g. within 3 months of surgery) increase the incidence of inadvertently puncturing the dura in patients with severe stenosis/ossification of the yellow ligament.
INADVERTENT LUMBAR INTRADURAL INJECTIONS OCCURRING DURING ATTEMPTED EPIDURAL INJECTIONS FOR ANESTHESIA DURING LABOR AND DELIVERY
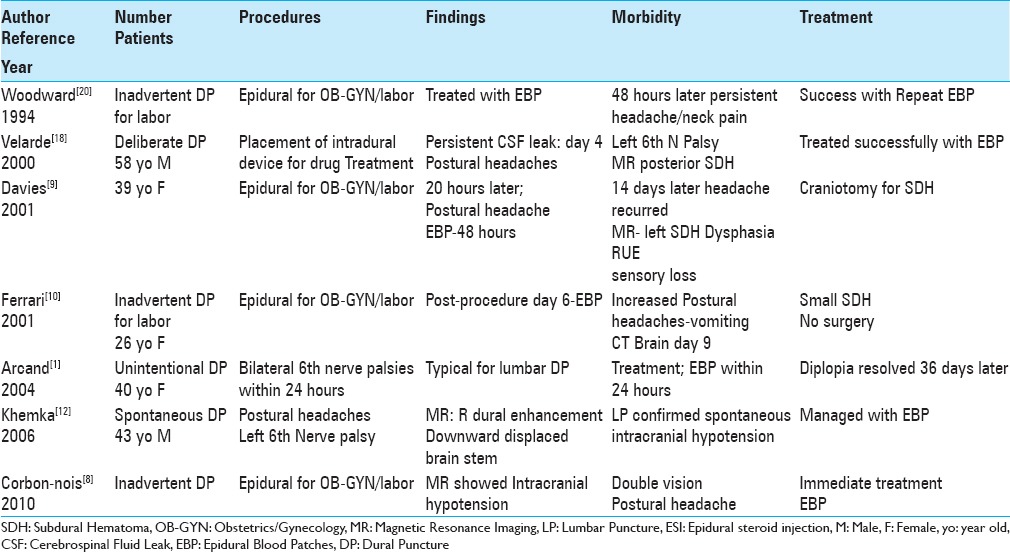
Inadvertent lumbar dural punctures resulted in intracranial hypotension, subdural hematomas, and/or 6th cranial nerve palsies in 4 patients undergoing attempted epidural spinal injections performed to deliver anesthesia during labor/delivery [Table 1].[8,9,10,20] The first patient developed intracranial hypotension alone, whereas the second patient exhibited both intracranial hypotension and double vision; both were successfully treated with epidural blood patches (EBP).[8,20] The third patient was a 39-year-old female who developed postural headaches 20 hours after her epidural injection; she required an EBP (epidural blood patch) 48 hours post- injection.[9] However, 14 days later, with headaches, expressive dysphasia, lack of coordination, and sensory loss in the right arm, she underwent an MR scan that documented a left SDH; she required a craniotomy, for which she required a craniotomy. The fourth patient, a 26-year-old female developed postural headaches 6 days after an epidural; she was first treated with an EBP.[10] Nine days later, with vomiting and continued postural headaches, the brain CT showed a small hemispheric SDH that did not require surgery.
Table 1.
Lumbar epidural spinal injections and inadvertent dural puncture (DP) lead to intracranial hypotension, subdural hematomas, and/or cranial nerve palsies
THREE CASES OF DOUBLE VISION/6TH CRANIAL NERVE PALSIES DUE TO DELIBERATE, INADVERTENT, OR SPONTANEOUS LUMBAR DURAL PUNCTURES
Three cases of double vision/6th cranial nerve palsies were variously attributed to deliberate, inadvertent, or spontaneous lumbar dural punctures [Table 1].[1,12,18] In the first case, a 58-year-old male developed intracranial hypotension/postural headaches and a left 6th cranial nerve palsy 4 days after placement of an intrathecal pain device (e.g., intradural/subarachnoid drug-delivering device for chronic back pain).[18] When the brain MR showed a small posterior SDH, he required an EBP for symptom resolution. In the second case, a 40-year-old female developed bilateral 6th nerve palsies 24 hours after an “unintentional dural puncture;” this was immediately treated with an EBP, and her diplopia resolved 36 days later.[1] In the third case, a 43-year-old male spontaneously developed a left 6th cranial nerve palsy accompanied by postural headaches/intracranial hypotension that correlated with MR scan findings of dural enhancement/downward displacement of the brain stem; for the intracranial hypotension, the patient was successfully managed with an EBP.[12]
CERVICAL EPIDURAL SPINAL INJECTIONS
Bureau et al. noted the “catastrophic complications” that may be seen with cervical ESI (BUREAU 2014).[4] To minimize these risks, they designed a 4-week randomized double blind controlled study to compare the safety/efficacy for cervical intrafacet steroid injections (noted to have fewer AE (Adverse Events)) vs. TFESI. Twenty-eight patients had facet injections alone vs. 28 who underwent cervical CT-fuided TFESI; facet injections significantly reduced the pain score by 45.3% vs. TFESI that showed a “nonsignificant” pain score reduction of just 9.8%. Furthemore, cervical facet injections effectively managed cervical radiculopathy use; and were a safer option than TFESI. The study by Wald et al. discussed/compared the safety/efficacy of CT-guided TFESI in the cervical spine using a posterior approach, but demonstrated no significant differences in pain relief when compared with other approaches.[19]
FOUR CERVICAL EPIDURAL STEROID INJECTIONS RESPONSIBLE FOR INTRAMEDULLARY SPINAL CORD INJURIES
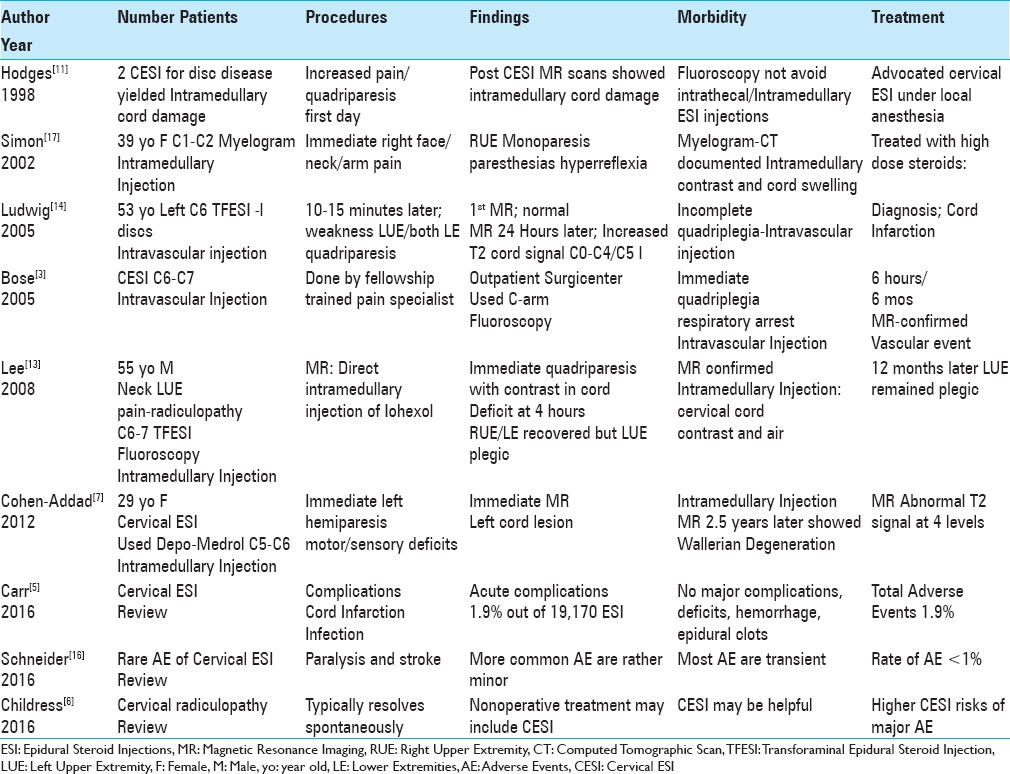
Four patients in 3 studies developed monoplegia/quadriplegia following intramedullary spinal cord injections occurring during cervical ESI (CESI) [Table 2].[7,11,13] The first two patients developed irreversible intramedullary cord damage after these injections.[11] The third patient, a 55-year-old-male, was immediately quadriparetic following a fluoroscopically-guided cervical transforaminal (TFESI) utilizing Iohexol; the MR study documented an intramedullary injection of the dye that directly correlated with his deficit.[13] The fourth patient, a 29-year-old female, had a CESI performed with Depo-Medrol at the C5-C6 level; she acutely developed a left-hemiparesis/hemisensory deficit that clearly corresponded with the C5-C6 MR-documented location of an intramedullary cord injection.[7]
Table 2.
Cervical epidural steroid injections (CESI) result in intramedullary cord lesions, intravascular injections, and 6th cranial nerve palsies
TWO CASES OF CERVICAL EPIDURAL STEROID INJECTIONS RESULTING IN INTRAVASCULAR VERTEBRAL ARTERY INJECTIONS AND SPINAL CORD STROKES
In two cases, CESI resulted in inadvertent intravascular vertebral artery injections, responsible for to irreversible quadriparesis/quadriplegia [Table 2].[3,14] In the first case, a 53-year-old male with multiple cervical disc “protrusions” underwent a left C5-C6 TFESI.[14] Within 10 to 15 minutes, he developed weakness in his left arm and both legs. Although the first emergent MR showed no changes, at 24 hours, the subsequent MR revealed a patchy increased cord signal extending from the odontoid to the C4-C5 level. This was consistent with diffuse vascular cord infarction, and correlated with the patient's incomplete quadriplegia. The second case involved a patient undergoing a C6-C7 CESI who immediately developed respiratory arrest/irreversible quadriplegia due to an inadvertent intravascular/vertebral injection resulting in a spinal cord stroke.[3]
ONE CASE OF CERVICAL MYELOGRAPHY LEADING TO AN INTRAMEDULLARY SPINAL CORD INJECTION/QUADRIPLEGIA
A 39-year-old female underwent a C1-C2 myelogram and immediately noted “right-side face, neck, and arm pain and paresthesias” accompanied by right arm weakness/hyperreflexia [Table 2].[17] The myelo-CT documented an intramedullary spinal cord injection; contrast was seen within the upper cord, resulting in marked cord swelling.[17] She was placed on a high-dose methylprednisolone protocol, but only showed mild symptomatic improvement.
THREE STUDIES MINIMIZE OR LARGELY DENY COMPLICATIONS OF CERVICAL EPIDURAL STEROID INJECTIONS
Three additional studies minimized or largely denied that significant complications resulted from cervical ESI [Table 2].[5,6,16] When Childress and Becker (2016) discussed the use of CESI to manage cervical radiculopathy, they barely acknowledged they “may have higher risks of serious complications.”[6] Although Carr et al. (2016) noted that cord infarction and infection may be potential complications attributed to CESI, they documented “no major complications” (e.g., neurologic deficits, significant hemorrhaging, or epidural hematomas) in their very large series.[5] Schneider et al. (2016) stated CESI rarely resulted in paralysis and stroke, and noted “the more common adverse events were rather minor, generally transient, and mostly occurred at incidences of less than 1%.” [Table 2].[16] Certainly, these latter studies indicate that complications are either not reported or under-reported in the spine literature.
CONCLUSION
Various types of lumbar and cervical dural punctures result in significant neurological injury. In the lumbar spine, these may occur during attempted epidural anesthesia for labor/delivery, while performing LESI/TLESI, during placement intradural pain devices, or spontaneously. They can result in intracranial hypotension, subdural hematomas, and double vision/6th cranial nerve palsies. In the cervical spine, all dural punctures were attributed to CESI resulting in monoplegia/quadriplegia directly attributed to intramedullary spinal cord injuries or vertebral artery injections/cord strokes. The neurological complications attributed to lumbar and more notably cervical ESI must be carefully considered when choosing to perfom any type of epidural injection, particularly for those with too often, minimal complaints.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
REFERENCES
- 1.Arcand G, Girard F, McCormack M, Chouinard P, Boudreault D, Williams S. Bilateral sixth cranial nerve palsy after unintentional dural puncture. Can J Anaesth. 2004;51:821–3. doi: 10.1007/BF03018456. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia A, Flamer D, Shah PS, Cohen SP. Transforaminal Epidural Steroid Injections for Treating Lumbosacral Radicular Pain from Herniated Intervertebral Discs: A Systematic Review and Meta-Analysis. Anesth Analg. 2016;122:857–70. doi: 10.1213/ANE.0000000000001155. [DOI] [PubMed] [Google Scholar]
- 3.Bose B. Quadriparesis following cervical epidural steroid injections: Case report and review of the literature. Spine J. 2005;5:558–63. doi: 10.1016/j.spinee.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Bureau NJ, Moser T, Dagher JH, Shedid D, Li M, Brassard P, et al. Transforaminal versus intra-articular facet corticosteroid injections for the treatment of cervical radiculopathy: A randomized, double-blind, controlled study. AJNR Am J Neuroradiol. 2014;35:1467–74. doi: 10.3174/ajnr.A4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr CM, Plastaras CT, Pingree MJ, Smuck M, Maus TP, Geske JR, et al. Immediate Adverse Events in Interventional Pain Procedures: A Multi-Institutional Study. Pain Med. 2016 doi: 10.1093/pm/pnw051. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Childress MA, Becker BA. Nonoperative Management of Cervical Radiculopathy. Am Fam Physician. 2016;93:746–54. [PubMed] [Google Scholar]
- 7.Cohen-Adad J, Buchbinder B, Oaklander AL. Cervical spinal cord injection of epidural corticosteroids: Comprehensive longitudinal study including multiparametric magnetic resonance imaging. Pain. 2012;153:2292–9. doi: 10.1016/j.pain.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corbonnois G, O’Neill T, Brabis-Henner A, Schmitt E, Hubert I, Bouaziz H. Unrecognized dural puncture during epidural analgesia in obstetrics later confirmed by brain imaging. Ann Fr Anesth Reanim. 2010;29:584–8. doi: 10.1016/j.annfar.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Davies JM, Murphy A, Smith M, O’Sullivan G. Subdural haematoma after dural puncture headache treated by epidural blood patch. Br J Anaesth. 2001;86:720–3. doi: 10.1093/bja/86.5.720. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari L, De Sevin F, Vigué JP, Granry JC, Preckel MP. Intracranial subdural hematoma after obstetric dural puncture. Ann Fr Anesth Reanim. 2001;20:563–6. doi: 10.1016/s0750-7658(01)00424-5. [DOI] [PubMed] [Google Scholar]
- 11.Hodges SD, Castleberg RL, Miller T, Ward R, Thornburg C. Cervical epidural steroid injection with intrinsic spinal cord damage. Two case reports. Spine. 1998;23:2137–42. doi: 10.1097/00007632-199810010-00020. [DOI] [PubMed] [Google Scholar]
- 12.Khemka S, Mearza AA. Isolated sixth nerve palsy secondary to spontaneous intracranial hypotension. Eur J Neurol. 2006;13:1264–5. doi: 10.1111/j.1468-1331.2006.01505.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Lee JK, Seo BR, Moon SJ, Kim JH, Kim SH. Spinal cord injury produced by direct damage during cervical transforaminal epidural injection. Reg Anesth Pain Med. 2008;33:377–9. doi: 10.1016/j.rapm.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Ludwig MA, Burns SP. Spinal cord infarction following cervical transforaminal epidural injection: A case report. Spine. 2005;30:E266–8. doi: 10.1097/01.brs.0000162401.47054.00. [DOI] [PubMed] [Google Scholar]
- 15.Manchikanti L, Pampati V, Hirsch JA. Retrospective cohort study of usage patterns of epidural injections for spinal pain in the US fee-for-service Medicare population from 2000 to 2014. BMJ. 2016;6:e013042. doi: 10.1136/bmjopen-2016-013042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider B, Zheng P, Mattie R, Kennedy DJ. Safety of epidural steroid injections. Expert Opin Drug Saf. 2016;15:1031–9. doi: 10.1080/14740338.2016.1184246. [DOI] [PubMed] [Google Scholar]
- 17.Simon SL, Abrahams JM, Sean Grady M, LeRoux PD, Rushton SA. Intramedullary injection of contrast into the cervical spinal cord during cervical myelography: A case report. Spine. 2002;27:E274–7. doi: 10.1097/00007632-200205150-00026. [DOI] [PubMed] [Google Scholar]
- 18.Velarde CA, Zuniga RE, Leon RF, Abram SE. Cranial nerve palsy and intracranial subdural hematoma following implantation of intrathecal drug delivery device. Reg Anesth Pain Med. 2000;25:76–8. doi: 10.1016/s1098-7339(00)80016-4. [DOI] [PubMed] [Google Scholar]
- 19.Wald JT, Maus TP, Geske JR, Carter RE, Diehn FE, Kaufmann TJ, et al. Safety and efficacy of CT-guided transforaminal cervical epidural steroid injections using a posterior approach. AJNR Am J Neuroradiol. 2012;33:415–9. doi: 10.3174/ajnr.A2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodward WM, Levy DM, Dixon AM. Exacerbation of post-dural puncture headache after epidural blood patch. Can J Anaesth. 1994;41:628–31. doi: 10.1007/BF03010004. [DOI] [PubMed] [Google Scholar]
- 21.Yang S, Werner BC, Cancienne JM, Hassanzadeh H, Shimer AL, Shen FH, et al. Preoperative epidural injections are associated with increased risk of infection after single-level lumbar decompression. Spine J. 2016;16:191–6. doi: 10.1016/j.spinee.2015.07.439. [DOI] [PubMed] [Google Scholar]


