Abstract
The boreal forest is one of the largest terrestrial biomes on Earth. Conifers normally dominate the tree layer across the biome, but other aspects of ecosystem structure and dynamics vary geographically. The cause of the conspicuous differences in the understory vegetation and the herbivore–predator cycles between northwestern Europe and western North America presents an enigma. Ericaceous dwarf shrubs and 3– to 4-year vole–mustelid cycles characterize the European boreal forests, whereas tall deciduous shrubs and 10-year snowshoe hare–lynx cycles characterize the North American ones. We discuss plausible explanations for this difference and conclude that it is bottom-up: Winter climate is the key determinant of the dominant understory vegetation that then determines the herbivore–predator food-web interactions. The crucial unknown for the twenty-first century is how climate change and increasing instability will affect these forests, both with respect to the dynamics of individual plant and animal species and to their community interactions.
Keywords: climate, food webs, dwarf and tall shrubs, population cycles, small mammals
The boreal forest biome covers some 11% of the Earth's terrestrial surface and constitutes about 25% of the Earth's closed canopy forests. Despite its low species diversity compared with that in many other biomes at lower latitudes, the boreal forest has attracted a substantial attention because of its important role in timber production and its crucial role in the global carbon cycle (Bonan et al. 1995) but also as a provider of local ecosystem services for people living in the boreal zone (Esseen et al. 1997, Chapin et al. 2006). Although most reviews have focused on gross biome scale structural and functional generalities across North America and Eurasia, few have touched on some conspicuous geographic ecosystem differences and why these should exist in the first place (Bonan and Shugart 1989). The need for such comparisons was recognized some time ago (Krebs et al. 2001).
The boreal ecosystems of northwestern Europe (with a focus on Fennoscandia) and western North America (with a focus on the Yukon) provide a good case for the first cross-continental comparison, because these two regions have been subjected to intense ecological research over many decades. In both regions, the dominant trees are conifers, especially spruce trees. However, beyond the trees, the visual vegetation difference between these two regions is striking (figure 1). The layer of deciduous tall shrubs that predominates in boreal North America is virtually missing in Europe, where a layer of ericaceous dwarf shrubs predominates. Other aspects of the food web are also markedly different between the two regions, and the most striking are those due to the key herbivores. In North America, the snowshoe hare (Lepus americanus) exhibits a high-amplitude 9- to 10-year population cycle (figure 2a; Krebs et al. 2014a). In Europe, a guild of small rodents (Arvicoline voles) exhibits 3- to 5-year population cycles (figure 2b; Sundell et al. 2013; see also Hansson and Henttonen 1988).
Figure 1.

(a) A typical view of the boreal forest in the southwestern Yukon in the valley east of Kluane Lake. Tall shrubs consisting of dwarf birch and willow are interspersed among the mature white spruce (photo by Alice Kenney). (b) A typical view of the boreal forest in central Norway, where the understory is covered by bilberry shrubs (photo by Petter Wabakken).
Figure 2.
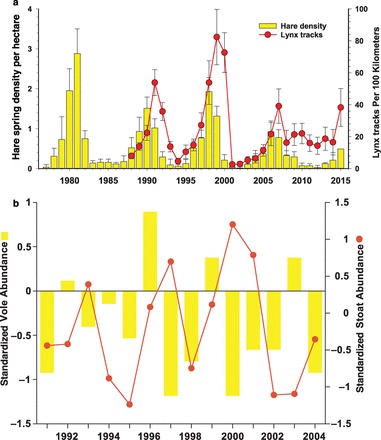
(a) The dynamics of the snowshoe-hare population density in spring at Kluane Lake, Yukon from live trapping of control grids and the lynx density estimated from snow tracking during the previous winter (Krebs et al. 2014a). (b) An example of the dynamics of voles and stoats from the central Finnish boreal forest (Sundell et al. 2013).
Here, we elaborate on these ecological differences in order to understand their underlying cause. We argue that the regional characteristics of the vegetation are mainly determined by large-scale geographic bioclimatic domains. In turn, both regional bioclimate and the resultant vegetation set the stage for the characteristic structure and dynamics of higher trophic levels in the food web, mainly in a bottom-up fashion, but with important modifying trophic feedback from predators to herbivores (Legagneux et al. 2014). Although such a causal path from climate to vegetation to trophic food-web functions constitutes our main thesis, we also discuss the possible roles of other factors—in particular, the long-term historical contingencies and impacts of more recent human interventions.
Regional contrasts in climate
Every ecology textbook shows a diagram of how biomes of the Earth map onto temperature and rainfall (Whittaker 1975). Accordingly, climate is considered to be the most significant force shaping the boreal forest (Bonan and Shugart 1989). In our two focal regions, there is a marked contrast in climate, particularly in winter temperatures and precipitation. The dynamics of the air stream over the Atlantic are the main reason that mean annual temperatures in northwestern Europe are 15–20°C warmer than those at the same latitude in western North America (Seager 2006). For example, just north of Umeå in Sweden (64°15′N, 19°46′E), the mean annual temperature was 1.8 degrees Celsius (°C; 1980–2009), with an average of –9.5°C and 14.6°C in January and July, respectively (Kreyling et al. 2012). Annual precipitation averaged 623 millimeters, with approximately 40% of this occurring as snow, resulting in a maximum average snow depth of 76.5 centimeters (cm; varying between 43 cm and 113 cm, 1980–2010; Lehtonen et al. 2013). At a comparable latitude in western North America (Dawson City, Yukon, 64°02′N, 139°07′ W), the mean annual temperature was –4.4°C (1971–2000, Canadian Climate Normals), with an average of –26.7°C and 15.6°C in January and July, respectively. Annual precipitation averaged 324 millimeters, with approximately 38% of this falling as snow and an average maximum snow depth in February of 54 cm. At Kluane Lake, Yukon, the average February snow depth from 1985 to 1997 varied from 5 cm to 30 cm (Krebs et al. 2001, figure 2.4).
Thus, western North America winter temperatures are about 15–20°C colder than those of northwestern Europe, total precipitation is half that of northwestern Europe, and snow depth is approximately 70% that of northwestern Europe. Importantly, snow depth in western North America only increases slowly over winter, with that in November being only 23 cm deep at Dawson City (10 cm at Kluane Lake), but average temperatures in early winter are –17.9°C. The severe cold and late snowfall of the interior western North America result in large areas within the boreal zone being underlain by ground frost or even discontinuous permafrost, whereas in northwestern Europe, permafrost is largely absent (Hinzman et al. 2006).
Another key feature connected to these regional differences in winter climate is snow texture. The snowpack of European boreal forest contains relatively dense snow because of snow falling at temperatures just below freezing and because of the formation of ice crusts owing to thaw–freeze cycles during the relatively mild winters. In North America, snow typically falls at temperatures well below zero, and freeze–thaw cycles are relatively rare, giving rise to a soft, powder-like snowpack.
Regional contrasts in food-web structure
The marked differences between these two boreal forest ecosystems can be most readily appreciated if we partition them into broad trophic levels and food webs.
Vegetation
The soils in both of the two boreal regions are nutrient poor, and the vegetation in the forest layer shows broad similarities to many of the same species of plants present. White spruce (Picea glauca) dominates the western North America boreal forest tree layer, with a minor component of quaking aspen (Populus tremuloides) and balsam poplar (Populus balsamifera). Scots pine (Pinus sylvestris), Norway spruce (Picea abies), mountain birch (Betula pubescens), and downy birch (B. pendula) dominate the boreal forests of northwestern Europe, with a minor component of European aspen (Populus tremula).
In the forest understory, the vertical structure and dominant plant-growth form differ markedly between these two forest ecosystems. In western North America, gray willow (Salix glauca) and dwarf birch (Betula pumila var. glandulifera) form a dominant deciduous shrub layer 0.6 meters to 2 meters high (Krebs et al. 2001), and about two-thirds of the landscape is in early to intermediate successional stages after forest fires. Beneath this tall shrub layer, the vegetation is usually sparse (mosses cover approximately 20% of the forest floor and dwarf shrubs less than 15%). In early succession after fire in the Yukon, grasses can dominate in 8%–9% of the area before shrubs invade. In northwestern Europe, the tall shrub layer is absent, except for some narrow ecotones along riverbeds (e.g., see Palviainen et al. 2005, Wardle et al. 2012). A short-statured layer of dwarf shrubs (0–0.5 meters high) forms the dominant understory vegetation. Up to 98% of the dwarf shrubs comprise ericaceous species such as bilberry (Vaccinium myrtillus), lingonberry (Vaccinium vitis-idaea), black crowberry (Empetrum hermaphroditum), feather mosses (Hylocomium splendens, Pleurozium schreberi, Ptilium crista-castrensis), and reindeer lichens (Cladonia spp.) on the forest floor (Nilsson and Wardle 2005, Wardle et al. 2012). Although the dwarf shrubs make up less than 5% of the standing biomass, they can contribute to almost half the net primary productivity of the European forest (Kulmala et al. 2011). In early successional stages after fires or clearcutting, grasses—especially Avenella flexuosa—can become a co-dominant component of the understory vegetation (Palviainen et al. 2005). However, forest fires play a minor role (Miller et al. 2008, Ohlson et al. 2011).
Herbivores
We can gain a better appreciation of the two food webs (figure 3a and 3b) and the key players in the trophic dynamics if we quantify the abundance of the vertebrate species in these two ecosystems (figure 4). Small- to medium-sized mammals (small rodents to hares) are most important in terms of trophic flows in both food webs. The moose (Alces alces) is now important in northwestern Europe but is nearly negligible in the Kluane region of the Yukon. The relative importance of the small- to medium-sized herbivores is strikingly different between the two regions. Although the mountain hare (Lepus timidus) and the red squirrel (Sciurus vulgaris) are negligible parts of the trophic pyramid of northwestern Europe, their equivalents in North America, the snowshoe hare and the American red squirrel (Tamiasciurus hudsonicus), have key roles as primary consumers. The snowshoe hare is, however, much more important than the red squirrel in terms of food-web dynamics because of its strong population cycles and importance as prey for predators (figure 3a). Small rodents, represented by several species of voles (Myodes and Microtus spp.), are clearly most abundant in northwestern Europe, where they are the main prey for many small- and medium-sized predators (figure 3b).
Figure 3.
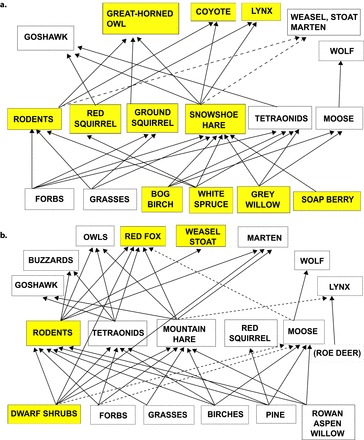
(a) The food web of the boreal forest in the Kluane region of the Yukon in western North America and (b) in central Norway in northwestern Europe. The major trophic species are indicated by yellow shading.
Figure 4.
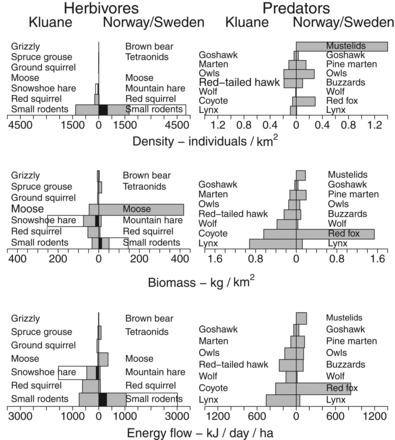
Trophic pyramids for herbivores and carnivores from western North America and northwestern Europe. See the supplemental material for details.
Predators
The boreal community predators span a body size range from weasels (approximately 0.03 kilograms, kg–0.05 kg) to bears (approximately 70 kg–350 kg). There are four pronounced differences between the western North America and northwestern European predator communities. First, the two large predators that are common and trophically important (figures 3 and 4) in western North America are altogether missing in northwestern Europe: the Canada lynx (Lynx canadensis) and coyote (Canis latrans). In the northern boreal forest, both of these predators are specialists on snowshoe hares. The Eurasian lynx (Lynx lynx) is much larger than the Canada lynx (16 kg–34 kg versus 5 kg–17 kg, respectively) and acts like a generalist predator that frequently includes ungulates such as roe deer (Capreolus capreolus) and reindeer (Rangifer tarandus) in their diet. The Eurasian lynx has now recolonized most parts of northwestern Europe after having been driven to near-extinction by the 1920s (Rueness et al. 2003). The Eurasian lynx also appears to have its stronghold in the southern boreal regions, where the diversity of prey species is higher and roe deer (as important prey) are more common than in the northern boreal regions (Elmhagen et al. 2015). Second, the red fox (Vulpes vulpes) in Europe is the most abundant and important generalist predator in this food web, whereas the red fox in the North American boreal forest is rare. Third, the wolf (Canis lupus) is another large carnivore that is a dominant predator on ungulates in North America but has been largely absent in Europe until the last decades (Chapron et al. 2014). Fourth, estimates of abundance and therefore trophic flows in the boreal small mustelids—ermine (Mustela ereminea) and least weasels (M. nivalis)—are low in North America (Boonstra and Krebs 2006) and very uncertain and possibly underestimated in Europe. However, it is clear that these specialist predators on voles are more important in Europe (Sundell et al. 2013) than in North America (figure 4).
Explaining the differences in food-web structure
We propose that the profound difference in winter climate is the key factor permitting the understory shrub layer to be distinct in these two boreal regions and that this then selects for the herbivore species that consume it.
Climate–vegetation interaction
Here, we outline how unstable ambient winter temperatures in combination with a shallow snowpack limits ericaceous dwarf shrubs, and we focus on bilberry although the findings apply to the other species as well. Like many boreal forest species, bilberry has a circumpolar distribution. However, although bilberry can survive moderately severe cold (–20 to –35°C), even with shallow snowpack (Ögren 1996), ambient conditions must remain stable for extended period unless it is insulated by a deep snowpack. In the alpine, boreal, and Arctic regions of northwestern Europe, the bilberry prefers topographic sections of the landscape where a fairly deep snow accumulates in the winter (Rasmus et al. 2011) and is absent from habitat such as ridges blown clear of snow. Bilberry is subjected to substantial dieback in winters with little snow and periods of approximately 0°C with return to subsequent winter conditions because of the loss of winter dormancy (dehardening) and the subsequent loss of freeze tolerance, winter desiccation, and resumption of metabolism (Ögren 1996, Bokhorst et al. 2011). Experimental warming (Bokhorst et al. 2011) or removal of snow cover (e.g., Kreyling et al. 2012) causes both extensive aboveground dieback and belowground root loss. The combination of very low temperatures, little snow particularly in early winter, and the variability of snow conditions in spring in western North America may thus exceed the tolerance limits of bilberries. Consequently, in North America, the bilberry is only found in relatively mild climates in mountain areas from southern British Columbia to Arizona (vander Kloet 1988). In Eurasia, bilberry is found throughout Great Britain, the alpine areas of Europe (e.g., the Alps and the Pyrenees), Fennoscandia, and farther east (Ritchie 1956).
In contrast to the ericaceous dwarf shrubs, tall deciduous shrubs (Salix and Betula) are highly tolerant to very low ambient temperatures in the winter. Their distribution ranges extend northward deep into the Arctic tundra, where they constitute the dominant growth form under favorable conditions mainly set by permafrost, soil hydrology, and ambient summer temperature (Walker et al. 2005). In particular, the combination of relatively high summer temperatures and the moist active layer that develops above frozen soil during the growing season appears to promote the formation of closed canopies (thickets) of tall shrubs (Pajunen 2009).
Therefore, the difference in winter conditions between these two boreal areas may actually have initiated the difference in the understory vegetation. As soon as a continuous dense understory layer of ericaceous dwarf shrubs is established, these dwarf shrubs are able to limit tree regeneration through allelopathic chemicals (Mallik 2003, Nilsson and Wardle 2005). This primarily concerns Empetrum and Calluna, but there is also evidence for bilberry. It is possible that these allelopathic traits limit the establishment of tall shrubs such as willows, especially on well-drained soils in regions without ground frost or in habitats without high groundwater tables (riparian plains or mires). For instance, wherever the tundra lacks permafrost, extensive dwarf shrub heaths dominate the landscape in sub- and low-Arctic Europe (Oksanen and Virtanen 1995).
Vegetation–herbivore interactions
We propose that the different roles of the small mammalian herbivores in the two regions—in particular, hares and voles—can be explained as bottom-up responses to the different understory vegetation. The tall shrubs that protrude through the snowpack in winter are staple winter food for hares (Smith et al. 1988). However, the palatable biomass of tall shrubs is physically out of reach of voles that have a subnivean lifestyle. In winter, voles have access to dwarf shrubs in the subnivean space, the loose “hoar layer” that develops in the snowpack just aboveground under stable winter conditions. But hares do not make deep feeding craters in snow (Formozov 1946) and are therefore unable to exploit plants in the lowest layers of understory vegetation in winter.
The abundant European dwarf shrubs include highly palatable species, such as the bilberry, that are important food for voles. Indeed, for the northwestern European bank vole (Myodes glareolus) and gray-sided vole (M. rufocanus), shoots of bilberry dominate their winter diets (Hansson 1985). The large-bodied gray-sided vole is a true dwarf-shrub specialist in winter that exhibits high cyclic peak densities in Vaccinium-rich habitats in both tundra and boreal forest in northern Fennoscandia (Hansson and Henttonen 1985a).
Another attribute that may contribute to the importance of voles in northwestern Europe is that Microtus voles become important in early successional stages (after fires and clearcutting), when grasses replace the dwarf shrubs, especially in the more mesic and eutrophic sections of the forest (Henttonen et al. 1977). On the other hand, early to midsuccessional stages in North American boreal forest are especially rich in tall shrubs and prime habitats for hares (Krebs et al. 2001).
Explaining the difference in trophic dynamics
There has been a century-long debate about the role of different trophic interactions (i.e., plant–herbivore and herbivore–predator) in vole and hare population cycles (Korpimäki and Krebs 1996, Krebs 2011). Although both European voles (Ericsson 1977) and the snowshoe hare (Smith et al. 1988) have substantial impact on dwarf shrubs and tall shrubs, respectively, there has been a view emerging that predator–prey interactions are most decisive in setting the amplitude of vole and hare cycles (Krebs et al. 2014b). Moreover, these predator–prey cycles have an overall central function that drives a host of pulsed indirect fluxes and interactions in the food webs (Ims and Fuglei 2005, Krebs et al. 2014a).
Specialist predator–prey dynamics
Theory suggests that the delay in the numerical responses of the specialist predators introduces a time lag in their population dynamics relative to their prey that generates cycles (Murdoch and Oaten 1975). Specialized predators regarded as essential for 3- to 5-year cycles centered on voles in northwestern Europe are the small mustelids (figure 2b; Henttonen et al. 1987). In western North America, the most essential specialist hare predators (figures 2a and 3a) for the 10-year cycles are the lynx, coyote, and great-horned owl (O'Donoghue et al. 1998, Rohner et al. 2001). The different cycle lengths (i.e., 3–5 years versus 10 years) are most likely a matter of allometry: The small-sized, short-lived, and reproductively fecund voles and small mustelids generate the shortest cycle lengths (Calder 1983).
As evident from figures 3 and 4, there are also other herbivores and predators that may also have importance for the overall dynamics in the two boreal ecosystems. In western North America, terrestrial and arboreal squirrels are abundant. However, ground squirrels hibernate all winter and are not available to predators then. They are nevertheless exposed to predators during summer and consequently exhibit a population cycle that is synchronized with the hare cycle (figure 5), presumably owing to an alternative prey mechanism. Red squirrels are most difficult for mammal predators to capture and consequently show dynamics driven by seed masting in spruce seed, their main food. This dynamic is independent of the hare–predator cycle (figure 5; Boonstra et al. 2001).
Figure 5.
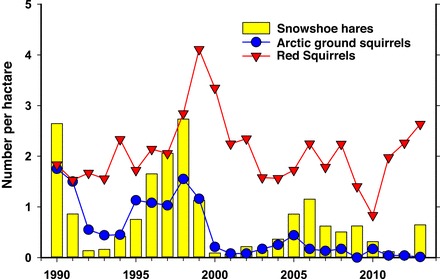
Spring abundance of snowshoe hares, Arctic ground squirrels, and red squirrels in the Kluane region of the Yukon in western North America (Boonstra et al. 2001, Krebs et al. 2014a).
Generalist predator–prey interactions
In northwestern Europe, the red fox is the most abundant generalist predator. It exhibits a population cycle that tracks the vole cycle with a 1-year delay (figure 6; Henden et al. 2009)—the dynamics expected for a specialist predator. However, in terms of diet, the red fox is clearly a generalist (and scavenger on, e.g., moose) in northwestern European boreal forest (Lindström and Hörnfeldt 1984). Moreover, studies suggest that the red fox by itself does not play any decisive role in generating the vole cycles in this area (Marcström et al. 1988, Lindström et al. 1994). However, the red fox does have a significant impact on the mountain hare as alternative prey (Angelstam et al. 1984). Lindström and colleagues (1994) found that mountain hare populations increased 40%–100% after sarcoptic mange had greatly reduced the red fox population during the 1980s but that the hare population declined within 1–2 years after the fox populations had recovered. Therefore, in northwestern Europe, the red fox acts to keep medium-sized boreal herbivores at low densities (Kauhala et al. 2003) and also contributes by the alternative prey mechanism to convey the pulse of the 3–5 year vole cycles sometimes seen in hares and forest grouse (Angelstam et al. 1984, Hansson and Henttonen 1988, Marcström et al. 1988).
Figure 6.
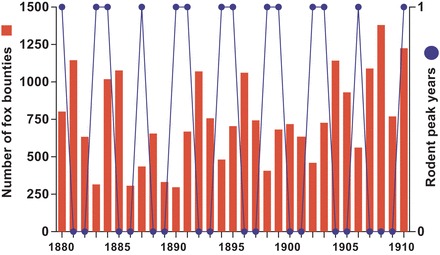
Typical population dynamics of red foxes and small rodents in the boreal zone of northwestern Europe. Red fox dynamics are depicted as the number of fox bounties paid in the county of Sør-Trøndelag (63°N) in Norway during the period 1880–1910 (Henden et al. 2009), whereas the rodent dynamics are scored at a binary scale (high and low) for mid-Norway (Steen et al. 1990).
Mesopredator release
Some interactions among the different predator species may also influence the dynamics of the medium-sized herbivores. In particular, the red fox in northwestern Europe appears to have been under less pressure from larger predators that are known to competitively exclude or kill foxes (Palomares and Caro 1999). However, because the Eurasian lynx has recolonized most parts of northwestern Europe and is now suppressing the red fox, prey dynamics are shifting again, with mountain hares and grouse populations increasing (Elmhagen et al. 2010) and possibly disentangling hares and grouse from the vole cycle. Because of the lack of specialist hare predators in northwestern Europe, a 10-year cycle like that of the snowshoe hare would not be expected for the mountain hare in Europe.
Climate effects on trophic dynamics
The very different winter-climate and snow-cover characteristics of the two geographic regions are also likely to influence the interactions between herbivores and predators. The deep, soft snow conditions could have acted as a selective pressure on both lynx and hares in western North America to evolve relatively smaller body sizes and larger feet (snowshoes) than those of their European counterparts. The inability to travel effectively in soft snow may also explain why the red fox is an insignificant player in the North American boreal ecosystem. On the other hand, the hard snow in Europe may render the red fox a much more efficient hare predator in this region. Moreover, because of the deeper and harder snowpack in Europe, vole populations can escape regulation by supranivean generalist predators such as the red fox (Hansson and Henttonen 1985b). Vole populations can therefore increase to high densities before being caught by the delayed-density-dependent predation by small mustelids. Thus, snowpack characteristics may be crucial for the distinct vole–predator cycles in Europe.
In essence, a key difference between the cycle-generating predator–prey interactions of boreal Europe and America lies in their physical relationship to the snow pack (figure 7). The one between mustelids and voles is mainly subnivean, whereas that between the snowshoe hare and its predators is supranivean. The last one has resulted in a specialized “snowshoe” niche not apparent in northwestern Europe.
Figure 7.
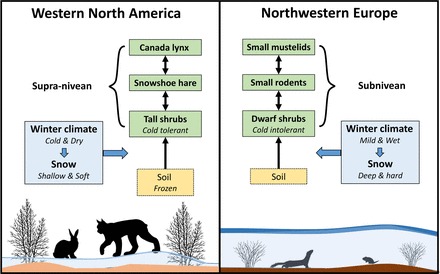
A proposed explanation for how winter climate—acting mainly through temperature—and snow quantity and quality give rise to the different vegetation compositions and structures and to food-web dynamics in the boreal forests of western North America and of northwestern Europe.
According to our proposal of the interactions among climate, vegetation, and herbivores, the lack of vole population cycles in North America is therefore due to the lack of subnivean primary production during winter. Vole populations in the North American boreal forest are thus limited by bottom-up effects during winter.
Finally, considering the importance of snow for boreal forest ecosystem dynamics, it is worth noting that changing snow conditions are likely to be among the most rapid ecological impacts of climate warming in boreal and Arctic ecosystems (Callaghan et al. 2011). In Europe, rodent cycles appear to already have become dampened in shorter winters with unfavorable snow conditions (Ims et al. 2008). The formation of ice due to freeze–thaw cycles during winter may be limiting vole availability to bilberries and other edible food items by encrusting them in ice and therefore limiting the peak phase of the cycles. Other climate variables are now simultaneously changing in the boreal regions of Europe (e.g., decreased snow depths, increased summer and autumn temperatures, increased summer rainfall), and these interact with deteriorating snow conditions to diminish vole cycles over much of the region (Korpela et al. 2013).
Historical contingencies and impacts of humans
Here, we consider whether the regional differences described above have always been there during the Holocene (last 7000 years) and are solely the result of the biogeography of the key species or whether there are other processes that have caused the differences between the two regions. The similarities in species or subspecies (e.g., figure 3) suggest that the species pool was largely the same and that the differences have evolved as a response to climatic and/or biological conditions. Could what we now see be a human artifact of the consequences of land use for forestry and agriculture going back several hundreds or thousands of years?
Human landscape modification
The boreal ecosystem we see in northwestern Europe today has been strongly affected by human modification. In contrast, western North America does not have any long-term agricultural history, and the related exploitation of the forests has been lacking. Consequently, in the Canadian boreal forest, there are large areas of relatively pristine forest. Human agriculture started having an impact on the southern portions of Fennoscandia about 6000 years ago, but it was not until about 4000 years ago that the impacts of agriculture—the clearing of land, the grazing of livestock, and the harvesting of fodder from forests—became more pronounced (Esseen et al. 1997, Framstad et al. 2013). Farming practices in northwestern Europe traditionally employed free-ranging livestock, with intensive grazing suppressing the shrub layer. Human populations increased rapidly from 1700 AD onward. By 1900, the northwestern European boreal forest was open in large areas following the grazing of livestock, slash-and-burn land clearing, tar and charcoal making, and firewood and timber extraction. Framstad and colleagues (2013) argued that by 1900, the total amount of old-stand boreal forest in Fennoscandia had declined to approximately 20% of that present prior to the advent of the major expansion of agriculture in the Bronze Age. Östlund and colleagues (1997) calculated that over the last two centuries, 96% of all boreal forests in Finland and Sweden had been harvested for timber. Today, the boreal forest in northwestern Europe is characterized by cultivated forests with single-species stands where deciduous trees have been cut and the ground in some areas is managed to increase regeneration. Since 1900, boreal-forest growing stock has doubled in Norway, increased 70% in Finland, and increased 85% in Sweden (Framstad et al. 2013).
The higher proportion of the early successional stage of the forest may have increased the importance of grass-eating Microtus voles in the northwestern European forest compared with that in the original pristine forest (Boström and Hansson 1981). Microtus voles have larger bodies and higher population densities than most Myodes species and may therefore be particularly important drivers of the predator-driven cycle in the food web (Henttonen et al. 1987). In addition, ungulates such as wild reindeer and moose, the three top carnivore species (wolf; wolverine, Gulo gulo; and European lynx), and the omnivorous brown bear (Ursus arctos) became functionally extinct in northwestern Europe during the 1800s to 1900s or earlier owing to extermination by humans. Later, the creation of increased forage for moose from forestry clearcuts and pine monocultures in the 1960s and the introduction of a selective moose-hunting regime culling a low proportion of reproductively active females in the 1970s led to very high moose densities (more than one moose per square kilometer). These are intensely hunted (approximately 30% of the population harvested yearly). The increase in the amount of carrion from overabundant ungulate populations has contributed to a bottom-up boost of generalist mesopredators such as the red fox (Henden et al. 2014) and possibly a top-down limitation of hares and grouse (Ehrich et al. 2012). Finally, the low number of apex predators (especially wolves) in northwestern Europe favored the increase in moose and red fox populations.
Long-term historical contingencies
Obviously then, the observed differences in moose and red fox densities today between the two focal areas may be attributed to human activity. But what about the differences in vegetation? The boreal forest ecosystem in its current form was formed after the last Pleistocene deglaciation, and it is one of the few ecosystems whose development we understand well. The climatic conditions in western North America have remained remarkably stable over the last 7000 years (Gajewski et al. 2014). These conditions were distinctly different from those in northwestern Europe, where especially the summers were warmer and drier from about 8000 to 4000 years ago compared with those from 4000 years onward (Miller et al. 2008).
One way of establishing what the vegetation looked like in the past is to use evidence from pollen and macrofossil analysis from lake and bog cores. For North America, the tree pollen over the last 7000 years has changed little (Gajewski et al. 2014). For Europe, however, there was a marked change about 4000 years ago, when spruce invaded from the east (about 6000 years ago into Finland) to the west (about 2000–3000 years ago into Sweden) and the abundance and distribution of the north temperate trees such as oak (Quercus robur), lime (Tilia cordata), and elm (Ulmus glabra) decreased (Miller et al. 2008).
However, pollen diagrams cannot help us discern what was going on in the shrub layer (John Birks, University of Bergen, Bergen, Norway, personal communication, 11 November 2014). Paleoecology relies heavily on species that are wind pollinated, and the various boreal conifer and wind-pollinated deciduous tree species produce such vast amounts of pollen that they swamp pollen produced from other species. The ericaceous dwarf shrubs (Vaccinium spp.) and tall willow shrubs (Salix spp.) are insect pollinated. Therefore, although these shrubs are present to a minor degree in the pollen and macrofossil record from northwestern Europe (Seppä and Birks 2002), this information does not tell us about their actual abundance in plant communities.
An alternative approach is to use the evidence from chronosequence data. A chronosequence is a set of sites that share similar attributes but differ in age. Chronosequences from northern Sweden (where the disturbance regime is accurately known back to 5350 BP) show that there have been three constants in the vegetation: the tree layer, the dwarf ericaceous understory layer, and the feather moss layer (Wardle et al. 2012). There was no tall shrub layer in these forests. Because these long-term chronosequences depend on lack of fire for setting back the successional sequence, perhaps this has produced an artifact. However, fire-related disturbances play a minor role in northwestern European forests (Miller et al. 2008, Ohlson et al. 2011), in contrast to what occurs in western North American forests (Bergeron et al. 2001). Finally, virtually all Fennoscandian literature on present-day forests indicates the predominance of the Vaccinium understory (e.g., Framstad et al. 2013).
Therefore, the difference in the understory layers between these two regions with the abundant dwarf shrubs in northwestern Europe versus the tall shrubs in boreal North America is thousands of years old. The fact that there are voles in northwestern Europe that are specialized on dwarf shrubs (such as the gray-sided vole) but none in western North America also indicates the long-term constancy of the understory vegetation in European boreal forests.
Conclusions
We contend that the fundamental regional differences we have highlighted in this article—the contrasting understory vegetation and the keystone herbivore–predator interactions that drive ecological dynamics with different pulse rates in the two systems—do not result from different degrees of human intervention. Instead, we propose that these differences are ancient and caused by different winter climates that mainly act bottom-up by first shaping the understory vegetation, then the dominant herbivores, and finally, the dominant predators (figure 7). Winter climate, acting through snowpack characteristics, also has a direct influence on the key predator–prey interaction type and strength that contributes to shaping the dynamics, such as population cycle period and amplitude. We recognize that recent human impacts in the boreal forest of northwestern Europe have shaped present-day forest-stand structure, the distribution of successional stages, and some aspects of the structure of the food web that do not have North American counterparts, but these impacts are not primary. Humans in northwestern Europe have also caused increases of mesopredators (especially red fox) and ungulates (especially moose) resulting from the release of top-down limitations by apex predators (especially wolves) as well as bottom-up boosts from land use (especially forestry and agriculture).
Our syntheses and proposed explanations are focused on a comparison between two localities within the northern boreal forest: the Yukon representing western North America and Fennoscandia representing northwestern Europe. This is because these localities for decades have been hotspots for ecological research, particularly aiming at understanding boreal food-web dynamics. However, the boreal biome is vast and includes regions with bioclimates, historical contingencies, and human impacts that are both similar to and different from our two focal regions. Future studies should draw on this interregional variability to provide syntheses that are more geographically comprehensive in their analysis of putative drivers of boreal food-web dynamics and thereby better able to disentangle the relative importance of continental biography, historical contingencies, and current bioclimate. Our proposal that regional characteristics of the vegetation are mainly set by large-scale geographic bioclimatic domains, which in turn set the stage for the characteristic structure and dynamics of higher trophic levels, largely accords with the view that has emerged from recent comparisons of ecosystems within the Arctic tundra (Ims et al. 2013). We believe that further comparisons between boreal forest and adjacent Arctic and alpine tundra within the same geographic region will improve our overall understanding of these important biomes.
A Circumpolar Boreal Vegetation Map would be an important first step forward to a better understanding of the ecological dynamics of the entire boreal forest biome (Talbot and Meades 2011). However, the next major challenge is to obtain adequate data on food-web dynamics. Indeed, it takes decades of comprehensive research and monitoring to achieve the population time series needed to establish what are the key trophic interactions and overall dynamics of the food web. The establishment of a circumpolar monitoring program for the boreal forest, akin to what is now under implementation for the Arctic tundra (Christensen et al. 2013), could be a vehicle for building adequate data for future analyses.
Finally, the importance of winter in determining the structure and dynamics of boreal food webs, which we have highlighted by our comparison between northwestern Europe and western North America, implies that the boreal forest will be subjected to dramatic changes in the coming decades with ongoing and intensifying climate change. Therefore, there is an urgent need for supporting long-term research and monitoring with a food-web approach in these northern regions so that we can detect and properly understand these changes. To accomplish the food-web approach, we need reliable estimates on densities (not just abundance) that incorporate spatial models (see Krebs et al. 2011 for their application to rodents in northern Canada) and the diet of small mammals in the boreal forest as well as specific experiments on winter ecosystems to fully understand the interactions among snow condition, vegetation, and small herbivores and their impact on boreal forest ecosystem dynamics.
Supplemental material
The supplemental material is available online at Supplementary Data.
Acknowledgments
This article is part of the BEcoDyn project funded by Hedmark University College and a grant from the Norwegian Research Council (NFR project no. 221056) to HPA. The Natural Sciences and Engineering Research Council of Canada provided funding to support the Yukon research reported here.
References
- Angelstam P, Lindtröm E, Widén P. Role of predation in short-term population fluctuations of some birds and mammals in Fennoscandia. Oecologia. 1984;62:199–208. doi: 10.1007/BF00379014. [DOI] [PubMed] [Google Scholar]
- Bergeron Y, Gauthier S, Kafka V, Lefort P, Lesieur D. Natural fire frequency for the eastern Canadian boreal forest: Consequences for sustainable forestry. Canadian Journal of Forest Research. 2001;31:384–391. [Google Scholar]
- Bokhorst SF, Bjerke JW, Street LE, Callaghan TV, Phoenix GK. Impacts of multiple extreme winter warming events on sub-Arctic heathland: Phenology, reproduction, growth, and CO2 flux responses. Global Change Biology. 2011;17:2817–2830. [Google Scholar]
- Bonan GB, Shugart HH. Environmental factors and ecological processes in boreal forests. Annual Review of Ecology and Systematics. 1989;20:1–28. [Google Scholar]
- Bonan GB, Chapin FS, Thompson SL. Boreal forest and tundra ecosystems as components of the climate system. Climatic Change. 1995;29:145–167. [Google Scholar]
- Boonstra R, Krebs CJ. Population limitation of the northern red-backed vole in the boreal forests of northern Canada. Journal of Animal Ecology. 2006;75:1269–1284. doi: 10.1111/j.1365-2656.2006.01149.x. [DOI] [PubMed] [Google Scholar]
- Boonstra R, Boutin S, Byrom A, Karels TJ, Hubbs AH, Stuart-Smith K, Blower M, Antpoehler S. The role of red squirrels and Arctic ground squirrels. In: Krebs CJ, Boutin S, Boonstra R, editors. Ecosystem Dynamics of the Boreal Forest: The Kluane Project. Oxford University Press; 2001. pp. 179–214. [Google Scholar]
- Boström U, Hansson L. Small rodent communities on mires: Implications for population performance in other habitats. Oikos. 1981;37:216–224. [Google Scholar]
- Calder WA. An allometric approach to population cycles of mammals. Journal of Theoretical Biology. 1983;100:275–282. [Google Scholar]
- Callaghan TV, et al. The changing face of Arctic snow cover: A synthesis of observed and projected changes. Ambio. 2011;40:17–31. [Google Scholar]
- Chapin FS, Oswood MW, Cleve KV, Viereck LA, Verbyla DL. Alaska's Changing Boreal Forest. Oxford University Press; 2006. [Google Scholar]
- Chapron G, et al. Recovery of large carnivores in Europe's modern human-dominated landscapes. Science. 2014;346:1517–1519. doi: 10.1126/science.1257553. [DOI] [PubMed] [Google Scholar]
- Christensen T, et al. The Arctic Terrestrial Biodiversity Monitoring Plan. Conservation of Arctic Flora and Fauna (CAFF) International Secretariat; 2013. CAFF Monitoring Series Report no. 7. [Google Scholar]
- Ehrich D, Henden JA, Ims RA, Killengren ST, Lecomte N, Pokrovsky IG, Skogstad G, Sokolov AA, Sokolov VA, Yoccoz NG. The importance of willow thickets for ptarmigan and hares in shrub tundra: The more the better? Oecologia. 2012;168:141–151. doi: 10.1007/s00442-011-2059-0. [DOI] [PubMed] [Google Scholar]
- Elmhagen B, Ludwig G, Rushton SP, Helle P, Lindén H. Top predators, mesopredators, and their prey: Interference ecosystems along bioclimatic productivity gradients. Journal of Animal Ecology. 2010;79:785–794. doi: 10.1111/j.1365-2656.2010.01678.x. [DOI] [PubMed] [Google Scholar]
- Elmhagen B, Kindberg J, Hellström P, Angerbjörn A. A boreal invasion in response to climate change? Range shifts and community effects in the borderland between forest and tundra. Ambio. 2015;44:39–50. doi: 10.1007/s13280-014-0606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson L. The influence of voles and lemmings on the vegetation in a coniferous forest during a 4-year period in northern Sweden. Wahlenbergia. 1977;4:1–114. [Google Scholar]
- Esseen P-A, Ehnström B, Ericson L, Sjöberg K. Boreal forests. Ecological Bulletins. 1997;46:16–47. [Google Scholar]
- Formozov AM. The Snow Cover as an Environmental Factor and Its Importance in the Life of Mammals and Birds. University of Alberta, Boreal Institute; 1946. 1970. Pryehodko W, Pruitt O, trans. Occasional Paper no 1. [Google Scholar]
- Framstad E, de Wit H, Mäkipää R, Larjavaara M, Vesterdal L, Karltun E. Biodiversity, Carbon Storage and Dynamics of Old Northern Forests. Nordic Council of Ministers; 2013. [Google Scholar]
- Gajewski K, Bunbury J, Vetter M, Kroeker N, Khan AH. Paleoenvironmental studies in southwestern Yukon. Arctic 67 Supplement. 2014;1:58–70. [Google Scholar]
- Hansson L. Clethrionomys food: Specific, generic, and regional characteristics. Annales Zoologici Fennici. 1985;22:315–318. [Google Scholar]
- Hansson L, Henttonen H. Regional differences in cyclicity and reproduction in Clethrionomys species: Are they related? Annales Zoologici Fennici. 1985a;22:277–288. [Google Scholar]
- Hansson L, Henttonen H. Gradients in density variations of small rodents: The importance of latitude and snow cover. Oecologia. 1985b;67:394–402. doi: 10.1007/BF00384946. [DOI] [PubMed] [Google Scholar]
- Hansson L, Henttonen H. Rodent dynamics as community processes. Trends in Ecology and Evolution. 1988;3:195–200. doi: 10.1016/0169-5347(88)90006-7. [DOI] [PubMed] [Google Scholar]
- Henden J, Ims RA, Yoccoz NG. Nonstationary spatio-temporal small rodent dynamics: Evidence from long-term Norwegian fox bounty data. Journal of Animal Ecology. 2009;78:636–645. doi: 10.1111/j.1365-2656.2008.01510.x. [DOI] [PubMed] [Google Scholar]
- Henden JA, Stien A, Bårdsen BJ, Yoccoz NG, Ims RA. Community-wide carnivore response resulting from partial ungulate migration. Journal of Applied Ecology. 2014;51:1525–1533. [Google Scholar]
- Henttonen H, Kaikusalo A, Tast J, Viitala J. Interspecific competition between small rodents in subarctic and boreal ecosystems. Oikos. 1977;29:581–590. [Google Scholar]
- Henttonen H, Oksanen T, Jortikka A, Haukisalmi V. How much do weasels shape microtine cycles in the northern Fennoscandian taiga? Oikos. 1987;50:353–365. [Google Scholar]
- Hinzman LD, Viereck LA, Adoms PC, Ramovsky VE, Yoshikawa K. Climate and permafrost of the Alaskan boreal forest. In: Chapin FS, Oswood MW, Cleve KV, Viereck LA, Verbyla DL, editors. Alaska's Changing Boreal Forest. Oxford University Press; 2006. pp. 39–61. [Google Scholar]
- Ims RA, Fuglei E. Trophic interaction cycles in tundra ecosystems and the impact of climate change. BioScience. 2005;55:311–322. [Google Scholar]
- Ims RA, Henden J-A, Killengren ST. Collapsing population cycles. Trends in Ecology and Evolution. 2008;23:79–86. doi: 10.1016/j.tree.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Ims RA, et al. Terrestrial ecosystems. In: Meltofte H, editor. Arctic Biodiversity Assessment: Status and Trends in Arctic Biodiversity. Conservation of Arctic Flora and Fauna, Arctic Council; 2013. pp. 384–440. [Google Scholar]
- Kauhala K, Helle P, Hiltunen M. Population dynamics of mountain hare Lepus timidus populations in Finland. Wildlife Biology. 2003;11:299–307. [Google Scholar]
- Korpela K, Delgado M, Henttonen H, Korpimäki E, Koskela E, Ovaskainen O, Pietiäinen H, Sundell J, Yoccoz NG, Huitu O. Non-linear effects of climate on boreal rodent dynamics: Warm winters do not negate high-amplitude cycles. Global Change Biology. 2013;13:697–710. doi: 10.1111/gcb.12099. [DOI] [PubMed] [Google Scholar]
- Korpimäki E, Krebs CJ. Predation and population cycles of small mammals. BioScience. 1996;46:754–764. [Google Scholar]
- Krebs CJ. Of lemmings and snowshoe hares: The ecology of northern Canada. Proceedings of the Royal Society B. 2011;278:481–489. doi: 10.1098/rspb.2010.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs CJ, Boutin S, Boonstra R. Ecosystem Dynamics of the Boreal Forest: The Kluane Project. Oxford University Press; 2001. [Google Scholar]
- Krebs CJ, Boonstra R, Gilbert BS, Reid DG, Kenney AJ, Hofer EJ. Density estimation for small mammals from live trapping grids: Rodents in northern Canada. Journal of Mammalogy. 2011;92:974–981. [Google Scholar]
- Krebs CJ, Boonstra R, Boutin S, Sinclair ARE, Smith JNM, Gilbert BS, Martin K, O'Donoghue M, Turkington R. Trophic dynamics of the boreal forests of the Kluane Region. Arctic 67, Supplement. 2014a;1:71–81. [Google Scholar]
- Krebs CJ, et al. What factors determine cyclic amplitude in the snowshoe hare cycle? Canadian Journal of Zoology. 2014b;92:1039–1048. [Google Scholar]
- Kreyling J, Haie M, Laudon H. Absence of snow cover reduces understory plant cover and alters plant community composition in boreal forests. Oecologia. 2012;168:577–587. doi: 10.1007/s00442-011-2092-z. [DOI] [PubMed] [Google Scholar]
- Kulmala L, Pumpanen J, Kolari P, Muukkonen P, Hari P, Vesala T. Photosynthetic production of ground vegetation in different-aged Scots pine (Pinus sylvestris) forests. Canadian Journal of Forest Research. 2011;41:2020–2030. [Google Scholar]
- Legagneux P, et al. Climate and body-size shape structure and functioning of Arctic ecosystems. Nature Climate Change. 2014;4:379–383. [Google Scholar]
- Lehtonen I, Venäläinen A, Ikonen J, Puttonen N, Gregow H. Some features of winter climate in northern Fennoscandia. Finnish Meteorological Institute Reports. 2013;3:1–20. [Google Scholar]
- Lindström ER, Andren H, Angelstam P, Cederlund G, Hornfeldt B, Jaderberg L, Lemnell PA, Martinsson B, Skold K, Swenson JE. Disease reveals the predator: Sarcoptic mange, red fox predation, and prey populations. Ecology. 1994;75:1042–1049. [Google Scholar]
- Lindström ER, Hörnfeldt B. Vole cycles, snow depth, and fox predation. Oikos. 1984;70:156–160. [Google Scholar]
- Mallik AU. Conifer regeneration problems in boreal and temperate forests with ericaceous understory: Role of disturbance, seedbed limitation, and keystone species change. Critical Reviews in Plant Sciences. 2003;22:341–366. [Google Scholar]
- Marcström V, Kenward RE, Engren E. The impact of predation on boreal tetraonids during vole cycles: An experimental study. Journal of Animal Ecology. 1988;57:859–872. [Google Scholar]
- Miller PA, Giesecke T, Hickler T, Bradshaw RHW, Smith B, H Seppä, Valdes PJ, Sykes MT. Exploring climatic and biotic controls on Holocene vegetation change in Fennoscandia. Journal of Ecology. 2008;96:247–259. [Google Scholar]
- Murdoch WW, Oaten A. Predation and population stability. Advances in Ecological Research. 1975;9:1–131. [Google Scholar]
- Nilsson MC, Wardle DA. Understory vegetation as a forest ecosystem driver: Evidence from the northern Swedish boreal forest. Frontiers in Ecology and the Environment. 2005;3:421–428. [Google Scholar]
- O'Donoghue M, Boutin S, Krebs CJ, Zuleta G, Murray DL, Hofer EJ. Functional responses of coyotes and lynx to the snowshoe hare cycle. Ecology. 1998;79:1193–1208. [Google Scholar]
- Ögren E. Premature dehardening in Vaccinium myrtillus during a mild winter: A cause for winter dieback? Functional Ecology. 1996;10:724–732. [Google Scholar]
- Ohlson M, Brown KJ, Birks HJB, Grytnes J-A, Hörnberg G, Niklasson M, H Seppä, Bradshaw RHW. Invasion of Norway spruce diversifies the fire regime in boreal European forests. Journal of Ecology. 2011;99:395–403. [Google Scholar]
- Oksanen L, Virtanen R. Topographical, altitudinal, and regional patterns in continental and suboceanic heath vegetation of northern Fennoscandia. Acta Botanica Fennica. 1995;153:1–80. [Google Scholar]
- Östlund L, Zackrisson O, Axelsson AL. The history and transformation of a Fennoscandian boreal forest landscape since the 19th century. Canadian Journal of Forestry Research. 1997;27:1198–1206. [Google Scholar]
- Pajunen AM. Environmental and biotic determinants of growth and height of Arctic willow shrubs along a latitudinal gradient. Arctic, Antarctic, and Alpine Research. 2009;41:478–485. [Google Scholar]
- Palomares F, Caro T. Killing among mammalian carnivores. American Naturalist. 1999;153:492–508. doi: 10.1086/303189. [DOI] [PubMed] [Google Scholar]
- Palviainen M, Finér L, Mannerkoski H, Piirainen S, Starr M. Responses of ground vegetation species to clear-cutting in a boreal forest: Aboveground biomass and nutrient contents during the first 7 years. Ecological Research. 2005;20:652–660. [Google Scholar]
- Rasmus S, Lundell R, Saarinen T. Interactions between snow, canopy, and vegetation in a boreal coniferous forest. Plant Ecology and Diversity. 2011;4:55–65. [Google Scholar]
- Ritchie JC. Biological flora of the British Isles: Vaccinium myrtillus L. Journal of Ecology. 1956;44:290–298. [Google Scholar]
- Rohner C, Doyle FI, Smith JNM. Great horned owls. In: Krebs CJ, Boutin S, Boonstra R, editors. Ecosystem Dynamics of the Boreal Forest: The Kluane Project. Oxford University Press; 2001. pp. 339–376. [Google Scholar]
- Rueness EK, Jorde PE, Hellborg L, Stenseth NC, Ellegren H, Jakobsen KS. Cryptic population structure in a large, mobile mammalian predator: The Fennoscandian lynx. Molecular Ecology. 2003;12:2623–2633. doi: 10.1046/j.1365-294x.2003.01952.x. [DOI] [PubMed] [Google Scholar]
- Seager R. The source of Europe's mild climate. American Scientist. 2005;94:334–341. [Google Scholar]
- H Seppä, Birks HJB. Holocene climate reconstructions from the Fennoscandian tree-line area based on pollen data from Toskaljavri. Quaternary Research. 2002;57:191–199. [Google Scholar]
- Smith JNM, Krebs CJ, Sinclair ARE, Boonstra R. Population biology of snowshoe hares II: Interactions with winter food plants. Journal of Animal Ecology. 1988;57:269–286. [Google Scholar]
- Sundell J, O'Hara RB, Helle P, Hellstedt P, Henttonen H, Pietiäinen H. Numerical response of small mustelids to vole abundance: Delayed or not. Oikos. 2013;122:1112–1120. [Google Scholar]
- Talbot SS, Meades WJ. Conservation of Arctic Flora and Fauna (CAFF) International Secretariat. CAFF Flora Group; 2011. Circumboreal Vegetation Map (CBVM): Mapping the Concept Paper. Strategy Series Report no. 3. [Google Scholar]
- Vander Kloet SP. The genus Vaccinium in North America. Agriculture Canada, Research Branch; 1988. Publication no. 1828. [Google Scholar]
- Walker DA, et al. The circumpolar Arctic vegetation map. Journal of Vegetation Science. 2005;16:267–282. [Google Scholar]
- Wardle DA, Jonsson M, Bansal S, Bardgett RD, Gundale MJ, Metcalfe DB. Linking vegetation change, carbon sequestration, and biodiversity: Insights from island ecosystems in a long-term natural experiment. Journal of Ecology. 2012;100:16–30. [Google Scholar]
- Whittaker RH. Communities and Ecosystems. 2nd ed. Macmillan; 1975. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


