Abstract
Background
The introduction of direct-acting antivirals (DAAs) created a major paradigm shift in the treatment of chronic hepatitis C. Currently, there is little “real-world” data regarding hepatitis C virus (HCV) treatment outcomes in the human immunodeficiency virus (HIV)/HCV-coinfected population.
Methods
This retrospective cohort study examined HCV treatment outcomes of HIV/HCV-coinfected patients at a large, urban, Ryan White-funded clinic caring for an underserved population. All HIV patients initiating HCV treatment from January 1, 2013 to November 30, 2015 were included in the analysis. The primary end point was sustained virologic response 12 weeks after the end of therapy (SVR12).
Results
A total of 172 patients initiated HCV treatment within the study period: 79% were male, 83% were black, 95% were HCV genotype 1, 79% were HCV treatment naive, and 16% had cirrhosis. At baseline, median CD4 was 494 cells/μL (interquartile range, 316–722) and 92% had HIV ribonucleic acid less than 40 copies/mL. The most common DAA initiated was ledipasvir/sofosbuvir (LDV/SOF) (85%), with 92% receiving 12 weeks of treatment. Overall, SVR12 was 93% by intention-to-treat analysis and 98% by per-protocol analysis. The majority of patients on LDV/SOF did not report any adverse effect. One patient in the ribavirin plus SOF group discontinued treatment due to adverse effect.
Conclusions
In a cohort of mainly black, male, HIV/HCV-coinfected patients at a large, urban, Ryan White clinic, HCV treatment with DAAs resulted in high SVR12 rates and was well tolerated despite real-world challenges including medication access barriers and drug interaction concerns.
Keywords: direct-acting antivirals, hepatitis C treatment, HIV/HCV coinfection
Coinfection with human immunodeficiency virus (HIV) and hepatitis C virus (HCV) is a major public health concern. Worldwide, an estimated 2.3 million people are coinfected with HIV and HCV, with overall coinfection prevalence in HIV-infected individuals of 6.2% [1]. Furthermore, the odds of HCV infection are 6 times higher in people living with HIV than their HIV-negative counterparts [1]. In the United States, coinfection prevalence is significantly higher, with estimated rates of 25% reported by the Centers for Disease Control and Prevention, especially those with a history of injection drug use (IDU) [2]. In addition, the HCV prevalence among United States-based reports for non-IDU HIV men who have sex with men (MSM) is 7.51% [3] with evidence suggesting an increase in incidence of HCV among this group of patients [4]. Human immunodeficiency virus/HCV coinfection increases the risk for liver disease, liver failure, and liver-related death [2, 5]. In the era of highly active antiretroviral therapy (ART), a meta-analysis found increased risk of mortality with HIV/HCV coinfection compared with HIV infection alone [6], underscoring the need for highly effective HCV treatment for HIV-coinfected patients in the real world.
It is fortunate that groundbreaking changes in HCV treatment have occurred, with the approval of several novel direct-acting antiviral (DAA) agents with improved efficacy, shorter treatment duration, fewer adverse effects, and convenient dosing and administration. Although many DAA trials have been conducted in the monoinfected population, only a few have been completed in the HIV/HCV-coinfected population. After 12 to 24 weeks of treatment with a DAA regimen in HIV/HCV-coinfected patients, rates of sustained virologic response 12 weeks posttreatment (SVR12) have ranged from 91% to 98% primarily in genotype 1 infection, regardless of fibrosis (Fib) stage and treatment experience [7–10].
Although the DAA trials have reported remarkable success, concern has been raised as to their real-world applicability, particularly in the HIV/HCV-coinfected population [11]. Trials conducted in the HIV/HCV-coinfected population have included a relatively small number of participants and excluded a substantial segment of the coinfected population due to strict eligibility criteria. Numerous factors including comorbid medical and psychiatric conditions, substance abuse, drug-drug interactions especially with ART, and restrictions placed by payer sources may affect access to HCV treatment and outcomes in the real world.
The primary objective of this retrospective cohort study was to determine the effectiveness of HCV treatment with all-oral DAA regimens in a real-world population of patients with HIV coinfected with HCV, including patients with compensated cirrhosis and those in whom previous treatment with an HCV regimen had failed.
METHODS
Patients
We performed a retrospective cohort study at Grady Health System’s Infectious Disease Program (IDP) clinic in Atlanta, Georgia. The IDP clinic is a large, urban Ryan White-funded HIV/acquired immune deficiency syndrome clinic in Atlanta, Georgia serving approximately 5800 underinsured, predominantly black, male patients, approximately 11% of whom are coinfected with HCV. The IDP HCV treatment program sees HIV/HCV-coinfected patients who are referred by their primary care providers. The team includes 2 physicians and a nurse practitioner experienced in the management of HCV, 2 infectious diseases clinical pharmacists, and a pharmacy case manager. Standard criteria for referral for HCV treatment in the IDP population included suppressed HIV viral load, free from active substance abuse for at least 6 months, no signs or symptoms suggestive of decompensated cirrhosis, and creatinine clearance above 30 mL/min. All patients had an initial visit with the HCV provider, in addition to a visit with the clinical pharmacist for treatment education and initiation. Subsequent visits were scheduled with either the HCV provider, primary HIV provider, or clinical pharmacist. Patients with HIV treated for HCV infection at the IDP clinic from January 1, 2013 to November 30, 2015 were identified from a pharmacy database and electronic medical records. Medical records of individuals aged ≥18 years or older were screened for the following inclusion criteria: (1) HIV and HCV coinfection, confirmed by current or prior detectable HIV ribonucleic acid (RNA) and quantitative HCV RNA, respectively; and (2) HCV treatment with an all-oral DAA regimen. Pregnancy was the only exclusion criteria. The study was not limited by cirrhosis or prior HCV treatment status or specific HCV genotype. All demographic, clinical, and laboratory data were obtained through the medical records in accordance with study protocol.
The study was approved by the institutional review board of Emory University and the Grady Research Oversight Committee. The study design, data collection, statistical analysis, and writing of the manuscript were conducted by the authors.
Study Assessments
Baseline data abstracted from the chart were those closest to the HCV treatment initiation date. Demographic data included age, gender, race, height, and weight. Baseline laboratory tests included serum creatinine, aminotransferase, platelets, serum HCV RNA, HCV genotype, serum HIV RNA, and CD4 count. Other baseline assessments included (1) liver Fib staging based on biopsy and/or (2) noninvasive diagnostic test results such as abdominal magnetic resonance imaging (MRI), computed tomography (CT) scan, ultrasound, fibroscan, fibrosure, aspartate aminotransferase-to-platelet index score, and Fib-4 score. Cirrhosis was determined by biopsy, FibroScan score ≥13.5 kPa, and/or results of imaging studies (ie, MRI, CT scan, ultrasound). Patients without any of the aforementioned tests were categorized as cirrhotic if they had a Fib-4 score ≥3.25. Serum HCV RNA was measured with the Abbott RealTime HCV Assay, with a lower limit of quantification (LLOQ) of 12 IU/mL. Human immunodeficiency virus RNA was measured by Abbott Realtime HIV-1 Assay with a LLOQ of 40 copies/mL. Direct-acting antiviral regimen, dose, and treatment duration, in addition to ART and drug-drug interactions encountered were obtained from medical records, as were prior HCV treatment(s) and response. Hepatitis C virus medication payer source and refill history were identified by a pharmacy database.
Assessments during and after treatment included standard laboratory testing and measurement of serum HCV RNA at week 4, end of HCV treatment, and 12 weeks posttreatment and clinic notes regarding adverse events including hospitalizations.
Study End Points
The primary efficacy end point was the rate of SVR12. The main secondary efficacy end points were rate of rapid virologic response measured at week 4 of treatment (RVR) and rate of end of treatment virologic response. In all cases, virologic response was defined as the absence of quantifiable HCV RNA in serum (<12 IU/mL). The primary safety end point was any adverse event leading to permanent discontinuation of treatment.
Statistical Analysis
Statistical programs in SPSS version 20 were used for all analyses. Missing response data at posttreatment week 12 were inferred from the next available HCV RNA measurement with the use of the next-value-carried-backward approach. The primary analysis was done in the intention-to-treat population (ITT), which included all study participants. Patients lost to follow up were considered to have virologic failure in the ITT analysis. An additional per-protocol analysis was performed that excluded patients who either discontinued therapy or were lost to follow up. Differences between categorical variables across selected groups were assessed using Pearsons’s χ2 tests or Fisher exact test. Odds ratios (ORs) from univariate and multivariate logistic regression analyses were used to determine association between selected independent variables and SVR12. In all analyses, P values of <.05 and 95% confidence intervals (CIs) were used to establish statistical significance.
RESULTS
Patients
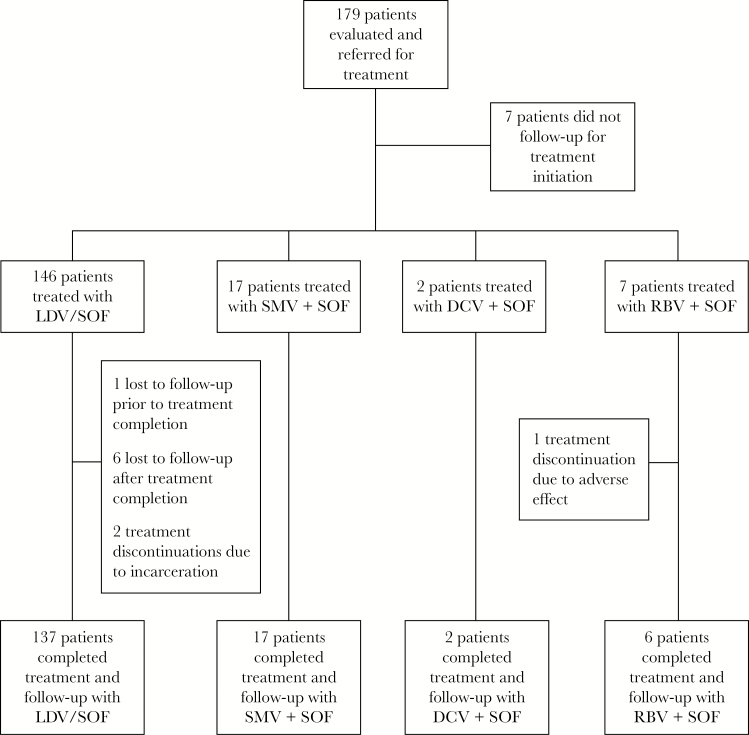
A total of 172 patients started HCV treatment from January 1, 2013 to November 30, 2015 (Figure 1). Overall, 83% were black, 79% were male, 16% had compensated cirrhosis, 95% had genotype 1 infection, and 21% had failed previous HCV treatment (Table 1). The median CD4 count at baseline was 494 cells/µL, and 92% had an undetectable HIV RNA.
Figure 1.
Patient flowchart of hepatitis C virus treatment groups. Abbreviations: DCV, daclatasvir; LDV, ledipasvir; RBV, ribavirin; SMV, simeprevir; SOF, sofosbuvir.
Table 1.
Baseline Patient Characteristics
| Characteristic | Total (N = 172) |
|---|---|
| Median age (range), yr (IQR) | 55 (51–60) |
| Male sex, no. (%) | 136 (79) |
| Race, no. (%) | |
| Black | 142 (82.5%) |
| White | 22 (13.4%) |
| Hispanic | 6 (3.5%) |
| Asian | 0 (0%) |
| Other | 1 (0.6%) |
| HCV acquisition risk factor, no. (%) | |
| Heterosexual | 74 (43%) |
| MSM | 55 (32%) |
| IDU | 35 (20%) |
| IDU and MSM | 8 (5%) |
| Median BMI (kg/m2; IQR) | 26 (23–29) |
| HCV genotype, no. (%) | |
| 1 | 163 (94.8%) |
| 1a | 85 (49.4%) |
| 1b | 34 (19.8%) |
| Unknown | 44 (25.6%) |
| 2 | 8 (4.7%) |
| 3 | 1 (0.6%) |
| Median HCV RNA (IQR), log10 IU/mL | 6.29 (5.87–6.66) |
| Cirrhosis, no. (%) | 28 (16%) |
| Fib-4 ≥3.25 | 35 (20%) |
| Median Scr, µmol/L (IQR) | 88.4 (79.6–106.1) |
| Median ALT, U/L (IQR) | 38 (27–58) |
| Median Platelets, ×103/µL (IQR) | 188 (151–229) |
| Treatment experienced, no. (%) | 36 (21%) |
| Nonresponder | 26 (72.2%) |
| Relapse | 4 (11.1%) |
| Partial responder | 3 (8.3%) |
| Unknown past response | 3 (8.3%) |
| Previous HCV treatments, no. (%) (n= 41) | |
| IFN/RBV or PEG-IFN/RBV | 35 (85.3%) |
| PEG-IFN alone | 1 (2.4%) |
| PEG-IFN/RBV + TPV or BOC | 4 (9.7%) |
| Treatment unknown | 1 (2.4%) |
| Characteristic | Total (N = 172) |
| HIV-1 RNA | |
| Undetectable, no. (%) | 158 (92%) |
| Detectable and <100 copies/mL, no. (%) | 8 (5%) |
| 100–399 copies/mL | 5 (3%) |
| >400 copies/mL | 1 (1%) |
| Median CD4+ count (IQR), cells/µL | 494 (315–706) |
| HIV-1 Treatment, no./total | |
| Protease inhibitor | 82 (48%) |
| Darunavir + ritonavir | 41 |
| Atazanavir + ritonavir | 30 |
| Lopinavir + ritonavir | 9 |
| Atazanavir | 2 |
| NNRTI | 53 (31%) |
| Efavirenz | 29 |
| Rilpivirine | 20 |
| Nevirapine | 1 |
| Etravirine | 3 |
| Integrase inhibitor | 68 (40%) |
| Raltegravir | 38 |
| Dolutegravir | 29 |
| Elvitegravir - cobicistat | 1 |
| NRTI | |
| Tenofovir (TDF) | 129 (75%) |
| Abacavir | 35 (20%) |
| Lamivudine or emtricitabine | 159 (92%) |
| Zidovudine | 7 (4%) |
| Didanosine | 1 (0.01%) |
| CCR5 Inhibitor | |
| Maraviroc | 1 (0.01%) |
| Tenofovir (TDF) + protease inhibitor (with ritonavir) | 53 (31%) |
Abbreviations: ALT, alanine aminotransferase; BMI, body mass index; BOC, boceprevir; CCR5, chemokine receptor 5; Fib, fibrosis; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IDU, injection drug use; IFN, interferon-α; IQR, interquartile range; MSM, men whom have sex with men; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PEG-IFN, pegylated IFN-α; RBV, ribavirin; RNA, ribonucleic acid; Scr, serum creatinine; TDF, tenofovir disoproxil fumarate; TPV, telaprevir.
All patients were prescribed a sofosbuvir-containing regimen, with the majority receiving the combination of ledipasvir and sofosbuvir (LDV/SOF) (85%). The remaining patients received simeprevir plus sofosbuvir (SMV + SOF) (10%), ribavirin + sofosbuvir (RBV + SOF) (4%), or daclatasvir plus sofosbuvir (DCV + SOF) (1%). Before hepatitis C treatment initiation, 32 of 172 patients (19%) required a modification to their ART mainly due to drug interactions between SMV and ritonavir, and LDV/SOF and elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate. In addition, rosuvastatin and gastric acid suppressants were the most the common drugs noted to interact with LDV/SOF (see Supplementary Table S1).
Medication payer sources included patient assistance programs (PAPs) (37%), Medicare Part D (33%), Medicaid (23%), and private insurance (7%). All patients required insurance approval for treatment and/or needed drug company to assist with copays or with the full cost of the medication. The clinical pharmacist and pharmacy case manager were involved in all patient cases to ensure treatment access, medication counseling, drug interaction screening and management, and tracking medication refill and pick-up for patients who used the onsite IDP clinic pharmacy, including those patients receiving treatment through drug company PAP. A total of 159 patients (92%) used the IDP clinic pharmacy, whereas the remainder used retail pharmacies. Of the 159 patients, 157 (99%) had 100% refill pick up of HCV medications.
Effectiveness
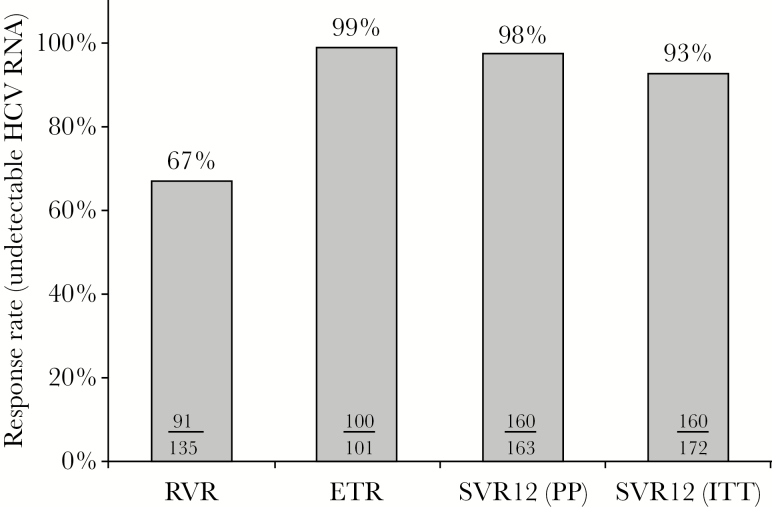
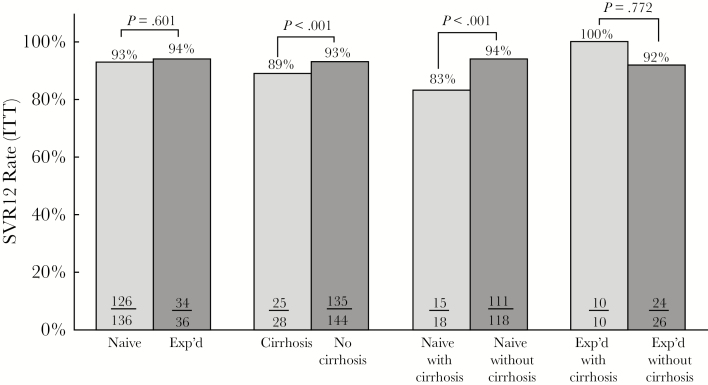
According to ITT analysis, 160 of 172 patients (93%) achieved SVR12 (Figure 2). The SVR12 rate by ITT and per-protocol analysis was similar in patients that were HCV treatment naive and treatment experienced (see Figure 3 and Supplementary Table S2). However, SVR12 rate by ITT analysis was significantly lower for patients with cirrhosis compared with those without cirrhosis. This finding was also observed in the treatment-naive patients with cirrhosis compared with those without cirrhosis. In the per-protocol analysis, 98% of the patients achieved SVR12 and no difference was noted between patients with cirrhosis and those without cirrhosis. The RVR rate was lower in patients with cirrhosis than those without cirrhosis (46% vs 72%, P < .001). Patients with genotype 1 achieved a higher SVR12 rate of 93% (152 of 163) compared with 88% (7 of 8) in genotype 2 (see Supplementary Table S3). Those treated with LDV/SOF had an SVR12 of 92% (135 of 146).
Figure 2.
Overall virologic response rates. Abbreviations: ETR, end of treatment response; HCV, hepatitis C virus; ITT, intention to treat; PP, per protocol; RNA, ribonucleic acid; RVR, rapid virologic response; SVR12 (PP), sustained virologic response 12 weeks posttreatment (PP).
Figure 3.
Sustained virologic response (SVR)12 rate by intention to treat (ITT) for hepatitis C virus (HCV) treatment naive and experienced with and without cirrhosis. Abbreviations: SVR12 (ITT), SVR 12 weeks posttreatment (ITT); Exp’d, HCV treatment experienced; naive, HCV treatment naive.
In univariable logistic regression analysis (Table 2), HCV treatment-naive status was positively associated with achievement of SVR12 (OR, 3.08; 95% CI, 1.24–7.69). This association was maintained in the multivariable model (OR, 6.26; 95% CI, 1.92–20.4).
Table 2.
Univariable and Multivariable Analysis With Sustained Virologic Response 12 Weeks After Completion
| Factors | Univariate (Unadjusted) | Multivariate (Adjusted) | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Age, yr | 0.99 | 0.94–1.05 | .70 | 0.98 | 0.90–1.06 | .71 |
| Gender, female | 1.87 | 0.74–4.74 | .19 | 1.67 | 0.42–6.68 | .46 |
| Race, black | 2.28 | 0.80–6.52 | .12 | 1.97 | 0.46–8.50 | .36 |
| HCV genotype, 1a | 0.48 | 0.29–1.17 | .11 | 0.28 | 0.13–1.31 | .11 |
| Body mass index (kg/m2) | 1.06 | 0.96–1.16 | .24 | 1.03 | 0.93–1.14 | .62 |
| Treatment naive | 2.89 | 1.18–7.07 | .02 | 6.93 | 2.61–10.4 | .01 |
| CD4 <200 cells/µL | 0.69 | 0.18–2.62 | .58 | 0.27 | 0.32–2.24 | .23 |
| HIV-1 RNA <40 copies/mL | 1.08 | 0.23–5.11 | .93 | 0.74 | 0.06–9.41 | .81 |
| HCV RNA (IU/mL) | 1.00 | 0.99–1.01 | .95 | 1.00 | 0.99–1.01 | .99 |
| Fib-4 score ≥3.25 | 0.79 | 0.29–2.15 | .65 | 0.52 | 0.12–2.22 | .38 |
| Platelets, ×103/µL | 1.00 | 0.99–1.00 | .39 | 0.99 | 0.98–1.00 | .06 |
| ALT | 1.00 | 0.99–1.01 | .82 | 1.01 | 0.98–1.04 | .50 |
| AST | 0.99 | 0.98–1.01 | .20 | 0.98 | 0.96–0.99 | .04 |
Abbreviations; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; Fib, fibrosis; HCV, hepatitis C virus; HIV, human immunodeficiency virus; OR, odds ratio; RNA, ribonucleic acid.
In total, 12 patients (7%) in the ITT analysis did not achieve SVR12. Of these patients, 2 failed to achieve SVR12 and were considered treatment failures, 1 discontinued treatment due to adverse effects, 2 discontinued treatment due to incarceration, and 1 patient discontinued treatment after 2 months of therapy and was lost to follow up. The remaining 6 patients completed treatment based on pharmacy refill report but did not follow up for 12-week posttreatment evaluation.
The 2 treatment failures were in the LDV/SOF-treated group (see Supplementary Table S4). Both patients achieved RVR. Because both missed end-of-treatment appointments, virologic breakthrough or relapse could not be determined. Both patients maintained HIV virologic suppression during and after completion of hepatitis C treatment. Posttreatment NS5A resistance testing indicated resistance to LDV; however, baseline NS5A resistance testing was not performed for either patient. Posttreatment NS5B resistance mutations were not identified in either patient.
Safety
Overall, 60 patients (35%) reported an adverse effect (see Supplementary Table S5). Among the patients receiving LDV/SOF, the majority (72%) did not report any adverse effects. The most commonly reported adverse effects in this group included fatigue, headache, nausea, vomiting, and insomnia. Likewise, fatigue was the most commonly reported adverse effect in those receiving SMV + SOF and RBV + SOF. There were no deaths due to medication adverse effect. However, 1 of the 7 patients in the RBV + SOF group discontinued treatment prematurely because of an adverse event. The patient developed significant nausea and vomiting resulting in acute kidney injury after 20 days of treatment. The patient was hospitalized for 2 days and made a full recovery after medication discontinuation and supportive care.
DISCUSSION
The introduction of DAAs has completely transformed the management of HCV in HIV/HCV-coinfected individuals. Multiple clinical trials have demonstrated comparable rates of SVR12 in HIV/HCV-coinfected and HCV-monoinfected individuals, which was not attainable with previous interferon-based HCV treatment [7–10, 12–14]. However, the applicability of these trials to wider populations has been questioned, because real-world effectiveness can be influenced by many factors such as adherence, drug interactions, comorbidities, medication access, and loss to follow-up. Issues that arise with these types of real-world factors in HIV/HCV-coinfected individuals have not been effectively captured in clinical trials due to rigorous eligibility criteria raising the concern for data gaps that impair the translation of clinical trials to clinical practice. Saeed et al [11] evaluated eligibility criteria from 5 HCV treatment efficacy trials to determine the percentage of patients from the Canadian Co-infection Cohort that would qualify for each of these studies. They determined that only 5.9% of patients would have been eligible for the NCT01479868 trial, 9.8% in the PHOTON-1, 6.3% in TURQUOISE-I, 8.1% in ION-4, and 43% in the ALLY-2 trial [11]. Data from real-world settings need to be obtained to determine whether the efficacy of HCV treatment can be maintained when a larger and more diverse spectrum of patients are treated.
To our knowledge, this is one of the largest single-center, real-world, cohort studies of HIV/HCV-coinfected patients. Patients in this study were mainly black, and 75% of participants reported sexual contact (43 % heterosexual, 32% MSM) as their risk factor for acquiring HCV rather than IDU. The demographics, social economic status, and HCV risk factors make this population different from clinical trial populations. The overall observed SVR12 rate of 93% by ITT in this study is similar or better than those previously reported in clinical and observational trials [7–10, 12–18]. ION-4 was a multicenter, single-arm, open-label study of 335 HIV patients coinfected with HCV genotype 1 or 4 treated with LDV/SOF for 12 weeks [7]. In ION-4, 20% of the patients had cirrhosis and the majority of patients (55%) had been previously treated for HCV. Overall, 322 patients (96%) achieved SVR12. High SVR12 rates (≥94%) were reported for treatment naive and experienced, with or without cirrhosis. Unlike the results from ION-4, the SVR12 rates by ITT analysis in this study favored patients without cirrhosis. In addition, in the univariate and multivariate analysis, HCV treatment-naive status was associated with greater odds of achieving SVR12. Of note, this study included a larger proportion of black patients (83%) compared with those enrolled in ION-4 (34%). Historically, black patients have been underrepresented in clinical trials and had lower sustained virologic response rates compared with whites [19]. Unlike the results from ION-4, which reported lower SVR12 rates in blacks than patients of other races (90% vs 99%, P < .001), this study observed high SVR12 rates despite a large percentage of black patients. Another interesting observation in ION-4 was a RVR rate of 99%, whereas patients in this study had a much lower overall RVR rate of 67%, which was even lower in patients with cirrhosis. A similar observation was reported in a real-world all-oral DAA prospective cohort study in HIV/HCV-coinfected patients with cirrhosis, where 52% of patients achieved RVR [20]. Despite a lower RVR rate, patients in this study had a high SVR12 rate, which may challenge the clinical utility of testing for RVR. In addition, this study included 48% of patients who were on an ART regimen containing protease inhibitors, a population that was excluded from the ION-4 trial. We also noted overall cure rates higher than those previously reported in other real-world cohort studies; however, this may be associated with a lower proportion of our patients being treated with regimens that included RBV and/or interferon, as well as having a higher proportion of patients who completed HCV treatment [15, 17].
As successful as DAAs have been, enthusiasm for these therapies has been tempered by the challenges both patients and clinicians face with respect to medication access and reimbursement, a real-world issue not addressed in clinical trials. Many insurance companies and government programs in the United States currently approve payment only for patients with advanced liver fibrosis or those with other “highest priority” indications for treatment as outlined in national treatment guidelines [21]. Patients without insurance rely on pharmaceutical companies’ PAP to obtain medication; however, these programs also have restrictions that potentially limit access to therapy and do not pay for the cost of clinical visits and laboratory monitoring required for treatment. The process of obtaining insurance approval or accessing medication through PAP can be daunting, complicated, and time consuming. In addition, even when drug approval occurs, it may occur after a substantial delay, potentially resulting in loss to follow up. Despite these challenges, no patient in this study was denied treatment due to lack of medication access. In fact, a large proportion of patients were uninsured, but they were still able to obtain access to DAAs through PAP. The high rates of medication acquisition and subsequent success with DAA therapy in this study likely reflect the fact that all the patients were treated in the context of a multidisciplinary hepatitis C clinic model. In previous real-world studies, higher cure rates were found to be partially associated with attendance at follow-up clinic visits [22]. In this study, the majority of patients (92%) obtained HCV medication refills at the clinic pharmacy, and of this group approximately 99% of patients picked up all their refills.
Another real-world barrier to timely initiation of HCV therapy is the potential for drug-drug interactions, especially in the HIV-coinfected population. Patients infected with HIV are often on a number of medications in addition to ART that may interact with DAAs [23]. In this study, several patients required medication modifications due to drug interactions with the selected HCV treatment regimen. Overall, 40% of the patients required either a dose modification or medication change due to a potential drug interaction with HCV treatment. Antiretrovirals were modified in 19% of patients, especially those patients on a ritonavir-boosted protease inhibitor ART regimen who were treated with SMV + SOF and patients on elvitegravir/cobicistat/tenofovir disoproxil fumarate/emtricitibine treated with LDV/SOF.
In addition to efficacy, DAAs are attractive because of their excellent safety profile. Similar to clinical trial results, the frequency of adverse effects overall was low in this study, and only 1 discontinuation of HCV therapy was observed in a patient receiving RBV + SOF. Of the HCV combination therapies used, LDV/SOF was found to have the lowest occurrence of adverse effects.
Although this study may address clinicians’ concerns regarding the generalizability of the results from clinical research trials, it is not without its own limitations. The limitations for this study include the small number of patients treated with DAAs other than LDV/SOF, being a single-center site, few patients lost to follow up before obtaining SVR12 laboratory results, the small percentage of patients with cirrhosis, and the retrospective design. In addition, this study did not specifically evaluate adherence to clinic visits during treatment or posttreatment.
CONCLUSIONS
In conclusion, in this real-world cohort of largely black, male, HIV/HCV-coinfected patients, all-oral HCV DAA regimens were highly effective and well tolerated despite real-world challenges including medication access barriers and potential drug interactions. These encouraging results underscore the value of treating HIV/HCV-coinfected patients in the real world using a multidisciplinary approach.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
Financial support. L. M. received funding from Emory University’s Open Access Publishing Fund for the publication of this manuscript. L. M. received grant funding from Gilead Sciences.
Potential conflicts of interest. M. P. served as a consultant to ViiV Healthcare. L. M. served on an advisory board for Bristol Myers Squibb. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Platt L, Easterbrook P, Gower E, et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis 2016; 16:797–808. [DOI] [PubMed] [Google Scholar]
- 2. National Center for HIV/AIDS, Viral Hepatitis, STD and TB Prevention and Division of Viral Hepatitis. HIV and viral hepatitis. Available at: http://www.cdc.gov/hiv/pdf/library/factsheets/hiv-viral-hepatitis.pdf. Accessed September 1, 2016. [Google Scholar]
- 3. Jordan AE, Perlman DC, Neurer J, et al. Prevalence of hepatitis C virus infection among HIV + men who have sex with men: A systematic review and meta-analysis. Int J STD AIDS 2017; 28:145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ward C, Lee V. Experience of acute hepatitis C and HIV co-infection in an inner city clinic in the UK. J Int AIDS Soc 2014; 17(Suppl 3):19639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Limketkai BN, Mehta SH, Sutcliffe CG, et al. Relationship of liver disease stage and antiviral therapy with liver-related events and death in adults coinfected with HIV/HCV. JAMA 2012; 308:370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen TY, Ding EL, Seage Iii GR, Kim AY. Meta-analysis: increased mortality associated with hepatitis C in HIV-infected persons is unrelated to HIV disease progression. Clin Infect Dis 2009; 49:1605–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Naggie S, Cooper C, Saag M, et al. Ledipasvir and sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med 2015; 373:705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wyles DL, Ruane PJ, Sulkowski MS, et al. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med 2015; 373:714–25. [DOI] [PubMed] [Google Scholar]
- 9. Rockstroh JK, Nelson M, Katlama C, et al. Efficacy and safety of grazoprevir (MK-5172) and elbasvir (MK-8742) in patients with hepatitis C virus and HIV co-infection (C-EDGE CO-INFECTION): a non-randomised, open-label trial. Lancet HIV 2015; 2:e319–27. [DOI] [PubMed] [Google Scholar]
- 10. Sulkowski MS, Eron JJ, Wyles D, et al. Ombitasvir, paritaprevir co-dosed with ritonavir, dasabuvir, and ribavirin for hepatitis C in patients co-infected with HIV-1: a randomized trial. JAMA 2015; 313:1223–31. [DOI] [PubMed] [Google Scholar]
- 11. Saeed S, Strumpf EC, Walmsley SL, et al. How generalizable are the results from trials of direct antiviral agents to people coinfected with HIV/HCV in the real world? Clin Infect Dis 2016; 62:919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Osinusi A, Townsend K, Kohli A, et al. Virologic response following combined ledipasvir and sofosbuvir administration in patients with HCV genotype 1 and HIV co-infection. JAMA 2015; 313:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Luetkemeyer AF, McDonald C, Ramgopal M, et al. 12 weeks of daclatasvir in combination with sofosbuvir for HIV-HCV coinfection (ALLY-2 Study): efficacy and safety by HIV combination antiretroviral regimens. Clin Infect Dis 2016; 62:1489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sulkowski MS, Naggie S, Lalezari J, et al. Sofosbuvir and ribavirin for hepatitis C in patients with HIV coinfection. JAMA 2014; 312:353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saxena VFF, Sise M, Lim JK, et al. Safety and efficacy of sofosbuvir-containing regimens in heaptitis C infected patients with reduced renal function: real-world experience from HCV-TARGET. In: 50th Annual Meeting of the European Association for the Study of the Liver; April 22–26, 2015; Vienna, Austria. [Google Scholar]
- 16. Dieterich DT, Bacon B, Flamm S, et al. Final evaluation of HCV patients treated with 12 week sofosbuvir +/- simeprevir regimens in TRIO network: academic and community treatment of a real-world, heterogeneous population. In: American Association for the Study of Liver Diseases; 2015; Boston, Masachussetts. [Google Scholar]
- 17. Del Bello D, Cha A, Sorbera M, et al. Real-world sustained virologic response rates of sofosbuvir-containing regimens in patients coinfected with hepatitis C and HIV. Clin Infect Dis 2016; 62:1497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hawkins C, Grant J, Ammerman LR, et al. High rates of hepatitis C virus (HCV) cure using direct-acting antivirals in HIV/HCV-coinfected patients: a real-world perspective. J Antimicrob Chemother 2016; 71:2642–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saab S, Jackson C, Nieto J, Francois F. Hepatitis C in African Americans. Am J Gastroenterol 2014; 109:1576–84. [DOI] [PubMed] [Google Scholar]
- 20. Sogni P, Gilbert C, Lacombe K, et al. All-oral direct-acting antiviral regimens in HIV/Hepatitis C virus-coinfected patients with cirrhosis are efficient and safe: real-life results from the prospective ANRS CO13-HEPAVIH cohort. Clin Infect Dis 2016; 63:763–70. [DOI] [PubMed] [Google Scholar]
- 21. AASLD-IDSA. When and whom to initiate HCV therapy. Recommendations for testing, managing, and treating hepatitis C. Available at: http://www.hcvguidelines.org/full-report/hcv-testing-and-linkage-care Accessed 30 August 2016. [Google Scholar]
- 22. Lakshmi S, Alcaide M, Palacio A, et al. Improving HCV cure rates in HIV-coinfected patients—a real-world perspective. Am J Manag Care 2016; 22(6 Spec No):SP198–204. [PMC free article] [PubMed] [Google Scholar]
- 23. MacBrayne CE, Kiser JJ. Pharmacologic considerations in the treatment of hepatitis C virus in persons with HIV. Clin Infect Dis 2016; 63(Suppl 1):S12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.