Abstract
In this report we describe the first human case of hypertryptophanemia confirmed to be due to tryptophan 2,3-dioxygenase deficiency. The underlying etiology was established by sequencing the TDO2 gene, in which there was compound heterozygosity for two rare variants: c.324G>C, p.Met108Ile and c.491dup, p.Ile165Aspfs*12. The pathogenicity of these variants was confirmed by molecular-level studies, which showed that c.491dup does not produce soluble protein and c.324G>C results in a catalytically less efficient Met108Ile enzyme that is prone to proteolytic degradation. The biochemical phenotype of hypertryptophanemia and hyperserotoninemia does not appear to have significant clinical consequences.
Keywords: hypertryptophanemia, hyperserotoninemia, tryptophan 2, 3-dioxygenase, TDO2
1. Introduction
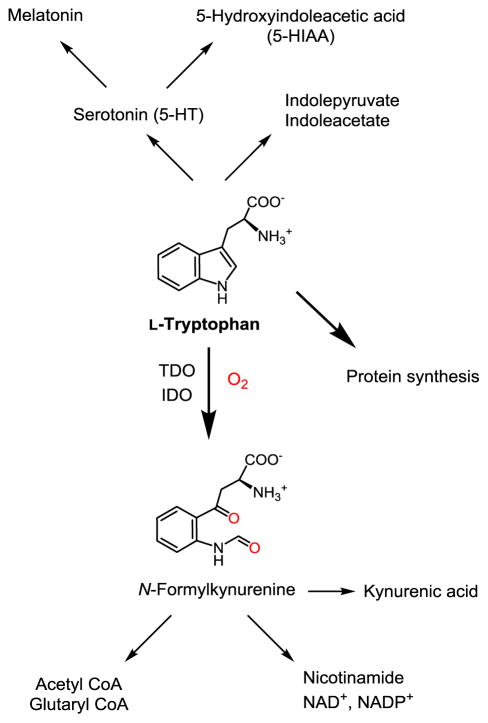
L-Tryptophan (L-Trp) is an essential aromatic amino acid. It is a precursor to the neurotransmitters serotonin and melatonin, as well as nicotinamide which is also supplied from niacin in the diet. The overall metabolic pathway is summarized in Figure 1: about 95% is catabolized via the kynurenine pathway. The first rate-limiting step in this to N-formylkynurenine is catalyzed by two heme-containing enzymes: L-tryptophan 2,3-dioxygenase (TDO, EC 1.13.11.11) and indoleamine 2,3-dioxygenase (IDO, EC1.13.11.52). TDO, encoded by the TDO2 gene, is mainly expressed in the liver, has high substrate specificity for L-tryptophan, and its activity is increased by the substrate, whereas IDO, encoded by the IDO1 gene, has more widespread tissue distribution, less substrate specificity, and its activity is increased by inflammation [1]. A third enzyme, IDO2, encoded by the IDO2 gene catalyzes the same reaction; although its relative activity is less than IDO, recent work suggests that it has an important immunoregulatory role [2]. TDO activity is thought to be quantitatively more important than IDO activity under normal physiological conditions: using knockout mouse models, it was estimated to account for 75% of tryptophan oxidation [3], and TDO2−/− mice had plasma tryptophan concentrations 9.3 times controls [4], compared with only 1.3 times for IDO1−/− [5].
Figure 1.
Fate of L-tryptophan: TDO/IDO-mediated kynurenine pathway is the principle route of catabolism.
Hypertryptophanemia has rarely been reported in the medical literature. Tada et al. described a 9-year-old girl with significant hypertryptophanuria and parental consanguinity [6]. She had growth and developmental delay, ataxia, and photosensitive pellagra-like rash with hyperpigmentation, all features clinically suggestive of Hartnup disease. However, unlike that disorder, her fasting plasma tryptophan was high rather than low, at 91 μmol/L (controls 51–81 μmol/L, N = 10), and after tryptophan loading became higher, and for a longer time, than controls, with less kynurenine excretion. There was also no accompanying neutral aminoaciduria. The suggested explanation was a block in the conversion of tryptophan to kynurenine. A 7-year-old boy with somewhat similar clinical and biochemical findings was described by Wong et al., though the fasting tryptophan level was more normal [7]. In this case the renal clearance of tryptophan was documented as being normal, and the photosensitive skin rash was responsive to nicotinamide therapy. Much higher plasma tryptophan levels were observed in two adult siblings, who also had marked hypertryptophanuria, but without other significant aminoaciduria [8,9,10]. Fasting plasma tryptophan in the brother at age 23 was 476.7 μmol/L (mean of 3 measurements), and 406.9 μmol/L at follow up 10 years later. The sister at age 22 had similar levels: the mean of two measurements was 472.5 μmol/L. Both had markedly increased levels of urinary indoles (indolelactate, indolepyruvate, indoleacetate and 5-HIAA), but normal or low kynurenine, 3-hydroxykynurenine, kynurenic acid and xanthurenic acid. They had some similar psychiatric features including descriptions of emotional lability, and hypersexuality, but otherwise their clinical descriptions were very different; a complicating factor in the sister was possible congenital rubella. She had more severe intellectual disability throughout childhood as well as profound sensorineural hearing loss. The brother’s growth and development was apparently normal until adolescence when he developed hyperacusis and hyperalgesia, and joint issues including ulnar drift of fingers and limited elbow extension as well hyperflexibility, which may have been a familial trait. He was also noted to have high myopia, strabismus, and ocular hypertelorism. Neither sibling had ataxia or skin rashes.
Tryptophan is not routinely measured or reported in most neonatal metabolic screening programs, though an exception was in Manchester UK where paper chromatography of amino acids was used from 1961–1990. Persistently elevated plasma tryptophan (>49 μmol/L) was observed in 12/1,196,113 infants screened (1:99,743), but no consistent adverse effects were apparent, and it was concluded that it was a benign condition [11]. Tryptophanuria, as part of the characteristic pattern of urinary neutral amino acids seen in the renal transport defect Hartnup disease, was reported in 56/2,469,929 (1:44,106) infants in the Quebec urine newborn screening program, but it was never found to be isolated, or secondary to hypertryptophanemia [12].
In this report we describe an apparently asymptomatic adult who has significant chronic hypertryptophanemia and hyperserotoninemia, and we provide evidence that it is due to deficient activity of the TDO enzyme.
2. Methods
TDO2 sequencing
Genomic DNA was extracted from EDTA anti-coagulant peripheral blood using an Autopure LS System and Gentra Puregene Blood Kit reagents (Quiagen, Toronto, Canada). Bi-directional Sanger sequencing of TDO2 was undertaken with primers designed by NCBI Primer-BLAST software (sequences are available upon request), the HotStar Plus amplification system (Qiagen, Toronto, ON), and resolution using a 3130xL Genetic Analyzer (Life Technologies, Burlington, ON). Sequence subtraction was performed using Mutation Surveyor V4.05 software (SoftGenetics, State College, PA) and variants analyzed using Alamut Software (Interactive Biosoftware, San Diego, CA).
Cloning, expression and purification of human TDO
The human TDO gene was purchased from DNASU. The N- and C- terminal truncated variant of TDO (40–390) was amplified by PCR using site specific primers (forward primer, 5′-GGAATTCCATATGCTTATCTATGGGAACTACCTG-3′; reverse primer, 5′-AACCGCTCGAGTTAATATAGAAATTTGTGAATGGTTG-3′ and was introduced into pET28a expression vector (Novagen) which was modified to have a TEV cleavage site behind the N-terminal polyhistidine tag using restriction enzymes NdeI and XhoI. c.324G>C and c.491dup variants were generated by quick change method (Stratagene). All the constructs generated were verified by DNA sequencing.
Escherichia coli BL21(DE3) cells which were transformed with each construct were grown in Luria-Bertani medium at 37°C until OD600 reached 0.3, 160 mg of δ-aminolevulinic acid and 14 mg of ferrous ammonium sulfate (Mohr’s salt) per liter of culture volume were supplemented. Temperature was lowered down to 20°C and cell culture was continued for 18 h. Cells were harvested by centrifugation at 8,000×g for 20 min, suspended in 50 mM Tris-HCl, 200 mM NaCl (pH 7.4) and disrupted by an LM20 cell disruptor from Microfludizer. After centrifugation at 30,000×g for 1 h, the supernatant was loaded onto a Ni-charged affinity chromatography column (GE Healthcare) and purified by gradient elution with 50 mM Tris-HCl, 200 mM NaCl, 500 mM imidazole (pH 7.4). The elution fraction was desalted using Sephadex G25 column (GE Healthcare) with 50 mM Tris-HCl, 200 mM NaCl, 10% (v/v) glycerol. The purified proteins were frozen by liquid nitrogen or dry ice with ethanol bath and stored at −80°C for further use.
HeLa cell expression and qPCR
The native TDO in HeLa cells was knocked down by siRNA. Then, the full-length wild-type TDO and the two variants c.491dup and c.324G>C, were transfected into the HeLa cell line by using lipofectamine 2000. Quantitation of the gene expression was achieved by qPCR analysis.
Enzyme characterization and activity assays
The catalytically active form of ferrous TDO was prepared from the ferric enzyme by adding sodium dithionite under O2-free conditions and used for the Michaelis-Menten kinetics analyses on an Aglient 8453 spectrophotometer. The steady-state kinetics experiments were carried out as previously described [13,14,15]. The initial rate was obtained by monitoring the formation of N-formylkynurenine at 321 nm with the known extinction coefficient 3,150 M−1cm−1 [16]. Schlenk line and a gloveless anaerobic chamber (COY Laboratory Products) equipped with a digital O2 analyzer (ppm) were used to keep the anaerobicity. The anaerobic chamber was filled with 95% N2, 5% H2 and a catalyst that removes trace oxygen by promoting water production from H2 and O2.
Isothermal titration calorimetry (ITC) measurements
Desalting and buffer exchange of purified protein were carried out using Sephadex G-25 column (GE Healthcare) equilibrated with the ITC buffer containing 200 mM Tris-HCl, 200 mM NaCl, and 5% glycerol (pH 8.0). Microcal VP-ITC system (Malvern Instruments) was used to conduct the ITC measurements under aerobic or anaerobic conditions. The anaerobic experiments were carried out inside the COY anaerobic chamber. For αMTrp, a total of 45 injections with a reference power of 15 μcal/s and a stirring speed of 155 rpm were performed after the temperature was equilibrated to 20 °C. ITC measurements for L-Trp binding to TDO were carried out with identical settings except injection volumes and the number of injections. The variant enzyme required higher concentration of L-Trp or αMTrp to reach saturation in the ITC experiment. The ITC data were processed and analyzed using non-linear least square curve fitting with Origin version 7.0 (OriginLab Corp.) software by using one-site or two-site binding models.
Psychological evaluation was done using the Center for Epidemiological Studies in Depression Scale (CES-D) [17], Sf-36v2 Health Survey (Optum Inc.), the Pittsburgh Sleep Quality Index [18], and the Psychiatric Diagnostic Screening Questionnaire [19]. Informed consent for this investigation was obtained from the proband and her parents.
3. Case Report
An infant female was found to have hypertryptophanemia on newborn screening [20]. (At that time, in 1986, Alberta, Canada, filter paper blood spots were initially screened for hyperphenylalaninemia and other amino acid disorders using a thin-layer chromatography method). The tryptophan concentration was estimated at 129 μmol/L from the blood spot taken at three days of life, and confirmed to be high at 479 μmol/L in a follow up plasma sample at 21 days of age (normal 11–64 μmol/L). She was mainly breast fed with occasional formula supplements. No unusual urinary indole metabolites were found. At three months a 50 mg/Kg oral tryptophan load resulted in a rise in plasma tryptophan from a baseline of 295 μmol/L to 503 μmol/L at 1 hour, 544 μmol/L at 2 hours and 495 μmol/L at 4 hours. Serum serotonin was also high: 1.6 μmol/L at baseline rising to 2.1 μmol/L at 1 hour and 4 hours (normal 0.6–1.2 μmol/L). The baby became somnolent during the test.
The significance and cause of the baby’s hypertryptophanemia were unknown at the time, but there was some concern because of the previous reports of hypertryptophanemia associated with developmental issues [6,7,8,9,10]. She was therefore placed on a low protein diet, with supplementation using an amino acid mixture lacking tryptophan. Plasma tryptophan on this diet over the first two years averaged 126 μmol/L, range 16–265 μmol/L (n=13). As her growth, development, and general health was clearly normal at 2 years of age, the diet was relaxed, then discontinued. Subsequent intermittent plasma tryptophan measurements to age 26 averaged 239 μmol/L, range 161–385 μmol/L (n=12, normal <51 μmol/L), on a diet containing approximately 1g/Kg of protein per day. Reevaluation at age 26 revealed elevated serum serotonin: mean 2.024 μmol/L, range 1.75–2.16 μmol/L, n=4, (normal 0.17–1.174 μmol/L) and a normal plasma melatonin at 27 ng/L (normal 11–135 ng/L). 24 hour urinary 5-HIAA measurements were in the high normal range (mean 37 μmol/day, n=3, normal <41 μmol/L. She had completed university education. Height, weight and BMI were normal. The only health issues in the past had been bilateral inguinal hernia repairs at age 6, and tonsillectomy and adenoidectomy at age 9. Her parents were unrelated, and had completely normal tryptophan levels: father 30 μmol/L, and mother 23 μmol/L. Her one brother declined testing.
At age 28, she was considering a pregnancy, so sought advice regarding possible teratogenic risks. Since there was little information in the literature, we sought to determine if the tryptophan and serotonin levels could be normalized by dietary means. Food records on an unrestricted diet showed an average protein intake of 1.1 g/Kg/day. Restriction of natural protein to 0.8 g/Kg/day (close to daily requirements for an adult) did not reveal any difference in tryptophan and serotonin values compared to an unrestricted diet. Further natural protein restriction was undertaken. To avoid protein deficiency, we supplemented with tryptophan free protein formulas. The goal was to maintain total protein intake between 0.8 to 1 g/Kg/day, but at times actual intake dropped to 0.6 g/Kg/day. Various combinations of natural and medical protein ratios were trialed, but natural protein was never lower than 0.3 g/Kg/day. Plasma tryptophan normalized on a few occasions but was not sustained. Serotonin could not be normalized and always remained elevated. Low tryptophan and low serotonin could not be achieved in conjunction; for example, plasma tryptophan between 46–75 μmol/L correlated with serotonin levels between 1.4 to 2.7 μmol/L. When compared to an unrestricted diet, average tryptophan values were lower with protein restriction but a sustained decrease in serotonin levels could not be achieved. The lowest serotonin achieved was 1.4 μmol/L and highest was 2.7 μmol/L with an average of 1.9 μmol/L (n=21) with no obvious correlation with protein intake. She has subsequently had two first trimester miscarriages (10 weeks and 8 weeks). She had normal serum albumin and blood pressure, and was being supplemented continually and preconceptually with a daily prenatal multivitamin containing 18mg niacinamide.
Psychological testing did not reveal any significant abnormalities. The CES-D score was in the “no depressive symptoms” range. The Sf-36v2 Health Survey scores were all in the normal range across all of the scales and the two summary scores related to physical and mental health. The Pittsburgh Sleep Quality Index score indicated “good sleep quality”. The Psychiatric Diagnostic Screening Questionnaire revealed no indication of any psychiatric problems in the past 2 weeks or 6 months.
4. Results
Sequencing of the TDO2 gene in the proband and her parents revealed that she is a compound heterozygote for a paternally inherited variant c.491dup, p.Ile165Aspfs*12, and a maternally inherited variant c.324G>C, p.Met108Ile. Expression studies were therefore done to characterize the effects of each variant on transcription, translation, and enzyme activity. We expressed TDO2 in E. coli BL21(DE33). The two variants were generated by site-directed mutagenesis.
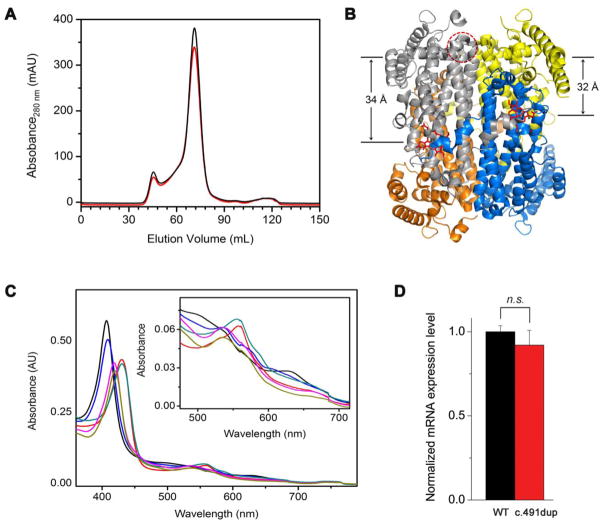
The c.491dup variant results in a frame shift and introduces a premature stop codon 10 amino acids distal to Ile165Asp, resulting in a predicted truncated protein only 43% of its normal length. The E. coli expression system did not produce a soluble protein, so we also examined expression in a human cell line. We overexpressed the wild-type TDO and the c.491dup variant in HeLa cells. The wild-type expression generated TDO with anticipated catalytic activity, but the variant expression did not. We then analyzed the mRNA expression levels by qPCR, which revealed similar mRNA expression levels in both c.491dup and wild-type genes (Figure 2D), suggesting a problem in translation or protein stability rather than in transcription.
Figure 2.
(A) Superdex-200 elution profile of the wild-type enzyme (black trace) and Met108Ile (red trace). (B) Structural representation of the distance between Met108 and the heme center (34 Å) in the same subunit and adjunct unit (32 Å). The coordinates were taken from 5TIA.PDB [21]. (C) The optical spectra of Met108Ile at the ferric (black trace), ferrous (red) oxidation state, ferric-CN complex (magenta), and in the presence of L-Trp with the ferric (blue), ferrous (cyan), and ferric-CN complex (brown), respectively. The inset is a zoomed-in view of the α/β band region. These spectra are indistinguishable from those of the wild-type enzyme. (D) The mRNA expression level of c.491dup and the native enzyme in HeLa cells as quantitated by qPCR.
Both the wild-type and Met108Ile variant yielded soluble and catalytically active proteins. Gel filtration chromatography on a Superdex-200 column (Figure 2A) revealed that Met108Ile is predominately a homotetramer as reported for the native human enzyme [21], so the protein quaternary structure appears unaffected. Figure 2C shows the Soret and the α/β bands under various conditions. The Soret peak of the heme center at the ferric, ferrous, and ferric-CN complex states were 407, 430, and 418 nm, respectively. In the presence of L-Trp, the absorbance maxima were 409, 430, and 420 nm, respectively. These absorbance features are identical to the wild-type enzyme and similar to those reported for the hemin-reconstituted human TDO [22]. There was no noticeable difference between the variant and the wild-type enzyme in the reduced and oxidized oxidation states in the response of the exogenous ligand binding to the heme, either in the absence or presence of L-Trp (Figure 2C). Analysis of the catalytic properties showed that the Met108Ile enzyme had a kcat value of 0.66 ± 0.04 s−1, similar to 0.54 ± 0.02 s−1 for the wild-type, but the Km value was noticeably increased at 236 ± 39 μM, compared to 132 ± 25 μM for the wild-type.
We next examined L-Trp interaction with Met108Ile and wild-type TDO. To avoid turnovers during the analysis of the substrate binding experiments by ITC, we employed two strategies. In the first set of experiments, we treated the TDO proteins with excess sodium cyanide. After a column filtration, the resulting TDO proteins were catalytic inactive, even though L-Trp is able to reduce the Fe ion and activate the ferric form of TDO [23], as the heme was blocked from O2 binding by the cyanide ligand [13]. The second approach was to perform isothermal titration calorimetry (ITC) experiments in an O2-free anaerobic chamber.
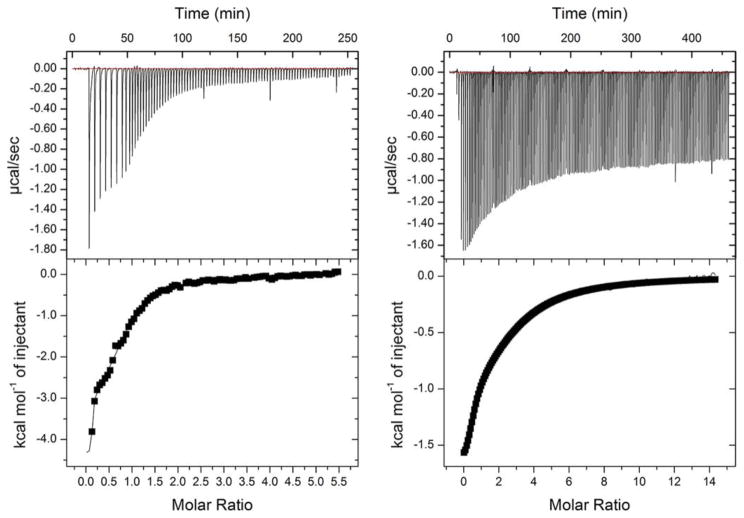
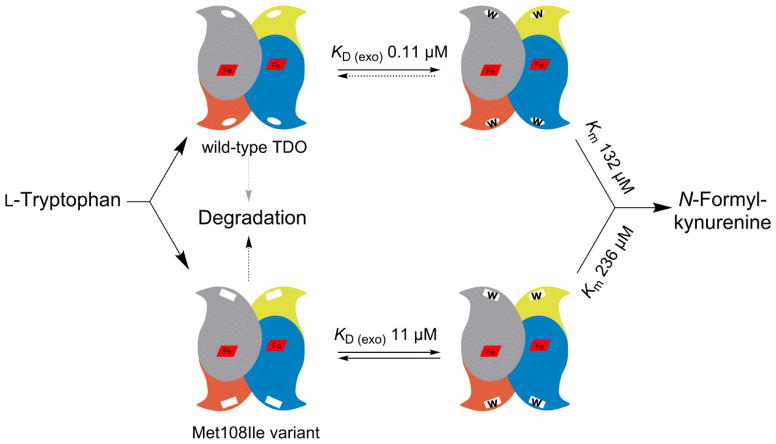
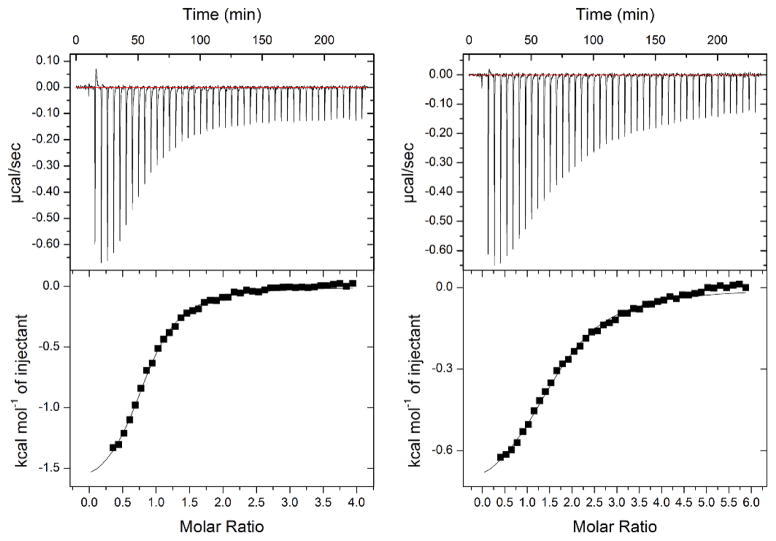
The variant and wild-type TDO proteins were reduced to the ferrous oxidation state with 1 mM sodium dithionite under anaerobic conditions. Full reduction of the heme Fe was verified by UV-Vis spectroscopy which showed a complete transition of the Soret band from 407 nm to 430 nm. Figure 3 shows that the wild-type enzyme has two binding sites for L-Trp with KD values of 0.11 ± 0.08 μM and 30 ± 1.7 μM - i.e. one site has 273-fold tighter binding than the other. The Met108Ile variant protein showed substantially larger KD values at 11 ± 4.4 μM and 220 ± 13, which is only a 20-fold difference, accounted for by the high affinity binding site having 100-fold reduced affinity, the other site less than 8-fold decreased. The lower affinity site has been documented to be the active site; the higher being a non-catalytic exo site [21].
Figure 3.
Isothermal titration calorimetry (ITC) binding assays of L-Trp show two binding sites with distinct affinities to the wild-type TDO (left panel) and Met108Ile variant (right panel). The experiments were performed under O2-free conditions in an anaerobic chamber.
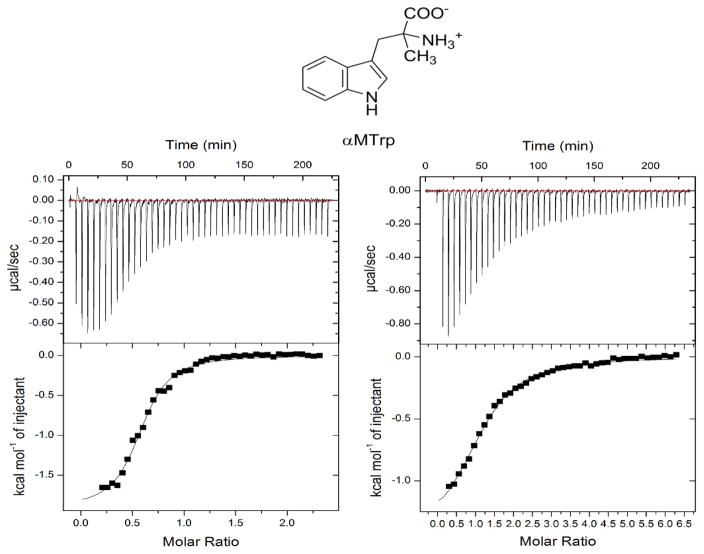
To further characterize this relatively major effect of the Met108Ile change on the exo site we performed anaerobic binding experiments with αMTrp, an L-Trp analogue that mainly binds to the exo site [21]. We first confirmed that αMTrp is not a competitive inhibitor of TDO at physiologically relevant L-Trp concentrations. Only at a very high concentration of 4 mM αMTrp was a mixed-type weak inhibition pattern observed in which Vmax is decreased but Km is increased (data not shown). As expected, ITC measurements showed evidence of a single binding site (Figure 4). The wild-type enzyme (KD, 5.8 ± 0.7 μM) has 6.4-fold higher affinity for αMTrp than the Met108Ile variant (KD, 37 ± 2.7 μM). We also tested αMTrp by locking the active site with 2 M sodium cyanide as an oxygen surrogate.
Figure 4.
ITC binding assays of αMTrp show a single binding sites with distinct affinities to the wild-type TDO (left panel) and Met108Ile variant (right panel). The chemical structure of αMTrp is shown at the top of the figure.
5. Discussion
The observation of chronic hypertryptophanemia associated with hyperserotoninemia and borderline high 5-HIAA without any excessive excretion of indole metabolites, led us to hypothesize that a deficiency of the enzyme TDO could be responsible. As it is mainly expressed in the liver, direct measurement of activity would require a liver biopsy, which was not justified by the clinical circumstances. We therefore took the approach of sequencing the TDO2 gene, discovering that the individual was a compound heterozygote for two variants that in subsequent expression experiments were shown to be deleterious. The variants are rare: c.491dup, p.Ile165Aspfs*12 was reported heterozygote in 1/121,218 chromosomes, and c.324G>C, p.Met108Ile was not listed in the ExAc database [24]. The Met108 position is highly conserved in mammals.
The c.491dup, p.Ile165Aspfs*12 variant did not express soluble protein, likely because of a problem in transcription or instability, but the c.324G>C, p.Met108Ile variant did express catalytically active protein with a normal quaternary structure. As this variant is more than 30 Å away from the closest heme (Figure 2B), the electronic structure of the catalytic heme center is unlikely to be altered. However, it does have a remote allosteric effect as evidenced by an increased Km, and therefore its predicted activity would be significantly reduced at anticipated physiological L-Trp concentrations. (Human intrahepatic L-Trp concentrations do not appear to be well documented in the literature, but in rats, the concentration is similar to plasma [25]). More importantly is a markedly increased susceptibility for degradation. The crystal structure of holo-TDO recently became available and detailed functional analysis showed that the second high-affinity non-catalytic exo L-Trp binding site functions as a protein degradation signal via ubiquitin dependent proteasomal degradation [21]. TDO has a very short half-life: in mice estimated at 2–3 hours compared to 2–3 days for total liver protein [26], so this explains a mechanism whereby the substrate increases in vivo activity. Our data show that the Met108Ile variant decreases the affinity for L-Trp at the exo site by two orders of magnitude. This would therefore predict a greatly accelerated rate of degradation. The significantly altered binding of αMTrp at this site compared with the wild-type enzyme further confirms this rather unusual mutational mechanism.
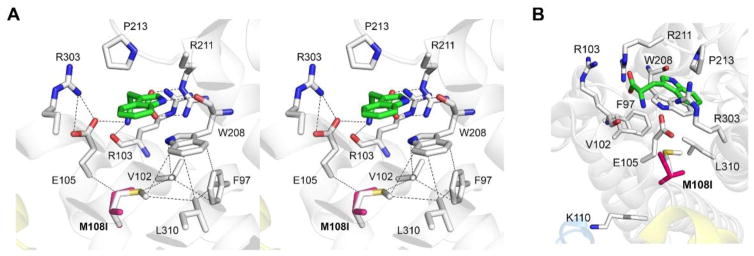
Met108 is located near the high-affinity L-Trp binding site (Figure 6A), so we propose the following mechanism to explain the altered binding characteristics of the Met108Ile protein. The aromatic ring of L-Trp is flanked between Trp208 and Pro213, and the ring nitrogen atom forms a hydrogen bond with the carbonyl oxygen of Trp208. The carboxyl group of L-Trp forms a salt-bridge with Arg211. The amino group is stabilized by hydrogen bonds with the main chain oxygen atom of Arg103 and Glu105, whose position is fixed through interaction with Arg303. The Met108Ile alteration would perturb the van der Waals interaction to the Cα carbon of Glu105; thereby the H-bonding between Glu105 and the amino group of L-Trp would be affected. Trp208 is supported by the hydrophobic residues: Phe97, Val102, Leu310, and Met108. The Met108Ile variant would disrupt the tight packing below Trp208 and affect the binding efficiency of L-Trp to the signaling site of the enzyme.
Figure 6.
(A) A stereoview of the non-catalytic signaling site for L-Trp (i.e., exo site) in human TDO (coordinates were taken from 5TIA.PDB, [21]). L-Trp bound is shown as green carbons. The magenta Met108Ile in silico variant is overlaid with Met108. Dashed lines represent hydrogen bonds or van der Waals interactions. (B) Met108 is pointed towards the exo site rather than the potential ubiquitination site at Lys110.
We noticed that Met108 is close to one of the newly identified ubiquitination site in human TDO, i.e., Lys110 [21]. Even though both Met108 and Lys110 are located in the same helix (Figure 6B), it is not likely that the Met108Ile change can affect the residue of Lys110 because the residue of Met108 is heading toward the exo site but Lys110 is protruding the opposite side.
Figure 7 illustrates our understanding of the molecular mechanism of Met108Ile pathology. The protein is less active at physiological L-Trp concentrations, and is very much more vulnerable to protein degradation. It is possible that there may be some residual activity in vivo; if so, L-Trp levels in our case may not be as high as it might be in an individual with biallelic null mutations. In conclusion there is strong molecular and biochemical evidence to conclude that TDO deficiency is the explanation for the individual’s chronic hypertryptophanemia and resulting hyperserotoninemia.
Figure 7.
Schematic model comparing the Met108Ile TDO variant with the wild-type enzyme. The variant has a higher Km; but more importantly shows a hundredfold reduction of L-Trp binding affinity to the non-catalytic exo regulatory site, predicting a greatly increased rate of proteolytic degradation.
TDO deficiency has not been previously documented in humans, but a knock-out mouse model was developed by Kanai et al. [4]. TDO2−/− mice had mean plasma tryptophan levels of 360 μmol/L, or 9.3 times that of TDO2+/+ controls, and 20 times the concentration in brain tissue. Brain serotonin was twice that of controls, and 5-HIAA 5 times; plasma serotonin levels were not reported. Interestingly, behavioral differences were observed: TDO2−/− mice seemed to exhibit less anxiety in elevated plus maze and open field tests than WT controls. Also they demonstrated increased adult neurogenesis. Serotonin has numerous essential functions both within the central nervous system and without, and modulates most human behavioral processes [27]. Therefore we wondered whether the hyperserotoninemia observed in our case might have any discernable consequences, but we were unable to find any significant behavioral, physical or pathological effects either in routine clinical assessments or more detailed psychological evaluations.
We wondered whether the altered tryptophan metabolism in our case might be a cause of recurrent pregnancy loss. There is considerable literature on the role of tryptophan and metabolites in pregnancy, recently reviewed by Badawy et al [28]. Some of the many potential factors that might be considered include toxic or teratogenic effects of high levels of tryptophan and/or serotonin, depletion of kynurenine and other metabolites that may have a role in fetal immune tolerance, and decreased nicotinamide production. On the other hand, placental and peripheral IDO and IDO2 activity might increase kynurenine and metabolite concentrations, particularly in the context of elevated tryptophan levels, and the individual was on niacinamide supplements. The frequency of two consecutive first trimester miscarriages in the general population is around 5%, and there are a variety of possible causes, though the etiology remains unknown after thorough investigation in about half the cases [29]. It is notable that in none of the knockout mouse models TDO−/−, IDO−/− and IDO2−/−, is there any apparent adverse effect on fertility or ability to maintain pregnancy [30]. Therefore in summary we do not believe that any conclusion can be reached on this point at the present time on the basis of a single case.
It remains speculative whether the previous case reports of hypertryptophanemia [6,7,8,9,10] were due to TDO deficiency, but the biochemical data presented seem to be generally consistent with that conclusion. Although the hypertryptophanemia was associated with significant, but rather different clinical problems in several cases, this is likely due to ascertainment bias. Unbiased ascertainment by newborn screening as in our case and by Cleary et al. [11] strongly suggests that this is a benign biochemical variant of no clinical significance, though additional data from similarly affected individuals, particularly with regard to pregnancy loss, would be helpful to document this more conclusively.
In summary, we have documented the first human case of TDO deficiency. It results in the biochemical phenotype of persistent hypertryptophanemia and hyperserotoninemia, but without any discernable clinical phenotype.
Figure 5.
Binding of αMTrp to the cyanide complex of wild-type (left panel) and Met108Ile variant (right panel).
Highlights.
The first clinical case report of human tryptophan 2,3-dioxygenase deficiency
Biochemical phenotype is hypertryptophanemia and hyperserotoninemia, but no clearly discernable clinical phenotype. Ascertainment was by newborn screening
Compound heterozygosity for two rare TDO2 variants: c.324G>C, p.Met108Ile and c.491dup, p.Ile165Aspfs*12
p.Met108Ile has an increased Km and is prone to proteolytic degradation via a greatly decreased KD at an exo tryptophan-binding regulatory site. c.491dup does not produce soluble protein
Acknowledgments
We would like to thank David Sinasac, Jillian Parboosingh, and Ryan Lamont for helpful advice and discussions, and Rhonda Klock for technical assistance. We are grateful for the interest and participation of the proband and her parents in this study. This work was supported in part by NIH grants R01GM108988, R01GM107529, R21MH107985, and NSF grants CHE-1623856 (to A.L.).
Abbreviations
- TDO
tryptophan 2,3-dioxygenase
- ITC
isothermal titration calorimetry
- L-Trp
L-tryptophan
- 5-HIAA
5-hydroxyindoleacetic acid
- αMTrp
α-methyltryptophan
Footnotes
ORCID numbers: F.L. 00-0002-3235-9996, A.L. 0000-0002-4182-8176.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Badawy AA-B. Tryptophan availability for kynurenine pathway metabolism across the life span: Control mechanisms and focus on aging, exercise, diet and nutritional supplements. Neuropharmacology. 2017;112:248–263. doi: 10.1016/j.neuropharm.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Prendergast GC, Metz R, Muller AJ, Merlo LMF, Mandik-Nayak L. IDO2 in immunomodulation and autoimmune disease. Front Immunol. 2014;5 doi: 10.3389/fimmu.2014.00585. article 585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maeta A, Sano M, Fukuwatari T, Funakoshi H, Nakamura T, Shibata K. Contributions of tryptophan 2,3-dioxygenase and indoleamine 2,3-dioxygenase to the conversion of D-tryptophan to nicotinamide analyzed by using tryptophan 2,3-dioxygenase-knockout mice. Biosci Biotechnol Bioch. 2014;78:878–881. doi: 10.1080/09168451.2014.905185. [DOI] [PubMed] [Google Scholar]
- 4.Kanai M, Funakoshi H, Takahashi H, Hayakawa T, Mizuno S, Matsumoto K, Nakamura T. Tryptophan 2,3-dioxygenase is a key modulator of physiological neurogenesis and anxiety-related behavior in mice. Mol Brain. 2009;2:8. doi: 10.1186/1756-6606-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackman KA, Brait VH, Wang Y, Maghzal GJ, Ball HJ, McKenzie G, De Silva TM, Stocker R, Sobey CG. Vascular expression, activity and function of indoleamine 2,3-dioxygenase-1 following cerebral ischaemia-reperfusion in mice. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:471–481. doi: 10.1007/s00210-011-0611-4. [DOI] [PubMed] [Google Scholar]
- 6.Tada K, Ito H, Wada Y, Arakawa K. Congenital hypertryptophanuria with dwarfism. Tohoku J Exp Med. 1963;80:118–134. [PubMed] [Google Scholar]
- 7.Wong PW, Forman P, Tabahoff B, Justice P. A defect in tryptophan metabolism. Pediatr Res. 1976;10:725–730. doi: 10.1203/00006450-197608000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Snedden W, Mellor CS, Martin JR. Hypertryptophanemia and indoleketonuria in two mentally subnormal siblings. N Engl J Med. 1982;307:1405. doi: 10.1056/NEJM198211253072219. [DOI] [PubMed] [Google Scholar]
- 9.Snedden W, Mellor CS, Martin JR. Familial hypertryptophanemia, tryptophanuria and indoleketonuria. Clin Chim Acta. 1983;131:247–256. doi: 10.1016/0009-8981(83)90094-3. [DOI] [PubMed] [Google Scholar]
- 10.Martin JR, Mellor CS, Fraser FC. Familial hypertryptophanemia in two siblings. Clin Genet. 1995;47:180–183. doi: 10.1111/j.1399-0004.1995.tb03956.x. [DOI] [PubMed] [Google Scholar]
- 11.Cleary MA, Imrie J, Bridge C, Wraith JE, Walter JH. Tryptophanaemia: outcome of 12 infants identified in the newborn period. J Inherit Metab Dis. 1998;21(suppl 2):27. [Google Scholar]
- 12.Auray-Blais C, Cyr D, Drouin R. Quebec neonatal mass urinary screening programme: from micromolecules to macromolecules. J Inherit Metab Dis. 2007;30:515–521. doi: 10.1007/s10545-007-0607-x.and Auray-Blais C. personal communication. 2016.
- 13.Geng J, Dornevil K, Liu A. Chemical rescue of the distal histidine mutants of tryptophan 2,3-dioxygenase. J Am Chem Soc. 2012;134:12209–12218. doi: 10.1021/ja304164b. [DOI] [PubMed] [Google Scholar]
- 14.Fu R, Gupta R, Geng J, Dornevil K, Wang S, Zhang Y, Hendrich MP, Liu A. Enzyme reactivation by hydrogen peroxide in heme-based tryptophan dioxygenase. J Biol Chem. 2011;286:26541–26554. doi: 10.1074/jbc.M111.253237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta R, Fu R, Liu A, Hendrich MP. EPR and Mossbauer spectroscopy show inequivalent hemes in tryptophan dioxygenase. J Am Chem Soc. 2010;132:1098–1109. doi: 10.1021/ja908851e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishimura Y, Nozaki M, Hayaishi O, Nakamura T, Tamura M, Yamazaki I. The oxygenated form of L-tryptophan 2,3-dioxygenase as reaction intermediate. J Biol Chem. 1970;245:3593–3602. [PubMed] [Google Scholar]
- 17.Radloff LS. The CES-D scale: A self report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 18.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 19.Zimmerman M, Mattia JI. A self-report scale to help make psychiatric diagnoses: the Psychiatric Diagnostic Screening Questionnaire. Arch Gen Psychiatry. 2001;58:787–794. doi: 10.1001/archpsyc.58.8.787. [DOI] [PubMed] [Google Scholar]
- 20.McCoy EE, Ferreira P. Hypertryptophanemia and hyperserotoninemia – detection in a newborn screening program for PKU. Clin Res. 1987;85:212A. [Google Scholar]
- 21.Lewis-Ballester A, Forouhar F, Kim SM, Lew S, Wang Y, Karkashon S, Seetharaman J, Batabyal D, Chiang BY, Hussain M, Correia MA, Yeh SR, Tong L. Molecular basis for catalysis and substrate-mediated cellular stabilization of human tryptophan 2,3-dioxygenase. Sci Rep. 2016;6:35169. doi: 10.1038/srep35169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basran J, Rafice SA, Chauhan N, Efimov I, Cheesman MR, Ghamsari L, Raven EL. A kinetic, spectroscopic, and redox study of human tryptophan 2,3-dioxygenase. Biochemistry. 2008;47:4752–4760. doi: 10.1021/bi702393b. [DOI] [PubMed] [Google Scholar]
- 23.Batabyal D, Yeh SR. Human tryptophan dioxygenase: a comparison to indoleamine 2,3-dioxygenase. J Am Chem Soc. 2007;129:15690–15701. doi: 10.1021/ja076186k. [DOI] [PubMed] [Google Scholar]
- 24.Exome Aggregation Consortium (ExAC); Cambridge, MA: [Accessed Feb 2017]. ( http://exac.broadinstitute.org) [Google Scholar]
- 25.Pawlak D, Tankiewicz A, Matys T, Buczko W. Peripheral distribution of kyneurenine metabolites and activity of kyneurinie pathway enzymes in renal failure. J Physiol Pharmacol. 2003;54:175–189. [PubMed] [Google Scholar]
- 26.Schimke RT, Doyle D. Control of enzyme levels in animal tissues. Annu Rev Biochem. 1970;39:929–976. doi: 10.1146/annurev.bi.39.070170.004433. [DOI] [PubMed] [Google Scholar]
- 27.Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Badawy AAB, Namboodiri AMA, Moffet JR. The end of the road for the tryptophan depletion concept in pregnancy and infection. Clin Sci. 2016;130:1327–1333. doi: 10.1042/CS20160153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shahine L, Lathi R. Recurrent pregnancy loss. Evaluation and treatment. Obstet Gynecol Clin North Am. 2015;42:117–134. doi: 10.1016/j.ogc.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Jusof FF. PhD Thesis. University of Sydney; 2015. Tryptophan-catabolising enzymes in animal models. ( https://ses.library.usyd.edu.au/bitstream/2123/13697/1/JUSOF%20Felicita%20-%20Final%20Thesis.pdf)and Jusof FF. personal communication. 2017.