Abstract
Blood-contacting devices, such as intravascular catheters, suffer from challenges related to thrombus formation and infection. Nitric oxide (NO) is an endogenous antiplatelet and antimicrobial agent. Exogenous release of NO from various polymer matrices has been shown to reduce thrombosis and infection of/on implantable medical devices. However, the clinical applications of such materials have been hindered due to factors such as NO donor leaching and thermal instability. In this study, a novel approach is demonstrated in which one lumen of commercial dual lumen catheters is dedicated to the NO release chemistry, allowing the other lumen to be available for clinical vascular access. A composite consisting of poly(ethylene glycol) (PEG) and S-nitroso-N-acetylpenicillamine (SNAP) is used to fill the NO-releasing lumen of commercial 7 French silicone catheters. Physiological levels of NO are released from the SNAP-PEG catheters for up to 14 d, as measured by chemiluminescence NO analyzer (in PBS buffer at 37 °C). PEG facilitates the NO release from SNAP within the lumen by increasing the water absorption and slowly dissolving the solid SNAP-PEG composite. In a CDC biofilm bioreactor, the SNAP-PEG catheters are found to reduce > 97% bacterial adhesion as compared to the PEG controls for single bacterial species including E. coli and S. aureus. SNAP-PEG and PEG control catheters were implanted in rabbit veins for 7 h (single lumen) and 11 d (dual lumen) to evaluate their hemocompatibility properties. Significant reductions in thrombus formation on the SNAP-PEG vs. PEG controls were observed, with ca. 85% reduction for 7 h single lumen catheters and ca. 55% reduction for 11 d dual lumen catheters.
Keywords: antimicrobial, catheters, hemocompatibility, nitric oxide, poly(ethylene glycol), S-nitrosothiols
Table of Contents/Abstract Graphic
1. Introduction
Two major challenges with blood-contacting medical devices (e.g., intravascular (IV) catheters, extracorporeal circuits) are related to thrombus formation and infection.1–3 When blood comes in contact with biomedical devices like IV catheters the coagulation cascade is initiated, where plasma proteins adsorb (e.g., fibrinogen) to the surface, platelets become activated and adhere, and ultimately a thrombus forms.3–4 This thrombus formation can prevent normal clinical use of the catheters (occlusion that prevents blood removal or drug/nutrient infusion), and can also lead to other potentially life-threatening conditions such as deep vein thrombosis or pulmonary embolism.5 Catheter-related bloodstream infections are another significant concern, where 11% of the 1.7 million hospital acquired infections that occur in the United States are associated with catheters, and such catheter-related infections lead to nearly 28,000 deaths each year.1, 6 Commercial heparin-coated catheters are available, but fail to fully address the issues related to infection because these surfaces only indirectly reduce infection by their reduction in clots (since bacteria can become trapped within the clots).7–9 Catheters containing active antibacterial agents (e.g., silver) are available, but have been reported to have similar rates of infection as standard catheters.10 In clinical practice, anticoagulant (e.g., heparin, citrate) and antibiotic lock solutions are also used.1, 11–12 Lock solutions containing anticoagulants are employed to prevent local thrombus formation, but have had similar rates of thrombosis and fail to address issues related to microbial infection.11, 13 Catheter replacement is the only solution in many cases, which can further increase chances of infection.14–15 Development of polymers that can resist both thrombosis and infection is still a prominent area of research with the potential for significant clinical benefits.
One promising approach to improving the hemocompatibility of medical devices has been the development of polymers with localized nitric oxide (NO) delivery. Nitric oxide is a biologically-active innate gas molecule with many important physiological roles. Healthy endothelial cells that line blood vessels release NO with a surface flux of 0.5 – 4 × 10−10 mol cm−2 min−1 that prevents platelet activation.16 Macrophages and nasal epithelial cells also produce NO that is a potent broad-spectrum antimicrobial agent.17–18 Low levels of NO release (nM concentrations) act as a signaling agent and have also been effective in dispersing microbial biofilms.19–20 Nitric oxide is highly reactive and has a short half-life under physiological conditions,21 so NO donor molecules such as N-diazeniumdiolates and S-nitrosothiols (RSNOs) have been widely studied. RSNOs such as S-nitrosoglutathione (GNSO) and S-nitrosoalbumin are recognized as an endogenous reservoir of NO.22–24 Synthetic RSNOs such as S-nitroso-N-acetylpenicillamine (SNAP) and S-nitroso-N-acetylcysteine (SNAC) have also been studied as NO release agents due to their stability.25 Heat, light (340 and 590 nm), and metal ions (e.g., Cu+) are the major catalysts for initiating NO release from RSNOs and forming a disulfide.24–27
Polymers with localized NO delivery have shown promising antithrombotic and antimicrobial properties.28–42 To date, the most common approaches to incorporating RSNOs, such as SNAP, within polymers for blood-contacting devices has been non-covalently dispersal within the biomedical polymer39–45 or synthetically binding the RSNO functional group to the polymer backbone.27, 46–49 Recent reports have shown that SNAP incorporated within silicone-polyurethane copolymers have long-term NO release, excellent storage stability, and can significantly reduce thrombus formation and bacterial adhesion on extracorporeal circuits39 and catheters.40–41 Despite the potential benefits of NO-releasing polymers, clinical application of NO-releasing catheters has not occurred to date. Some of the limitations have been related to the thermal sensitivity of NO donor molecules, challenging synthesis procedures, or leaching of NO donors/byproducts.39, 45, 48–51 The thermal sensitivity of NO donor molecules poses complications related to typical extrusion used to fabricate catheters and medical tubing, where the temperatures of molten polymers (> 150 °C) would be detrimental to maintaining the RSNO functionality.52
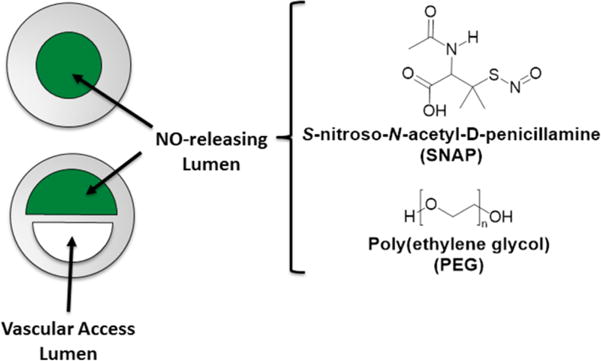
In this study, a novel method of fabricating catheters with NO release is investigated. One lumen of commercial single and dual lumen silicone catheters is filled with a composite mixture of poly(ethylene glycol) (PEG) and SNAP (Fig. 1). PEG is a polymer matrix used for various pharmaceutical purposes, such as drug release applications and as a suppository base, due to its water solubility, low toxicity, stability, and non-volatility.53–55 PEG polymers are available in a range of various molecular weights which at room temperature are viscous liquids (low Mn) to hard waxy solids (high Mn).56 GSNO and SNAC have previously been incorporated in liquid poly(ethylene glycol) (PEG) with a low molecular weight (average Mn 200 and 400) for potential topical applications.51, 57 The PEG matrix creates a stabilization effect where the geminate recombination reaction of the two radicals (RS• and NO•) within the PEG cage is favored due to the microviscosity of the PEG solvent, leading to slower NO release rates in comparison to the RSNOs in water. However, due to the use of low molecular weight PEGs employed in these prior studies the RSNO-PEG matrix still required low temperature storage in the freezer to maintain the RSNO stability.
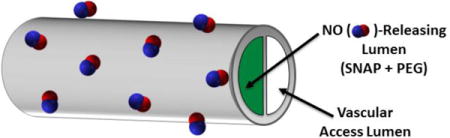
Fig. 1.
Configuration of single and dual lumen catheters with 1 lumen filled with the mixture of S-nitroso-N-acetyl-D-penicillamine (SNAP) and poly(ethylene glycol) (PEG). Single lumen catheter tubing was Tygon 3350 with I.D. 0.79 mm and O.D. 2.38 mm. Cook dual lumen silicone catheters have an O.D. 2.34 mm and lumen dimensions of (maximum width × height): NO-releasing lumen (1.27 × 0.64 mm) and vascular access lumen (1.14 × 0.48 mm).
Here, SNAP and high molecular weight PEGs (Mn 2,000 to 35,000) form a composite that is used to fill catheter lumens. The hydrophilic PEGs provide a matrix for filling catheter lumens with SNAP and facilitates the NO release under physiological conditions. Initial in vitro feasibility studies evaluated the water uptake and NO release properties were conducted using single lumen catheter tubing filled with the SNAP-PEG composite material. The SNAP-PEG composite was further used to fill one lumen of a 7 French commercial dual lumen silicone catheters, allowing the other lumen to provide for vascular access. These clinically relevant catheters were further evaluated for their antimicrobial activity using a CDC biofilm reactor and their hemocompatibility in a rabbit model.
2. Materials and Methods
2.1 Materials
N-Acetyl-D-penicillamine (NAP), sodium chloride, potassium chloride, sodium phosphate dibasic, potassium phosphate monobasic, ethylenediaminetetraacetic acid (EDTA), tetrahydrofuran (THF), Chelex® 100 resin, and sulfuric acid were purchased from Sigma-Aldrich (St. Louis, MO). BioUltra grade poly(ethylene glycol) (PEG) with the following molecular weights (Mn) were also purchased from Sigma Aldrich: 2,000; 4,000; 10,000; and 35,000. Methanol, hydrochloric acid, sulfuric acid, and Saint-Gobain Tygon® Formula 3350 Silicone Tubing (I.D. = 0.79 mm, O.D. = 2.38 mm) were obtained from Fisher Scientific (Pittsburgh, PA). Dual lumen silicone rubber catheters were a gift from Cook Medical Inc. (Bloomington, IN). Dow Corning RTV 3140 silicone rubber sealant was purchased from Ellsworth Adhesives (Germantown, WI). All aqueous solutions were prepared with 18.2 MΩ deionized water using a Milli-Q filter (Millipore Corp., Billerica, MA). Phosphate buffered saline (PBS), pH 7.4, containing 10 mM sodium phosphate, 138 mM NaCl, 2.7 mM KCl, and 100 μM EDTA was used for all in vitro experiments. SNAP was synthesized following previously described methods,39, 58 where an equimolar ratio of sodium nitrite and NAP were dissolved in a 1:1 mixture of water and methanol containing 2 M HCl and 2 M H2SO4, followed by precipitation over an ice bath. The green SNAP crystals were collected by filtration, rinsed with water, and dried.
2.2 Catheter Preparation
Initial studies were conducted using single lumen silicone catheter tubing (Tygon® 3350) with approximate length of 4 cm and an I.D. of 0.79 mm and O.D. of 2.38 mm. Cook dual lumen silicone catheters have an overall O.D. 2.34 mm and lumen dimensions of (maximum width × height): NO-releasing lumen (1.27 × 0.64 mm) and vascular access lumen (1.14 × 0.48 mm). Catheter lumens were filled with a solid SNAP-PEG composite containing 20, 30, or 40 wt% SNAP. To fill the catheter lumens, various solutions of SNAP and PEG were prepared by dissolving in 1 mL methanol. For example, the 40 wt% SNAP-PEG solution consisted of 200 mg SNAP and 300 mg PEG in 1 mL methanol. Catheter lumens were filled with the SNAP-PEG solution using a 1 mL syringe, and the methanol was allowed to evaporate under ambient conditions in order to form a solid SNAP-PEG composite within the catheter lumen. Catheters were filled with the SNAP-PEG solution and dried until a constant weight was obtained in order to completely fill the lumen. Both ends of the SNAP-PEG lumen were sealed with the RTV silicone rubber and dried in the dark under ambient conditions. Chelex® pretreatment was based on a previously reported method,35 PEG was dissolved in methanol and allowed to react with Chelex® 100 resin (5 g/100mL) overnight. The resulting supernatant solution was transferred to a new vial and used to prepare the SNAP-PEG solutions described above for filling the catheter lumen. The SNAP control catheters (without PEG) were prepared by dissolving 200 mg/mL SNAP in methanol, and repeating the process described above to a constant weight. The PEG control catheters for bacteria and rabbit studies were filled with only PEG using the same filling procedure described above.
2.3 NO release measurements
The real-time NO release from catheters was measured by a chemiluminescence Nitric Oxide Analyzer (NOA), model 280i (Sievers, Boulder, CO). The NOA had a cell pressure of 7.2 Torr and an oxygen pressure of 7.1 psi. The NOA was calibrated prior to sample analysis using a 2-point calibration (0 and 45 ppm NO calibration gas). To measure the NO release, a catheter sample was placed in 4 mL of PBS buffer with EDTA in the NOA sample cell and incubated at 37 °C. Nitric oxide liberated from the sample was continuously swept from the solution and headspace of the sample cell by purging the buffer with a nitrogen sweep gas (200 mL/min), and then this stream flowed into the chemiluminescence detection chamber. Catheters were incubated in PBS at 37 °C and the NO release was measured at various time points during a 14 d period. After the rabbit experiments, some of the SNAP-PEG catheters were tested for NO release post-blood exposure.
2.4 Water uptake experiments
Catheter samples weighed and photographed after preparation and incubated in PBS at 37 °C. Each day the catheters were weighed and pictures of the whole catheter were taken. The % water uptake was calculated using the initial weight (W0) and the weight at each time point (Wt) as follows: water uptake (wt%) = (Wt − W0)/W0 × 100. The approximate rate of SNAP-PEG dissolution within the catheter lumen was determined from the photos using ImageJ software, where the green pixels (solid SNAP-PEG) were used to quantitate a 2-D representation of the solid SNAP-PEG remaining at each time during a 2-week period.
2.5 Mass Spectrometry Detection of SNAP in PBS
In order to assess any leaching of SNAP and its degradation products, catheters (4 cm lengths, n=6) filled with 40 wt% SNAP-PEG composite were soaked in 4 mL PBS at 37 °C for 14 d. Prior to the experiment catheters were rinsed several times in PBS (which was discarded) in order to wash away any residual SNAP-PEG on the catheter surface. After 14 days, the PBS soaking solutions were analyzed by liquid chromatography-tandem mass spectrometry (6520 Accurate-Mass Q-TOF LC/MS, Agilent Technologies, CA) for quantification of any SNAP, NAP, and NAP disulfide products present. Using a previously reported method,40 the experiments were conducted using a reversed-phase column (ZORBAX RRHD Eclipse Plus C18, 2.1 × 50 mm). The gradient was obtained with eluent A (water with 0.1% formic acid) and eluent B (95% acetonitrile, 0.1% formic acid). After a 15 μL sample injection a linear change of eluent mixtures from 100% A to 0% A over 10 min was carried out with a flow rate of 0.4 mL/min. The mass spectrometer used electrospray ionization in the negative ion mode, and the detected species were [M − H]−.
2.6 In vitro antimicrobial testing
E. coli ATCC 25922 and S. aureus ATCC 25923 were purchased from American Type Culture Collection (ATCC, USA). Lyophilized bacteria were reconstituted in LB broth and grown overnight at 37 °C at 100 rpm. The overnight grown bacteria cultures were washed with PBS buffer 3 times and re-suspended in PBS to a concentration of ~108 CFU/mL. Catheters (4 cm in length) were mounted aseptically on the coupon holders of the CDC biofilm bioreactor (Biosurface Technologies, Bozeman, MT). The bioreactor was inoculated with ~ 1 × 106 CFU/mL for 1 h. The bioreactor was supplemented with 10% LB media by a peristaltic pump with continuous flow rate of 100 mL/h. The CDC bioreactor was incubated at 37 °C and shear force was generated by magnetic stirring. After 3 d of biofilm growth, the catheters were removed and rinsed in sterile PBS. In order to disperse any bacteria attached to the surface for plate counting, each catheter was homogenized in PBS to form a homogeneous cell suspension.
In order to compare the extent of biofilm formation on the filled lumen vs. vascular access lumen of the dual lumen catheters, the outer wall of each lumen was swabbed with dry sterile cotton swabs. The bacteria pellet was collected from the swab by centrifugation at using the Swab Extraction Tube System (Fisher Scientific) and re-suspended in PBS. To assess cell viability, all bacteria solutions were 10-fold serially diluted in sterile PBS and plated on LB agar plates. Plates were incubated overnight at 37 °C followed by CFU counting.
Biofilm structure was also imaged by using a Live/Dead BacLight Bacterial Viability Kit (Invitrogen, Carsbad, CA) with a fluorescent microscope. Catheter samples were stained with the fluorescent dye and the surface was visualized using an inverted fluorescent microscope (Olympus 1 × 17, Center Valley, PA) equipped with Fluorescence Illumination System (X-Cite 120, EXFO) and filters for SYTO-9 (excitation = 488 nm/emission = 520 nm) and Propidium Iodide (excitation = 535 nm/emission = 617 nm). Images were obtained at random locations on the catheter surface using an oil immersed 60× objective lens.
2.7 Rabbit Model
All animals were cared for by the standards of the Institutional Animal Care and Use Committee (IACUC) at the University of Michigan. The surgical area was sanitized and dedicated to the purpose of performing surgery. The rabbit protocols employed in this study were similar to those used in previous studies.33, 40, 59 A total of 16 New Zealand white rabbits (Myrtle’s Rabbitry, Thompson’s Station, TN) were studied. All rabbits (2.5–3.5 kg) were initially anesthetized with intramuscular injections of 5 mg/kg xylazine injectable (AnaSed® Lloyd Laboratories Shenandoah, Iowa) and 30 mg/kg ketamine hydrochloride (Hospira, Inc. Lake Forest, IL). No systemic anticoagulation was administered to the rabbits.
Acute studies (7 h)
Maintenance anesthesia was administered via isoflurane gas inhalation at a rate of 1.5–3% via mechanical ventilation which was done via a tracheotomy and using an A.D.S. 2000 Ventilator (Engler Engineering Corp. Hialeah, FL). Peek inspiratory pressure was set to 15 cm of H2O and the ventilator flow rate was 8 L/min. To facilitate the maintenance of blood pressure stability, IV fluids of Lactated Ringer’s were given at a rate of 10 mL/kg/h. In order to monitor blood pressure and to collect intermittent blood samples for analysis during the experiment, each rabbit’s right carotid artery was cannulated using a 16-gauge IV angiocatheter (Jelco®, Johnson & Johnson, Cincinnati, OH). The blood pressure and derived heart rate were monitored by a Series 7000 monitor (Marquette Electronics Milwaukee, WI) while the animal body temperature was monitored with a rectal probe and maintained at 38 °C using a water-jacketed heating blanket. Blood samples were collected each hour in 1 mL syringes containing 40 U/mL of sodium heparin (APP Pharmaceuticals, LLC, Schamberg, IL) for blood-gas analysis using an ABL 825 blood-gas analyzer. One SNAP-PEG and 1 PEG control (5 cm length) were inserted into the external jugular veins for 7 h.
Chronic studies (11 d)
All surgical instruments were sterilized using steam sterilization and sterile drapes were employed to create a sterile field around the dorsal and ventral sides of rabbit neck. Maintenance anesthesia was administered via isoflurane gas inhalation at a rate of 1.5–3% via a face mask and using an A.D.S. 2000 Ventilator (Engler Engineering Corp. Hialeah, FL). The rabbits’ neck areas were cleaned with iodine and ethanol prior to incision. A modified rabbit venous model, originally developed by Klement et al,60 was used where the internal right and left jugular veins provided an access point to the common jugular veins and the tip of the catheter was placed at the entrance to the right atrium. By using the internal jugular veins, the common jugular vein blood flows were maintained over the catheter which enabled both thrombosis and microbial biofilm assessments. Under sterile conditions, a small skin incision (2 cm) was made over the right and left external jugular veins, and the internal jugular vein branches were isolated for the catheter insertion. Briefly, the internal jugular veins were ligated proximally and under distal occlusion, a small venotomy was made through which the catheters were introduced into the internal jugular veins and then advanced into the cranial vena cava. About 7 cm of the catheter length was inserted and then fixed to the vein at its entrance by two sterile silk sutures. A second skin incision (1 cm) was made on the dorsum of the neck. The remaining external portion (~6 cm long) of the catheters were then tunneled under the skin from the jugular vein, connected to vascular access ports, and sutured in place under the dorsal skin incision. Skin incisions were closed using absorbable sutures in a routine manner using uninterrupted stitches for the ventral incision and interrupted stitches for the dorsal incision. The incision sites were treated with Neosporin ointment. After removal from anesthesia, animals were placed in an oxygenated and 37 °C incubator for post-operative recovery. Animals were checked during 1–2 h recovery until they were able to maintain sternal recumbency before moving to the animal facility.
After surgery and recovery from anesthesia, the rabbits were housed individually with a respective cage card identifying the animal in the animal facility. Animal health was monitored during routine daily check-ups and weighing, and the skin incisions were examined for inflammation (redness). Four mg/kg Rimadyl (analgesic) was given for 2 days after surgery and 5mg/kg Baytril (antibiotic) was given for 4 days post-surgery. The vascular access lumen was filled with a saline lock solution, and each day it was aseptically flushed with 2 mL sterile saline.
2.8 Catheter explant and evaluation of hemocompatibility
Rabbits were given 400 IU/kg sodium heparin just prior to euthanasia to prevent necrotic thrombosis. The animals were euthanized using a dose of Fatal Plus (130 mg/kg sodium pentobarbital) (Vortech Pharmaceuticals Dearborn, MI). After 7 h or 11 d in rabbit veins the catheters were explanted and rinsed in sterile PBS. Pictures were taken of the exterior of the whole catheter and the interior of the vessel wall using a Nikon L24 digital camera. ImageJ software was used to quantitate the 2-D representation of clot formation for each catheter. After the 11 d chronic implantation, catheters were also assessed for viable bacteria on the surface of the catheters. The explanted chronic catheters were placed in 10 mL of sterile PBS and homogenized. The resulting homogenate was 10-fold serially diluted in sterile PBS. Triplicate aliquots of each dilution (50 μL) of each dilution were plated on agar plates. The agar plates were incubated at 37 °C for 24 h followed by calculation of colony forming units per catheter surface area (CFU/cm2). The remaining SNAP within the NO-releasing lumen was determined by UV-Vis spectrophotometer (Lambda 35, Perkin—Elmer, MA) after dilution in PBS and compared to the initial SNAP amount within fresh catheters, where the presence of S-NO group provides a characteristic absorbance maxima at 340 nm (εSNAP=1029 M−1 cm−1).27, 61
2.9 Statistical Analysis
Data are expressed as mean ± standard error of the mean (SEM). A Student’s t-test was used to compare results between the SNAP-PEG and control catheters, where values of p < 0.05 were considered statistically significant for all tests.
3. Results and Discussion
3.1 In Vitro NO release from SNAP-PEG catheters
Commercial catheter tubing was used in these studies to evaluate the potential benefits of dedicating one lumen of multi-lumen catheter to NO release chemistry and the other lumen for vascular access. Catheters were filled with SNAP-PEG dissolved in methanol and dried until obtaining a constant weight (approximately 8 mg of the solid SNAP-PEG per cm length of catheter) to ensure that the entire lumen was filled. The high molecular weight PEGs used are solids at room temperature and allow for the formation of a SNAP-PEG composite that fills the catheter lumen after the methanol evaporates. During the filling and drying process (several days) the SNAP-PEG formed a solid crystalline composite that fills the lumen. The crystalline form of SNAP within a polymeric matrix has been shown previously to have enhanced stability due to the formation of intermolecular hydrogen bonding.40 Indeed, in this prior work, CarboSil catheters doped with SNAP that formed crystals after solvent evaporation, had > 88% of the SNAP remaining after stored dry at 37 °C for 8 months.40 In the present work, the higher the viscosity of the SNAP-PEG solution in methanol allowed for more rapid filling the lumen to a constant weight. Any SNAP-PEG solutions with greater than 40 wt% SNAP could have the benefit of higher amounts of SNAP in the catheter lumen. However, increasing the wt% of SNAP would decrease the overall viscosity of the filling solution (when both SNAP and PEG are completely dissolved), owing to the limited solubility of SNAP in methanol of ~200 mg/mL, and would significantly extend the duration of time needed to fill the lumen completely.
Initial studies were conducted using single lumen silicone catheter tubing (Tygon® 3350) filled with 20 wt% SNAP in PEG. The water uptake of single lumen catheter tubing filled with PEGs of various molecular weights was recorded and compared to SNAP control (no PEG) catheters (Fig. 2). All of the SNAP-PEG catheters had a significantly higher absorption of water than the SNAP control catheters. PEG is a very hydrophilic polymer that draws water into the lumen of the catheter through the walls of the devices and gradually dissolves the SNAP-PEG composite. No significant differences in the rate of water uptake when using PEGs with different molecular weights was observed. Any loss in weight due to the NO released was considered insignificant in comparison to the increased weight due to the water uptake observed during these experiments. The NO release was also measured from these catheters under physiological conditions (incubated in PBS buffer at 37 °C) using chemiluminescence over 2 weeks. The catheters filled with SNAP-PEG composite had higher NO release levels than the corresponding SNAP control catheters due to the water absorption that dissolves the SNAP crystals and initiates NO release (Fig. 3A). The catheters prepared with PEG 4,000 consistently exhibited a greater NO flux than the other PEGs; therefore, further studies were conducted using the PEG 4,000 species.
Fig. 2.
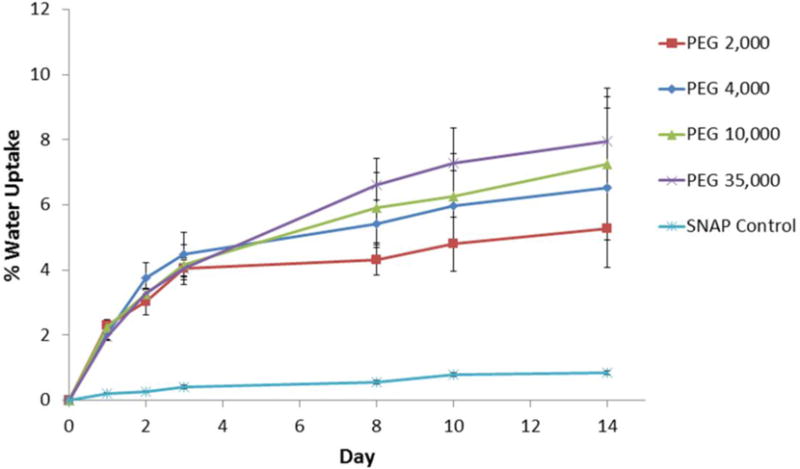
Water uptake (wt%) of single lumen silicone catheters filled with 20 wt% SNAP and 80 wt% of PEG with various molecular weights (2,000; 4,000; 10,000; or 35,000) during 10 d incubation in PBS buffer at 37 °C. The SNAP Control catheter was filled completely with SNAP without any PEG. Data represents the mean ± SEM (n=3).
Fig. 3.
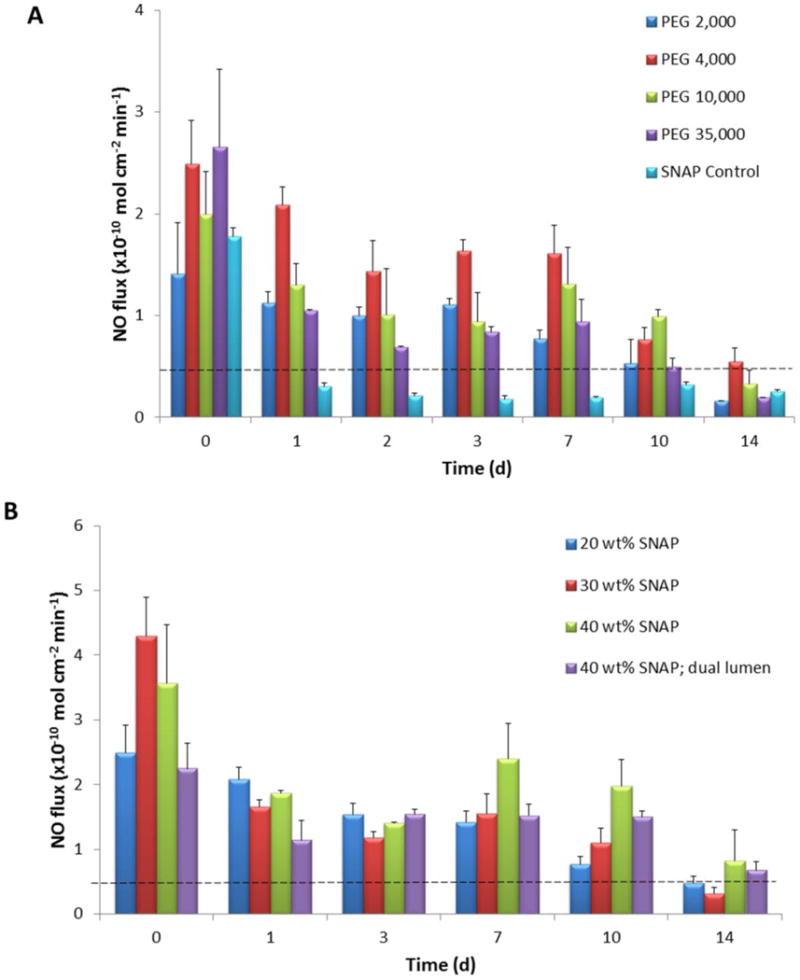
NO release single lumen catheters filled with 20 wt% SNAP and 80 wt% PEG with various molecular weights and SNAP control (completely filled with only SNAP) (A). NO release from single and dual lumen catheters filled with 20, 30, and 40 wt% SNAP in PEG 4,000 (B). NO release from catheters under physiological conditions (in PBS buffer at 37 °C) as measured by chemiluminescence. Data represents the mean ± SEM (n=3).
The NO release was measured from catheters prepared with 20, 30, or 40 wt% SNAP in PEG 4,000 with SNAP loadings of approximately 7, 10, and 14 μmol SNAP/cm length, respectively (Fig. 3B). All of the catheters release physiologically relevant levels of NO (0.5 –4 × 10−10 mol cm−2 min−1) and released their entire NO payload during the 14 d testing period. The catheters filled with higher wt% SNAP had higher NO fluxes on day 0 and days 10–14. Therefore, dual lumen catheters for bacteria and rabbit studies were prepared using 40 wt% SNAP in PEG 4,000, and the NO release levels were similar to that observed from the single lumen catheters. In order to monitor any leaching of SNAP and its degradation products (NAP and NAP disulfide), catheters filled with 40 wt% SNAP-PEG composite were incubated for 14 d in PBS at 37 °C. After the total of 14 days of soaking the total leaching of SNAP, NAP and NAP disulfide within the PBS was found to be 135 ± 55, 50 ± 16, and 416 ± 25 nmol per cm catheter length, respectively. These small amounts of SNAP, NAP, and NAP disulfide account for < 3% of the total SNAP initially incorporated within the lumen. This level of leaching is significantly lower than previous reports of SNAP-doped biomedical polymers (up to 47% of initial SNAP)39–40 because in this study the SNAP is entrapped within the catheter lumen. Indeed, the presence of these compounds within the soaking buffer are probably more likely attributed to residual SNAP on the catheter surface after fabrication where the catheter surface can easily be contaminated with the SNAP-PEG solution during the lumen filling process, or minor imperfections in the silicone seals placed on the ends of the catheter lumens after filling with the SNAP-PEG composite. Previous studies with SNAP-based polymers where SNAP is incorporated within the walls by a solvent impregnation method have exhibited much more significant true leaching of SNAP and its byproducts.39–40 In general, low levels of SNAP leaching is not a major concern since NAP has been clinically used to treat heavy metal poisoning and cystinuria for many years.62–63 These results further demonstrate that this catheter design localizes the NO release only from the catheter surface, without significant levels of precursor or product leaching.
The single lumen catheters with 20, 30, and 40 wt% SNAP in PEG 4,000 were also photographed over the 14 d testing period. Initially the lumen is completely filled with SNAP-PEG (dark green from SNAP), which gradually dissolves and becomes clear/transparent as PEG absorbs water (Fig. 4A). The rate of SNAP-PEG dissolution was approximated by using ImageJ software to calculate the 2-D area of dark green solid (SNAP-PEG) remaining at each time point. All of the SNAP-PEG filling was dissolved and the SNAP reservoir was nearly depleted by day 14, as indicated by the low NO flux levels observed (see Fig. 3). No significant differences in the rate of dissolution with different PEG amounts was observed (Fig 4B). PEG polymers also have been reported to have trace metal ions present,53 which could potentially catalyze the NO release from the SNAP-PEG catheters vs. SNAP control (without PEG). In order to assess the role of any potential trace metal ions in initiating NO release from these catheters, the PEG solutions were pre-treated with Chelex® resin in order to remove any trace divalent metal ions. Polymers have been purified previously with the Chelex® resin, which replaces the divalent metal ions with sodium ions.64 Catheters were filled with the SNAP-PEG composite with and without the Chelex® pretreatment and no significant differences in their NO release was observed (data not shown). This demonstrates that the water uptake and resulting dissolution of the SNAP-PEG filling has a greater role in initiating the NO release from SNAP than any trace metal ions present in the PEG polymer. When SNAP is in its solid/crystal form within the polymer it is quite stable due to crystal formation and intramolecular hydrogen bonding, while soluble SNAP within polymer matrices is less stable and can more rapidly release its NO.40, 65 As the PEG draws water into the catheter lumen and becomes hydrated, SNAP dissolves and can more rapidly release it’s NO payload resulting in the higher NO fluxes observed.
Fig. 4.
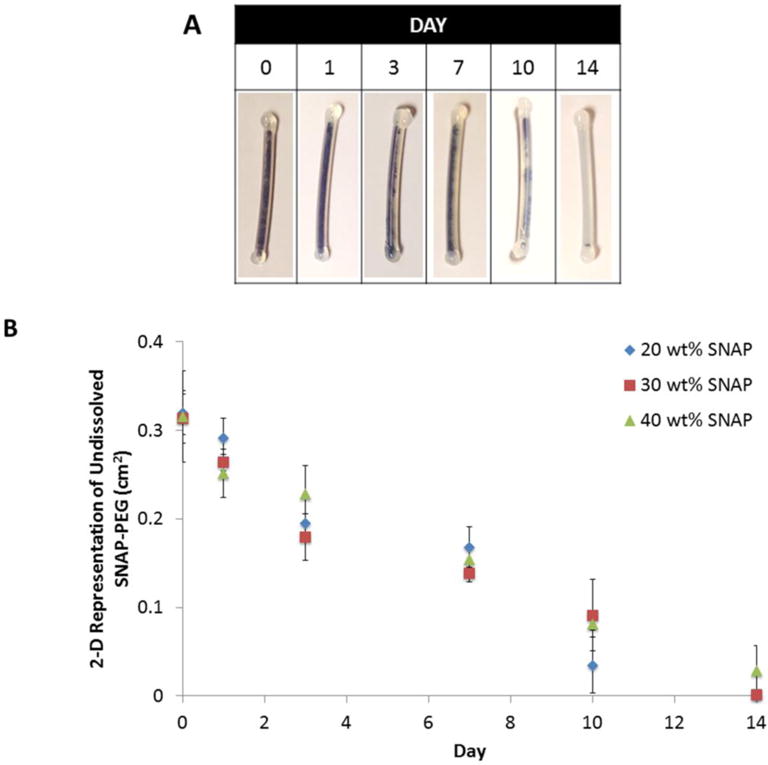
Representative images of the gradual dissolution of 40 wt% SNAP and PEG 4,000 within single lumen catheters (A). Approximate rate of SNAP-PEG dissolution in single lumen catheters (filled with 20, 30, and 40 wt% SNAP and PEG 4,000), where the undissolved SNAP-PEG was quantitated with ImageJ software (B). Data is the mean ± SEM (n=3).
3.2 In Vitro Bacteria Testing
An in vitro CDC bioreactor was used to assess the antimicrobial activity of the NO released from SNAP-PEG catheters because it provides shear forces and a renewable nutrient source that mimics an in vivo environment.66 The SNAP-PEG and PEG control catheters were placed in the CDC bioreactor with gram-negative strain E. coli or gram-positive strain S. aureus cultures for 3 d. Catheters were removed from the bioreactor and homogenized in sterile PBS. The homogenate solution was serially diluted and plate counting was used to determine the viable bacteria on catheter surfaces. It was found that there was > 98% reduction in viable bacteria on the surfaces of the SNAP-PEG catheters for both bacteria strains when compared to the corresponding control catheters (Fig. 5A,B). In order to assess any potential asymmetrical effects of NO release from the dual lumen catheters (filled lumen vs. blood access lumen), additional experiments were conducted where the outer surfaces of the NO-releasing and blood access lumens were cultured separately using sterile swabs and a Swab Extraction Tube System. The viable bacteria on the blood access lumen were slightly higher than the NO-releasing lumen; however, the difference was not significant (Fig. 5C). This demonstrates minimal asymmetry in the NO release around the catheter due to the high solubility and rapid diffusion of NO in silicone, as previously reported.67 Bacteria on the catheters’ surfaces were also fluorescently imaged with a fluorescence microscope after being stained with the Bacterial Live/Dead dye (Fig. 5D). The PEG control catheter surfaces are fully colonized by mostly viable E. coli or S. aureus cells (green). In contrast, the surfaces of the SNAP-PEG catheters are covered with significantly fewer live bacteria cells, and further many of the adhered bacteria are dead or membrane damaged cells (red) (Fig. 5D). This data corresponds well with previous reports that NO release from polymers can act as a signaling agent to disperse biofilms, prevent bacterial adhesion, and has significant broad-spectrum antimicrobial activity.20, 33, 35, 40–41, 68 The NO release from the SNAP-PEG catheters results in ca. 2 log reduction in bacteria on the surface, which correlates well with previously reported short-term in vitro studies with 2–3 log reduction in bacterial adhesion.33 Methods to achieve higher levels of therapeutic NO delivery from the catheters could further improve the antimicrobial properties and will have significant benefit for potential clinical applications. Further, a key element of using NO release is also to reduce and/or disrupt microbial biofilms that would make use of systemic antibiotic treatment much more effective. Indeed, in recent work from this group demonstrating that low NO release from the surface of catheters in conjunction with use antibiotics has a significant synergistic effect, with the antibiotic (gentamicin in that study).69 The antibiotic alone had no effect on the biofilm bacteria on the surface of the catheters, and NO release alone had 2–3 log unit reduction. However, when the two were combined, nearly 5 log unit reduction was achieved. Hence, in a clinical setting, where antibiotic use would be common, the effectiveness of NO release would be greatly enhanced.
Fig. 5.
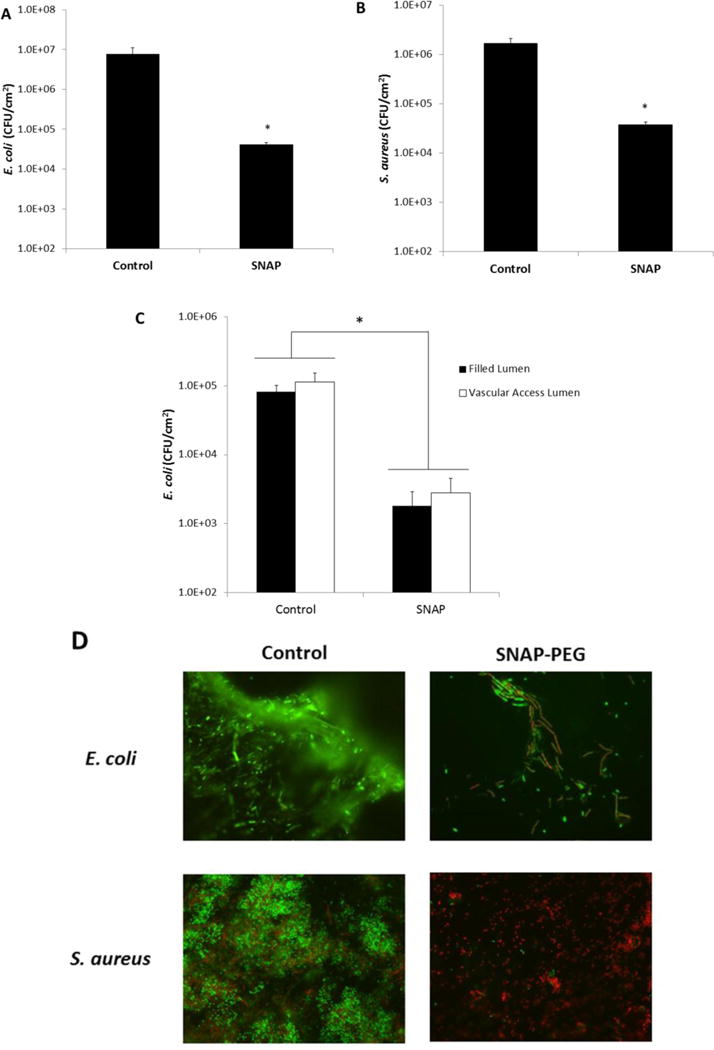
Control and 40 wt% SNAP-PEG catheters (4 cm lengths) were incubated at 37 °C in a CDC bioreactor for 3 d with E. coli or S. aureus. Viable bacteria counts of E. coli (A) and S. aureus (B) on control and SNAP-PEG catheters after as determined by plate counting. Viable bacteria counts on the filled lumen (filled with PEG control or 40 wt% SNAP-PEG) vs. the vascular access lumen of dual lumen catheters as determined with the swab extraction system and plate counting (C). Representative fluorescent micrographs comparing the bacteria on control and SNAP-PEG catheters after incubation at 37 °C in a CDC bioreactor containing E. coli or S. aureus, where bacterial LIVE/DEAD staining shows viable cells as green and dead or membrane damaged cells as red (D). Data represents the mean ± SEM (n=4). * p < 0.05, SNAP-PEG vs. Control.
3.3 Rabbit Model
Initial hemocompatibility testing of the single lumen SNAP-PEG catheters was conducted in an acute rabbit model where catheters (7 cm long) were inserted in the jugular veins of rabbits for 7 h. At the end of the experiment the catheters were removed by longitudinally cutting open the veins in order to prevent disruption of any formed thrombus on the catheter surfaces. The catheters were briefly rinsed and photographed. The 2-D representation of clot area was quantitated using NIH ImageJ software. The 40 wt% SNAP catheters had ca. 85% less clot area than the PEG control catheters, with clot areas of 0.11 ± 0.02 cm2 and 0.77 ± 0.13 cm2 respectively (Fig. 6).
Fig. 6.
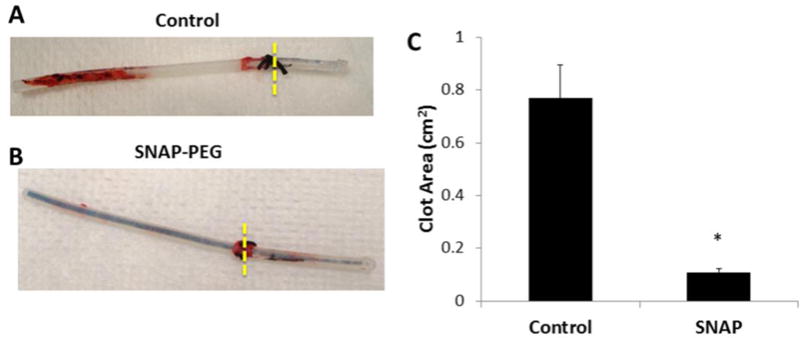
Representative images of single lumen catheters filled with PEG control (A) or 40 wt% SNAP-PEG (B) after implantation in rabbit veins for 7 h. Two-dimensional representation of thrombus formation on the catheters was quantitated using ImageJ software (C). Data represents the mean ± SEM (n=4). * p < 0.05, SNAP-PEG vs. Control.
In order to evaluate the catheters in a more clinically relevant model, chronic 11 d rabbit studies were conducted. For these experiments the dual lumen catheters were used (13 cm long), with one lumen dedicated to the either the 40 wt% SNAP-PEG or PEG control filling allowing the second lumen to be used as the vascular access lumen. As described in the Methods Section, 7 cm of the catheters was inserted in the jugular veins of rabbits under sterile operating conditions with one SNAP-PEG and one PEG control catheter in each rabbit. The remaining length of catheter (ca. 6 cm) was tunneled under the skin and connected to an implantable vascular access port (connected to the vascular access lumen of the catheter). Each day the blood access lumen was aseptically flushed with sterile saline. After 11 d, the catheters were removed from the rabbits under sterile conditions. As measured by UV-Vis, the NO-releasing lumen had 34 ± 5 % of the initial SNAP content remaining after the 11 d in rabbit veins. This remaining SNAP is expected to release NO for an additional 3 d as the in vitro NO release from the catheters lasts 14 d (see Fig. 3). The clot formation on the catheters and within the vessel was photographed and quantitated using ImageJ software (Fig. 7). The thrombus formation (dark red clots within the blood vessels shown below the catheters) around the PEG control catheters was significantly more than observed for the SNAP-PEG catheters. After 11 d implantation, the SNAP-PEG catheters had ca. 55% less clot than the controls (0.77 ± 0.14 cm2 and 1.70 ± 0.17 cm2, respectively). The entire catheter length was also cultured for viable bacteria using the plate counting method described above, and a ca. 90% reduction was observed for the SNAP-PEG catheters when compared to controls (an average of 6 ± 2 and 37 ± 16 CFU/cm2, respectively). The sterile operating conditions and administration of post-surgery antibiotics was effective in preventing any chance of adverse bacterial effects, such as severe illness or mortality of the rabbits, during the study. These results correlate well with previous chronic catheter studies using SNAP or diazeniumdiolated dibutylhexanediamine (DBHD/NONO) as NO donor molecules doped within Elasteon E2As polyurethane, where ca. 70 and 82% reduction in thrombus formation as well as ca. 90 and 95% reduction in bacterial adhesion were observed, respectively.41, 70
Fig. 7.
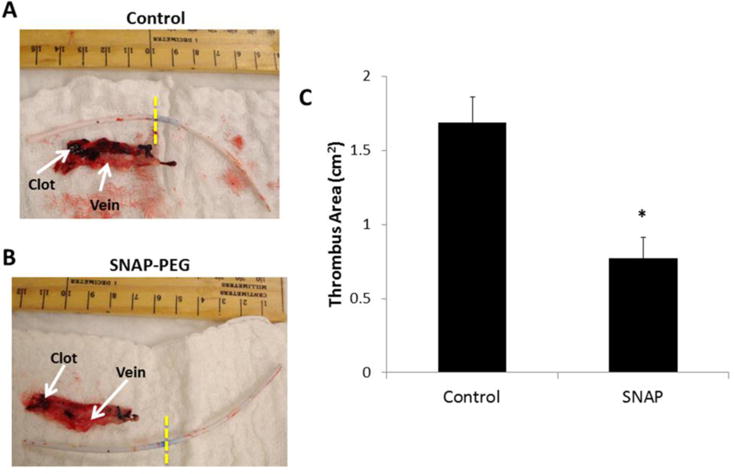
Representative images of thrombus formation (dark red clots in veins) on dual lumen catheters and vein interior after 11 d implantation in rabbit veins, where catheter left of yellow dashed lines was in the vein. The dark reddish-black areas are thrombus formed, and the red tissue is the explanted vein that the catheter was inserted within. The vascular access lumen was locked with saline, and the other lumen was filled with PEG control (A) or 40 wt% SNAP-PEG (B). Two-dimensional representation of thrombus formation was quantitated using ImageJ software (C). Data is the mean ± SEM (n=4). * p < 0.05, SNAP-PEG vs. Control.
4. Conclusions
The approach used in this study demonstrates a simple and feasible method to incorporate therapeutic NO delivery from commercial/existing multi-lumen catheters by dedicating one lumen to house the SNAP-PEG composite. This method has major advantages of overcoming the need of extruding catheters with the thermally unstable SNAP within the walls, as well as significantly reducing concerns of the NO donor and/or product leaching. In this study commercial silicone catheters were used, but the method of filling one lumen with the SNAP-PEG composite could also be applied to commercial multi-lumen catheters made of other polymers (e.g., polyurethanes) in order to improve their hemocompatibility. The proposed catheters can release physiological levels of NO for up to 14 d when soaked in PBC at 37 °C. The presence of PEG with SNAP in the NO-releasing lumen plays a critical role in facilitating the water absorption and subsequent dissolution of the SNAP-PEG composite within the NO-releasing lumen. This new NO release catheter design also has an advantage of essentially eliminating any substantive leaching of SNAP and/or its byproducts as they are completely sealed within the catheter lumen. The SNAP-PEG catheters exhibit significant antimicrobial activity, as tested in the CDC bioreactor, with > 97% reduction of viable E. Coli and S. aureus. The catheters prepared with the SNAP-PEG lumen also significantly decrease clotting in both the 7 h and 11 d rabbit models, as compared to the PEG controls. This new approach of employing a dedicated NO-releasing lumen within multi-lumen catheters has significant potential to improve the biocompatibility/antimicrobial properties of existing commercial catheters. Methods to increase the NO flux from these catheters (e.g., using optical fiber within the SNAP-PEG lumen to photo-release NO from the SNAP) could potentially further increase the NO release rates and thereby enhance the antimicrobial and hemocompatibility properties of the proposed devices. Future studies will also evaluate the shelf-life and sterilization stability of the SNAP-PEG composite which are critical for potential future clinical applications.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL128337, F32HL127981, K25HL111213).
References
- 1.Abad CL, Safdar N. Catheter-Related Bloodstream Infections. Infect Dis Special Ed. 2011;14:84–98. [Google Scholar]
- 2.Beathard GA. Catheter Thrombosis. 2001. pp. 441–445. (Seminars in Dialysis, Wiley Online Library). [DOI] [PubMed] [Google Scholar]
- 3.Ratner BD. The Catastrophe Revisited: Blood Compatibility in the 21st Century. Biomaterials. 2007;28:5144–5147. doi: 10.1016/j.biomaterials.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sefton MV, Gemmell CH, Gorbet MB. What Really Is Blood Compatibility? J Biomater Sci Polym Ed. 2000;11:1165–1182. doi: 10.1163/156856200744255. [DOI] [PubMed] [Google Scholar]
- 5.Goldhaber SZ, Fanikos J. Prevention of Deep Vein Thrombosis and Pulmonary Embolism. Circulation. 2004;110:e445–e447. doi: 10.1161/01.CIR.0000145141.70264.C5. [DOI] [PubMed] [Google Scholar]
- 6.O’Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, Masur H, McCormick RD, Mermel LA, Pearson ML, Randolph A, Weinstein RA. Guidelines for the Prevention of Intravascular Catheter–Related Infections. Clin Infect Dis. 2002;35:1281–1307. [Google Scholar]
- 7.Liang Y, Kiick KL. Heparin-Functionalized Polymeric Biomaterials in Tissue Engineering and Drug Delivery Applications. Acta Biomater. 2014;10:1588–1600. doi: 10.1016/j.actbio.2013.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tal MG, Ni N. Selecting Optimal Hemodialysis Catheters: Material, Design, Advanced Features, and Preferences. Tech Vasc Interv Radiol. 2008;11:186–191. doi: 10.1053/j.tvir.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Appelgren P, Ransjo U, Bindslev L, Espersen F, Larm O. Surface Heparinization of Central Venous Catheters Reduces Microbial Colonization In Vitro and In Vivo: Results from a Prospective, Randomized Trial. Crit Care Med. 1996;24:1482–1489. doi: 10.1097/00003246-199609000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y-M, Dai A-P, Shi Y, Liu Z-J, Gong M-F, Yin X-B. Effectiveness of Silver-Impregnated Central Venous Catheters for Preventing Catheter-Related Blood Stream Infections: A Meta-Analysis. Int J Infect Dis. 2014;29:279–286. doi: 10.1016/j.ijid.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Ivan DM, Smith T, Allon M. Does the Heparin Lock Concentration Affect Hemodialysis Catheter Patency? Clin J Am Soc Nephrol. 2010;5:1458–1462. doi: 10.2215/CJN.01230210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aslam S, Jenne K, Reed S, Ghannoum M, Mehta R, Darouiche R. N-Acetylcysteine Lock Solution Prevents Catheter-Associated Bacteremia in Rabbits. Int J Artif Organs. 2012;35:893–897. doi: 10.5301/ijao.5000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaffney AM, Wildhirt SM, Griffin MJ, Annich GM, Radomski MW. Extracorporeal Life Support. BMJ. 2010;341:982–986. doi: 10.1136/bmj.c5317. [DOI] [PubMed] [Google Scholar]
- 14.Kidane AG, Salacinski H, Tiwari A, Bruckdorfer KR, Seifalian AM. Anticoagulant and Antiplatelet Agents: Their Clinical and Device Application(S) Together with Usages to Engineer Surfaces. Biomacromolecules. 2004;5:798–813. doi: 10.1021/bm0344553. [DOI] [PubMed] [Google Scholar]
- 15.Jordan SW, Chaikof EL. Novel Thromboresistant Materials. J Vasc Surg. 2007;45:A104–A115. doi: 10.1016/j.jvs.2007.02.048. [DOI] [PubMed] [Google Scholar]
- 16.Vaughn MW. Estimation of Nitric Oxide Production and Reaction Rates in Tissue by Use of a Mathematical Model. Am J Physiol Heart Circ Physiol. 1998;274:H2163–H2176. doi: 10.1152/ajpheart.1998.274.6.H2163. [DOI] [PubMed] [Google Scholar]
- 17.Rouby J. The Nose, Nitric Oxide, and Paranasal Sinuses: The Outpost of Pulmonary Antiinfectious Defenses? Am J Respir Crit Care Med. 2003;168:265–266. doi: 10.1164/rccm.2305009. [DOI] [PubMed] [Google Scholar]
- 18.Fang FC. Mechanisms of Nitric Oxide-Related Antimicrobial Activity. J Clin Invest. 1997;99:2818–2825. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDougald D, Rice SA, Barraud N, Steinberg PD, Kjelleberg S. Should We Stay or Should We Go: Mechanisms and Ecological Consequences for Biofilm Dispersal. Nat Rev Microbiol. 2011;10:39–50. doi: 10.1038/nrmicro2695. [DOI] [PubMed] [Google Scholar]
- 20.Barraud N, Storey MV, Moore ZP, Webb JS, Rice SA, Kjelleberg S. Nitric Oxide- Mediated Dispersal in Single- and Multi-Species Biofilms of Clinically and Industrially Relevant Microorganisms. Microb Biotechnol. 2009;2:370–378. doi: 10.1111/j.1751-7915.2009.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hakim TS, Sugimori K, Camporesi EM, Anderson G. Half-Life of Nitric Oxide in Aqueous Solutions with and without Haemoglobin. Physiol Meas. 1996;17:267–277. doi: 10.1088/0967-3334/17/4/004. [DOI] [PubMed] [Google Scholar]
- 22.Hogg N. Biological Chemistry and Clinical Potential of S-Nitrosothiols. Free Radical Biol Med. 2000;28:1478–1486. doi: 10.1016/s0891-5849(00)00248-3. [DOI] [PubMed] [Google Scholar]
- 23.Al-Sa’doni H, Ferro A. S-Nitrosothiols: A Class of Nitric Oxide-Donor Drugs. Clin Sci. 2000;98:507–520. [PubMed] [Google Scholar]
- 24.Williams DLH. The Chemistry of S-Nitrosothiols. Acc Chem Res. 1999;32:869–876. [Google Scholar]
- 25.Bainbrigge N, Butler AR, Gorbitz CH. The Thermal Stability of S-Nitrosothiols: Experimental Studies and Ab Initio Calculations on Model Compounds. J Chem Soc, Perkin Trans. 1997;2:351–353. [Google Scholar]
- 26.McCarthy CW, Guillory RJ, Goldman J, Frost MC. Transition-Metal-Mediated Release of Nitric Oxide (No) from S-Nitroso-N-Acetyl-D-Penicillamine (SNAP): Potential Applications for Endogenous Release of No at the Surface of Stents Via Corrosion Products. ACS Appl Mater Interfaces. 2016;8:10128–10135. doi: 10.1021/acsami.6b00145. [DOI] [PubMed] [Google Scholar]
- 27.Frost MC, Meyerhoff ME. Controlled Photoinitiated Release of Nitric Oxide from Polymer Films Containing S-Nitroso-N-Acetyl-Dl-Penicillamine Derivatized Fumed Silica Filler. J Am Chem Soc. 2004;126:1348–1349. doi: 10.1021/ja039466i. [DOI] [PubMed] [Google Scholar]
- 28.Brisbois EJ, Handa H, Meyerhoff ME. Recent Advances in Hemocompatible Polymers for Biomedical Applications. In: Puoci F, editor. Advanced Polymers in Medicine. Springer International Publishing; 2015. pp. 481–511. Chapter 16. [Google Scholar]
- 29.Brisbois EJ, Bayliss J, Wu J, Major TC, Xi C, Wang SC, Bartlett RH, Handa H, Meyerhoff ME. Optimized Polymeric Film-Based Nitric Oxide Delivery Inhibits Bacterial Growth in a Mouse Burn Wound Model. Acta Biomater. 2014;10:4136–4142. doi: 10.1016/j.actbio.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Major TC, Brisbois EJ, Jones AM, Zanetti ME, Annich GM, Bartlett RH, Handa H. The Effect of a Polyurethane Coating Incorporating Both a Thrombin Inhibitor and Nitric Oxide on Hemocompatibility in Extracorporeal Circulation. Biomaterials. 2014;35:7271–7285. doi: 10.1016/j.biomaterials.2014.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Handa H, Major TC, Brisbois EJ, Amoako KA, Meyerhoff ME, Bartlett RH. Hemocompatibility Comparison of Biomedical Grade Polymers Using Rabbit Thrombogenicity Model for Preparing Nonthrombogenic Nitric Oxide Releasing Surfaces. J Mater Chem B. 2014;2:1059–1067. doi: 10.1039/C3TB21771J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Handa H, Brisbois EJ, Major TC, Refahiyat L, Amoako KA, Annich GM, Bartlett RH, Meyerhoff ME. In Vitro and in Vivo Study of Sustained Nitric Oxide Release Coating Using Diazeniumdiolate-Doped Poly(Vinyl Chloride) Matrix with Poly(Lactide-Co-Glycolide) Additive. J Mater Chem B. 2013;1:3578–3587. doi: 10.1039/C3TB20277A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren H, Colletta A, Koley D, Wu J, Xi C, Major TC, Bartlett RH, Meyerhoff ME. Thromboresistant/Anti-Biofilm Catheters Via Electrochemically Modulated Nitric Oxide Release. Bioelectrochemistry. 2015;104:10–16. doi: 10.1016/j.bioelechem.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Major TC, Handa H, Brisbois EJ, Reynolds MM, Annich GM, Meyerhoff ME, Bartlett RH. The Mediation of Platelet Quiescence by No-Releasing Polymers Via cGMP-Induced Serine 239 Phosphorylation of Vasodilator-Stimulated Phosphoprotein. Biomaterials. 2013;34:8086–8096. doi: 10.1016/j.biomaterials.2013.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vogt C, Xing Q, He W, Li B, Frost MC, Zhao F. Fabrication and Characterization of a Nitric Oxide-Releasing Nanofibrous Gelatin Matrix. Biomacromolecules. 2013;14:2521–2530. doi: 10.1021/bm301984w. [DOI] [PubMed] [Google Scholar]
- 36.VanWagner M, Rhadigan J, Lancina M, Lebovsky A, Romanowicz G, Holmes H, Brunette MA, Snyder KL, Bostwick M, Lee BP, Frost MC, Rajachar RM. S-Nitroso-N-Acetylpenicillamine (SNAP) Derivatization of Peptide Primary Amines to Create Inducible Nitric Oxide Donor Biomaterials. ACS Appl Mater Interfaces. 2013;5:8430–8439. doi: 10.1021/am4017945. [DOI] [PubMed] [Google Scholar]
- 37.Seabra AB, Da Silva R, De Souza GFP, De Oliveira MG. Antithrombogenic Polynitrosated Polyester/Poly(Methyl Methacrylate) Blend for the Coating of Blood-Contacting Surfaces. Artif Organs. 2008;32:262–267. doi: 10.1111/j.1525-1594.2008.00540.x. [DOI] [PubMed] [Google Scholar]
- 38.Seabra AB, Martins D, Simoes M, da Silva R, Brocchi M, de Oliveira MG. Antibacterial Nitric Oxide-Releasing Polyester for the Coating of Blood-Contacting Artificial Materials. Artif Organs. 2010;34:E204–E214. doi: 10.1111/j.1525-1594.2010.00998.x. [DOI] [PubMed] [Google Scholar]
- 39.Brisbois EJ, Handa H, Major TC, Bartlett RH, Meyerhoff ME. Long-Term Nitric Oxide Release and Elevated Temperature Stability with S-Nitroso-N-Acetylpenicillamine (SNAP)-Doped Elast-Eon E2as Polymer. Biomaterials. 2013;34:6957–6966. doi: 10.1016/j.biomaterials.2013.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wo Y, Li Z, Brisbois EJ, Colletta A, Wu J, Major TC, Xi C, Bartlett RH, Matzger AJ, Meyerhoff ME. Origin of Long-Term Storage Stability and Nitric Oxide Release Behavior of Carbosil Polymer Doped with S-Nitroso-N-Acetyl-D-Penicillamine. ACS Appl Mater Interfaces. 2015;7:22218–22227. doi: 10.1021/acsami.5b07501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brisbois EJ, Davis RP, Jones AM, Major TC, Bartlett RH, Meyerhoff ME, Handa H. Reduction in Thrombosis and Bacterial Adhesion with 7 Day Implantation of S-Nitroso-N-Acetylpenicillamine (SNAP)-Doped Elast-Eon E2as Catheters in Sheep. J Mater Chem B. 2015;3:1639–1645. doi: 10.1039/C4TB01839G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brisbois EJ, Major TC, Goudie MJ, Bartlett RH, Meyerhoff ME, Handa H. Improved Hemocompatibility of Silicone Rubber Extracorporeal Tubing Via Solvent Swelling-Impregnation of S-Nitroso-N-Acetylpenicillamine (SNAP) and Evaluation in Rabbit Thrombogenicity Model. Acta Biomater. 2016;37:111–119. doi: 10.1016/j.actbio.2016.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schanuel FS, Raggio Santos KS, Monte-Alto-Costa A, de Oliveira MG. Combined Nitric Oxide-Releasing Poly(Vinyl Alcohol) Film/F127 Hydrogel for Accelerating Wound Healing. Colloids Surf B. 2015;130:182–191. doi: 10.1016/j.colsurfb.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 44.Seabra AB, de Oliveira MG. Poly(Vinyl Alcohol) and Poly(Vinyl Pyrrolidone) Blended Films for Local Nitric Oxide Release. Biomaterials. 2004;25:3773–3782. doi: 10.1016/j.biomaterials.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 45.Joslin JM, Lantvit SM, Reynolds MM. Nitric Oxide Releasing Tygon Materials: Studies in Donor Leaching and Localized Nitric Oxide Release at a Polymer-Buffer Interface. ACS Appl Mater Interfaces. 2013;5:9285–9294. doi: 10.1021/am402112y. [DOI] [PubMed] [Google Scholar]
- 46.Gierke GE, Nielsen M, Frost MC. S-Nitroso-N-Acetyl-D-Penicillamine Covalently Linked to Polydimethylsiloxane (SNAP–PDMS) for Use as a Controlled Photoinitiated Nitric Oxide Release Polymer. Sci Technol Adv Mater. 2011;12:055007. doi: 10.1088/1468-6996/12/5/055007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCarthy CW, Goldman J, Frost MC. Synthesis and Characterization of the Novel Nitric Oxide (No) Donating Compound, S-Nitroso-N-Acetyl-D-Penicillamine Derivatized Cyclam (SNAP-Cyclam) ACS Appl Mater Interfaces. 2016 doi: 10.1021/acsami.5b12548. [DOI] [PubMed] [Google Scholar]
- 48.Riccio DA, Dobmeier KP, Hetrick EM, Privett BJ, Paul HS, Schoenfisch MH. Nitric Oxide-Releasing S-Nitrosothiol-Modified Xerogels. Biomaterials. 2009;30:4494–4502. doi: 10.1016/j.biomaterials.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y, Lee PI. Controlled Nitric Oxide Delivery Platform Based on S-Nitrosothiol Conjugated Interpolymer Complexes for Diabetic Wound Healing. Mol Pharmaceutics. 2009;7:254–266. doi: 10.1021/mp900237f. [DOI] [PubMed] [Google Scholar]
- 50.Riccio DA, Nugent JL, Schoenfisch MH. Stober Synthesis of Nitric Oxide-Releasing S-Nitrosothiol-Modified Silica Particles. Chem Mater. 2011;23:1727–1735. doi: 10.1021/cm102510q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shishido SM, de Oliveira MG. Polyethylene Glycol Matrix Reduces the Rates of Photochemical and Thermal Release of Nitric Oxide from S-Nitroso-N-Acetylcysteine. Photochem Photobiol. 2000;71:273–280. doi: 10.1562/0031-8655(2000)071<0273:pgmrtr>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 52.Sommer JG. Elastomer Molding Technology: A Comprehensive and Unified Approach to Materials, Methods and Mold Design for Elastomers. Elastech; 2003. [Google Scholar]
- 53.Harris JM. Poly(Ethylene Glycol) Chemistry: Biotechnical and Biomedical Applications. Springer Science & Business Media; 1992. [Google Scholar]
- 54.Gines J, Arias M, Moyano J, Sanchez-Soto P. Thermal Investigation of Crystallization of Polyethylene Glycols in Solid Dispersions Containing Oxazepam. Int J Pharm. 1996;143:247–253. [Google Scholar]
- 55.Fishburn CS. The Pharmacology of Pegylation: Balancing Pd with Pk to Generate Novel Therapeutics. J Pharm Sci. 2008;97:4167–4183. doi: 10.1002/jps.21278. [DOI] [PubMed] [Google Scholar]
- 56.Powell G. Polyethylene Glycol. Handbook of Water Soluble Gums and Resins. 1980;18:1–31. [Google Scholar]
- 57.Seabra AB, de Souza GFP, da Rocha LL, Eberlin MN, de Oliveira MG. S-Nitrosoglutathione Incorporated in Poly(Ethylene Glycol) Matrix: Potential Use for Topical Nitric Oxide Delivery. Nitric Oxide. 2004;11:263–272. doi: 10.1016/j.niox.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 58.Chipinda I, Simoyi RH. Formation and Stability of a Nitric Oxide Donor: S-Nitroso-N-Acetylpenicillamine. J Phys Chem B. 2006;110:5052–5061. doi: 10.1021/jp0531107. [DOI] [PubMed] [Google Scholar]
- 59.Yan Q, Major TC, Bartlett RH, Meyerhoff ME. Intravascular Glucose/Lactate Sensors Prepared with Nitric Oxide Releasing Poly(Lactide-Co-Glycolide)-Based Coatings for Enhanced Biocompatibility. Biosens Bioelectron. 2011;26:4276–4282. doi: 10.1016/j.bios.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klement P, Du YJ, Berry LR, Tressel P, Chan AKC. Chronic Performance of Polyurethane Catheters Covalently Coated with Ath Complex: A Rabbit Jugular Vein Model. Biomaterials. 2006;27:5107–5117. doi: 10.1016/j.biomaterials.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 61.Shishido SIM, Seabra AB, Loh W, Ganzarolli de Oliveira M. Thermal and Photochemical Nitric Oxide Release from S-Nitrosothiols Incorporated in Pluronic F127 Gel: Potential Uses for Local and Controlled Nitric Oxide Release. Biomaterials. 2003;24:3543–3553. doi: 10.1016/s0142-9612(03)00153-4. [DOI] [PubMed] [Google Scholar]
- 62.Aposhian HV. Penicillamine and Analogous Chelating Agents. Ann N Y Acad Sci. 1971;179:481–486. doi: 10.1111/j.1749-6632.1971.tb46924.x. [DOI] [PubMed] [Google Scholar]
- 63.Stephens A, Watts R. The Treatment of Cystinuria with N-Acetyl-D-Penicillamine, a Comparison with the Results of D-Penicillamine Treatment. QJM. 1971;40:355–370. [PubMed] [Google Scholar]
- 64.Xing Q, Yates K, Vogt C, Qian Z, Frost MC, Zhao F. Increasing Mechanical Strength of Gelatin Hydrogels by Divalent Metal Ion Removal. Sci Rep. 2014;4:4706. doi: 10.1038/srep04706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Oliveira MG, Shishido SM, Seabra AB, Morgon NH. Thermal Stability of Primary S-Nitrosothiols: Roles of Autocatalysis and Structural Effects on the Rate of Nitric Oxide Release. J Phys Chem A. 2002;106:8963–8970. [Google Scholar]
- 66.Williams DL, Bloebaum RD. Observing the Biofilm Matrix of Staphylococcus Epidermidis Atcc 35984 Grown Using the Cdc Biofilm Reactor. Microsc Microanal. 2010;16:143–152. doi: 10.1017/S143192760999136X. [DOI] [PubMed] [Google Scholar]
- 67.Mowery KA, Meyerhoff ME. The Transport of Nitric Oxide through Various Polymeric Matrices. Polymer. 1999;40:6203–6207. [Google Scholar]
- 68.Worley BV, Schilly KM, Schoenfisch MH. Anti-Biofilm Efficacy of Dual-Action Nitric Oxide-Releasing Alkyl Chain Modified Poly(Amidoamine) Dendrimers. Mol Pharmaceutics. 2015;12:1573–1583. doi: 10.1021/acs.molpharmaceut.5b00006. [DOI] [PubMed] [Google Scholar]
- 69.Ren H, Wu J, Colletta A, Meyerhoff ME, Xi C. Efficient Eradication of Mature Pseudomonas Aeruginosa Biofilm Via Controlled Delivery of Nitric Oxide Combined with Antimicrobial Peptide and Antibiotics. Frontiers in Microbiology. 2016;7:1260. doi: 10.3389/fmicb.2016.01260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brisbois EJ, Major TC, Goudie MJ, Meyerhoff ME, Bartlett RH, Handa H. Attenuation of Thrombosis and Bacterial Infection Using Dual Function Nitric Oxide Releasing Central Venous Catheters in a 9 Day Rabbit Model. Acta Biomater. 2016;44:304–312. doi: 10.1016/j.actbio.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


