Abstract
Background
Wedge resection in selected patients with early stage non-small cell lung cancer (NSCLC) is considered to be a valid treatment option. The aim of this study was to evaluate the recurrence patterns after wedge resection, to analyze the survival of patients under routine follow up and to recommend a follow-up regimen.
Methods
Retrospective analysis of 446 consecutive patients between May 2000 and December 2012 who underwent a wedge resection for clinical stage I NSCLC. All patients were followed up with a computed tomography (CT) scan with or without contrast. The recurrence was recorded as local (involving the same lobe of wedge resection), local-regional (involving mediastinal or hilar lymph nodes or a different lobe), or distant including distant metastasis and pleural disease.
Results
Median follow-up for survivors (n=283) was 44.6 months. 163 patients died: median overall survival of 82.6 months. 36 patients were diagnosed with new primary NSCLC and 152 with recurrence (79 local, 45 regional, and 28 distant). There was no difference in the incidence of recurrence detection detected by CT scans with vs. without contrast (p=0.18). The cumulative incidence of local recurrences at 1, 2 and 3- years was higher than the cumulative incidence for local, regional and distant recurrences: 5.2, 11.1 and 14.9% vs 3.7, 6.6 and 9.5% vs 2.3, 4.7, and 6.4%, respectively. Primary tumor diameter was associated with local recurrence in univariate analysis.
Conclusions
Wedge resection for early stage NSCLC is associated with a significant risk for local and regional recurrence. A long term follow-up using non-contrast CT scans at consistent intervals is appropriate to monitor for these recurrences.
Keywords: wedge resection, computed tomography, follow up, recurrence
A lobectomy is considered to be the optimal treatment in patients with clinical stage I non small cell lung cancer (NSCLC) [1]. In these patients, there is a 20–30% risk for disease recurrence, and recurrences usually present as distant disease in the first couple of years following surgery, rarely presenting at an earlier stage [2,3]. Given the preponderance of distant recurrences and the lack of curative treatments, some have questioned the benefit of close surveillance following surgical treatment [4]. Nonetheless, the NCCN guidelines recommend close follow-up after surgery with eventual transition to yearly screening [5].
Treatment of clinical stage I NSCLC with a wedge resection, on the other hand, has been mostly reserved for patients with poor lung function, multiple comorbidities, or previous lung surgery [6]. The reluctance to use this approach in fitter patients has been due to concerns about a higher risk of local and local-regional failure. Yet with the increasing use of computed tomography (CT) screening, there will be an expected tripling in the detection of early stage NSCLC [2,7], this has ignited a renewed interest for the broader use of sublobar resections as a possible treatment option. This interest has resulted in an ongoing prospective randomized trial (Cancer and Leukemia Group B 140503) that will compare the results of sublobar resections to lobectomy in early stage lung cancer.
The timing and benefit of post-operative surveillance in patients who undergo more limited resections is unknown; the expectation, however, would be that if there is a higher incidence of curable disease detected radiographically following surgery, the potential benefit of screening should be greater [8]. However, while some studies in these patients have shown that follow-up CT scans can be useful in detecting a new primary lung cancer, the data are less clear regarding detection of recurrences and the impact on overall survival [9,10]. In fact, some studies report that routine CT surveillance failed to detect asymptomatic recurrences or improve survival [11,12].
The primary aim of this study was to evaluate the patterns of recurrence in patients with early clinical stage NSCLC who were treated with a wedge resection, to analyze the survival of patients under routine follow up and to provide a rational CT scan surveillance regimen based on these patterns.
Materials and Methods
Patient Population
We performed a retrospective analysis of all patients between May 2000 and December 2012 who underwent a wedge resection for clinical stage I NSCLC. Patients with tumor completely excised and with negative margin were included in the analysis. Lymph nodes were not routinely sampled. 56% had no lymph node station sampled, 21% had 1 station sampled, 20% had 2–3 stations sampled, and 3% had 4–6 stations removed.
Indications for a wedge resection included patients with peripheral lesions less than 2 cm (n=364), previous anatomical lung resection (n=115), and poor pulmonary (n=24), cardiac function (n=80), or poor chronic renal failure (n=19). Staging was performed in accordance with the seventh edition of the AJCC Cancer Staging Manual [13]. Patients with previous surgically resected NSCLC were considered to have a new primary and were included in the study if the interval of time was more than 2 years and if there was no pathological correlation between these two cancers [14]. Reasons for exclusion included: recurrent lung cancers (n=49), synchronous cancers (n=154), any neoadjuvant treatment (n=16), any adjuvant treatment (n=31), completion lobectomy (n=15), satellite nodule in the same lobe (n=46) or in other lobes (n=40), incomplete resection, defined as microscopic or macroscopic residual tumor or margins involved by the tumor (n=135), carcinoid (n=69), and pure ground glass lesions with no solid component (n=38). In addition, patients who had scans done at an outside facility (n=44) were excluded if we were unable to review the images. Follow-up was conducted until April 2015. The study was approved by the Memorial Sloan Kettering Cancer Center Institutional review board.
CT Follow-up Protocol
All patients were regularly followed up with a computed tomography (CT) scan either with or without contrast (by physician choice). Scans were performed every 3 to 6 months for the first 2 years also according to physician preference, and then every 12 months. Other modalities of surveillance, such as positron emission tomography (PET), bronchoscopy or bone scans were not routinely performed. Each patient received also a physical examination and interval history at the time of each scan.
Recurrence and New Primary Lung Cancer
The type of recurrence, date of recurrence, imaging modality, and presence of symptoms were collected. Recurrence was classified as local if limited to the same lobe of the resection, local regional if involving the mediastinal or hilar lymph nodes or a different ipsilateral lobe from the location of the wedge resection, or distant including distant metastasis to other organs and diffuse pleural disease. Second primary tumors were recorded using the previously described criteria by Martini and Melamed [14]. Recurrences were documented with a biopsy and compared to the previous surgical specimen. A PET scan and brain magnetic resonance imaging (MRI) were used to complete the assessment of any recurrence.
Statistical Analysis
Descriptive statistics such as frequencies, medians, and ranges were utilized for patient and tumor characteristics. The primary endpoint was recurrence, which was analyzed using competing risks methods. New primary and death without recurrence or new primary were considered competing events. Time was calculated from date of surgery until recurrence, new primary, or death (whichever came first). Patients who were alive without recurrence or new primary were censored at the date of last available follow-up. Gray’s test was used to compare the cumulative incidence functions of subgroups in univariate analyses.
The univariate associations of patient and tumor characteristics with local recurrence were also analyzed using competing risks methods. In addition to new primary and death without recurrence or new primary, regional recurrence and distant recurrence were also competing events.
Overall survival (OS) for the whole cohort was estimated from date of surgery until death using the Kaplan-Meier method. OS for the comparisons of patients who recurred or had a second primary lung cancer were calculated from date of documented recurrence or second primary lung cancer until death. Patients who did not die during the study period were censored at date of last available follow-up.
All p-values were two-sided, and p<0.05 was considered significant. All statistical analyses were done in R (version 3.2.0, The R Foundation for Statistical Computing) including the “survival” and “cmprsk” packages [15,16].
Results
Demographics: Four hundred and forty-six patients were included in the analysis. Median age was 70 years, and female patients represented 57% of the study population. 110 patients had a previous NSCLC that was treated surgically and presented at this time with a biopsy-proven new primary NSCLC, and were consequently considered for limited surgical resection. Patient characteristics are summarized in Table 1. Median follow-up for survivors (n=283) was 44.6 months (range 0.8–145.4). 163 patients died, with a median OS of 82.6 months. Most follow-up CT scans were performed without contrast (n=318, 71%). During the first two years following surgery, the median interval between CT scan was 5.9 months; 14 patients had a scan every 3 months, 44 patients had a scan every 4 months, 367 patients had a scan every 6 months, and 21 patients every 8–12 months. After two years, all patients were scanned yearly.
Table 1.
Patient characteristics (n=446)
| Characteristic | No | % | ||
|---|---|---|---|---|
| Sex | T | |||
| Male | 190 | 43 | ||
| Female | 256 | 57 | ||
| Age, y median (range) | 70 (47–90) | |||
| ≤ 70 | 224 | 50 | ||
| > 70 | 222 | 50 | ||
| Median FEV1% (range) | 84 (25–161) | |||
| Median Diffusion capacity (range) | 75.5 (19.3–163.0) | |||
| Previous history of lung cancer | ||||
| No | 336 | 75 | ||
| Yes | 110 | 25 | ||
| Other cancers | ||||
| No | 239 | 54 | ||
| Yes | 207 | 46 | ||
| Surgical Approach | ||||
| Open or VATS converted | 137 | 31 | ||
| VATS | 309 | 69 | ||
| Side | ||||
| Right | 261 | 59 | ||
| Left | 185 | 41 | ||
| Location | ||||
| Right upper lobe | 149 | 33 | ||
| Right middle lobe | 14 | 3 | ||
| Right lower lobe | 99 | 22 | ||
| Left upper lobe | 122 | 27 | ||
| Left lower lobe | 62 | 14 | ||
| Median diameter of lesion cm (range) | 1.4 (0.1 – 6.5) | |||
| pT stage | ||||
| T1a | 316 | 71 | ||
| T1b | 53 | 12 | ||
| T2 | 77 | 17 | ||
| Median SUVmax (range)* | 2.8 (0 – 23) | |||
| Histology | ||||
| Adenocarcinoma | 252 | 57 | ||
| Lepidic | 115 | 26 | ||
| Squamous | 63 | 14 | ||
| Other | 16 | 4 | ||
| Vascular invasion | ||||
| No | 285 | 64 | ||
| Yes | 114 | 26 | ||
| Unknown | 47 | 11 | ||
| Visceral plaural inolvement | ||||
| No | 377 | 85 | ||
| Yes | 69 | 15 | ||
| Postoperative complication | ||||
| No | 383 | 86 | ||
| Yes | 63 | 14 | ||
| Median length of stay, days (range) | 3 (1 – 39) | |||
| CT scan | ||||
| With contrast | 128 | 29 | ||
| Without contrast | 318 | 71 | ||
| Interval follow-up first two years | ||||
| 3 | 14 | 3 | ||
| 4 | 44 | 10 | ||
| 6 | 367 | 82 | ||
| 8 | 10 | 2 | ||
| 12 | 10 | 2 | ||
| Treatment of recurrence/new primary | ||||
| Chemotherapy and radiotherapy | 23 | |||
| Radiotherapy/ablation | 68 | |||
| Surgery | 30 | |||
| Chemotherapy and surgery | 14 | |||
| None | 17 | |||
SUVmax=0, patients with no avid lesions
Recurrence Patterns: During follow-up, 36 (8%) patients were diagnosed with new primary NSCLC and 152 (34%) with recurrence. Only 10 of these patients were symptomatic; one patient with a new primary, and 9 patients with recurrence, 2 of whom had brain metastases.
New Primary Cancers: Twenty-seven new primary lung cancers were diagnosed as stage I, one as stage IIa, 4 as stage IIIa and 4 as stage IV. Eighteen cancers were located on the left side, and 15 metachronous cancers presented on the same site of the previous primary lung cancer. The median interval between surgery and new metachronous primary lung cancer was 43.4 months. For patients with stage I/II lung cancer, radiotherapy was used in 7 patients and surgery in 17 patients (wedge resection n=16, lobectomy n=1). Patients with stage IIIa were treated with chemotherapy and surgery (n=3) or chemotherapy only (n=1). Stage IV lung cancer presented with adrenal metastases (n=2), bone metastasis (n=1) and stomach metastasis (n=1); two patients were treated with palliative chemotherapy and two with supportive care.
Cancer Recurrence: 152 patients developed recurrent disease. Seventy-nine (52%) patients were diagnosed with a local recurrence. The median interval between surgery and local recurrence was 18.6 months (range 1.5 – 70.4). Forty-five (30%) patients presented with a local-regional recurrence: 18 (40%) of these patients recurred in the lymph nodes only, including mediastinal nodes (n=13) and hilar nodes (n=5). 7 patients recurred with a lung lesion in a different ipsilateral lobe. The median interval between surgery and regional recurrence was 16.6 months (range 3.6 – 80.1). Twenty-eight (18%) patients presented with distant (n=15) or combined local and distant recurrence (n=13) (Figure 1), and 2 of these patients presented with a solitary recurrence in the chest wall including one each involving the lung and the diaphragm. The most frequent metastatic site was bone (n=10), followed by brain (n=6), liver (n=5), adrenal glands (n=3), disseminated pleural disease with effusion (n=3), and pleura and diaphragm (n=1). The median interval between surgery and distant recurrence was 16.0 months (range 4.5 – 67.8).
Figure 1.
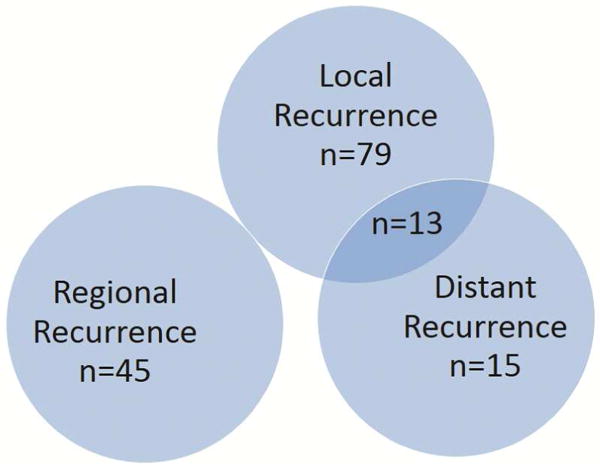
Ven diagram of the different pattern of recurrence
The cumulative incidence of all recurrences at 1, 2 and 3- years was 11.2%, 22.4% and 30.9%, respectively. The overall cumulative incidence of recurrence consistently increased over the course of the first 5 years, and plateaued after 5 years (Figure 2). The cumulative incidence of local recurrences at 1, 2 and 3- years was higher than the cumulative incidence for regional and distant recurrences: 5.2, 11.1 and 14.9% vs 3.7, 6.6 and 9.5% vs 2.3, 4.7, and 6.4%, respectively (Figure 3). There was no difference in the cumulative incidence of recurrence whether screening was done with CT scans with or without contrast (p=0.18, Figure 4). Patients scanned every 3–4 months have a higher CIR, 3-yr CIR 46.7% vs. 28.6% for 6–8 months vs. 21.4% for 12 months in the first two years.
Figure 2.
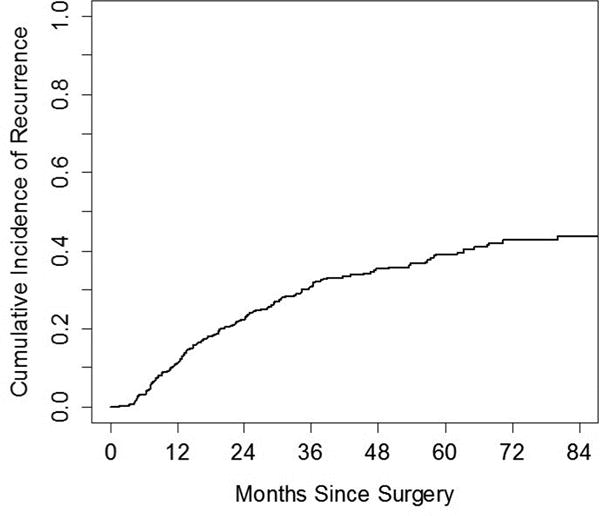
The cumulative incidence of recurrence in our study population (n=446)
Figure 3.
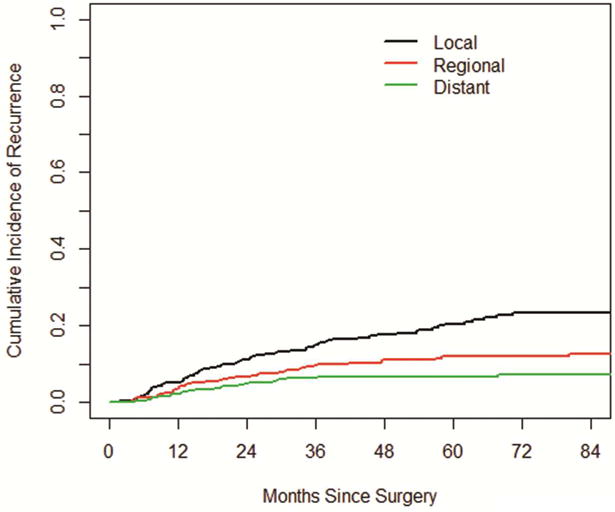
The cumulative incidence of local, regional and distant recurrences
Figure 4.
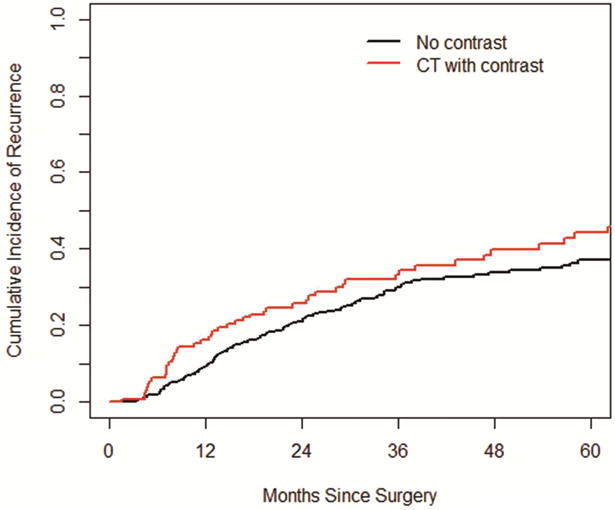
The cumulative incidence of recurrence between CT scans with or without contrast
Outcomes after Recurrence: OS in patients with a second primary lung cancer was significantly better when compared to patients with a recurrence (p=0.006). Patients with a new primary lung cancer had a median OS of 68.0 months (95% CI 68.0 – NA mo) following the diagnosis of the second primary. In patients with a recurrence, there was a significant difference in terms of median OS following the diagnosis of local, local regional, and distant recurrences: 40.5 months (95% CI 27.4 – 59.2 mo), 16.9 months (95% CI 13.8 – 36.4 mo) and 13.3 months (95% CI 8.6 – 19.4 mo), respectively (p<0.001). Patients with a distant recurrence were mainly treated with chemotherapy, while two patients were treated with surgery for solitary brain metastasis. Patients with regional recurrences were treated mainly with chemotherapy (n=17), combination of chemotherapy and radiotherapy (n=11), radiofrequency ablation and radiotherapy alone in 4 cases, surgery for 5 patients, and surgery in combination with chemotherapy (n=3). Fifteen patients with local recurrence were treated surgically, and 17 were treated with ablation or radiotherapy (Table 1).
Predictors of Local Recurrence: At univariate analysis only the diameter as a continuous variable was significantly associated with local recurrence (p<0.05). Dichotomizing tumor diameter at 2 cm, there was a trend of increased risk of local recurrence for tumors bigger than 2 cm (Table 2).
Table 2.
Univariate analysis of local recurrence
| Characteristics | HR | 95% CI | p value |
|---|---|---|---|
| Age (> 70 vs. ≤ 70) | 1.40 | (0.90 – 2.18) | 0.14 |
| Sex (female vs. male) | 0.94 | (0.61 – 1.46) | 0.79 |
| Previous lung cancer (yes vs. no) | 1.46 | (0.93 – 2.31) | 0.10 |
| Other cancer (yes vs. no) | 0.94 | (0.61 – 1.47) | 0.79 |
| SUVmax (continuous) | 1.02 | (0.96 – 1.09) | 0.54 |
| Diameter (continuous) | 1.38 | (1.13 – 1.69) | 0.002 |
| Size (cm) (> 2 vs. ≤ 2) | 1.56 | (0.95 – 2.56) | 0.082 |
| Histology | |||
| Lepidic vs. Adenoca | 0.94 | (0.55 – 1.62) | 0.82 |
| Squamous vs. Adenoca | 1.44 | (0.79 – 2.59) | 0.23 |
| Other vs. Adenoca | 0.65 | (0.16 – 2.61) | 0.55 |
| Visc Pleura involvement (yes vs. no) | 1.12 | (0.61 – 2.05) | 0.72 |
| Laterality (left vs. right) | 0.97 | (0.62 – 1.52) | 0.89 |
| Location of tumor | |||
| RML vs. RUL | 0.29 | (0.04 – 2.26) | 0.24 |
| RLL vs. RUL | 0.70 | (0.38 – 1.29) | 0.25 |
| LUL vs. RUL | 0.91 | (0.53 – 1.56) | 0.73 |
| LLL vs. RUL | 0.53 | (0.24 – 1.14) | 0.10 |
HR hazard ratio; CI confidence interval
Comment
In this study, we show that there is a high incidence of asymptomatic recurrent disease following a wedge resection for clinical stage I NSCLC, in fact much higher than what would be expected after a standard lobectomy. As previously published by this group sublobar resection is an independent risk for recurrence [17], like the micropapillary subtype component of resected adenocarcinoma which can increase the risk of local recurrence [18].
While metachronous cancers occurred in 8% of patients as expected based on previous lobectomy series [9], 34% of patients developed disease recurrence. Most (82%) of the recurrences were local and regional, while metastatic disease represented only 18% of all recurrences. The risk for developing recurrent disease appeared to plateau after 2 years for distant recurrences, while it appeared to plateau after 5 years for local and regional recurrences. Once detected and treated, patients with metachronous cancers lived longest, followed by patients with local, regional, and then distant recurrences. In this series, patients who developed a distant recurrence were treated primarily with chemotherapy, while patients with metachronous disease, local recurrences, and regional recurrences were mostly treated with curative intent using various combinations of radiation, ablation, chemotherapy, and sometimes surgery.
The main rationale for screening patients following resection for lung is to improve outcomes. Only a prospective randomized trial can definitively prove this link. In patients who undergo a lobectomy, NCCN guidelines support imaging for follow-up, yet there remains significant variability in the timing and type of imaging modalities [9]. Moreover, it remains controversial whether imaging is even associated with improved survival in patients who undergo an anatomic lung resection. With regards to the timing of disease recurrences, as previously reported from a study done in this institution, the risk of recurrence remained consistent for up to 4 years after anatomical lung resection [10].
While this study is incapable of establishing a causal association between close post-operative screening and survival following a wedge resection, there are some circumstantial findings that support a possible benefit. Firstly, 36 new primary lung cancers were discovered during follow-up in our population, and only one was symptomatic at the time of the CT scan. All of these tumors were detected by CT scan at an early stage and were amenable to a curative treatment. These findings were confirmed by previous studies [3,4,19]. Lamont et al. showed a 15% rate of newly detected primary lung cancer at an early stage, all of which were asymptomatic. They showed that the survival of patients with newly detected primary lung cancer was significantly higher compared to that of the patients with local recurrence [11].
With regards to recurrence detection, the majority of the recurrences were local and regional, asymptomatic, and re-treated with curative intent. While their survival was not as good as the metachronous cancers, patients with a local recurrence had a 5-year post-re-treatment survival rate of 32.0%. Presumably, had these patients not been screened and treated they would have progressed to an incurable stage. In our study the cumulative incidence of recurrence was higher in patients scanned every 4 months compared to the ones scanned every 6 or 12 months in the first two years. These data have to be analyzed very carefully because the groups are not homogenous and selection biases can effect this finding: the number of lepidic adenocarcinoma was significantly higher in the group scanned every 6 months and the squamous cell cancer was significantly higher in the group of patients scanned every 4 months.
The literature findings regarding screening are mixed. One meta-analysis showed an improved survival in patients who underwent close follow-up [20]. Westeel and Choi’s reports showed an improvement in survival of asymptomatic patients with recurrence [17,18], but other papers did not show any improvement in survival in patients under CT scan surveillance, regardless of the site of recurrence [19,21].
This paper did not evaluate if sublobar resection is equivalent or superior to a lobar resection for patients with NSCLC and did not analyze indications for sublobar resections. The primary limitation of this study is that it is retrospective, so proving a causal association between screening and outcome is not possible. Furthermore, the population in this study underwent a wedge primarily because of medical co-morbidities; it is unclear whether a healthier population would show a similar association. One could speculate, however, that if anything, more treatment options would be available to treat healthier patients with a metachronous or local/regional recurrence such that close screening might provide greater benefit.
In conclusion, there is a high incidence of potentially curable recurrences following a wedge for lung cancer that are detectable with a CT scan. Since the cumulative incidence of recurrence increases for the first 5 years following surgery, we feel that close monitoring with a CT scan with no contrast every 6 months for the first 5 years is supported by our data. In the routine practice this should allow to detect early recurrence potentially still curable.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the Fifty-second Annual Meeting of the Society of Thoracic Surgeons, Phoenix, AZ, Jan 23–27, 2016.
References
- 1.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995 Sep;60(3):615–22. doi: 10.1016/0003-4975(95)00537-u. discussion 622–3. [DOI] [PubMed] [Google Scholar]
- 2.Crino’ L, Weder W, Van Meerbeeck J, Felip E, ESMO Guidelines Working Group Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Onc. 2010;21(5):v103–v115. doi: 10.1093/annonc/mdq207. [DOI] [PubMed] [Google Scholar]
- 3.Sugimura H, Nichols FC, Yang P, Allen MS, Cassivi SD, Deschamps C, Williams BA, Pairolero PC. Survival after recurrent nonsmall cell lung cancer after complete pulmonary resection. Ann Thorac Surg. 2007;83:409–418. doi: 10.1016/j.athoracsur.2006.08.046. [DOI] [PubMed] [Google Scholar]
- 4.Rubins J, Unger M, Colice GL, American College of Chest Physicians Follow-up and surveillance of the lung cancer patient following curative intent therapy: ACCP evidence-based clinical practice guideline (2nd edition) Chest. 2007 Sep;132(3 Suppl):355S–367S. doi: 10.1378/chest.07-1390. [DOI] [PubMed] [Google Scholar]
- 5.Ettinger DS, Akerley W, Borghaei H, Chang AC, Cheney RT, Chirieac LR, D’Amico TA, Demmy TL, Ganti AK, Govindan R, Grannis FW Jr, Horn L, Jahan TM, Jahanzeb M, Kessinger A, Komaki R, Kong FM, Kris MG, Krug LM, Lennes IT, Loo BW Jr, Martins R, O’Malley J, Osarogiagbon RU, Otterson GA, Patel JD, Pinder-Schenck MC, Pisters KM, Reckamp K, Riely GJ, Rohren E, Swanson SJ, Wood DE, Yang SC, Hughes M, Gregory KM, NCCN (National Comprehensive Cancer Network) Non-small cell lung cancer. J Natl Compr Canc Netw. 2012 Oct 1;10(10):1236–71. doi: 10.6004/jnccn.2012.0130. [DOI] [PubMed] [Google Scholar]
- 6.Donington J, Ferguson M, Mazzone P, Handy J, Jr, Schuchert M, Fernando H, Loo B, Jr, Lanuti M, de Hoyos A, Detterbeck F, Pennathur A, Howington J, Landreneau R, Silvestri G, Thoracic Oncology Network of American College of Chest Physicians Workforce on Evidence-Based Surgery of Society of Thoracic Surgeons. American College of Chest Physicians and Society of Thoracic Surgeons consensus statement for evaluation and management for high-risk patients with stage I non-small cell lung cancer. Chest. 2012 Dec;142(6):1620–35. doi: 10.1378/chest.12-0790. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson FL, Austin Jh, Field JK, Jett JR, Keshavjee S, MacMahon H, Mulshine JL, Munden RF, Salgia R, Strauss GM, Sugarbaker DJ, Swanson SJ, Travis WD, Jaklitsch MT. Development of the American Association for Thoracic Surgery Guidelines for low dose computed tomography scans to screen for lung cancer in North America: reccomandations of the American Association for Thoracic Surgery Task Force for Lung Cancer Screenng nd Surveillance. J Thorac cardiovasc Surg. 2012;144:25–32. doi: 10.1016/j.jtcvs.2012.05.059. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura R, Kurishima K, Kobayashi N, Ishikawa S, Goto Y, Sakai M, Onizuka M, Ishikawa H, Satoh H, Hizawa N, Sato Y. Postoperative follow-up for patients with non-small cell lung cancer. Onkologie. 2010;33(1–2):14–8. doi: 10.1159/000264623. [DOI] [PubMed] [Google Scholar]
- 9.Lou F, Huang J, Sima CS, Dycoco J, Rusch V, Bach PB. Patterns of recurrence and second primary lung cancer in early-stage lung cancer survivors followed with routine computed tomography surveillance. J Thorac Cardiovasc Surg. 2013 Jan;145(1):75–81. doi: 10.1016/j.jtcvs.2012.09.030. discussion 81–2. [DOI] [PubMed] [Google Scholar]
- 10.Lou F, Sima CS, Rusch VW, Jones DR, Huang J. Differences in patterns of recurrence in early-stage versus locally advanced non-small cell lung cancer. Ann Thorac surg. 2014;98:1755–61. doi: 10.1016/j.athoracsur.2014.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamont JP, Kakuda JT, Smith D, Wagman LD, Grannis FW., Jr Systematic postoperative radiologic follow-up in patients with non-small cell lung cancer in stage IA. Arch Surg. 2002;137:935–939. doi: 10.1001/archsurg.137.8.935. [DOI] [PubMed] [Google Scholar]
- 12.Younes RN, Gross JL, Deheinzelin D. Follow-up in lung cancer: how often and for what porpoise. Chest. 1999;115:1494–9. doi: 10.1378/chest.115.6.1494. [DOI] [PubMed] [Google Scholar]
- 13.Edge SB, Byrd Dr, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th. New York: Springer-Verlag; 2009. [Google Scholar]
- 14.Martini N, Melamed Mr. Multiple primary lung cancers. J Thorac Cardiovasc Surg. 1975;70:606–612. [PubMed] [Google Scholar]
- 15.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. The Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 16.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 17.Ujiie H, Kadota K, Chaft JE, Buitrago D, Sima CS, Lee MC, Huang J, Travis WD, Rizk NP, Rudin CM, Jones DR, Adusumilli PS. Solid Predominant Histologic Subtype in Resected Stage I Lung Adenocarcinoma Is an Independent Predictor of Early, Extrathoracic, Multisite Recurrence and of Poor Postrecurrence Survival. J Clin Oncol. 2015 Sep 10;33(26):2877–84. doi: 10.1200/JCO.2015.60.9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nitadori J, Bograd AJ, Kadota K, Sima CS, Rizk NP, Morales EA, Rusch VW, Travis WD, Adusumilli PS. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. J Natl Cancer Inst. 2013 Aug 21;105(16):1212–20. doi: 10.1093/jnci/djt166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi SH, Kim YT, Kim SK, Kang KW, Goo JM, Kang CH, Kim JH. Positron emission tomography-computed tomography for post operative surveillance in non-small cell lung cancer. Ann Thorac Surg. 2011;92:26–32. doi: 10.1016/j.athoracsur.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Calman L, Beaver K, Hind D, Lorigan P, Roberts C, Lloyd-Jones M. Survival benefts from follow-up of patients with lung cancer: a systematic review and meta analysis. J thorac Onc. 2011;6:1993–2004. doi: 10.1097/JTO.0b013e31822b01a1. [DOI] [PubMed] [Google Scholar]
- 21.Westeel V, Choma D, Clement F, Woronoff-Lemsi MC, Pugin JF, Dubiez A, Depierre A. Relevance of intensive postoperative follow-up after surgery for non small-cell lung cancer. Ann Thorac Surg. 2000;70:1185–90. doi: 10.1016/s0003-4975(00)01731-8. [DOI] [PubMed] [Google Scholar]


