Abstract
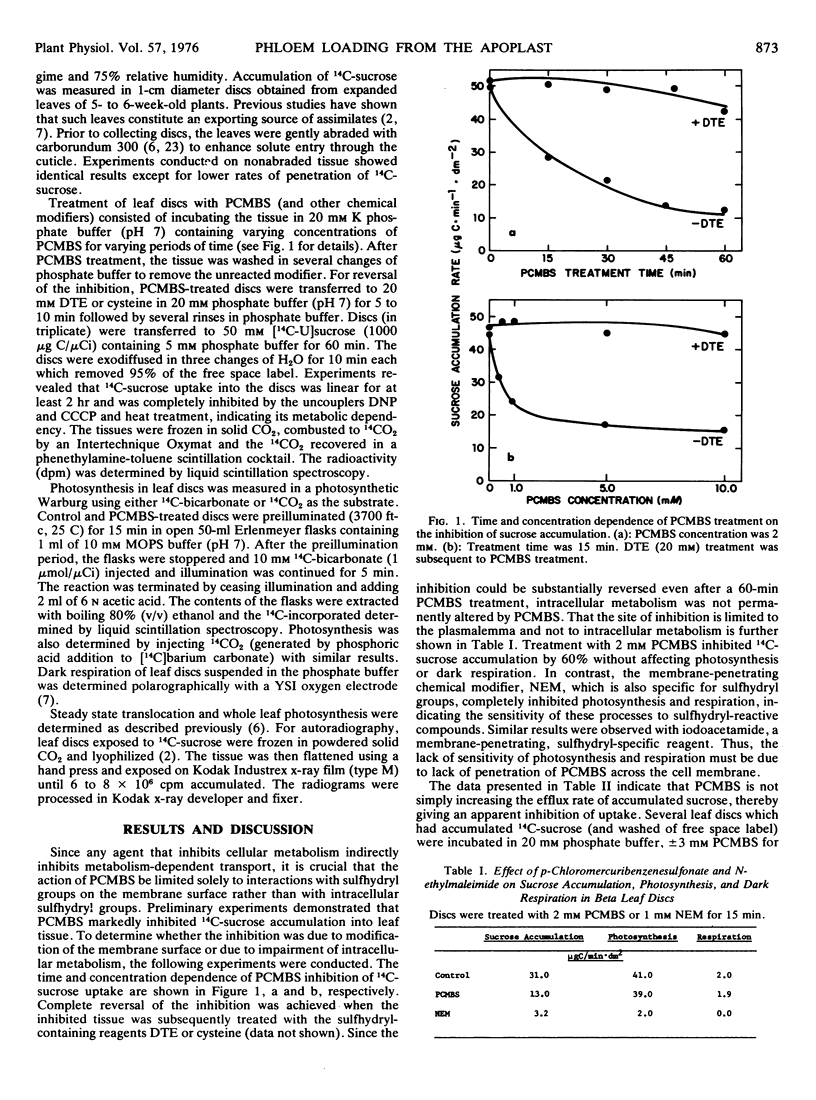
The water-soluble, sulfhydryl-specific, chemical modifier p-chloromercuribenzenesulfonic acid reversibly inhibited the accumulation of exogenously supplied 14C-sucrose into leaf discs of Beta vulgaris. P-Chloromercuribenzenesulfonic acid treatment did not inhibit photosynthesis or respiration or induce membrane leakage to sucrose, indicating that the site of inhibition was the plasmalemma. The active loading of sucrose and 14CO2-derived assimilates into the phloem and their translocation from the source leaf were inhibited by the nonpermeant modifier. Several nonpermeant sulfhydryl group modifiers also inhibited sucrose accumulation into leaf discs while two amino-reactive reagents had no effect. The results indicate that sugars are actively accumulated into the phloem from the apoplast and that membrane sulfhydryl groups may be involved.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cataldo D. A. Vein Loading: The Role of the Symplast in Intercellular Transport of Carbohydrate between the Mesophyll and Minor Veins of Tobacco Leaves. Plant Physiol. 1974 Jun;53(6):912–917. doi: 10.1104/pp.53.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows R. J., Geiger D. R. Structural and Physiological Changes in Sugar Beet Leaves during Sink to Source Conversion. Plant Physiol. 1974 Dec;54(6):877–885. doi: 10.1104/pp.54.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudemer Y., Latruffe N. Evidence for penetrant and non-penetrant thiol reagents and their use in the location of rat liver mitochondrial D(-)-beta-hydroxybutyrate dehydrogenase. FEBS Lett. 1975 Jun 1;54(1):30–34. doi: 10.1016/0014-5793(75)81061-1. [DOI] [PubMed] [Google Scholar]
- Geiger D. R., Cataldo D. A. Leaf structure and translocation in sugar beet. Plant Physiol. 1969 Jan;44(1):45–54. doi: 10.1104/pp.44.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Giaquinta R. T., Sovonick S. A., Fellows R. J. Solute distribution in sugar beet leaves in relation to Phloem loading and translocation. Plant Physiol. 1973 Dec;52(6):585–589. doi: 10.1104/pp.52.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Sovonick S. A., Shock T. L., Fellows R. J. Role of free space in translocation in sugar beet. Plant Physiol. 1974 Dec;54(6):892–898. doi: 10.1104/pp.54.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaguinta R. T., Dilley R. A., Anderson B. J. Light potentiation of photosynthetic oxygen evolution inhibition by water soluble chemical modifiers. Biochem Biophys Res Commun. 1973 Jun 19;52(4):1410–1417. doi: 10.1016/0006-291x(73)90658-x. [DOI] [PubMed] [Google Scholar]
- Giaquinta R. T., Geiger D. R. Mechanism of inhibition of translocation by localized chilling. Plant Physiol. 1973 Feb;51(2):372–377. doi: 10.1104/pp.51.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. T., Selman B. R., Bering C. L., Dilley R. A. Inhibition of coupling factor activity of chloroplast membranes by diazonium compounds. J Biol Chem. 1974 May 10;249(9):2873–2878. [PubMed] [Google Scholar]
- Goldner A. M., Schultz S. G., Curran P. F. Sodium and sugar fluxes across the mucosal border of rabbit ileum. J Gen Physiol. 1969 Mar;53(3):362–383. doi: 10.1085/jgp.53.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning B. E., Pate J. S., Minchin F. R., Marks I. Quantitative aspects of transfer cell structure in relation to vein loading in leaves and solute transport in legume nodules. Symp Soc Exp Biol. 1974;(28):87–126. [PubMed] [Google Scholar]
- Hare J. D. Distinctive alterations of nucleoside, sugar and amino acid uptake of sulfhydryl reagents in cultured mouse cells. Arch Biochem Biophys. 1975 Oct;170(2):347–352. doi: 10.1016/0003-9861(75)90128-9. [DOI] [PubMed] [Google Scholar]
- Knauf P. A., Rothstein A. Chemical modification of membranes. I. Effects of sulfhydryl and amino reactive reagents on anion and cation permeability of the human red blood cell. J Gen Physiol. 1971 Aug;58(2):190–210. doi: 10.1085/jgp.58.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komor E., Tanner W. The hexose-proton cotransport system of chlorella. pH-dependent change in Km values and translocation constants of the uptake system. J Gen Physiol. 1974 Nov;64(5):568–581. doi: 10.1085/jgp.64.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komor E., Tanner W. The hexose-proton symport system of Chlorella vulgaris. Specificity, stoichiometry and energetics of sugar-induced proton uptake. Eur J Biochem. 1974 May 2;44(1):219–223. doi: 10.1111/j.1432-1033.1974.tb03476.x. [DOI] [PubMed] [Google Scholar]
- Nelson S. O., Glover G. I., Magill C. W. The essentiality of sulfhydryl groups to transport in Neurospora crassa. Arch Biochem Biophys. 1975 Jun;168(2):483–489. doi: 10.1016/0003-9861(75)90278-7. [DOI] [PubMed] [Google Scholar]
- Sovonick S. A., Geiger D. R., Fellows R. J. Evidence for active Phloem loading in the minor veins of sugar beet. Plant Physiol. 1974 Dec;54(6):886–891. doi: 10.1104/pp.54.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West I., Mitchell P. Proton-coupled beta-galactoside translocation in non-metabolizing Escherichia coli. J Bioenerg. 1972 Aug;3(5):445–462. doi: 10.1007/BF01516082. [DOI] [PubMed] [Google Scholar]
- Ziegler H. Biochemical aspects of phloem transport. Symp Soc Exp Biol. 1974;(28):43–62. [PubMed] [Google Scholar]



