Abstract
Background
The durability of responses and long-term safety of pegylated interferon alpha-2a (PEG-IFN-a-2a) in patients with polycythemia vera and essential thrombocythemia have not been reported. Here, we present long-term efficacy and safety data from a single-center, prospective, phase 2 study, after 7 years of follow-up.
Methods
Patients older than 18 years who were diagnosed with essential thrombocythemia or polycythemia vera per 2001 World Health Organization criteria were eligble to enroll.
Responses were assessed every 3–6 months: Data were analyzed using descriptive statistics. The rate of leukemia transformation was compared with age- and gender-matched patients who were not treated with PEG-IFN-α-2a.
Findings
PEG-IFN-α-2a induced hematologic (80%) and molecular responses (63%) in 83 patients with essential thrombocythemia (n=40) and polycythemia vera (n=43), with median durations of 66 and 53 months, respectively. Thirty-nine percent of hematologic responders and 71% of molecular responders (JAK2V617F+) have maintained some response during follow-up: 48% maintained their best molecular response, including 9 of 10 patients with a complete molecular response. The incidence of major venous-thrombotic events during the study was 1.22/person-year. Overall, 22% of patients discontinued therapy due to treatment-related toxicity. While toxicity rates decreased over time, 5 patients experienced treatment-limiting G3/4 toxicities after 60 months on therapy. Rates of transformation to MF/AML were similar between patients treated with PEG-IFN-a-2a and those from a historical control series.
Interpretation
PEG-IFN-α-2a can induce durable hematologic and molecular remissions in patients with essential thrombocythemia and polycythemia vera. We suggest a starting dose of 45 mcg/week, and its combination with other drugs should be explored further in clinical trials.
Keywords: essential thrombocythemia, polycythemia vera, pegylated interferon alpha-2a, long term follow-up
Introduction
The two classical BCR-ABL–negative myeloproliferative neoplasms (MPN) essential thrombocythemia and polycythemia vera are clonal hematopoietic neoplasms characterized by an overproduction of mature blood elements, tendencies toward thrombosis and hemorrhage, extramedullary hematopoiesis (mild splenomegaly), and transformation to myelofibrosis (MF)/acute myeloid leukemia (AML) 1–3. The therapeutic approach primarily focuses on controlling blood counts and reducing the risk of thrombosis. For patients judged to be at high-risk of thrombosis, cytoreductive therapy is instituted (typically hydroxyurea). As an alternative to hydroxyurea, recombinant interferon-alpha is frequently suggested given its biologic, anti-proliferative, immunomodulating, and anti-clonal effects in these patients. However, its widespread use has been limited by high rates of discontinuation due to side effects 4–9. Pegylated forms of interferon have a better pharmacologic profile than short-acting interferons, resulting in a more convenient less-frequent schedule of injections, less immunogenicity and possibly less toxicity 10. In particular, several clinical studies of pegylated interferon alfa-2a (PEG-IFN-α-2a), including our own 11,12, have reported promising results in patients with essential thrombocythemia and polycythemia vera. PEG-IFN-α-2a induced complete hematologic response (CHR) in a great majority of patients and complete molecular remission (CMR) in a fraction of patients, with lower toxicity rates than expected. However, all studies had a relatively short follow-up and the durability of responses and long-term safety have not been reported.
Herein, we present long-term efficacy and safety data from a prospective phase 2 study of PEG-IFN-α-2a in 83 patients with advanced essential thrombocythemia and polycythemia vera after a median follow-up of 7 years, nearly twice as long as in previously reported studies.
Methods
Patient Selection
This is an ad hoc analysis of data from a prospective, open-label, single-center, phase 2 trial of PEG-IFN-α-2a in patients with essential thrombocythemia and polycythemia vera.11,12. Patients older than 18 years with either newly diagnosed or previously treated essential thrombocythemia or polycythemia vera according to the 2005 Polycythemia Vera Study Group criteria were eligble to enroll. Additional inclusion criteria included Eastern Cooperative Group Oncology status ≤ 2; adequate liver (total bilirubin ≤ 2.0 mg/dl) and renal function (serum creatinine ≤ 2.0 mg/dl); and normal cardiac function. Patients must have been off chemotherapy for at least one week prior to enrollment, but could be receiving hydroxyurea or anagrelide for up to 1 month after study entry. A washout period of one month was required for patients treated with continuous or chronic high doses of steroid. Exclusion criteria included pregnant or lactating women; history of another malignancy unless disease free for > 3 years); ischemic retinopathy; severe cardiac disease; history of medically significant psychiatric disease if not controlled, especially endogenous depression; a seizure disorder requiring anticonvulsant therapy; known infection with hepatitis B or C or HIV or other active systemic infection; renal disease requiring hemodialysis; or known autoimmune disease (except rheumatoid arthritis). All patients provided written informed consent. This study was approved by the Institutional Review Board at The University of Texas MD Anderson Cancer Center and conducted in accordance with the Declaration of Helsinki.
Study Procedures
PEG-IFN-α-2a was administered subcutaneously once weekly. The initial starting dose was 450 μg/week but was decreased in a stepwise manner due to toxicity to a final starting dose of 90 mcg/week: 3 patients were started at a dose of 450 μg/week, 3 at 360 μg/week, 19 at 270 μg/week, 26 at 180 μg/week, and 32 at 90 μg/week. Treatment was continued as long as the patients derived clinical benefit. During the study, the dose was modified based on toxicity or lack of efficacy. Any grade 3 or 4 event required therapy interruption. If the event resolved to grade 0 or 1 therapy could be resumed at a lower dose level. Persistent (≥ 2 weeks) significant grade 2 adverse events required dose reductions or discontinuation. Criteria for discontinuation included clearly documented disease progression (increasing transfusion requirement, splenomegaly, platelet or white blood cell counts, frequency of phlebotomy, or thromboembolic events) or no response within 6 months from the start of therapy despite dose escalation. The study is ongoing but not enrolling new patients.
Outcomes
The primary endpoint was hematologic response rate, as defined by European LeukemiaNet criteria 13. Complete hematologic response (CHR) was defined as normalization of blood counts (essential thrombocythemia: platelets ≤ 440 x 109/L; PV: hemoglobin < 15.0 g/L without phlebotomy) with complete resolution of palpable splenomegaly/symptoms in the absence of a thrombotic event. A partial hematologic response (PR) required at least a 50% reduction in the platelet count for essential thrombocythemia or a 50% reduction in the rate of phlebotomies or 50% reduction in spleen size by palpation for polycythemia vera. Secondary endpoints were to evaluate the toxicities in these patients as well as the bone marrow morphologic and molecular disease characteristics before and during therapy. Physical exam and blood counts were assessed every 3 months. Bone marrow aspiration and biopsy with quantitation of JAK2V617F and cytogenetics were performed at the start of therapy and every 3 to 6 months thereafter. 11,12 All patients who tested positive for the JAK2V617F mutation were evaluable for a MR if they had 2 or more adequate bone marrow samples while on therapy taken 3 months apart within the first year. Complete molecular remission (CMR; based solely on the assessment of a JAK2V617F allele burden) required undetectable JAK2V617F, while partial and minor molecular remissions (PMR, mMR) required reductions in baseline allele burden of >50% and 20–49%, respectively. All adverse events were graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events (NCI-CTAE v2.0).
Statistical Analysis
The analysis was based on an intention to treat population. A hematologic response rate of ≥ 35% was considered an indication of efficacy and justification for larger studies. An adverse event rate up to 20% was allowed. Only patients with a detectable JAK2V61F mutation at the start of therapy were evaluable for a molecular response.
Responses and clinical data were analyzed using descriptive statistics. The Kaplan-Meier method with log-rank test were used to define and compare the time to leukemia transformation from the date of diagnosis, with p-value < 0.05 as statistically significant. Fisher’s exact test was used to compare responses in different groups for categorical variable. The Mann-Whitney U or Kruskal-Wallis tests were used to compare continuous variables, as indicated. GraphPad Prism and SPSS v.23 were used for all analyses.
This study is registered with http://clinicaltrials.gov, number NCT00452023.
Role of the Funding Source
This research is supported in part by the MD Anderson Cancer Center Support Grant P30 CA016672 from the National Cancer Institute. Hoffman-LaRoche provided PEG-IFN-α-2a for five years but had no role in designing the study, data collection, analysis or interpretation of the data, or writing the final report. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Results
Patient characteristics/current status
Forty-three patients with polycythemia vera and 40 with essential thrombocythemia were enrolled in a phase 2 trial of PEG-IFN-α-2a between May 31, 2005 and October 13, 2009, and 32 (39%) patients are still on study (polycythemia vera n=14, essential thrombocythemia n=18). Table 1 shows baseline demographic and clinical characteristics, which were evenly distributed between the polycythemia vera and essential thrombocythemia groups. The median follow-up time was 83 months (IQR, 69–94 months). Twenty-six (31%) patients were older than 60 years. Sixty-three percent of patients had received some form of therapy (in addition to aspirin) prior to enrollment, including standard IFN-α (n=14) and PEG-IFN-α-2a (n=1). Eleven essential thrombocythemia patients tested positive for CALR (n=8, 10%) and MPL (n=3, 3%), and 9 (11%) were triple negative.
Table 1.
Demographic and clinical characteristics of the entire cohort.
| Characteristic | PV (n=43) | ET (n=40) | total (n=83) | p-value |
|---|---|---|---|---|
| Median age, (IQR) | 54 (44–63) | 52 (39–62) | 53.4 (43–62) | p = 0.41 |
|
| ||||
| Males, n (%) | 17 (40) | 12 (30) | 29 (35) | p = 0.49 |
| Females, n (%) | 26 (60) | 28 (70) | 54 (65) | p = 0.49 |
| High risk disease, n (%) | 14(33) | 16(40) | 30 (36) | p < 0.0001 |
| Time from diagnosis to study entry, months (IQR) | 50 (13–89) | 37 (14–115) | 42 (14–98) | p = 0.67 |
|
| ||||
| History of major thrombosis, n (%) | 2 (4.6) | 2 (5) | 4 (5) | p = 1.00 |
|
| ||||
| No. JAK2 V617F-positive patients (%) | 41 (95%) | 19 (48%) | 60 (72)* | p < 0.0001 |
|
| ||||
| JAK2 V617F allele burden, median (IQR) | 65 (34–78) | 23 (12–45) | 46 (23–76)* | p < 0.0001 |
|
| ||||
| Abnormal karyotype, n (%) | 2 (5) | 4 (10) | 6 (7) | p = 0.42 |
|
| ||||
| Median white blood cell count, 109/L (IQR) | 11.2 (8–16) | 7.2 (6–9) | 9 (6–13) | p < 0.0001 |
|
| ||||
| Median hemoglobin, g/dL (IQR) | 14.2 (13–15) | 13.1 (12–14) | 13.8 (12–15) | p = 0.06 |
|
| ||||
| Median platelet count, 109/L (IQR) | 496 (338–786) | 752 (473–1020) | 592 (389–938) | p < 0.0025 |
|
| ||||
| ¶ Significant splenomegaly, n (% of known) | 7/42 (16) | 0/39 (0) | 7/81 (8.6) | NA |
|
| ||||
| Median spleen size BCM in cm (IQR) | 11 (4–14) | NA | 11 (4–14) | NA |
|
| ||||
| Disease-related symptoms, n (%) | 19 (44) | 24 (60) | 43 (52) | p = 0.19 |
|
| ||||
| Phlebotomy, n (%) | 32 (74%) | NA | 32 | NA |
High risk disease = patients ≥ 60 or previous thrombosis;
Significant splenomegaly defined as a palpable spleen > 5 cm below costal margin (BCM); NA = not applicable
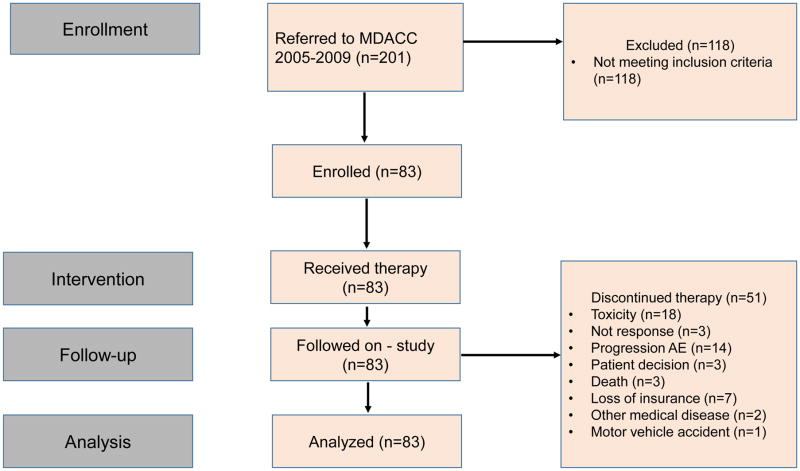
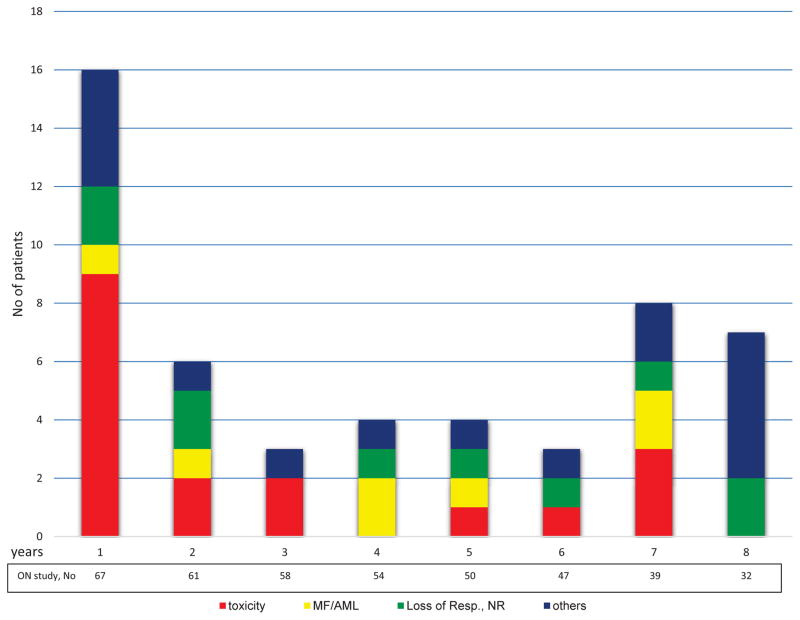
Yearly discontinuation rates varied from 5% to 19% (Figure 1), with a median discontinuation rate of 5 patients/year (range, 3–16 patients/year). The median PEG-IFN-α-2a exposure time was 87 months (IQR, 17–85 months). The median follow-up after PEG-IFN-α-2a discontinuation for patients with available follow-up information (n=44) was 31 months (IQR, 18–65), and 12 patients were followed for more than 60 months after discontinuation.
Figure 1.
Yearly discontinuation rates and reasons for treatment discontinuation. MF/AML= transformation to myelofibrosis/acute leukemia; Resp. = response, NR = no response; others [see explanation in the main text]
Hematologic response
All 83 patients were evaluable for a hematologic response, and the overall response rate was 80% (n=66) (Table 2), as previously reported12. The median duration of HR was 66 months (IQR, 35–83), and 26 (39%) were in HR at the time of last follow-up (all but 1 was a CHR). Neither achievement of a HR nor time to response was associated with age, gender, baseline clinical characteristics, splenomegaly, molecular status or JAK2V617F allele burden (Appendix p.1).
Table 2.
Summary of clinical efficacy in all enrolled patients
| Characteristics | Total, N= 83 | PV, N= 43 | ET, N= 40 | P-value | |
|---|---|---|---|---|---|
| Median total follow-up time, months (IQR) | 82.5 (69–94) | 83 (65–92) | 83 (74–95) | 0.43 | |
|
| |||||
| Median treatment duration, months (IQR) | 68.8 (15–85) | 59 (12–91) | 76 (25–83) | 0.49 | |
|
| |||||
| INITIAL RESPONSE | |||||
|
| |||||
| Hematologic Resp. (HR), n, (%) | Overall HR | 66 (80) | 34 (79) | 32 (80) | 1.00 |
|
| |||||
| CHR | 62 (75) | 33 (77) | 29 (73) | 0.35 | |
| PHR | 4 (5) | 3 (7) | 1 (3) | 0.61 | |
|
| |||||
| Median HR duration, months (IQR) | Overall | 65.7 (35–83) | 65 (43–87) | 58 (36–84) | 0.38 |
|
| |||||
| CHR | 67 (36–86) | 65 (33–82) | 58 (33–83) | 0.21 | |
| PHR | 30 (6–52) | 35 (2–58) | 25 | NA | |
|
| |||||
| Median time to response, months (IQR) | 4 (1.3–7.3) | 1.7 (1–4.7) | 4.8 (2.1–12.5) | 0.11 | |
|
| |||||
| Molecular Resp. (MR), n (%) | Overall MR | 35/55 (63) | 22 (63) | 13 (37) | 0.40 |
|
| |||||
| CMR | 10 (18) | 7 (20) | 3 (9) | 0.71 | |
| PMR | 20 (36) | 14 (40) | 6 (17) | 0.48 | |
| mMR | 5 (9) | 1 (3) | 4 (11) | 0.06 | |
|
| |||||
| Median MR duration, months (IQR) | Total | 53.4 (24–70) | 58 (38–77) | 57 (14–60) | 0.12 |
|
| |||||
| CMR | 69 (54–77) | 70 (59–77) | 54 (11–76) | 0.13 | |
| PMR | 49 (24–65) | 50 (22–68) | 47 (32–60) | 0.67 | |
| mMR | 18 (4–46.4) | 17.7 | 18 (6–51) | NA | |
|
| |||||
| Median time to MR, months (range) | 24 (12–35) | 24 (12–30) | 23 (12–42) | 0.99 | |
|
| |||||
| RESPONSE AT LAST FOLLOW-UP | |||||
|
| |||||
| Hematologic response, n (%) | Overall HR | 26/66 (39) | 13/34 | 13/32 | |
|
| |||||
| HR type, n (% of overall HR) | CHR | 25 (96) | 12 (92) | 13 (100) | |
|
| |||||
| Molecular response, n (%) | Overall MR | 25/35 (71) | 17 (68) | 8 (32) | |
|
| |||||
| MR subtype, n (% of overall MR) | CMR | 9 (36) | 6 (35) | 3 (38) | |
| PMR | 6 (24) | 5 (29) | 1 (13) | ||
Comments: The response at last follow-up is reported as seen at the time of last follow-up regardless of initial response.
Overall, 40 patients lost their response. Nineteen after dose reductions or drug holds due to intolerance or toxicity; one when he developed concurrent diffuse large B-cell lymphoma; and 20 due to progressive disease, despite being treated with the highest tolerable dose of PEG-IFN-α-2a. The median response duration among patients who lost their response was 46 months (IQR, 17–68). Among patients who were treated for at least 46 months, the median dose of PEG-IFN-α-2a was similar regardless of whether or not they lost their response (135 mcg/week vs 90 mcg/week, respectively, p=0.44). Remarkably, 7 patients (28%, 4 essential thrombocythemia, 3 polycythemia vera) have sustained their HR after discontinuation of PEG-IFN-α-2a (median time on therapy, 77 months [IWR, 56–98 months]; median response duration off study, 6 months [IQR, 4–34 months]).
Molecular response
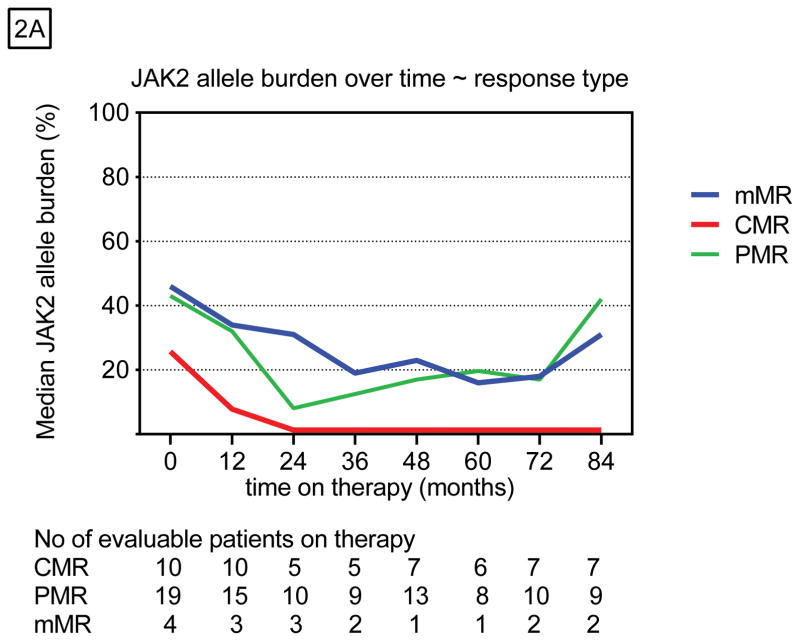
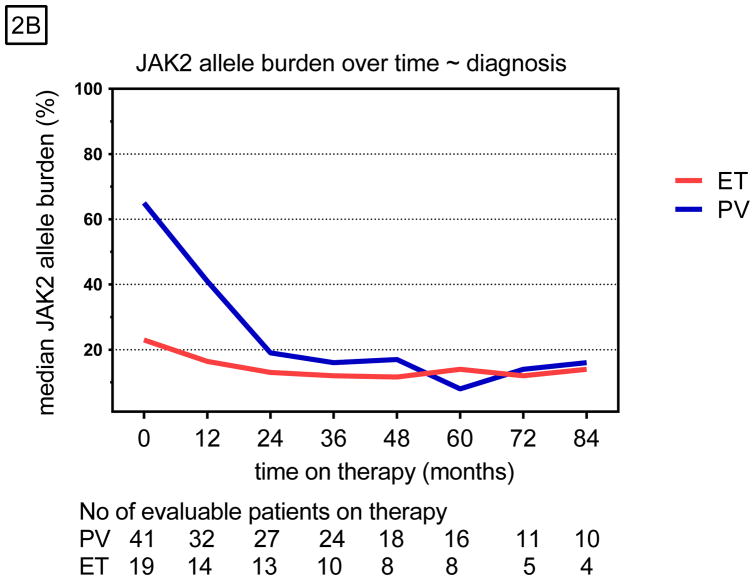
Of the 63 (76%) JAK2V617F-positive patients, 55 (87%) were evaluable for a molecular response (MR). Eight patients who discontinued therapy in the first year and did not have 2 or more representative samples were not evaluable for a MR. The JAK2V617F allele burden was reduced in 63% (n=35) of these patients (Table 2), and 10 of them had a CMR as their best response (Appendix p.1). Among all JAK2-positive patients, the median JAK2V617F allele burden was 43% at baseline and 12% at the time of best response (Figure 2A). Among those with CMR, the JAK2V617F allele burden decreased by 35% and remained at that level (Appendix p.2). Patients with PMR or mMR had maximum reductions in allele burden of 23% and 16%, which were not sustained. This reduction was statistically significant only among polycythemia vera patients (P<0.001) and those with a CMR (P<0.001), as previously reported 12 (Figure 2B).
Figure 2.
Molecular responses over time stratified by A) response type and B) diagnosis.
Molecular responses were achieved gradually over time, with a median time to response of 24 months, which did not differ by depth of response. Age was the only variable that significantly differed between patients with (median, 45 years) and without (median, 59 years) a MR (Appendix p.3). The response duration was longest among patients with a CMR (Table 2). Twenty-five patients have maintained at least a mMR, whereas 9 patients (6 polycythemia vera, 3 essential thrombocythemia) have lost their response completely. Four patients lost their response after the drug was withheld (median time from discontinuation to relapse 2 years. The other 5 lost their response while on therapy. Only one patient who achieved a CMR has relapsed after being off therapy for 16 months (CMR duration, 66 months). Among the 20 patients who achieved a PMR, 5 have sustained their best PMR, 7 are now in mMR, and 8 lost their response. Three of 5 patients have sustained their mMR.
Ninety-four percent (n=33) of molecular responders also achieved a HR. However, a direct correlation between MR and HR was not observed in all cases, confirming the suggestion that HR is not a useful objective response measure in essential thrombocythemia and polycythemia vera.14,15 For example, some patients who did not achieve a MR achieved a HR (n=11) and vice versa (2 patients with mMR never achieved a HR (Appendix p.3).
Disease–associated clinical complications
Over the course of the study, there were 8 major vascular thromboembolic (VTE) events, 3 of which were provoked by heart catheterization, elective chest surgery and angiogram. The incidence rate of major unprovoked VTE in enrolled patients during the entire study follow-up was 1.22 per 100 person-years. All 3 provoked VTEs occurred very shortly after starting therapy (median 2 months), and only 1 of these patients had achieved a HR. The other two patients continued therapy after the VTE, and one is still receiving treatment after more than 96 months without experiencing another VTE. Five patients (3 of whom were in CHR) experienced unprovoked VTEs (i.e., there was no discernable cause) after a median time on therapy of 38 months (range, 14–60 months) (Appendix p.3). Two of them were younger than 60 with no history of thrombosis. In addition, 1 patient had a serious unprovoked cerebrovascular hemorrhage while in CHR after 3 years on therapy.
Control of disease progression
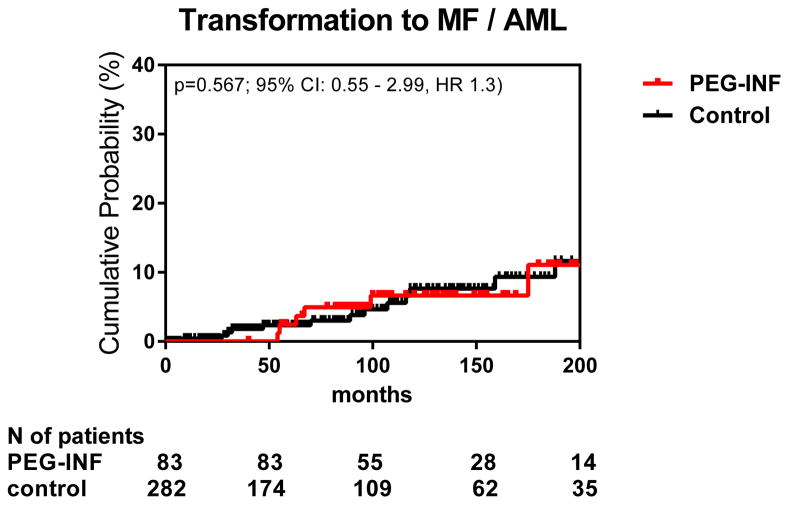
Seven (8%) patients had disease progression while on therapy: six had progression to MF and one transformed to AML (Appendix p.4). All seven patients were women (4 essential thrombocythemia/3 polycythemia vera) and the median time to transformation was 40 months (IQR, 18–61 mo). Two of the polycythemia vera patients had splenomegaly and 3 patients had platelet counts > 1000 x 109/L (2 essential thrombocythemia, 1 polycythemia vera) at the start of therapy. All transformations were documented with repeated bone marrow biopsies and met the IWG-MRT diagnostic criteria for post-essential thrombocythemia or post-polycythemia myelofibrosis or had bone marrow blasts > 20% (for acute myeloid leukemia).16 The cumulative incidence of MF progression among all enrolled patients at 5 years was 11%. However, given the small number of patients with progression to MF this number may not accurately reflect the true cumulative incidence. Cytogenetic analysis of BM biopsies at the time of progression showed no clonal evolution. Sequencing of a 48-gene panel in samples from 6 of 7 patients at baseline and time of transformation showed that two of these patients had acquired a mutation (1 DNMT3A, 1 ASXL1) at the time of transformation. The rate of transformation to MF/AML did not differ when compared with a cohort of age- and gender-matched historical patients not treated with PEG-IFN-α-2a (Figure 3). In addition, we observed no significant differences in disease duration, prior therapies, and cytogenetic/molecular features between patients with or without transformation. Five patients had been in HR at the last assessment before transformation (approximately 6 months earlier); one patient who transformed to AML (patient 7, Appendix) had been off therapy for 6 months prior to transformation for an unrelated reason (knee surgery). One patient was diagnosed with CML after transformation to MF. This patient had tested negative for the BCR-ABL transcript at least 3 times before and during the study. The BCR-ABL transcript was only detected after the patient had discontinued therapy due to progression to MF (biopsy confirmed).
Figure 3.
Cumulative probability of transformation to MF/AML (age, gender matched historical control)
Safety and Tolerability
The distribution of hematologic and non-hematologic adverse events (AE) is outlined in Table 5. Among all patients, fatigue (75%), muscle pain (52%), nausea/vomiting and diarrhea (44%), and depression (32%) were the most common AEs. Severe hematologic AEs (Grade 3/4 or recurrent events despite dose reductions) occurred in 89% (n=22/25) of patients with polycythemia vera and 83% (n=20/24) of those with essential thrombocythemia. The AE rate decreased with time on therapy, yet did not completely disappear (Figure 4). New G3/4 toxicities unrelated to dose occurred ≥24 months from start of therapy in 10–17% of patients annually. The most common late AEs were fatigue (prevalent in all years), anemia and neutropenia (highest in the 3rd & 6th year), and depression (highest in 4th–6th year). Three patients with essential thrombocythemia experienced 4 episodes of G4 neutropenia, 3 of which occurred on a dose < 90 mcg/week.
Figure 4.
Correlation of toxicities with time and dose.
Four patients (3 essential thrombocythemia, 1 polycythemia vera) developed autoimmune toxicities after a median time on therapy of 47.5 months (range, 26 – 78 months). All cases were biopsy proven and included hepatitis, central nervous system vasculitis, lupus nephritis and other presentations (Sjogren syndrome, dermatitis and vasculitis). Screening for auto-antibodies was only performed in patients with a clinical presentation suspicious for autoimmune disease (e.g., significant musculoskeletal or skin symptoms or any other atypical presentation). Thyroid function tests were done for all patients. None of the patients had a history of autoimmune disease, which was an exclusion criterion. Autoimmune thyroiditis, the most frequently reported autoimmune side effect of IFN-α 17, was observed in 15 patients (18%), but only 2 were severe (grade 3) enough to warrant treatment discontinuation.
Overall, the dose had to be adjusted over the course of treatment, either for toxicity or lack of efficacy, in all but 2 patients. Eighteen patients (22%) discontinued therapy due to drug-related toxicities, G1–2 in 8 (10%) or G3–4 in 10 (12%) (Figure 1). Discontinuation rates were not correlated with PEG-IFN-α-2a dosage (Appendix p.5). The median time on therapy for these patients was 11 months (range, 2–60), although 4 patients requested discontinuation after < 6 months. Half of these patients had more than 1 type of toxicity, with the most common being neuropsychiatric (n=5 patients), gastrointestinal (n=4), and hematologic (n=3).
An additional 12 patients had therapy held for >6 months due to toxicity, with a median time on hold of 29 months (range, 7–79). The most common toxicities leading to significant treatment interruptions were G3 neutropenia and multiple G2 toxicities, such as anemia, fatigue, musculoskeletal pain, diarrhea, neuropathy, and depression. Ten of 12 patients were rechallenged at a lower dose of PEG-IFN-a-2a, but because of persistent and recurrent toxicities (mostly G3 hematologic) they remained off therapy. The other two are being treated with a very low dose (45 mcg every 4–6 weeks), despite similar, though less severe, (only G1 non-hematologic) side effects (musculoskeletal, gastrointestinal, rash), which they have deemed tolerable. Other reasons for discontinuation included motor vehicle accident (n=1); loss to follow-up (n=3); death (n=3); other malignancy (n=2); and financial (n=7). Three patients died while on study, but none were thought to be related to the study drug. One patient died of central pontine myelinolysis due to rapid correction of G3 hyponatremia, one due to complications from severe aortic stenosis and pulmonary hypertension, and one as a consequence of a motor vehicle accident.
Long-term responders
Thirty-two (38%; essential thrombocythemia 18, polycythemia vera 14) patients are still enrolled in the study (Appendix p.6), with 24 on active treatment. Nineteen are in HR. Eighteen of 24 (75%) patients are on a dose ≤ 90 mcg per week. Eight patients are having therapy held (7 essential thrombocythemia, 1 polycythemia vera) due to toxicity (n=5) or for financial reasons (n=3). Two patients have been holding the drug for 6 and 6.5 years. While on hold, 3 of 8 patients have lost their response (2 HR, 1 MR). Despite losing their responses, all 3 patients are symptom-free and prefer to stay on the study with active observation.
Discussion
Our long-term follow-up of a phase 2 study of PEG-IFN-α-2a in 83 patients with essential thrombocythemia or polycythemia vera shows that hematologic and molecular responses are durable in some patients and provides some important additional observations. First, patients may continue to derive a clinical benefit from PEG-IFN-α-2a (i.e., remain symptom free with no organomegaly or thrombotic event) even after losing their hematologic or molecular response. Second, only CMRs are durable, and in selected cases, can be sustained after discontinuation of therapy. Third, the clinical activity of PEG-IFN-α-2a is not correlated with the JAK2 mutation status. Fourth, toxicities unrelated to dosage may develop and can be treatment limiting in some patients, even after a long exposure to the drug. Lastly, disease-related vascular complications and/or progression to MF can still occur on therapy.
Despite losing a HR, 13 patients continue to have significant clinical benefit and have elected to stay on study. We also found that neither molecular status nor achievement of a MR is a prerequisite for obtaining a HR or clinical benefit. For example, 11 (20%) JAK2-positive patients never achieved a MR, yet had HRs with a median duration of 35 months (range, 6–82). Similar findings have been reported by others18,19. In addition, 95% of JAK2-negative patients achieved a HR of similar duration to that of JAK2-positive patients, and half of them are still on study, suggesting that PEG-IFN-α-2a has similar clinical efficacy in JAK2-positive and -negative MPNs.
Nine of ten patients who achieved a CMR had durable remissions (median, 69 months), while only 7 patients with a PMR or mMR maintained their best response. These findings are in contrast to those of Kiladjian et al. 20, showing no increase in JAK2 allele burden during follow-up (median follow-up, 31.4 months) regardless of depth of response. This difference may be explained in part by our longer follow-up, but it also reinforces the hypothesis that resistance may occur during the course of therapy through the acquisition of additional mutations (e.g., TET2, DNMT3a, ASXL1, IDH1, IDH2, EZH2, TP53) in clones that are not suppressed by PEG-IFN-α-2a 11,21,22. Our finding that no clinical, demographic or treatment-related factors were associated with the achievement of a MR or its duration further strengthens this hypothesis. Additional molecular testing of patients treated for more than 24 months and after long-term follow-up is currently being performed in our patients to further investigate this question. Twenty eight percent (n=10) of patients with a MR achieved a CMR, similar to previously reported results 23, suggesting that PEG-IFN-α-2a may eradicate the JAK2 clone in selected cases, providing a “functional cure.” Moreover, 3 patients have maintained their CMR after discontinuation of therapy for 1.5, 4.5 and 6.5 years, similar to what has been reported by others 24,25. However, studies have shown endogenous erythroid colony formation activity and/or the presence of the JAK2V617F allele at very low levels in more sensitive assays (level of detectability, 0.1%) 11,25,26 in patients who achieved a CMR with PEG-IFN-α. Therefore, we cannot assume the complete disappearance of the JAK2V617F clone in our patients. On the other hand, a very important clinical observation is that patients who achieved a CMR (our level of detectability) derived the longest clinical benefit. It remains to be determined whether deeper molecular responses really translate into better clinical outcome compared with patients that only maintain hematologic control. The fact that patients who achieved a CMR did not experience disease progression may support this hypothesis.
Some reports have suggested that prolonged therapy with PEG-IFN-α could lead to a further reduction in JAK2V617F allele burden 20, a finding that was not confirmed in our study. In most of our patients, the JAK2V617F allele burden decreased within the first 24 months and subsequently increased over time; a continuous reduction was seen in only 3 patients (without a CMR) on long-term therapy.
The long-term safety data from our study shows that the type and severity of late adverse events are similar to those observed earlier in the course of therapy; however, new late adverse events do occur even after 60 months on treatment, are often unrelated to dose or response status, and are therapy-limiting. The overall discontinuation rate due to toxicity was 22%, which is similar to that reported in other studies with shorter follow-up 20.
Unexpectedly, we found that 10% of patients in our study experienced thrombotic events on therapy, which is in contrast to previous reports showing low rates of thrombosis during treatment with PEG-IFN-α-2a 20. One can only speculate as to why we have observed such a phenomenon. Similarly, treatment did not reduce the rate of transformation to MF/AML compared with our historical control cohort. Limitations of our study include its retrospective nature and the fact that only 44 of 83 patients were followed after discontinuation and some follow-ups were in the form of telephone conversations; therefore, rates of transformation to MF and thrombotic events may be underestimated. In addition, evaluation of bone marrow response was not a prespecified endpoint. However, changes in BM histology and response assessment with reticulin and trichrome staining are being evaluated as a separate study.27 In addition, because the JAK2 testing was not performed with the most highly sensitive method, patients with CMR may have minimal residual disease that was not detected with our assay.
Taken together, our finding suggest that PEG-IFN-a remains a viable treatment option, especially for younger patients who want to avoid prolonged cytotoxic therapy. Lower doses minimize side effects while retaining efficacy. Patients with a history of autoimmune diseases and those with mood disorders (e.g., depression or anxiety) should be monitored more closely for side effects. Future studies of PEG-IFN-α-2a or its combination with novel immunomodulatory drugs are needed to identify patients who would derive the most benefit. Furthermore, additional objective response criteria, such as objective measurement of spleen size and changes in bone marrow histology, and symptom/quality of life assessments before and after therapy should be used to better assess clinical benefit. While no formal consensus on the optimum dose or dosing schedule exists, from our experience and that of others28,29, a starting dose of 45 mcg weekly is the optimal dosing strategy to limit AEs and maximize response. Whether novel forms of interferon 30 would be better tolerated, allowing patients to remain on therapy longer is of interest.
Supplementary Material
Table 3.
Adverse events occurring in ≥ 10% of patients (related and unrelated)
| SAFETY | TYPE | ET, N (%) | PV, N (%) | GRADE 1–2 N (%) | GRADE 3 N (%) | GRADE 4 N (%) | GRADE 5 N (%) |
|---|---|---|---|---|---|---|---|
| PATIENTS WITH AE, N (%) | Any AE | 40 (100) | 43 (100) | 26 (33) | 53 (64) | 4 (5) | 3 (4) |
| Recurrent AE | 38 (95) | 36 (84) | 61 (84) | 13 (16) | |||
| AE SUBTYPES, N (%) | Musculoskeletal | 36 (90) | 37 (86) | 67 (92) | 6 (8) | ||
| Neurological | 26 (65) | 27 (63) | 51 (96) | 2 (4) | |||
| Psychological | 17 (43) | 21 (49) | 34 (89) | 4 (11) | |||
| GIT (except for LFT abn) | 25 (63) | 20 (47) | 43 (80) | 11 (20) | |||
| Dermatologic | 10 (25) | 8 (19) | 16 (89) | 2 (11) | |||
| Infection/fever | 13 (33) | 13 (30) | 23 (88) | 3 (12) | |||
| Respiratory | 10 (25) | 13 (30) | 21 (91) | 2 (9) | |||
| Cardiovascular | 5 (13) | 8 (19) | 10 (77) | 3 (23) | |||
| Hypothyroidism | 10 (25) | 5 (12) | 13 (87) | 2 (13) | |||
| SELECTED ABNORMAL LABS | Leukopenia/neutropenia | 17 (43) | 20 (47) | 16 (43) | 17 (46) | 4 (11) | |
| Thrombocytopenia | 15 (35) | 3 (7) a | 17 (94) | 1 (6) | |||
| Anemia | 16 (40) | 20 (47) | 35 (97) | 1 (3) | |||
| LFT elevation | 17 (43) | 10 (23) | 22 (82) | 5 (18) | |||
| DEATHS ON STUDY* | |||||||
| Central pontine myelinolysis | 1 (33) | ||||||
| Chronic cardiac disease | 1 (33) | ||||||
| Motor vehicle accident | 1 (33) |
None of the deaths were related to the study drug.
Table 4.
Summary of long-term safety
| Long-term safety | 3th year | 4th year | 5th year | >6th year | |
|---|---|---|---|---|---|
| New AE with RX> 24 mos | Total No of pts on RX | 60 | 53 | 50 | 41 |
| All grades | Patients, n (%) | 10 (17) | 6 (11) | 5 (10) | 10 (24) |
| Events, n | 13 | 14 | 10 | 18 | |
| Grade 1 – 2 | Patients, n (%) | 6 (60) | 2 (33) | 4 (80) | 9 (90) |
| Events, n (%) | 9 (69) | 10 (71) | 9 (90) | 17 (94) | |
| Grade 3 | Patients, n (%) | 4 (40) | 4 (67) | 1 (20) | 1 (10) |
| Events, n (%) | 4 (31) | 4 (29) | 1 (10) | 1 (6) | |
| Grade 4 | Patients, n (%) | 1 (17) | 2 (40) | 1 (10) | |
| Events, n (%) | 1 (7) | 2 (20) | 1 (6) | ||
| Deaths on Study | Patients, n (%) | 1 (6) |
Grade 4 toxicities occurred only in patients with ET.
Research in context.
Evidence before this study
Previous studies have shown that pegylated interferon is highly effective in patients with myeloproliferative neoplasms, particularly essential thrombocythemia and polycythemia vera. However, safety and tolerability has limited its use. All studies have shown a high rate of hematologic and molecular responses, with a continuous reduction in JAK2 allele burden, a unique finding not observed with other agents. All study reports were encouraging, showing disease control with no or minimal disease-related vascular complications and no progression to more aggressive myelofibrosis. However, reports of longer follow-up beyond 45 months, are lacking.
Added value of this study
In this long-term prospective analysis of patients with essential thrombocythemia and polycythemia vera treated with pegylated interferon alpha-2a, we report efficacy and safety data after a follow-up of 7 years, almost twice as long as in previously published studies. Our analysis confirmed the high efficacy (disease control with hematologic and molecular responses) of pegylated interferon alpha-2a, with a median response duration of up to 6 years. In contrast to earlier studies, a continuous reduction in JAK2 allele burden was not observed with longer follow-up. We could not identify any factors predictive of loss of response, which happens over time; however, the deepest responses lasted the longest. Late toxicities of a similar type and severity as those observed earlier on therapy do occur, regardless of dose, and may be treatment limiting. The discontinuation rate (~20%) was similar to that reported in studies with short term follow-up. Vascular events, and disease progression were observed while on therapy, and the rate of progression to MF or the blast phase (AML) was similar to that of a matched control group.
Implications of all the available evidence
Pegylated interferon can provide excellent disease control in patients with essential thrombocythemia and polycythemia vera, with an average response duration of 6 years. A deep response was achieved in some patients and seems to provide the longest benefit, including protection against vascular complications and disease progression. The long term toxicity rate is acceptable; however, late toxicities may occur and therefore patients should be carefully monitored.
Acknowledgments
Funding Source: This study was funded in part through the MD Anderson Cancer Center Support Grant P30 CA016672 from the National Cancer Institute
Funding
This research is supported in part by the MD Anderson Cancer Center Support Grant P30 CA016672 from the National Cancer Institute.
Footnotes
Author Contributions: SV and HK designed and coordinated the original clinical trial. LM, KP and SV analyzed the data. LM, SV, and KJN wrote the manuscript. JC, GB, MK, ZE, and HK enrolled patients and conducted the research. All authors participated in the discussion and have reviewed and approved the current manuscript.
Declaration of Interest: The authors declared no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dameshek W. Some speculations on the myeloproliferative syndromes [editorial]. Blood. 1951;6(4):372–375. Blood. 2016;127(6):663. [PubMed] [Google Scholar]
- 2.Marchioli R, Finazzi G, Landolfi R, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23(10):2224–32. doi: 10.1200/JCO.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 3.Wolanskyj AP, Schwager SM, McClure RF, Larson DR, Tefferi A. Essential thrombocythemia beyond the first decade: life expectancy, long-term complication rates, and prognostic factors. Mayo Clin Proc. 2006;81(2):159–66. doi: 10.4065/81.2.159. [DOI] [PubMed] [Google Scholar]
- 4.Hasselbalch HC, Larsen TS, Riley CH, Jensen MK, Kiladjian JJ. Interferon-alpha in the treatment of Philadelphia-negative chronic myeloproliferative neoplasms. Status and perspectives. Current drug targets. 2011;12(3):392–419. doi: 10.2174/138945011794815275. [DOI] [PubMed] [Google Scholar]
- 5.Silver RT. Recombinant interferon-alpha for treatment of polycythaemia vera. Lancet. 1988;2(8607):403. doi: 10.1016/s0140-6736(88)92881-4. [DOI] [PubMed] [Google Scholar]
- 6.Kanfer EJ, Price CM, Gordon AA, Barrett AJ. The in vitro effects of interferon-gamma, interferon-alpha, and tumour-necrosis factor-alpha on erythroid burst-forming unit growth in patients with non-leukaemic myeloproliferative disorders. European journal of haematology. 1993;50(5):250–4. doi: 10.1111/j.1600-0609.1993.tb00157.x. [DOI] [PubMed] [Google Scholar]
- 7.Gugliotta L, Bagnara GP, Catani L, et al. In vivo and in vitro inhibitory effect of alpha-interferon on megakaryocyte colony growth in essential thrombocythaemia. British journal of haematology. 1989;71(2):177–81. doi: 10.1111/j.1365-2141.1989.tb04251.x. [DOI] [PubMed] [Google Scholar]
- 8.Hino M, Futami E, Okuno S, Miki T, Nishizawa Y, Morii H. Possible selective effects of interferon alpha-2b on a malignant clone in a case of polycythemia vera. Annals of hematology. 1993;66(3):161–2. doi: 10.1007/BF01697629. [DOI] [PubMed] [Google Scholar]
- 9.Massaro P, Foa P, Pomati M, et al. Polycythemia vera treated with recombinant interferon-alpha 2a: evidence of a selective effect on the malignant clone. American journal of hematology. 1997;56(2):126–8. doi: 10.1002/(sici)1096-8652(199710)56:2<126::aid-ajh10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 10.Kiladjian JJ, Giraudier S, Cassinat B. Interferon-alpha for the therapy of myeloproliferative neoplasms: targeting the malignant clone. Leukemia. 2016;30(4):776–81. doi: 10.1038/leu.2015.326. [DOI] [PubMed] [Google Scholar]
- 11.Quintas-Cardama A, Abdel-Wahab O, Manshouri T, et al. Molecular analysis of patients with polycythemia vera or essential thrombocythemia receiving pegylated interferon alpha-2a. Blood. 2013;122(6):893–901. doi: 10.1182/blood-2012-07-442012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quintas-Cardama A, Kantarjian H, Manshouri T, et al. Pegylated interferon alfa-2a yields high rates of hematologic and molecular response in patients with advanced essential thrombocythemia and polycythemia vera. J Clin Oncol. 2009;27(32):5418–24. doi: 10.1200/JCO.2009.23.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barosi G, Birgegard G, Finazzi G, et al. Response criteria for essential thrombocythemia and polycythemia vera: result of a European LeukemiaNet consensus conference. Blood. 2009;113(20):4829–33. doi: 10.1182/blood-2008-09-176818. [DOI] [PubMed] [Google Scholar]
- 14.Alvarez-Larrán A, Pereira A, Cervantes F, et al. Assessment and prognostic value of the European LeukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia vera. Blood. 2012;119(6):1363–9. doi: 10.1182/blood-2011-10-387787. [DOI] [PubMed] [Google Scholar]
- 15.Barosi G, Mesa R, Finazzi G, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG-MRT consensus project. Blood. 2013;121(23):4778–81. doi: 10.1182/blood-2013-01-478891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barosi G, Mesa RA, Thiele J, et al. Proposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a consensus statement from the International Working Group for Myelofibrosis Research and Treatment. Leukemia. 2008;22(2):437–8. doi: 10.1038/sj.leu.2404914. [DOI] [PubMed] [Google Scholar]
- 17.Gisslinger H, Gilly B, Woloszczuk W, et al. Thyroid autoimmunity and hypothyroidism during long-term treatment with recombinant interferon-alpha. Clinical and experimental immunology. 1992;90(3):363–7. doi: 10.1111/j.1365-2249.1992.tb05852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuriakose E, Vandris K, Wang YL, et al. Decrease in JAK2 V617F allele burden is not a prerequisite to clinical response in patients with polycythemia vera. Haematologica. 2012;97(4):538–42. doi: 10.3324/haematol.2011.053348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stauffer Larsen T, Iversen KF, Hansen E, et al. Long term molecular responses in a cohort of Danish patients with essential thrombocythemia, polycythemia vera and myelofibrosis treated with recombinant interferon alpha. Leuk Res. 2013;37(9):1041–5. doi: 10.1016/j.leukres.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Kiladjian JJ, Cassinat B, Chevret S, et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood. 2008;112(8):3065–72. doi: 10.1182/blood-2008-03-143537. [DOI] [PubMed] [Google Scholar]
- 21.Kiladjian JJ, Masse A, Cassinat B, et al. Clonal analysis of erythroid progenitors suggests that pegylated interferon alpha-2a treatment targets JAK2V617F clones without affecting TET2 mutant cells. Leukemia. 2010;24(8):1519–23. doi: 10.1038/leu.2010.120. [DOI] [PubMed] [Google Scholar]
- 22.Verger E, Cassinat B. Clinical and molecular response to interferon-alpha therapy in essential thrombocythemia patients with CALR mutations. 2015;126(24):2585–91. doi: 10.1182/blood-2015-07-659060. [DOI] [PubMed] [Google Scholar]
- 23.Kiladjian JJ, Cassinat B, Turlure P, et al. High molecular response rate of polycythemia vera patients treated with pegylated interferon alpha-2a. Blood. 2006;108(6):2037–40. doi: 10.1182/blood-2006-03-009860. [DOI] [PubMed] [Google Scholar]
- 24.Larsen TS, Moller MB, de Stricker K, et al. Minimal residual disease and normalization of the bone marrow after long-term treatment with alpha-interferon2b in polycythemia vera. A report on molecular response patterns in seven patients in sustained complete hematological remission. Hematology (Amsterdam, Netherlands) 2009;14(6):331–4. doi: 10.1179/102453309X12473408860587. [DOI] [PubMed] [Google Scholar]
- 25.Utke Rank C, Weis Bjerrum O, Larsen TS, et al. Minimal residual disease after long-term interferon-alpha2 treatment: a report on hematological, molecular and histomorphological response patterns in 10 patients with essential thrombocythemia and polycythemia vera. Leukemia & lymphoma. 2015:1–7. doi: 10.3109/10428194.2015.1049171. [DOI] [PubMed] [Google Scholar]
- 26.Jovanovic JV, Ivey A, Vannucchi AM, et al. Establishing optimal quantitative-polymerase chain reaction assays for routine diagnosis and tracking of minimal residual disease in JAK2-V617F-associated myeloproliferative neoplasms: a joint European LeukemiaNet/MPN&MPNr-EuroNet (COST action BM0902) study. Leukemia. 2013;27(10):2032–9. doi: 10.1038/leu.2013.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masarova L, Yin CC, Cortes JE, et al. Histomorphological Responses after Therapy with Pegylated Interferon Alpha-2a in Patients with Essential Thrombocythemia (ET) and Polycythemia Vera (PV) Blood. 2016;128(22):4266. doi: 10.1186/s40164-017-0090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gowin K, Thapaliya P, Samuelson J, et al. Experience with pegylated interferon alpha-2a in advanced myeloproliferative neoplasms in an international cohort of 118 patients. Haematologica. 2012;97(10):1570–3. doi: 10.3324/haematol.2011.061390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ianotto JC, Boyer-Perrard F, Gyan E, et al. Efficacy and safety of pegylated-interferon alpha-2a in myelofibrosis: a study by the FIM and GEM French cooperative groups. Br J Haematol. 2013;162(6):783–91. doi: 10.1111/bjh.12459. [DOI] [PubMed] [Google Scholar]
- 30.Gisslinger H, Zagrijtschuk O, Buxhofer-Ausch V, et al. Ropeginterferon alfa-2b, a novel IFNalpha-2b, induces high response rates with low toxicity in patients with polycythemia vera. Blood. 2015;126(15):1762–9. doi: 10.1182/blood-2015-04-637280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.