Abstract
Acquired resistance to CDK4/6 small molecule inhibitors in breast cancer arises through mechanisms that are yet uncharacterized. In this study, we used a kinome-wide siRNA screen to identify kinases which when downregulated yields sensitivity to the CDK4/6 inhibitor ribociclib. In this manner, we identified PDK1 as a key modifier of ribociclib sensitivity in estrogen receptor-positive MCF-7 breast cancer cells. Pharmacological inhibition of PDK1 with GSK2334470 in combination with ribociclib or palbociclib, another CDK4/6 inhibitor, synergistically inhibited proliferation and increased apoptosis in a panel of ER+ breast cancer cell lines. Ribociclib-resistant breast cancer cells selected by chronic drug exposure displayed a relative increase levels of PDK1 and activation of the AKT pathway. Analysis of these cells revealed that CDK4/6 inhibition failed to induce cell cycle arrest or senescence. Mechanistic investigations showed that resistant cells coordinately upregulated expression of cyclins A, E and D1, activated phospho-CDK2 and phospho-S477/T479 AKT. Treatment with GSK2334470 or the CDK2 inhibitor dinaciclib was sufficient to reverse these events and restore the sensitivity of ribociclib-resistant cells to CDK4/6 inhibitors. Ribociclib in combination with GSK2334470 or the PI3Kα inhibitor alpelisib decreased xenograft tumor growth more potently than each drug alone. Taken together, our results highlight a role for the PI3K-PDK1 signaling pathway in mediating acquired resistance to CDK4/6 inhibitors.
Keywords: CDK4/6, PDK1, RNAi screen, ER+ breast cancer, cell cycle
INTRODUCTION
Small molecule inhibitors of the cyclin dependent kinases 4 and 6 (CDK4/6) demonstrated impressive activity in patients with ER-positive (ER+) HER2-negative (HER2-) breast cancer when combined with anti-estrogens (1–4). The CDK4/6 inhibitor palbociclib was approved by the FDA in 2015 for use in combination with letrozole for first line treatment of postmenopausal women with ER+ metastatic breast cancer. This was in part due to the outstanding results from the PALOMA-1 study, which demonstrated a marked improvement in progression-free survival (PFS) with the combination compared to letrozole alone (1). In the second line setting, the PALOMA-3 study demonstrated a remarkable improvement in PFS for the combination of fulvestrant/palbociclib compared to fulvestrant alone (4). Currently, two other CDK4/6 inhibitors, abemaciclib (LY2835219; Lilly) (5) and ribociclib (LEE011; Novartis) (2) are under clinical investigation in patients with ER+ breast cancer. Despite this positive clinical outcome, not all cancer patients benefit from CDK4/6 inhibition and a significant fraction of them eventually progress, underscoring the need to develop potent therapeutic combinations that circumvent drug resistance.
In seeking more effective rational drug combinations with CDK4/6 inhibitors, we utilized a high-throughput siRNA screen to identify kinases which, when inhibited, increased sensitivity to CDK4/6 inhibition. We identified upregulation of 3-phosphoinositide dependent protein kinase 1 (PDK1) as a mechanism of adaptation and eventual resistance to ribociclib. PDK1 has been implicated in important cellular processes including survival, metabolism and tumorigenesis. PDK1 is highly expressed in many human cancer cell lines (6) and breast tumors (7), suggesting a role for PDK1 in cancer progression. In a cohort of patients with ER+ breast cancer treated with endocrine therapy, high PDK1 expression was shown to predict for poor survival (8, 9). PDK1 functions downstream of PI3K and is required for the full activation of AKT (10) and other AGC kinases including serum glucocorticoid-dependent kinase (SGK), p90 ribosomal protein S6 kinase (RSK), p70 ribosomal protein S6 kinase (S6K), protein kinase C (PKC) and polo-like kinase 1 (PLK1) (11–13). At the plasma membrane, PDK1 binds via its pleckstrin homology (PH) domain to phosphatidylinositol 3,4,5 trisphosphate (PIP3), the product of PI3K, where it phosphorylates and activates AKT at T308 (10, 11, 14). For substrates of PDK1 lacking a PH domain, such as S6K, RSK and SGK, the interaction and subsequent activation depends on the hydrophobic motif of the target kinase binding to the PDK1-interacting fragment (PIF) rather than with PIP3 (15). Several PDK1-specific small molecules are in clinical development for the treatment of advanced cancers (16–18). Based on these data, we hypothesized that targeting the PI3K/PDK1 pathway in combination with CDK4/6 inhibitors will prolong the response to CDK4/6 inhibition and provide a novel treatment option for patients with ER+ metastatic breast cancer.
Herein, we demonstrate that genetic and pharmacological inhibition of PI3K/PDK1 in combination with CDK4/6 inhibition synergistically blocked cell proliferation and increased apoptosis of ER+ breast cancer cells in vitro and in vivo. In ribociclib-resistant cell lines, we observed that the PI3K/PDK1 pathway mediates cell survival and proliferation through upregulation of AKT and non-AKT targets of PDK1, all of which culminates in aberrant cell cycle progression in the presence of CDK4/6 inhibition. Inhibition of PDK1 with the small molecule GSK2334470 re-sensitized ribociclib-resistant cells to CDK4/6 inhibitors. These results provide a rationale for co-targeting of the PI3K/PDK1 and CDK4/6 pathways in patients with ER+ metastatic breast cancer.
MATERIALS AND METHODS
Cell lines and reagents
Parental lines were obtained from American Type Cell Culture (ATCC) within the past 10 years (2006 – 2014) and maintained in 10% FBS (Gibco). Cell lines were authenticated by ATCC prior to purchase by the STR method. Cell lines were not authenticated after purchase. Mycoplasma testing was conducted for each cell line before use. All experiments were performed less than 2 months after thawing early passage cells. All experiments were performed in IMEM/10% FBS/0.002% Phenol red under estrogen-containing conditions unless otherwise noted. To generate ribociclib resistant ER+ cell lines, MCF-7, T47D, HCC1428, and HCC1500 cells were cultured in the presence of progressively increasing concentrations of ribociclib starting at 10 nM. Cells were deemed resistant when they grew at the same rate as parental cells in 1000 nM of ribociclib. For the experiments outlined herein, resistant cells were removed from drug for at least 24–48 h prior to re-treatment. Ribociclib and alpelisib were obtained through a Materials Transfer Agreement (MTA) with Novartis. Fulvestrant was obtained from the Vanderbilt Chemotherapeutic Pharmacy. GSK2334470 and palbociclib were obtained from Selleckchem (Houston, TX). Abemaciclib (LY2835219) was obtained from MedChemExpress (Monmouth, NJ). Primary antibodies for immunoblot analyses were from Cell Signaling Technology (Danvers, MA) except for cyclin D1 (Santa Cruz Biotechnology, M-20; sc-718). The S477/T479 P-AKT antibody was a kind gift from Dr. Wenyi Wei (Harvard Medical School) (19).
Immunohistochemistry
Primary antibodies for IHC were from Cell Signaling Technology (Danvers, MA). See supplementary materials for assay details.
RNAi screen
MCF-7 cells were screened using the Dharmacon Human siGENOME Protein Kinase siRNA Library (GU-003505) available through the Vanderbilt High Throughput Screening (HTS) Facility as described in Supplementary Methods. Secondary validation was performed with two independent siRNAs against PDK1. siPDK1.1: GUGAGGAAAUGGAAGGAUAUU; siPDK1.2: CAAGAGACCUCGUGGAGAAUU.
Cell proliferation assays
Cells were seeded in IMEM/10% FBS for proliferation in 2D growth assays and counted or fixed/stained with crystal violet as described previously (20). Media and inhibitors were replenished every 2–3 days; after 10–21 days, adherent cells were trypsinized and counted using a Coulter Counter or fixed/stained with crystal violet followed by image analysis of the plates using an Odyssey Infrared Imaging System (LI-COR Biosciences). For siRNA experiments, cells were transfected with siRNAs targeting PDK1.1, PDK1.2, CDK4 (Santa Cruz Biotechnology) or a non-silencing control (Santa Cruz Biotechnology) using Lipofectamine RNAiMax Transfection Reagent (Invitrogen) according to the manufacturer’s protocol. The next day, cells were reseeded for proliferation assays and/or immunoblot analyses as described previously (20). 3D growth assays were conducted in growth factor-reduced Matrigel (BD Biosciences) as described previously (21). Phase-contrast pictures were taken using an Olympus CK40 microscope and colonies were counted using the GelCount scanning software.
β-galactosidase staining
Cells (2×105) were plated in triplicate in 6-well plates and treated with DMSO, 1 μM ribociclib, 1 μM GSK2334470 or the combination for 72 h. Cells were stained with β-galactosidase at pH 6.0 following the manufacturer’s protocol (Cell Signaling Technology #9860). Cells were photographed and β-galactosidase positive cells were counted using a light field microscope.
Flow cytometry
Cells (1×106) were plated in serum-free media and treated 24 h later with inhibitors. For cell cycle analyses, cells were treated for 24 h, then washed with PBS and fixed in 99% methanol for 3 h at −20°C. Cells were incubated with 0.1 mg/ml RNase A (Qiagen) and 40 μg/mL propidium iodide (PI; Sigma-Aldrich) for 10 min at room temperature. For apoptosis assays, cells were treated for 72 h and then washed and stained using the Alexa Fluor® 488 Annexin V/Dead Cell Apoptosis Kit according to the manufacture’s protocol (Thermo Scientific). Fluorescence-activated cell sorting (FACS) analysis was performed on the LSRFortessa X-20 Cell Analyzer (BD Biosciences) and the data were analyzed with FlowJo software.
Immunoblot analysis
Cells were lysed with RIPA buffer (150 mM NaCl, 1.0% IGEPAL®, 0.5% sodium deoxycholate, 0.1% SDS, and 50 mM Tris, pH 8.0. [Sigma], and 1x protease inhibitor cocktail [Roche]). Lysates (20 μg) were resolved by SDS-PAGE and transferred to nitrocellulose membranes; these were first incubated with primary antibodies at 4°C overnight, followed by incubation with HRP-conjugated anti-rabbit or anti-mouse secondary antibodies (Santa Cruz Biotechnology) for 1 h at room temperature. Immunoreactive bands were visualized by enhanced chemiluminescence (Thermo Scientific).
Xenograft studies
Mouse experiments were approved by the Vanderbilt Institutional Animal Care and Use Committee. Female ovariectomized athymic mice (Harlan Sprague Dawley) were implanted with a 14-day-release 17β-estradiol pellet (0.17 mg; Innovative Research of America, Sarasota, FL). The following day, 1×107 MCF-7 cells suspended in IMEM and Matrigel (BD Biosciences, San Jose, CA) at 1:1 ratio were injected subcutaneously (s.c.) into the right flank of each mouse. Approximately 4 weeks later, mice bearing tumors measuring ≥150 mm3 were randomized to treatment with 1) vehicle (control), 2) ribociclib (75 mg/kg/day via orogastric gavage), 3) GSK2334470 (100 mg/kg 3 times per week via intraperitoneal injection), or 4) both drugs. In a second animal experiment, mice harboring MCF-7 xenografts as above were treated with 1) vehicle, 2) fulvestrant (5 mg per week; s.c.), 3) fulvestrant and alpelisib (BYL719; Novartis; 35 mg/kg/day via orogastric gavage), 4) fulvestrant and ribociclib (as above), or 5) fulvestrant, alpelisib, and ribociclib for 6 weeks. Animal weights and tumor diameters (with calipers) were measured twice weekly and tumor volume was calculated with the formula: volume = width2 x length/2. After 6 weeks, tumors were harvested and snap-frozen in liquid nitrogen or fixed in 10% neutral buffered formalin followed by embedding in paraffin for immunohistochemistry. Tumors were harvested 4 h after the last dose of ribociclib or alpelisib or 24 h after the last dose of GSK2334470 or fulvestrant. Frozen tumors were homogenized using the TissueLyser II (Qiagen).
Statistical Analyses
Unless otherwise indicated, significant differences (p<0.05) were determined by ANOVA using GraphPad Prism software.
RESULTS
PDK1 siRNA oligonucleotides sensitize ER+ breast cancer cells to CDK4/6 inhibitors
We used an arrayed siRNA library targeting 714 kinases and related proteins to identify targetable molecules whose downregulation in combination with a CDK4/6 inhibitor would induce synthetic lethality in ER+ MCF-7 breast cancer cells (Fig. 1A). Following siRNA transfection, in half of the plates (n=3), cells were treated with an IC20 concentration of ribociclib (0.25 μM) and the other half with vehicle (DMSO). We chose to administer an IC20 concentration to cells so that the synergy between drug and siRNA-mediated gene knockdown would be more apparent. Cell viability was measured in triplicate six days later using a high-throughput cell viability assay. Experiments were performed three times to allow for assessment of variation of viability data in statistical analysis. Data amongst the biological replicates were highly reproducible and ultimately combined for the final analysis and hit selection (Supplementary Fig. S1A).
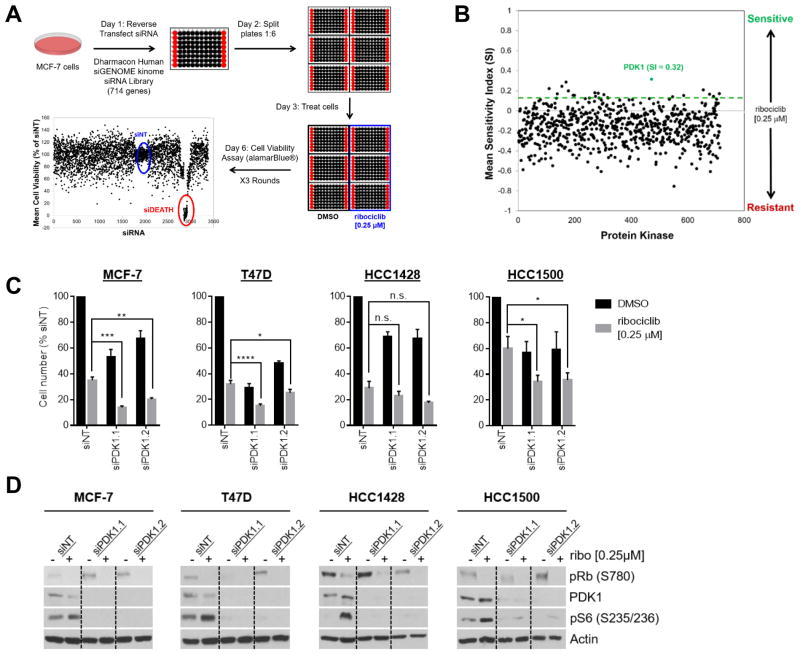
Figure 1. Kinase screen identifies PDK1 siRNA as sensitizer to CDK4/6 inhibitor.
(A) Overview of high-throughput screening (HTS) method. MCF-7 cells were reverse transfected with siRNA in 96-well plates. Each plate contained 80 individual siRNAs, indicated in black, and supplemented with controls, indicated in red [no siRNA, non-targeting control siRNA (siNT), and siDEATH positive control]. Transfected cells were divided into 6 replicate plates. Half of the plates (n=3) were treated with DMSO (vehicle control) and half (n=3) with 0.25 μM of ribociclib. Cell viability was assessed after 72 h of drug exposure using the AlamarBlue® reagent (Invitrogen). The experiment was repeated 3 times. (B) Scatter plot of the mean sensitivity index (SI) scores for 714 protein kinases and kinase-related proteins averaged across the three screening trials. A cutoff SI >0.15 (indicated by dotted line) was used for hit selection. The position of PDK1 (SI score 0.32) is noted. (C) ER+ MCF-7, T47D, HCC1428 and HCC1500 breast cancer cell lines were transfected with one of two siRNAs targeting PDK1 (siPDK1.1 and siPDK1.2), and a non-targeting control siRNA (siNT) and treated with DMSO (vehicle control) or 0.25 μM ribociclib for 72 h. Knockdown of PDK1 decreased cell proliferation and this effect was enhanced upon simultaneous treatment with ribociclib. (D) Immunoblot analyses of the cells following PDK1 knockdown and treatment for 72 h with DMSO or 0.25 μM ribociclib. Experiments were performed in full serum condition in the presence of endogenous estrogen (in IMEM/10% FBS/0.002% Phenol red).
To account for plate-to-plate variability, median centered global normalization was performed across all siRNAs against the non-targeting control siRNAs (siNT) in each plate. The sensitivity index (SI) score, which measures the influence of siRNA-induced gene knockdown on drug sensitivity, was calculated for each siRNA in each of the experiments after treatment with ribociclib (Supplementary Fig. 2S and Fig. 1B), as previously described (22–24). The SI scores range from −1 to +1, with positive values indicating sensitizing effects. The Z-score was calculated from the SI values. Genes with an SI ≥0.15 and a Z-score of >3 were considered significantly altered. In brief, individual knockdown of 15/714 (2.1%) kinases sensitized MCF-7 cells to ribociclib (Fig. 1B and Table 1). PDK1 was identified as the top siRNA sensitizing MCF-7 cells to ribociclib (SI=0.32). Notably, the most sensitizing siRNAs were those that alone had minimal effect on cell viability. Addition of ribociclib significantly decreased cell viability compared to the non-targeting control, consistent with a synergistic interaction. Some siRNAs, like PIK3CA for example, inhibited cell viability per se, such that the addition of ribociclib could not reduce viability any further, as indicated by an SI score of 0.08.
Table 1. Top siRNAs that most significantly sensitized MCF-7 cells to ribociclib as compared to non-sensitizing PIK3CA siRNA.
Data represent the mean of three different experiments performed in triplicate. Genes with an SI ≥0.15 and a Z-score >3 were considered significant. The concentration of ribociclib [0.25 μM] used in the screen corresponded to an inhibitory concentration of 20% (IC20).
| Rank | Gene | SI value1 | Z-score2 | Rc/Cc3 | Rd/Cc4 |
|---|---|---|---|---|---|
| 1 | PDK1 | 0.32 | 4.60 | 0.93 ± 0.12 | 0.68 ± 0.15 |
| 2 | DLG1 | 0.29 | 4.36 | 0.98 ± 0.10 | 0.73 ± 0.12 |
| 3 | DCAMKL1 | 0.25 | 4.08 | 0.93 ± 0.19 | 0.69 ± 0.21 |
| 4 | CDKN1B | 0.22 | 4.56 | 0.90 ± 0.26 | 0.65 ± 0.18 |
| 6 | DYRK1A | 0.20 | 3.79 | 1.0 ± 0.10 | 0.73 ± 0.09 |
| 7 | HUNK | 0.18 | 3.50 | 0.80 ± 0.22 | 0.59 ± 0.20 |
| 8 | ILK | 0.18 | 3.02 | 0.89 ± 0.17 | 0.68 ± 0.17 |
| 9 | YES1 | 0.17 | 3.61 | 0.84 ± 0.20 | 0.64 ± 0.15 |
| 10 | CRKL | 0.17 | 3.94 | 0.84 ± 0.23 | 0.59 ± 0.20 |
| 11 | TLK1 | 0.17 | 3.10 | 1.0 ± 0.17 | 0.79 ± 0.18 |
| 12 | KCNH8 | 0.16 | 4.40 | 0.82 ± 0.20 | 0.59 ± 0.09 |
| 13 | EPHA4 | 0.16 | 3.06 | 0.95 ± 0.18 | 0.74 ± 0.20 |
| 14 | EPHB1 | 0.16 | 3.15 | 0.92 ± 0.16 | 0.68 ± 0.18 |
| 15 | RELA | 0.15 | 3.64 | 0.84 ± 0.32 | 0.64 ± 0.27 |
| 16 | ICK | 0.15 | 3.64 | 0.91 ± 0.18 | 0.67 ± 0.17 |
| 37 | PIK3CA | 0.09 | 3.57 | 0.51 ± 0.12 | 0.38 ± 0.10 |
SI value = Expected combined effect - Observed combined effect
Z-score was calculated as follows:
Rc/Cc = the viability effect of siRNA without drug compared to siNT control
Rd/Cc = the viability effect of siRNA with drug compared to siNT control
To validate the results of the screen, we knocked down PDK1 using two independent siRNAs, each in combination with 0.25 μM ribociclib, in MCF-7, T47D, HCC1428, and HCC1500 ER+ breast cancer cells. Individually, ribociclib treatment and PDK1 siRNA transfection inhibited proliferation of all four cell lines (Fig. 1C). However, combined inhibition of CDK4/6 (with ribociclib) and of PDK1 (with siRNA) led to a statistically significant reduction in cell proliferation in MCF-7, T47D, and HCC1500 cell lines, consistent with the results of the kinome screen. This effect was greater in PIK3CA-mutant cell lines (MCF-7 and T47D) than PIK3CA wild–type cell lines (HCC1428 and HCC1500). Knockdown of PDK1 resulted in decreased phosphorylation of S6, a downstream effector of the PDK1 target p70S6K (Fig. 1D and Supplementary Fig. S1B). To subscribe CDK4-specificity to the effects of ribociclib, we treated MCF-7 cells with CDK4 and PDK1 siRNA oligonucleotides, individually and in combination. Treatment with both siRNAs inhibited cell viability more potently than each alone while simultaneously reducing levels of PDK1, CDK4, and P-Rb (Supplementary Fig. 1C), suggesting the effects of ribociclib may extend to other CDK 4/6 inhibitors.
Pharmacological blockade of PDK1 and CDK4/6 synergistically inhibits ER+ breast cancer cell proliferation
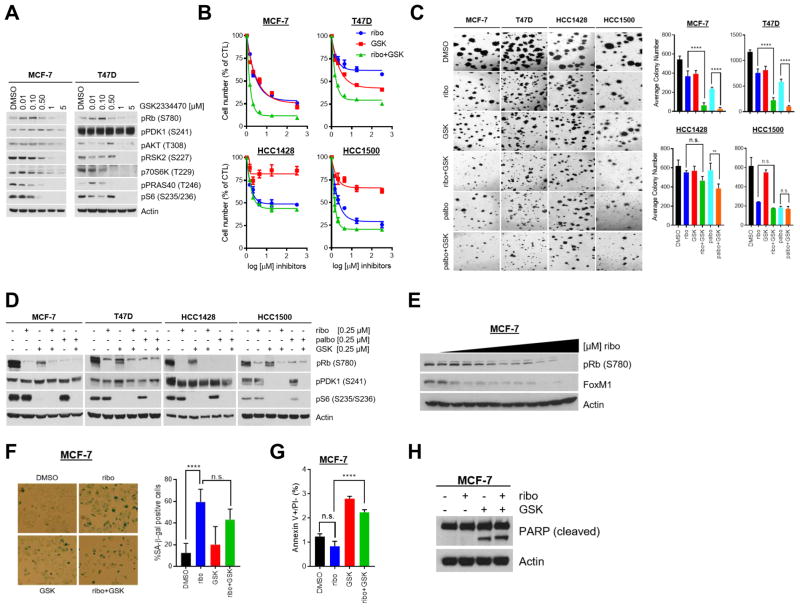
We next examined the effect of pharmacological inhibition of PDK1 in combination with CDK4/6 inhibitors. GSK2334470 is a highly specific small molecule inhibitor of PDK1 with a published inhibitory activity in the nanomolar range (16, 25). GSK2334470 suppresses T-loop phosphorylation and subsequent activation of the PDK1 substrates AKT, S6K, RSK2, and SGK in vitro. Treatment of MCF-7 and T47D cells with GSK2334470 resulted in a dose-dependent decrease in phosphorylation of known PDK1 substrates and downstream signal transducers such as P-S6 and P-PRAS40 (Fig. 2A). Growth of MCF-7, T47D, and HCC1500 was inhibited by ribociclib and GSK2334470 alone in a dose dependent fashion; however, treatment with the combination resulted in greater growth inhibition (Fig. 2B). By the Chou-Talalay (26, 27) method, the effect of drug combination was synergistic in the cell lines we examined (Supplementary Fig. 3A). It has been proposed that results in two-dimensional (2D) cell culture may not accurately reflect the in situ architecture and growth rates of cancers in vivo (28–30). Thus, we next extended our findings to cells growing in Matrigel in three-dimensional (3D) culture. Under these conditions, GSK2334470 enhanced the anti-proliferative effect of both ribociclib and palbociclib against MCF-7, T47D, and HCC1500 cells (Fig. 2C). Of note, the combined effect of CDK4/6 and PDK1 inhibitors in PIK3CA wild-type HCC1428 and HCC1500 cells was less pronounced than in PIK3CA-mutant MCF-7 and T47D cells despite similar reduction of P-Rb and P-S6 (Fig. 2D).
Figure 2. Combined PDK1 and CDK4/6 inhibition reduces ER+ breast cancer cell proliferation.
(A) ER+ MCF-7 and T47D cells were treated for 24 h with DMSO or increasing concentrations of GSK2334470. Lysates were prepared for immunoblot analysis with the indicated antibodies. GSK2334470 treatment diminished the expression of downstream targets of PDK1. (B) Cells were treated with DMSO or increasing concentrations of ribociclib, GSK2334470, or the combination for 7–10 days, after which nuclei were stained with DAPI and counted on the Molecular Devices ImageXpress instrument. Data are presented as the percent of cells remaining compared to the control (CTL, DMSO-treated). In all cell lines, combined inhibition of PDK1 and CDK4/6 was most effective than each drug alone. (C) Cells seeded in Matrigel were treated with DMSO or 0.25 μM ribociclib ± 0.25 μM GSK2334470. After 10–21 days, colonies were stained with the MTT reagent, photographed (left) and counted (right) using the GelCount® reader. In MCF-7 and T47D cells, specifically, combined inhibition of PDK1 and CDK4/6 was more effective than single-agent inhibition. (D) Cells were treated with DMSO, 0.25 μM ribociclib ± 0.25 μM GSK2334470, or 0.25 μM palbociclib ± 0.25 μM GSK2334470 for 24 h, after which lysates were prepared for immunoblot analysis with the indicated antibodies. Only combined inhibition of PDK1 and CDK4/6 led to concomitant decreases in P-Rb and P-S6. (E) MCF-7 cells were treated for 72 h with DMSO or increasing concentrations of ribociclib. Immunoblot analysis of the lysates showed that ribociclib decreased P-Rb and FoxM1 levels. (F) MCF-7 cells were treated with DMSO or 1 μM ribociclib ± 1 μM GSK2334470 for 72 h and analyzed for senescence by β-galactosidase staining. Ribociclib alone or in combination with GSK2334470 induced senescence compared to DMSO-treated cells. Data represent the average percent of senescence-associated (SA)-β-galactosidase positive cells per 5 high-power fields. (G) MCF-7 cells were treated with DMSO or 1 μM ribociclib ± 1 μM GSK2334470 for 72 h, stained with annexin V and propidium iodide, and analyzed by FACS. GSK2334470 alone or in combination with ribociclib increased the percent of apoptotic cells compared to DMSO-treated cells. (H) MCF-7 cells were treated with DMSO or 1 μM ribociclib ± 1 μM GSK2334470 for 72 h. Immunoblot analysis of these lysates revealed PARP cleavage only when cells were treated with GSK2334470 alone or in combination with ribociclib. Unless noted, media and drugs were replenished every 2–3 days (**, P < 0.01; ****, P < 0.0001 by ANOVA).
Combination therapies with CDK4/6 inhibitors are also being evaluated in other advanced solid tumors (REF 31). To test whether these findings in ER+ breast cancer cells can be translated to other tumor types, we treated triple negative breast cancer, ovarian/endometrial, melanoma, and glioblastoma cell lines with ribociclib, GSK23334470, or the combination. Results showed that the combination induced greater inhibition of cell viability compared to each drug alone (Supplementary Fig. S3B,C). These observations suggest that PDK1 plays a role in mediating resistance to CDK4/6 inhibition in a variety of tumor types where CDK4/6 inhibitors are being investigated clinically (31).
In addition to cell cycle arrest, CDK4/6 inhibitors can induce senescence through regulation of FoxM1-mediated transcription (32). Consistent with this, we observed a decrease in FoxM1 levels and an increase in senescence-associated (SA) β-galactosidase positive cells upon treatment with ribociclib, which was unaffected by the PDK1 inhibitor (Fig. 2E,F). Treatment with GSK2334470 alone or in combination with ribociclib induced apoptosis as measured by increased annexin V staining (Fig. 2G) and poly (ADP) ribose polymerase (PARP) cleavage (Fig. 2H), compared to DMSO or ribociclib treated MCF-7 cells. These findings suggest that inhibition of PDK1 with GSK2334470 induces apoptosis without counteracting the effect of ribociclib on tumor cell senescence resulting in the synergistic growth inhibition of ER+ breast cancer cells.
Inhibition of PI3K/PDK1 enhances the anti-tumor effect of ribociclib in vivo
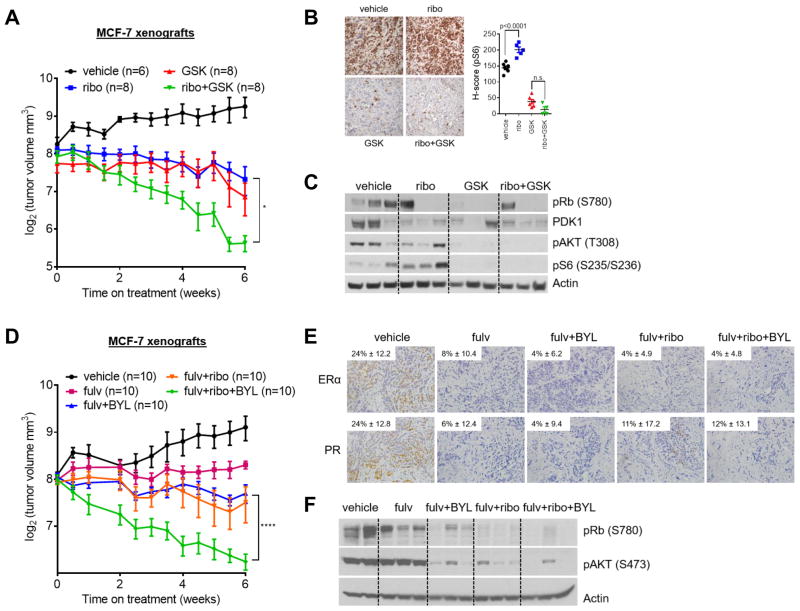
To validate the efficacy of combined inhibition of CDK4/6 and PDK1 in vivo, we established MCF-7 xenografts in athymic ovariectomized female nude mice supplemented with a 14-day release, 17β-estradiol pellet to support initial tumor establishment. Once tumors reached a volume of ≥150 mm3 and the estrogen pellet had expired, a state mimicking estrogen deprivation in patients treated with anti-estrogens, mice were randomized to treatment with vehicle, ribociclib, GSK2334470, or the combination of both drugs. After six weeks of treatment, the combination induced a statistically superior anti-tumor effect compared to each drug alone (Fig. 3A). The combination of ribociclib and GSK2334470 induced clear objective tumor regressions as early as two weeks in all mice with three xenografts treated with the combination achieving a complete response. After 6 months of follow up on no therapy, none exhibited a tumor recurrence (Supplementary Fig. S4A). During treatment, mice in all four groups displayed stable body weight (Supplementary Fig. S4B), and no signs of toxicity.
Figure 3. Pharmacological inhibition of PI3K/PDK1 enhances the effect of ribociclib in vivo.
(A) MCF-7 cells were injected s.c. into athymic ovariectomized female mice, each supplemented with a short-term, 14-day release 17β-estradiol pellet. Mice bearing tumors ≥150 mm3 were randomized to vehicle, ribociclib, GSK2334470 or the combination of ribociclib and GSK2334470 for 6 weeks. Data are presented as log2 of mean tumor volume in mm3 (*, P < 0.05 vs. single-agent ribociclib or GSK2334470). Numbers in parenthesis represent the number of mice per treatment arm. (B) Representative images of tumor sections from A and quantitative comparison of P-S6 histoscores (H-score). GSK2334470 ± ribociclib inhibited P-S6; single agent ribociclib increased P-S6 levels. (C) Xenografts from A were homogenized after the last dose of drug treatment and tumor lysates were subjected to immunoblot analysis for the indicated antibodies. (D) MCF-7 cells were injected into mice as in A. Mice bearing tumors ≥150 mm3 were randomized to vehicle, fulvestrant, BYL719 and fulvestrant, ribociclib and fulvestrant, or fulvestrant, BYL719, and ribociclib for 6 weeks. The triple combination was most effective at decreasing tumor volume compared to single-agent therapy or double-combinations. Data are presented as log2 of mean tumor volume in mm3 (****, P < 0.0001 vs. fulvestrant, fulvestrant and BYL719, or fulvestrant and ribociclib). (E) Representative images of tumor sections from D and quantitative comparison of ER and PR histoscores (H-score) confirming target inhibition with fulvestrant. (F) Xenografts from D were homogenized after the last dose of drug treatment and tumor lysates were subjected to immunoblot analysis for the indicated antibodies.
We next assessed pharmacodynamic biomarkers of drug action by immunohistochemistry (IHC) of tumor sections and immunoblot analysis of tumor lysates. Treatment with ribociclib modestly increased S235/236 P-S6 after six weeks of treatment whereas GSK2334470 alone or in combination with ribociclib reversed this effect (Fig. 3B,C). Both agents decreased Rb phosphorylation at the S780 CDK4 site (33), while treatment with GSK2334470 alone or in combination with ribociclib decreased T308 P-AKT (Fig. 3C). These data suggest that activation of S6 compensates for inhibition of CDK4/6 and that the combined blockade of PDK1 and CDK4/6 limits such activation.
PI3K activates PDK1, which then activates AKT and mTOR to increase tumor cell proliferation and survival (11). While PDK1 inhibitors remain in preclinical development (16–18), pharmacological blockade of the PI3K/PDK1 signaling pathway can be achieved with PI3K inhibitors currently in registration clinical trials (34). Further, since CDK4 inhibitors are approved for use in combination with anti-estrogens in ER+ breast cancer (1, 4), we extended our findings to such combination ± the PI3Kα specific inhibitor alpelisib (BYL719) (35) in ovariectomized nude mice bearing ER+ MCF-7 xenografts (Fig. 3D). Similar to the results with GSK2334470, addition of alpelisib markedly increased the anti-tumor effect of fulvestrant/ribociclib against established MCF-7 xenografts and inhibited ER, PR, and S473 P-AKT (Fig. 3D–F).
CDK4/6 inhibition results in upregulation of phosphorylated PDK1
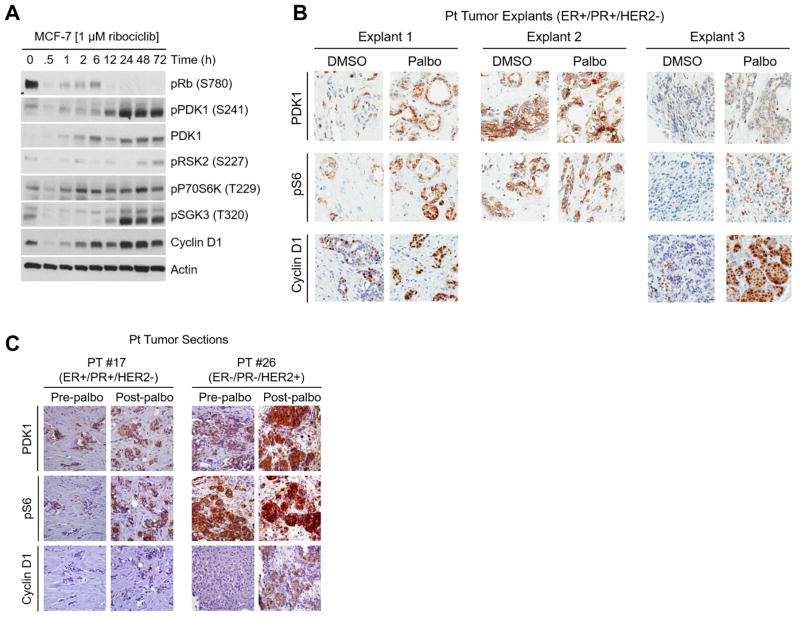
PDK1 siRNA was the top hit sensitizing MCF-7 cells to ribociclib in the siRNA kinome screen (Fig. 1B and Table1). Further, pharmacological inhibition of PDK1 markedly enhanced the antitumor effect of ribociclib in vivo while blocking S6 activation observed in tumors treated with ribociclib alone (Fig. 3A–C), suggesting that PDK1 function counteracts the response to CDK4/6 inhibitors. To test this possibility, we examined the expression of PDK1 and its known targets after short- and long-term treatment with CDK4/6 inhibitors. In MCF-7 cells treated with ribociclib, we observed an induction in total PDK1 protein levels followed by subsequent increase in S241 P-PDK1 and concomitant increase in the PDK1 targets: S227 P-RSK2, T227 P-70S6K, T320 P-SGK3, and cyclin D1 (Fig. 4A). The levels of PDK1 mRNA was variable with ribociclib treatment; however, there was a statistically significant increased seen after 72 h of treatment (Supplementary Fig. S5A), suggesting the induction of PDK1 requires, in part, new mRNA synthesis. In contrast, treatment with palbociclib and abemaciclib showed an induction of S241 P-PDK1 as early as 1 h after drug exposure (Supplementary Fig. S5B) without an increased in total PDK1 protein. We also examined PDK1 mRNA and protein levels in HCC1428, HEC1B, and LN229 to determine the effects of CDK4/6 inhibition in different cancer types. HCC1428 did not show an induction of PDK1 mRNA or protein, whereas, HEC1B, a human endometrial adenocarcinoma cell line, showed an increased in PDK1 mRNA but not PDK1 protein. In contrast, the glioma cell line LN229 showed an increased in PDK1 mRNA with concomitant increase in P-PDK1 levels but not total PDK1 (Supplementary Fig. S5C). These results suggest there is heterogeneity in the response to CDK4/6 inhibition across breast cancer cell lines and different cancer types.
Figure 4. CDK4/6 inhibition increases PDK1 expression in ER+ breast cancer cells and in primary tumor explants.
(A) MCF-7 cells were treated with ribociclib over a time course up to 72 h. Cell lysates were prepared and subjected to immunoblot analyses with the indicated antibodies as described in the Methods. (B) Patient tumor explants were treated with DMSO or palbociclib for 48 h. Representative IHC for PDK1, S235/236 P-S6 and cyclin D1 is shown. Tumor explant 2 exhibited high basal levels of PDK1 and P-S6, while explants 1 & 3 exhibited drug-induced increases in PDK1, P-S6, and cyclin D1 levels. (C) PDK1, P-S6, and cyclin D1 IHC analysis of serial primary tumor sections from two patients before treatment and on the 7th day of treatment with palbociclib.
To extend these findings to primary human cancers, we examined tumor explants obtained from patients with newly diagnosed ER+ breast cancer undergoing surgical resection. Tumor explants were treated ex vivo with palbociclib for 48 h. Consistent with the data with cell lines, treatment with palbociclib resulted in an increase in PDK1, S235/236 P-S6, and cyclin D1 levels in 2/3 primary tumor explants (Fig. 4B). Similarly, serial tumor samples from two patients with metastatic breast cancer treated with palbociclib for 7 days as part of a clinical trial (NCT01522989) showed an increase in PDK1, S235/236 P-S6, and cyclin D1 levels compared to the baseline (pre-treatment) biopsy (Fig. 4C).
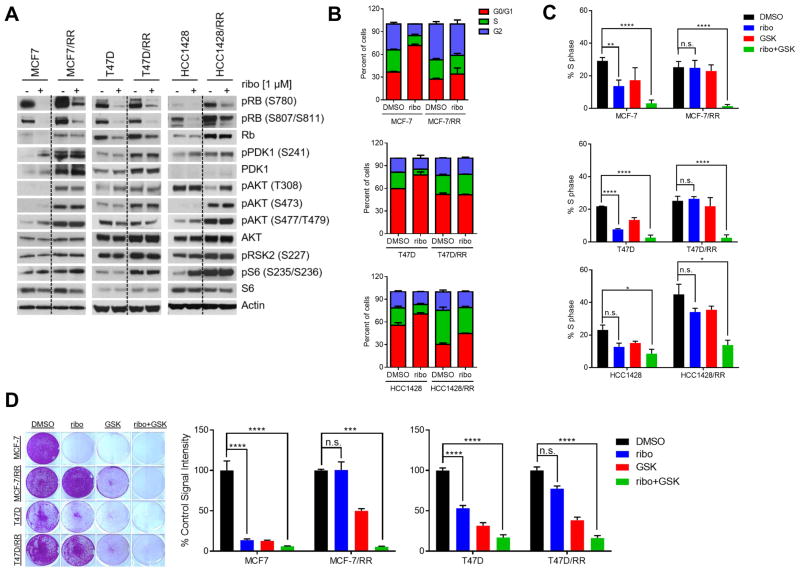
We also treated MCF-7, T47D, HCC1428, and HCC1500 cells long-term with progressively increasing concentrations (up to 1 μM) of ribociclib to develop cells that may recapitulate the acquired resistance observed in patients treated with CDK4/6 inhibitors. The ribociclib-resistant cells (MCF-7/RR, T47D/RR, HCC1428/RR, and HCC1500/RR) exhibited an IC50 at least 20-fold higher than that of their parental counterparts and displayed cross-resistance to the CDK4/6 inhibitors palbociclib and abemaciclib (Supplementary Fig. S5D). The increased content of S241 P-PDK1 observed upon short-term treatment was sustained in the resistant cell lines (Fig. 5A). Further, immunoblot analysis of ribociclib-resistant cells showed incomplete inhibition of Rb phosphorylation in the presence of drug and increased levels of S227 P-RSK2 (target of PDK1), T308 P-AKT (target of PDK1), S235/236 P-S6 (downstream effector of the PDK1-activated kinase P70S6K) compared to parental drug-sensitive cells (Fig. 5A). Cell cycle analysis revealed that CDK4/6 inhibition failed to induce G1 cell cycle arrest and a reduction in S phase in MCF-7/RR and T47D/RR as compared to MCF-7 and T47D parental cell lines (Fig. 5B,C). Consistent with these observations, MCF-7/RR and T47D/RR cells continued to proliferate in the presence of ribociclib (Supplementary Fig. S5E). Importantly, however, genetic and pharmacologic inhibition of PDK1 in combination with ribociclib re-sensitized MCF-7/RR and T47D/RR cells to ribociclib (Supplementary Fig. S6A,B and Fig. 5D). We also examined inhibitors along the PI3K/AKT/mTOR signaling pathway in combination with ribociclib and observed similar growth inhibition of MCF-7/RR and T47D/RR cells as with combined PDK1 and CDK4/6 inhibition (Supplementary Fig. S6C). Furthermore, combined CDK4/6 and PDK1 inhibition significantly reduced the percentage of ribociclib-resistant cells in S-phase (Fig. 5C) which, unlike in parental cells, was relatively unaffected by either single agent. These results suggest that enhanced PDK1 expression and PI3K/PDK1/AKT/mTOR signaling mediate acquired resistance to CDK4/6 inhibition by maintaining progression through the cell cycle.
Figure 5. PDK1 inhibition restores sensitivity to CDK4/6 blockade in drug-resistant cells.
(A) Lysates from parental MCF-7, T47D, HCC1428 and ribociclib-resistant cells treated ± 1 μM ribociclib were analyzed by immunoblot with the indicated antibodies. Resistant cells were removed from drug for 24 h prior to ribociclib treatment for this analysis. (B) Parental and ribociclib-resistant (RR) cells were serum starved for 24 h, treated with 1 μM ribociclib for 24 h, stained with propidium iodide, and then analyzed by FACS. (C) Cells were serum starved for 24 h as in A, treated with drugs for 24 h, stained with propidium iodide, and then analyzed by FACS. In all cases, combined treatment with ribociclib and GSK2334470 markedly reduced the percent of cells in S-phase (*, P ≤ 0.05; **, P ≤ 0.01; ****, P ≤ 0.0001). (D) MCF-7 and T47D parental and RR cells were seeded at low density in 12-well plates and treated with fresh media and drugs every 2–3 days. Resistant cells were removed from ribociclib for 24 h prior to drug treatment. Cells were treated with 1 μM of each drug in all experiments. After 10–21 days, cell monolayers were stained with crystal violet and subjected to image analysis as indicated in the Methods. Each bar represents the mean image signal intensity ± SD of triplicate wells (***, P ≤ 0.001; ****, P ≤ 0.0001).
PDK1 promotes cell cycle progression in CDK4/6-resistant cell lines through increased CDK2/cyclin E/cyclin A
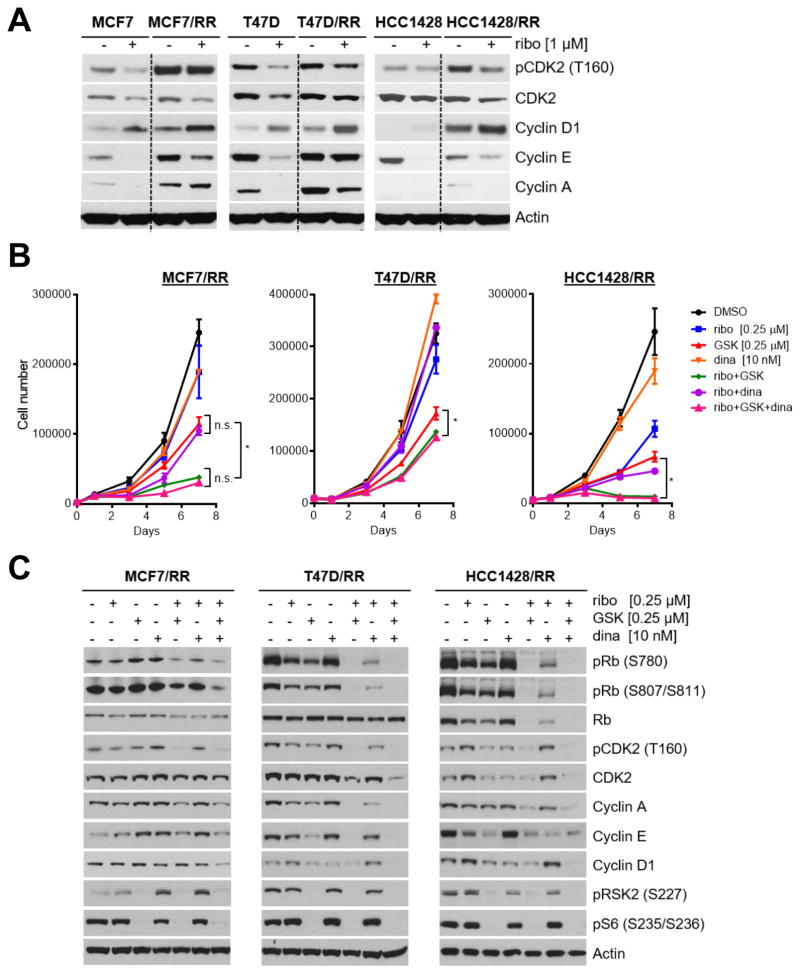
Phosphorylated PDK1 levels were increased upon CDK4/6 inhibition (Fig. 4A and Supplementary Fig. S5B). Ribociclib-resistant cells progressed through the cell cycle in presence of ribociclib (Fig. 5B,C). This continued progression into S phase suggested the ribociclib-resistant cells may exhibit increased or sustained expression of S-phase cyclins and/or CDKs. Indeed, cyclin A, cyclin E and activated T160 P-CDK2 levels were reduced upon ribociclib treatment in parental drug-sensitive cells but not, or not as potently, inhibited in all three drug-resistant cell lines (Fig. 6A). Additionally, MCF-7, T47D and HCC1428 ribociclib-resistant cells exhibited sustained phosphorylation of AKT at S477/T479 (Fig. 4A), a CDK2-dependent phosphorylation site required for full kinase activity, which is limited to the S-phase of the cell cycle (19). Consistent with recent studies (36), we also observed sustained increased expression of cyclin D1 in all three cell lines with acquired resistance to CDK4/6 inhibition (Fig. 6A).
Figure 6. Expression of cell cycle cyclins and CDKs is sustained in ribociclib-resistant cells.
(A) MCF-7, T47D, HCC1428 parental and ribociclib-resistant (RR) cells were treated ± ribociclib for 24 h. Cell lysates were then prepared and analyzed by immunoblot with the indicated antibodies. (B) Cells were plated in triplicate in 12-well plates and treated with ribociclib, GSK2334470 and dinaciclib alone or in combination as indicated. Cells were trypsinized and counted on days 0, 1, 3, 5, and 7 of treatment. Media and drugs were replenished on days 3 and 5. Ribociclib/GSK2334470 or the triple combination of ribociclib/GSK2334470/dinaciclib was most effective at inhibiting cell growth compared to single agent or ribociclib/dinaciclib (* P ≤ 0.05). (C) RR cells were treated with ribociclib, GSK2334470, dinaciclib or combinations of these drugs for 24 h; cell lysates were analyzed by immunoblot for the indicated proteins. Resistant cells were removed from ribociclib for 24 h prior to drug treatment. The combination of ribociclib/GSK2334470 or the triple combination was most effective at diminishing expression of cyclins and CDKs.
CDK2 regulates cell cycle progression through its interactions with both cyclin E and cyclin A. CDK2/cyclin E kinase activity is important for the G1 to S transition and phosphorylation of Rb (37). During S-phase and persisting through G2, active CDK2/cyclin A complexes phosphorylate E2F to promote transcription (38). To determine whether CDK2 promotes cell survival and proliferation in the context of CDK4/6 inhibitor resistance, we treated our ribociclib-resistant cells with dinaciclib (SCH 727965), a potent inhibitor of CDK2, CDK5, CDK1 and CDK9 with IC50 of 1 nM, 1 nM, 3 nM and 4 nM in cell-free assays, respectively (39). MCF-7/RR, T47D/RR, and HCC1428/RR cells continued to proliferate in the presence of ribociclib and also showed relative insensitivity to dinaciclib (Fig. 6B). Inhibition of PDK1 with GSK2334470 or of CDK2 with dinaciclib re-sensitized the drug-resistant cells to ribociclib (Fig. 6B). The combination of ribociclib/GSK2334470 inhibited MC-7/RR, T47D/RR, and HCC1428/RR cell proliferation more potently than ribociclib/dinaciclib. Furthermore, the combination of ribociclib/GSK2334470, but not ribociclib/dinaciclib, completely abrogated phosphorylation of Rb, S6, and RSK2 and expression of cyclin D1, cyclin A and cyclin E (Fig. 6C). Finally, addition of dinaciclib to ribociclib/GSK2334470 did not result in further inhibition of cell proliferation. Taken together, these results suggest the PI3K/PDK1 signaling pathway mediates acquired resistance to CDK4/6 inhibition via an aberrant upregulation of S-phase cyclins and CDKs, which can be blocked with a PDK1 inhibitor.
DISCUSSION
The combination of an anti-estrogen and the CDK4/6 inhibitor palbociclib has recently emerged as an effective option for the treatment of advanced ER+ breast cancer (1, 4). Despite the impressive results of randomized trials with palbociclib, some patients do not respond to therapy and those who do eventually progress, underscoring the need to discover mechanisms of de novo or acquired resistance to CDK4/6-targeted drugs. Using a kinome-wide siRNA screen, we identified PDK1 as the top RNA whose down regulation sensitized ER+ breast cancer cells to CDK4/6 inhibition. RNAi-mediated knockdown of PDK1 or CDK4 inhibited growth of ER+ breast cancer cells, but dual knockdown synergistically inhibited cell proliferation and suppressed PI3K/PDK1/AKT/mTOR signaling. Pharmacological inhibition of PDK1 with GSK2334470 in combination with CDK4/6 inhibitors synergistically inhibited proliferation and increased apoptosis of ER+ breast cancer cells and xenografts. In MCF-7 cells with acquired resistance to ribociclib, we observed an upregulation of phosphorylated and total PDK1 protein levels and subsequent activation of AGC kinases as a mechanism of escape from CDK4/6 inhibition. The upregulation of PDK1, P-S6, and cyclin D1 upon inhibition of CDK4/6 was confirmed in primary breast tumor biopsies. Furthermore, we observed aberrant cell cycle progression in the ribociclib-resistant cells via upregulation of the S-phase cyclins/CDKs (P-CDK2, cyclin E, and cyclin A) with a concomitant increased in phosphorylated AKT at S477/T479, a CDK2-dependent phosphorylation required for full AKT kinase activity and limited to the S-phase of the cell cycle (19). Pharmacological inhibition of PDK1 or CDK2 re-sensitized the ribociclib-resistant cells to CDK4/6 inhibitors; however, ribociclib/GSK2334470 inhibited MCF-7/RR and T47D/RR cell proliferation better than ribociclib/dinaciclib in part through complete abrogation of P-Rb, P-S6, P-RSK2, P-CDK2, cyclin A, cyclin E, and cyclin D1.
A prior study showed that CDK4/6 inhibitors can restore sensitivity to PI3K inhibitors in PIK3CA-mutant cells (40) and that early adaptation and acquired resistance to CDK4/6 inhibition can be prevented by co-treatment with PI3K inhibitors (36). Using an open-ended screen, our study identified PDK1 as the top RNA sensitizing ER+ breast cancer cells to CDK4/6 inhibition. PIK3CA siRNAs were included in the siRNA library used in the screen and the combination of ribociclib and alpelisib potently inhibited cell growth in vitro and in vivo. Of note, however, PIK3CA siRNA did not score as sensitizing to ribociclib based on our calculation of the sensitivity index which measures the influence of siRNA-induced gene knockdown on drug sensitivity (22, 24, 41). Based on the sensitivity index method, sensitizing siRNAs have a small effect on cell viability alone with a greater effect in combination with ribociclib. Thus, we suspect that because PIK3CA siRNAs had a significant effect on MCF-7 cell viability by themselves, they were not identified by our screen.
These results are in partial agreement with those recently reported by Herrera-Abreu et al. In their study, PI3K inhibitors prevented resistance to CDK4/6 inhibitors but did not restore sensitivity to CDK4/6 inhibitors once resistance has been acquired (36). In contrast, we show in the study herein that inhibition of PDK1 with GSK2334470 can re-sensitize ribociclib-resistant cells to CDK4/6 inhibition similar to parental drug-sensitive cells. CDK4/6 inhibitors are being explored in other solid tumors (31). There are preclinical studies that support the combination of a CDK4/6 with BRAF and MEK inhibitors in melanoma (42) and colorectal cancer (43), respectively. CDK4/6 inhibition can also overcome resistance to vemurafenib in BRAFV600E mutant melanoma cell lines (44). As a result, there are several clinical trials in registration exploring the combination of MAPK and PI3K pathway inhibitors in combination with CDK4/6 inhibitors in advanced solid tumors (www.clinicaltrials.gov). Our studies suggest the combination of CDK4/6 and PDK1 inhibitors may be effective in these tumor types.
PDK1 functions downstream of PI3K and is crucial for the activation of AKT and many other AGC kinases including PKC, S6K, SGK, and RSK (11, 13). Our data suggest PDK1 may be a compelling therapeutic target in order to inhibit AKT and non-AKT targets. This is further supported by a recent study demonstrating complete inhibition of PI3K/PDK1/AKT/mTOR signaling when a PDK1 inhibitor is added to a PI3K inhibitor in PIK3CA-mutant breast cancer cells resistant to PI3K inhibition (45). These data also suggest some plausible reasons for the overall lack of substantial tumor regressions in in patients with PIK3CA-mutant breast cancer treated with single agent PI3K inhibitors (46). Our studies suggest that the combination of PDK1 and CDK4/6 may be effective in both PIK3CA wild-type and mutant breast cancers as well as other solid tumors in which CDK4/6 inhibitors are being explored clinically.
The ribociclib-resistant cells we generated displayed cross-resistance to the CDK4/6 inhibitors palbociclib and abemaciclib. Further, the ribociclib-resistant cell lines failed to display G1 arrest, a reduction in S phase, and senescence compared to parental drug-sensitive cells upon drug treatment. The resistant cells exhibited significantly higher levels of phosphorylated CDK2 and cyclin E, consistent with a previous report (36). In this study, we also showed that ribociclib-resistant cells have increased levels of cyclin D1, cyclin A and CDK2-dependent S477/T479 P-AKT. Interestingly, while pharmacological inhibition of PDK1 or of CDK2 re-sensitized the ribociclib-resistant cells to CDK4/6 inhibition, the combination of ribociclib/GSK2334470 inhibited MCF-7/RR, T47D/RR, and HCC1428/RR cell proliferation better than ribociclib/dinaciclib. Furthermore, ribociclib/GSK2334470 but not ribociclib/dinaciclib completely abrogated P-Rb, P-S6, P-RSK2, P-CDK2, cyclin A, cyclin D1 and cyclin E, levels, further suggesting the PI3K/PDK1 pathway maintains cell cycle progression in cells with acquired resistance to CDK4/6 inhibitors.
Finally, the combination of GSK2334470 and ribociclib inhibited growth of established MCF-7 xenografts in nude mice more potently than either drug alone. Previous clinical studies with non-specific PDK1 inhibitors have been disappointing due to dosing issues (47). However, with the discovery of more selective PDK1 inhibitors like GSK2334470 (16, 25) or SNS-229 and SNS-510 (18), they may prove to be more efficacious when combined with CDK4/6 inhibitors. Our studies suggest co-targeting of PI3K/PDK1 and CDK4/6 may overcome resistance to CDK4/6 inhibitors and is worthy of further translational and clinical investigation in patients with ER+ breast cancer.
Supplementary Material
Acknowledgments
The authors acknowledge colleagues at Novartis Pharmaceuticals Corporation for providing ribociclib and alpelisib for the in vitro and in vivo work. The authors also acknowledge NIH/NCI funding through the Breast SPORE grant P50 CA98131 and the Cancer Center Support Grant P30 CA68485 to the Vanderbilt-Ingram Cancer Center. The authors thank the Brock Family Fellowship award for supporting this study (V.M. Jansen).
Financial Support: This work was supported by the Susan G. Komen for the Cure Foundation grant SAC100013 (C.L. Arteaga), a grant from the Breast Cancer Research Foundation (C.L. Arteaga), a Research Specialist Award 1R50CA211206 (J.A. Bauer), a Conquer Cancer Foundation of ASCO Young Investigator Award supported by the Breast Cancer Research Foundation 8364 (V.M. Jansen), Komen Post-Doctoral Award PDF 15329319 (V.M. Jansen), the Vanderbilt Clinical Oncology Research Career Development Program 2K12CA090625-17 (V.M. Jansen).
Footnotes
Conflict of Interest: The authors disclose no potential conflicts of interest.
References
- 1.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 2.Infante JR, Shapiro G, Witteveen P, Gerecitano JF, Ribrag V, Chugh R, et al. A phase I study of the single-agent CDK4/6 inhibitor LEE011 in pts with advanced solid tumors and lymphomas. Journal of Clinical Oncology. 2014:32. [Google Scholar]
- 3.Shapiro G, Rosen LS, Tolcher AW, Goldman JW, Gandhi L, Papadopoulos KP, et al. A first-in-human phase I study of the CDK4/6 inhibitor, LY2835219, for patients with advanced cancer. Journal of Clinical Oncology. 2013:31. [Google Scholar]
- 4.Turner NC, Huang Bartlett C, Cristofanilli M. Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N Engl J Med. 2015;373:1672–3. doi: 10.1056/NEJMc1510345. [DOI] [PubMed] [Google Scholar]
- 5.Patnaik A, Rosen LS, Tolaney SM, Tolcher AW, Goldman JW, Gandhi L, et al. Efficacy and Safety of Abemaciclib, an Inhibitor of CDK4 and CDK6, for Patients with Breast Cancer, Non-Small Cell Lung Cancer, and Other Solid Tumors. Cancer discovery. 2016;6:740–53. doi: 10.1158/2159-8290.CD-16-0095. [DOI] [PubMed] [Google Scholar]
- 6.Fry MJ. Phosphoinositide 3-kinase signalling in breast cancer: how big a role might it play? Breast Cancer Research. 2001;3:304–12. doi: 10.1186/bcr312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin HJ, Hsieh FC, Song H, Lin J. Elevated phosphorylation and activation of PDK-I/AKT pathway in human breast cancer. Br J Cancer. 2005;93:1372–81. doi: 10.1038/sj.bjc.6602862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mihaly Z, Kormos M, Lanczky A, Dank M, Budczies J, Szasz MA, et al. A meta-analysis of gene expression-based biomarkers predicting outcome after tamoxifen treatment in breast cancer. Breast Cancer Res Treat. 2013;140:219–32. doi: 10.1007/s10549-013-2622-y. [DOI] [PubMed] [Google Scholar]
- 9.Gyorffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li QY, et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010;123:725–31. doi: 10.1007/s10549-009-0674-9. [DOI] [PubMed] [Google Scholar]
- 10.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PRJ, Reese CB, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase B alpha. Curr Biol. 1997;7:261–9. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 11.Mora A, Komander D, van Aalten DMF, Alessi DR. PDK1, the master regulator of AGC kinase signal transduction. Seminars in Cell & Developmental Biology. 2004;15:161–70. doi: 10.1016/j.semcdb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 12.Tan J, Li ZM, Lee PL, Guan PY, Aau MY, Lee ST, et al. PDK1 Signaling Toward PLK1-MYC Activation Confers Oncogenic Transformation, Tumor-Initiating Cell Activation, and Resistance to mTOR-Targeted Therapy. Cancer discovery. 2013;3:1156–71. doi: 10.1158/2159-8290.CD-12-0595. [DOI] [PubMed] [Google Scholar]
- 13.Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Bio. 2010;11:9–22. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- 14.Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB, et al. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science. 1998;279:710–4. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- 15.Collins BJ, Deak M, Arthur JSC, Armit LJ, Alessi DR. In vivo role of the PIF-binding docking site of PDK1 defined by knock-in mutation. Embo J. 2003;22:4202–11. doi: 10.1093/emboj/cdg407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Najafov A, Sommer EM, Axten JM, Deyoung MP, Alessi DR. Characterization of GSK2334470, a novel and highly specific inhibitor of PDK1. The Biochemical journal. 2011;433:357–69. doi: 10.1042/BJ20101732. [DOI] [PubMed] [Google Scholar]
- 17.Medina JR. Selective 3-Phosphoinositide-Dependent Kinase 1 (PDK1) Inhibitors: Dissecting the Function and Pharmacology of PDK1. J Med Chem. 2013;56:2726–37. doi: 10.1021/jm4000227. [DOI] [PubMed] [Google Scholar]
- 18.Hansen S, Enquist J, Iwig J, Binnerts ME, Jamieson G, Fox JA, et al. Abstract C198: PDK1 inhibitors SNS-229 and SNS-510 cause pathway modulation, apoptosis and tumor regression in hematologic cancer models in addition to solid tumors. Mol Cancer Ther. 2015;14:C198. [Google Scholar]
- 19.Liu P, Begley M, Michowski W, Inuzuka H, Ginzberg M, Gao D, et al. Cell-cycle-regulated activation of Akt kinase by phosphorylation at its carboxyl terminus. Nature. 2014;508:541–5. doi: 10.1038/nature13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller TW, Hennessy BT, Gonzalez-Angulo AM, Fox EM, Mills GB, Chen H, et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest. 2010;120:2406–13. doi: 10.1172/JCI41680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiang B, Muthuswamy SK. Using three-dimensional acinar structures for molecular and cell biological assays. Method Enzymol. 2006;406:692–701. doi: 10.1016/S0076-6879(06)06054-X. [DOI] [PubMed] [Google Scholar]
- 22.Swanton C, Marani M, Pardo O, Warne PH, Kelly G, Sahai E, et al. Regulators of mitotic arrest and ceramide metabolism are determinants of sensitivity to paclitaxel and other chemotherapeutic drugs. Cancer Cell. 2007;11:498–512. doi: 10.1016/j.ccr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Bauer JA, Ye F, Marshall CB, Lehmann BD, Pendleton CS, Shyr Y, et al. RNA interference (RNAi) screening approach identifies agents that enhance paclitaxel activity in breast cancer cells. Breast cancer research : BCR. 2010;12:R41. doi: 10.1186/bcr2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye F, Bauer JA, Pietenpol JA, Shyr Y. Analysis of high-throughput RNAi screening data in identifying genes mediating sensitivity to chemotherapeutic drugs: statistical approaches and perspectives. BMC genomics. 2012;13(Suppl 8):S3. doi: 10.1186/1471-2164-13-S8-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medina JR, Becker CJ, Blackledge CW, Duquenne C, Feng Y, Grant SW, et al. Structure-based design of potent and selective 3-phosphoinositide-dependent kinase-1 (PDK1) inhibitors. J Med Chem. 2011;54:1871–95. doi: 10.1021/jm101527u. [DOI] [PubMed] [Google Scholar]
- 26.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacological reviews. 2006;58:621–81. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 27.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Advances in enzyme regulation. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 28.Edmondson R, Broglie JJ, Adcock AF, Yang LJ. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay Drug Dev Techn. 2014;12:207–18. doi: 10.1089/adt.2014.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pickl M, Ries CH. Comparison of 3D and 2D tumor models reveals enhanced HER2 activation in 3D associated with an increased response to trastuzumab. Oncogene. 2009;28:461–8. doi: 10.1038/onc.2008.394. [DOI] [PubMed] [Google Scholar]
- 30.Pampaloni F, Reynaud EG, Stelzer EHK. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Bio. 2007;8:839–45. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- 31.O’Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016;13:417–30. doi: 10.1038/nrclinonc.2016.26. [DOI] [PubMed] [Google Scholar]
- 32.Anders L, Ke N, Hydbring P, Choi YJ, Widlund HR, Chick JM, et al. A systematic screen for CDK4/6 substrates links FOXM1 phosphorylation to senescence suppression in cancer cells. Cancer Cell. 2011;20:620–34. doi: 10.1016/j.ccr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitagawa M, Higashi H, Jung HK, SuzukiTakahashi I, Ikeda M, Tamai K, et al. The consensus motif for phosphorylation by cyclin D1-Cdk4 is different from that for phosphorylation by cyclin A/E-Cdk2. Embo J. 1996;15:7060–9. [PMC free article] [PubMed] [Google Scholar]
- 34.Mayer IA, Abramson V, Formisano L, Balko JM, Estrada MV, Sanders M, et al. A Phase Ib Study of Alpelisib (BYL719), a PI3Kalpha-specific Inhibitor, with Letrozole in ER+/HER2-Negative Metastatic Breast Cancer. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-16-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Juric D, Rodon J, Gonzalez-Angulo AM, Burris HA, Bendell J, Berlin JD, et al. BYL719, a next generation PI3K alpha specific inhibitor: Preliminary safety, PK, and efficacy results from the first-in-human study. Cancer Res. 2012:72. [Google Scholar]
- 36.Herrera-Abreu MT, Palafox M, Asghar U, Rivas MA, Cutts RJ, Garcia-Murillas I, et al. Early Adaptation and Acquired Resistance to CDK4/6 Inhibition in Estrogen Receptor-Positive Breast Cancer. Cancer Res. 2016;76:2301–13. doi: 10.1158/0008-5472.CAN-15-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donjerkovic D, Scott DW. Regulation of the G1 phase of the mammalian cell cycle. Cell Res. 2000;10:1–16. doi: 10.1038/sj.cr.7290031. [DOI] [PubMed] [Google Scholar]
- 38.Bertoli C, Skotheim JM, de Bruin RAM. Control of cell cycle transcription during G1 and S phases. Nat Rev Mol Cell Bio. 2013;14:518–28. doi: 10.1038/nrm3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parry D, Guzi T, Shanahan F, Davis N, Prabhavalkar D, Wiswell D, et al. Dinaciclib (SCH 727965), a Novel and Potent Cyclin-Dependent Kinase Inhibitor. Mol Cancer Ther. 2010;9:2344–53. doi: 10.1158/1535-7163.MCT-10-0324. [DOI] [PubMed] [Google Scholar]
- 40.Vora SR, Juric D, Kim N, Mino-Kenudson M, Huynh T, Costa C, et al. CDK 4/6 Inhibitors Sensitize PIK3CA Mutant Breast Cancer to PI3K Inhibitors. Cancer Cell. 2014;26:136–49. doi: 10.1016/j.ccr.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bauer JA, Ye F, Marshall CB, Lehmann BD, Pendleton CS, Shyr Y, et al. RNA interference (RNAi) screening approach identifies agents that enhance paclitaxel activity in breast cancer cells. Breast Cancer Research. 2010:12. doi: 10.1186/bcr2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwong LN, Costello JC, Liu HY, Jiang S, Helms TL, Langsdorf AE, et al. Oncogenic NRAS signaling differentially regulates survival and proliferation in melanoma. Nat Med. 2012;18:1503–U96. doi: 10.1038/nm.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ziemke EK, Dosch JS, Maust JD, Shettigar A, Sen A, Welling TH, et al. Sensitivity of KRAS-Mutant Colorectal Cancers to Combination Therapy That Cotargets MEK and CDK4/6. Clinical Cancer Research. 2016;22:405–14. doi: 10.1158/1078-0432.CCR-15-0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yadav V, Burke TF, Huber L, Van Horn RD, Zhang YY, Buchanan SG, et al. The CDK4/6 Inhibitor LY2835219 Overcomes Vemurafenib Resistance Resulting from MAPK Reactivation and Cyclin D1 Upregulation. Mol Cancer Ther. 2014;13:2253–63. doi: 10.1158/1535-7163.MCT-14-0257. [DOI] [PubMed] [Google Scholar]
- 45.Castel P, Ellis H, Bago R, Toska E, Razavi P, Carmona FJ, et al. PDK1-SGK1 Signaling Sustains AKT-Independent mTORC1 Activation and Confers Resistance to PI3Kalpha Inhibition. Cancer Cell. 2016;30:229–42. doi: 10.1016/j.ccell.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maira SM. PI3K Inhibitors for Cancer Treatment: Five Years of Preclinical and Clinical Research after BEZ235. Mol Cancer Ther. 2011;10:2016. doi: 10.1158/1535-7163.MCT-11-0792. [DOI] [PubMed] [Google Scholar]
- 47.Mateo J, De Bono JS, Ramanathan RK, Lustberg MB, Zivi A, Basset D, et al. A first-in-human phase I trial of AR-12, a PDK-1 inhibitor, in patients with advanced solid tumors. Journal of Clinical Oncology. 2013:31. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.