Abstract
Anorexia nervosa (AN) is an illness that frequently begins during adolescence and involves weight loss. Two groups of adolescent girls (AN-A, weight-recovered following AN) and (HC-A, healthy comparison) completed a functional magnetic resonance imaging task involving social evaluations, allowing comparison of neural activations during self-evaluations, friend-evaluations, and perspective-taking self-evaluations. Although the two groups were not different in their whole-brain activations, anxiety and body shape concerns were correlated with neural activity in a priori regions of interest. A cluster in medial prefrontal cortex and the dorsal anterior cingulate correlated with the body shape questionnaire; subjects with more body shape concerns used this area less during self than friend evaluations. A cluster in medial prefrontal cortex and the cingulate also correlated with anxiety such that more anxiety was associated with engagement when disagreeing rather than agreeing with social terms during self-evaluations. This data suggests that differences in the utilization of frontal brain regions during social evaluations may contribute to both anxiety and body shape concerns in adolescents with AN. Clinical follow-up was obtained, allowing exploration of whether brain function early in course of disease relates to illness trajectory. The adolescents successful in recovery used the posterior cingulate and precuneus more for friend than self evaluations than the adolescents that remained ill, suggesting that neural differences related to social evaluations may provide clinical predictive value. Utilization of both MPFC and the precuneus during social and self evaluations may be a key biological component for achieving sustained weight-recovery in adolescents with AN.
Keywords: Eating disorders, social cognition, self-perception, fMRI, identity
Introduction
Anorexia nervosa (AN) involves altered perceptions about one's body shape and weight and an inability to maintain one's body weight, and typically begins in adolescence or young adulthood.(Nagl et al., 2016) Differences in self-identity have been proposed as a core feature of this illness (Fairchild and Cooper, 2010; Stein and Corte, 2007), and neurobiological differences in midline cortical structures associated with self-perception have been observed repeatedly in adult women with eating disorders when thinking about themselves and others. (McAdams et al., 2016; McAdams and Krawczyk, 2014; McAdams et al., 2015) However, whether neural differences in self-perception also contribute to AN in adolescents has not been established. Additionally, the relationships between the neural differences in self-perception and specific clinical symptoms in AN is unknown.
Here, we consider these clinical questions by comparing the neural activations during self and other evaluations in adolescent girls with AN (AN-A) and healthy adolescent girls (HC-A) using the same functional magnetic resonance imaging (MRI) task we recently examined in adults with and recovered from AN. (McAdams et al., 2016) This imaging task, the Social Identity-V2 task, involves reading and evaluating statements presented with different perspectives. Neurodevelopmental differences in the ability to consider oneself and others have been observed in adolescents compared to adults (Pfeifer et al., 2013; Pfeifer et al., 2007; Pfeifer et al., 2009); here we considered whether AN affects neural activations during perspective-taking and mentalization in adolescents.
We hypothesized that eating disorder (ED) clinical symptoms in adolescence would be related to neural activations in regions that differed in adults with AN because many adolescents with AN go on to be adults with AN. Thus, we also explored whether clinical outcomes following treatment of AN in adolescents could be related to neural activations, by collecting clinical follow-up from the AN-A subjects for a year after the scan, and dividing into recovered (AN-AR) and ill (AN-AI) groups based on the course of the disease. We then assessed whether any of the task-related neural activations, obtained soon after treatment, differed based on clinical outcome.
Methods
Participants
Subjects provided written informed consent to participate, approved by the UT Southwestern Institutional Review Board. A total of 42 adolescent girls (13-19 years) participated: 18 healthy comparison (HC-A) recruited from the community and 24 recently treated for AN (AN-A) recruited from both treatment centers and the community. All AN-A subjects met DSM IV criteria, excluding amenorrhea, within prior year but were weight recovered at the scan to minimize the possibility of effects due to current starvation.
Clinical Measures
Subjects aged 18-19 were interviewed using the Structured Clinical Interview for DSM-IV (First, 2002). Patient-participants aged 13-17 and their parents were interviewed using the Kiddie Schedule for Affective and Mood Disorders (Kaufman et al., 1997). The HC-A group, aged 13-19, were screened for psychiatric disorders, and excluded if there was any history of treatment for any mental illnesses. All AN-A participants had been in either a partial hospital and/or intensive outpatient program for AN during the prior 6 months. Treatment duration was defined as the total number of days of involvement in a specialty eating disorder treatment program, defined as an inpatient, residential, partial hospital, or intensive outpatient level of care, prior to the MRI scan.
All subjects completed a structured interview for current depression (Quick Inventory of Depressive Symptoms, QIDS-CR (Bernstein et al., 2010)), and anxiety (Structured Interview Guide for the Hamilton Anxiety Scale, SIGH-A (Shear et al., 2001)). The Wechsler Abbreviated Scale of Intelligence (WASI (Wechsler, 1999)) provided an estimate of intelligence. Self-report measures provided information about current eating symptoms (Eating Attitudes Test, EAT (Garner et al., 1982)) and body shape concerns (Body Shape Questionnaire, BSQ (Rosen et al., 1996)).
Both in-person office visits and phone calls to parents were utilized to determine clinical outcomes, with a minimum of twelve and up to forty-eight months of follow-up obtained after completing the MRI study for 19 of the 24 participants in the AN-A group; five were lost to follow-up. Variability in length of follow-up related to both availability of participants, and acquisition of funding for outcomes was received more than a year after many participants had completed the study. Subjects were designated as recovered if they had succeeded in sustaining weight-recovery and adequate control of eating disorder symptoms such that they remained at either an outpatient or no treatment level for the twelve months following completion of the study, and maintained work and school activities since completing the MRI scan. Subjects were designated as relapsed or ill if they had lost significant weight, or required return to a higher level of care (intensive outpatient, partial hospital, residential, or inpatient) for additional treatment within the first year after completing the MRI study.
Neuroimaging Task, MRI Acquisition and Processing
The Social Identity-V2 task (McAdams et al., 2016) involves reading and responding to statements related to thinking about oneself (Self, ex. “I believe I am deceitful”), one's friend (Friend, “I believe my friend is moody”), or what one's friend thinks of them (Reflected, “My friend believes I am proud”). In the Friend and Reflected conditions, the term friend was personalized with the actual name of a female friend. Each statement was presented above the terms Agree and Disagree for 4 s, with a response selected each trial, followed by a jittered fixation period of 4, 6, or 8 s. There were 48 statements in each of three runs; 16 statements for each condition resulting in a total duration of 8 minutes per run. All statements for each condition were sequential, and condition order pseudorandomized across runs. During each trial, each subject provided a rating of agreement using a hand-held button, providing behavioral data that included a reaction time and response (Agree or Disagree).
Images were acquired with a 3T Philips Achieva MRI scanner, using a 1-shot gradient T2*-weighted echoplanar (EPI) image sequence sensitive to blood oxygen level-dependent (BOLD) contrast with a repetition time (TR) of 2 s. For Social Identity-V2, the echo time (TE) was 35 ms, and the flip angle was 70°; volumes were composed of 36 axial slices (4 mm thick, no gap). Each slice was acquired with a 22.0 cm2 field of view, a matrix size of 64×64 and a voxel size of 3.4×3.4×4 mm. Functional images were acquired during 4 runs (3 for Social Identity-V2, each 480 s). High resolution MP-RAGE 3D T1-weighted images were acquired for anatomical localization with the following imaging parameters: TR = 2100 ms, TE = 3.7 ms; slice thickness of 1mm with no gap, a 12° flip angle, and 1 mm3 voxels.
Functional MRI task data were analyzed using Statistical Parametric Mapping software (SPM8, Wellcome Department of Imaging Neuroscience London, www.fil.ion.ucl.ac.uk/spm) run in Matlab 2012 (http://www.mathworks.com) and viewed in both SPM and xjview (http://www.alivelearn.net/xjview8/). Preprocessing began with motion-correction performed by aligning each image spatially to the first volume of acquisition, using least-squares minimization to determine the best-fit for a six-parameter, rigid-body spatial transformation. The realigned functional images were normalized to the MNI standard template, and spatially smoothed with a 6 mm full-width at half-maximum 3D Gaussian kernel. The voxel time-series was high pass filtered (128 s). An event-related design extracted the BOLD signal during the 4-s presentation of each statement or image and a general linear model created contrast images of each event (events: Self-Agree, Self-Disagree, Friend-Agree, Friend-Disagree, Reflected-Agree, Reflected-Disagree). Evoked activation was assessed using multiple regression analysis set as boxcar functions. Each regressor was convolved with a canonical hemodynamic response function (HRF) provided in SPM8 and entered into the modified general linear model (GLM). Parameter estimates (e.g. β values) were extracted from this GLM analysis for each regressor. The within-subject first level analysis evaluated the three a priori task contrasts (Self – Friend, Self-Agree – Self-Disagree, Reflected – Self) for each subject.
Group Statistical Comparisons
Demographic and clinical measures (Age, BMI, WASI, QIDS, SIGH-A, EAT, BSQ) were compared using two-tailed independent-sample t-tests (Criterion p < 0.05) and the behavioral measures (mean reaction time and percentage with agreement) were examined with a two-way mixed ANOVA examining both condition (Self, Friend, Reflected) and group (AN-A, HC-A). All statistical analyses were conducted with the IBM Statistical Package for Social Sciences (SPSS, v.23) Two sets of comparisons were conducted: the AN-A and HC-A groups to compare diagnosis and the AN-AR and AN-AI groups to consider clinical course.
Task-related neural activations and clinical group differences in the three a priori contrasts were established using an across-subjects, whole-brain voxel-wide comparison in Matlab SPM 8 to complete two-sample t-tests comparing the AN-A and HC-A groups (threshold: cluster-corrected pfwe< 0.05 with voxel-wise p < 0.005, uncorrected). Age was a covariate of no interest for these across-group whole-brain analyses.
Region of Interest Analyses
Parameter estimates from the five a priori ROIs (McAdams et al., 2016) were extracted and exported to SPSS for across-group t-tests and correlations. Both t-tests and Pearson's correlations were completed in SPSS, correcting for multiple comparisons (corrected threshold: p value of 0.01 (p = 0.05 / 5 regions)) to determine if specific clinical symptoms (QIDS-CR, SIGH-A, EAT, BSQ) related to activations within these ROIs.
To consider whether neural differences might be related to clinical outcome, parameter estimates were also extracted from the areas showing differential activation in the task (Table 2) as well as the a priori ROIs and compared for the AN-AI and AN-AR groups using t-tests. The p value was Bonferroni-corrected for multiple comparisons based on the total number of regions considered for each contrast (Self - Friend, 5 regions, p = 0.01; Self-Agree – Self-Disagree, 4 regions, p = 0.0125; Reflected – Self, 8 regions, p = 0.006).
Table 2.
Clusters differentially activated by Social Identity-V2 task contrasts in adolescents.a
| Region | Conditionb | Volume (mm3) | Cluster Size | Peak Z Score | MNI Coordinates | β Values (Mean(SD)) | Statistics | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| x | y | z | AN-A | HC-A | T | P | |||||
|
| |||||||||||
| Self – Friend | |||||||||||
|
| |||||||||||
| Left Parietal | Self | 3,650 | 79 | 4.25 | -48 | -32 | 36 | 1.13(0.9) | 0.51(1.2) | 1.90 | 0.06 |
| Left Middle Temporal Gyrus | Friend | 4,528 | 98 | 4.14 | -52 | -4 | -12 | -1.34(1.2) | -1.10(1.6) | -0.54 | 0.60 |
| Posterior Cingulate-Precuneus | Friend | 8,778 | 190 | 3.99 | -8 | -56 | 4 | -1.39(2.2) | -1.57(2.6) | 0.24 | 0.81 |
| Right Parietal | Self | 4,435 | 96 | 3.82 | 40 | -44 | 52 | 1.23(1.2) | 0.72(1.2) | 1.36 | 0.18 |
|
| |||||||||||
| Self-Agree – Self-Disagree | |||||||||||
|
| |||||||||||
| Dorsal Anterior Cingulate | Disagree | 5,221 | 113 | 4.17 | 12 | 24 | 32 | -0.43(0.5) | -0.37(0.7) | -0.30 | 0.77 |
| Left Parietal | Disagree | 4,297 | 93 | 3.98 | -44 | -32 | 28 | -0.30(0.4) | -0.34(0.4) | 0.32 | 0.75 |
| Left Insula | Disagree | 4,158 | 90 | 3.55 | -36 | 28 | -4 | -0.45(0.5) | -0.35(0.6) | -0.60 | 0.55 |
|
| |||||||||||
| Reflected – Self | |||||||||||
|
| |||||||||||
| Right Occipital Lobe | Self | 2,218 | 48 | 5.41 | 8 | -88 | 12 | -1.68(2.0) | -1.60(2.0) | -0.14 | 0.89 |
| Right Lingual Lobe | Self | 2,680 | 58 | 5.31 | 4 | -72 | 0 | -2.43(1.9) | -1.58(2.7) | -1.18 | 0.25 |
| Right Parietal | Self | 6,791 | 147 | 4.59 | 28 | -20 | 52 | -0.67(1.0) | -0.99(1.2) | -0.95 | 0.35 |
| Left Parietal | Self | 6,376 | 138 | 3.85 | -36 | -48 | 56 | -0.71(0.9) | -0.81(1.2) | 0.31 | 0.76 |
| Precuneus | Reflected | 3,742 | 81 | 3.65 | -16 | -56 | 32 | 1.21(2.3) | 1.43(2.0) | -0.34 | 0.74 |
Threshold was individual voxel P < 0.005 and cluster-corrected PFWE < 0.05. Volume of each voxel is 46.2 mm3. Peak Z Score refers to the z-score at the MNI coordinates for the specified anatomical location (x,y,z).
Condition in which the extracted parameter estimates were larger.
Results
Clinical and Behavioral Measures
The two groups did not differ in age, BMI at the time of the scan, or intelligence quotient (Table 1). They differed in all clinical measures, with the AN-A group showing higher levels of depression and anxiety, as well as cognitions related to body shape and eating behaviors. Five of the AN-A group had the binge-purge subtype of AN; the rest had the restricting subtype. Twenty of the AN-A group and none of the HC-A group were taking psychotropic medications at the time of the scan.
Table 1. Participant Characteristics.
| Measure | Participant Group | Statistical Comparisons | ||||
|---|---|---|---|---|---|---|
| HC-A (n = 18) | AN-A (n = 24) | |||||
| Mean(SD) | Range | Mean(SD) | Range | T | P | |
| Age(years) | 16.1(2.3) | 13-19 | 16.4(2.0) | 13-19 | 0.55 | 0.59 |
| ED Onset Age | NA | NA | 14.1(1.9) | 11-17 | NA | NA |
| BMI at Scan | 21.2(4.2) | 16-32 | 19.5(1.9) | 16-22 | 1.75 | 0.09 |
| Lowest BMI | NA | NA | 16.7(1.9) | 13-21 | NA | NA |
| Duration of initial ED Treatment (days)a | NA | NA | 90.4(32.5) | 14-147 | NA | NA |
| Intelligence (WASI) | 114.3(13.8) | 93-136 | 110.5(18.1) | 52-136 | 0.76 | 0.45 |
| Eating Attitudes Test (EAT) | 4.1(5.4) | 0-21 | 24.5(16.4) | 3-58 | 5.07 | <0.01 |
| EAT-Dieting | 1.6(2.9) | 0-9 | 13.4(10.0) | 0-33 | 4.86 | <0.01 |
| EAT-Bulimia | 0.6(0.9) | 0-3 | 5.0(4.2) | 0-14 | 4.38 | <0.01 |
| EAT-Oral | 1.9(2.8) | 0-11 | 6.2(4.3) | 0-15 | 3.64 | <0.01 |
| Quick Inventory of Depression (QIDS-CR) | 2.4(2.9) | 0-10 | 8.0(5.1) | 0-18 | 4.15 | <0.01 |
| Structured Clinical Interview for Anxiety (SIGH-A) | 2.1(3.4) | 0-13 | 10.0(5.8) | 0-21 | 5.15 | <0.01 |
| Body Shape Questionnaire | 58.7(29.1) | 34-122 | 122.1(45.7) | 35-188 | 5.15 | <0.01 |
Treatment duration was unavailable for two participants.
Response (Agree or Disagree) and reaction time for each condition (Self, Friend, and Reflected) were obtained each trial. The AN-A group was slower for all stimuli compared to the HC-A group (reaction time, AN-A 2282, HC-A 1931, F(1,37) 13.63, p = 0.001), but there were no differences in the amount of agreement (AN-A 0.51, HC-A 0.50, F(1,37) = 0.31, p = 0.58). There were no differences in reaction time (Self 2122, Friend 2045, Reflected 2151, F(2, 74) = 12.52, p < 0.001) or percent agreement (Self 0.52, Friend 0.48, Reflected 0.53, F(2,74) = 5.25, p = .007) based on task condition. Both groups had quicker reaction times and agreed less for Friend stimuli than for Self and Reflected stimuli which did not differ from one another. Group and stimuli type did not significantly interact to influence reaction time (F(2,74) = 1.68, p = 0.19) or agreement (F(2,74) = 0.91, p = 0.41). There were similar numbers of trials with agreement and disagreement across conditions and subjects (Supplemental Table S1).
Effects of Task Condition and Group
The Self – Friend contrast enables identification of neural regions utilized more for self-evaluations and friend-evaluations. Direct evaluation of oneself activated regions in the bilateral parietal cortex, whereas evaluations of one's friend activated the left middle temporal gyrus and the posterior cingulate/precuneus (Table 2, Figure S1). The Self-Agree – Self-Disagree contrast isolates neural activations related to self-relevance of the stimuli. No areas had more activity during self-agreement than self-disagreement; three areas were more active during self-disagreement than self-agreement: the dorsal anterior cingulate, left insula, and left inferior parietal lobe (Table 2, Figure S2). Finally, in the Reflected – Self contrast, one cluster was more active during the Reflected, 3rd person appraisals (precuneus) than the Self, 1st person appraisals whereas four regions (bilateral parietal and two occipital regions) were more active for Self, direct 1st person appraisals than for Reflected, 3rd person appraisals (Table 2, Figure S3).
No significant differences were observed in whole-brain comparisons of the AN-A and HC-A groups for any task contrast. The parameter estimates extracted from all of the regions differentially activated by task conditions also did not differ based on clinical group (Table 2), although the left parietal region from the Self – Friend contrast did show a trend-level difference with the AN-A group showing more activation than the HC-A group.
Region of Interest Analyses
Five ROIs were previously identified as showing differences in these three task contrasts in comparisons of healthy women and women with and recovered from anorexia nervosa (McAdams et al., 2016); no differences in these ROIs were observed in the adolescent population here (Supplemental Table S2). Parameter estimates were extracted for these ROIs and correlated with clinical symptom scales (QIDS, SIGH-A, EAT, BSQ). The MPFC-dACC cluster (10718 mm3, MNI -12, 48, 20) from the adult comparison of Self – Friend was significantly correlated with the BSQ (Figure 1A, r = -0.44, p = 0.004), and the same relationship was trending for significance for both the AN-A and HC-A groups considered separately (AN-A r = -0.46, p = 0.02; HC-A r = -0.43, p = 0.08, z = -0.11, p = 0.91). The MPFC-Cing ROI (6283 mm3, MNI 8, 48, 20) from the adult study for Self-Agree – Self-Disagree correlated with the SIGH-A for the AN-A group (Figure 1B, r = -0.51, p = 0.01) but not for the HC-A (r = -0.37, p = 0.13), with the trend remained for the whole group (r = -0.33, p = 0.03, z = -0.52, p = 0.60).
Figure 1. Symptoms correlate with utilization of medial prefrontal cortex.
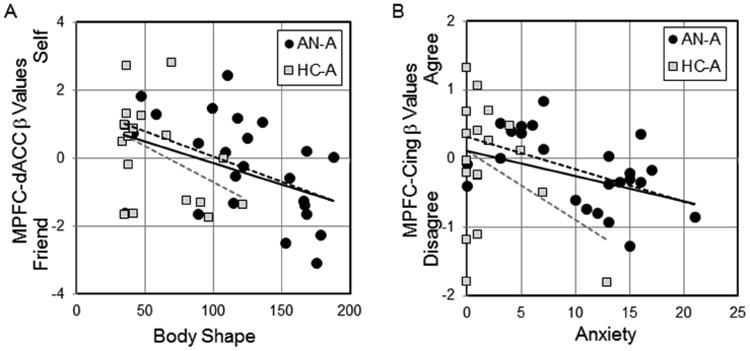
A. The body shape questionnaire correlated with neural responses in the Self – Friend cluster from the adult study. Adolescents that scored high on the body shape questionnaire, both AN-A and HC-A, had more activation of the MPFC-dACC cluster when considering their friend than when considering themselves. Regression lines for AN-A (dashed black, r = -0.46, p = 0.02), HC-A (dashed gray, r = -0.43, p = 0.08), and the entire group (solid, r = -0.44, p = 0.004) are shown. B. Participants in the AN-A group reporting higher levels of anxiety activated the MPFC-Cing cluster more when disagreeing with statements about themselves than when agreeing with statements. Regression lines for AN-A (dashed black, r = -0.51, p = 0.01), HC-A (dashed gray, r = -0.51, p = 0.03), and the entire group (solid, r = -0.37, p = 0.13) are shown.
Clinical Outcomes
Clinical outcomes were available for 19 of the 24 AN-A patient-participants; eleven remained recovered (AN-AR) and eight required additional treatment (AN-AI). Two subjects in the AN-AR group and one in the AN-AI group had the B/P subtype. Nine subjects in the AN-AR group and seven subjects in the AN-AI group were taking psychotropic medications. The demographic, clinical and neural data obtained at the time of the MRI scan were compared; the EAT was the only scale that differed, with the AN-AR group reporting increased symptoms relative to the AN-AI group (Table 3).
Table 3. Clinical symptoms sorted by clinical outcomes.
| Participant Group | Statistical Comparisons | |||||
|---|---|---|---|---|---|---|
| Measure | AN-AI (n = 8) | AN-AR (n = 11) | ||||
| Mean(SD) | Range | Mean(SD) | Range | T | P | |
| Age(years) at Scan | 16.8(1.8) | 13-18 | 15.7(2.1) | 13-19 | 1.10 | 0.29 |
| ED Onset Age | 13.6(2.5) | 11-17 | 13.7(1.5) | 12-16 | -0.11 | 0.91 |
| BMI at Scan | 19.4(1.2) | 18-22 | 19.4(2.5) | 16-22 | -0.003 | 0.99 |
| Lowest BMI, Lifetime | 16.6(1.7) | 14-19 | 16.4(2.1) | 13-20 | 0.30 | 0.77 |
| BMI at Follow-Up | 20.2(1.2) | 16-26 | 20.2(0.4) | 19-23 | -0.05 | 0.97 |
| Duration of Initial ED Treatment (days) | 90.6(38.6) | 14-147 | 86.2(30.2) | 28-138 | -0.27 | 0.79 |
| Mean Follow-Up Period After Scan (months) | 21.0(10.1) | 14-45 | 20.8(9.5) | 12-42 | 0.04 | 0.97 |
| Intelligence (WASI) at Scan | 109.5(26.5) | 52-136 | 111.0(14.2) | 78-134 | -0.16 | 0.88 |
| Eating Attitudes Test (EAT) at Scan | 14.5(13.6) | 4-36 | 29.6(16.6) | 3-58 | -2.10 | 0.05 |
| EAT-Dieting Subscale | 6.8(7.6) | 0-18 | 16.0(9.3) | 0-29 | -2.31 | 0.03 |
| EAT-Bulimia Subscale | 3.0(3.3) | 0-8 | 5.6(4.9) | 0-14 | -1.31 | 0.21 |
| EAT-Oral Control Subscale | 4.8(4.1) | 0-12 | 7.9(4.7) | 0-15 | -1.53 | 0.15 |
| Quick Inventory of Depression (QIDS-CR) at Scan | 9.0(5.0) | 0-16 | 7.5(5.0) | 3-17 | 0.66 | 0.52 |
| Structured Clinical Interview for Anxiety (SIGH-A) at Scan | 11.5(5.7) | 0-17 | 8.2(5.0) | 3-15 | 1.35 | 0.19 |
| Body Shape Questionnaire at Scan | 92.5(48.2) | 35-167 | 130.7(36.9) | 58-176 | -1.96 | 0.07 |
We conducted exploratory ROI analyses to see whether clinical outcome related to the neural activations measured early in recovery within either the task-related regions (Table 2) or the a priori ROIs (Table S2), correcting for multiple comparisons for each contrast. These analyses identified only one region, the cluster in the posterior cingulate extending into the precuneus (PC-PreC obtained from main effect of task for Self-Friend contrast) that differed significantly based on outcomes. This cluster was more active in response to Friend relative to Self evaluations amongst those that succeeded in maintaining weight-recovery compared to those that remained ill (Figure 2A, PC-PreC, AN-AI, -0.20, AN-AR, -2.83, t(17) = 3.25, p = 0.005, Cohen's d = 1.58, effect size r = 0.62). Amongst the a priori ROIs (Figure 2B, Supplemental Table S1), we also found trending differences for both the MPFC-Cing cluster (mean parameter estimates, AN-AI, -0.35, AN-AR, 0.12, t(17) = -1.92, p = 0.07, Cohen's d = 0.96, effect size r = 0.43) and the MPFC-dACC cluster (mean, AN-AI, 0.27, AN-AR -0.87, t(17) = 1.72, p = 0.10, Cohen's d = 0.84, effect-size r = 0.39). Clinical outcomes were not related to activations for the Reflected – Self clusters.
Figure 2. Clinical outcomes and neural activations in task and a priori ROIs.
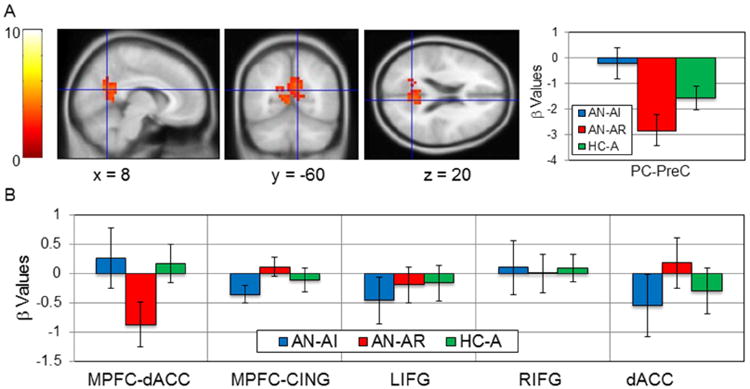
A. The task-related cluster in the precuneus and posterior cingulate cluster from the Self-Friend contrast (Table 2), is shown in the left panels, with parameter estimates extracted on right for the AN-AI (blue), AN-AR (red), and HC-A (green) groups. This cluster was significantly more active for Friend than Self evaluations in the AN-AR group compared to the AN-AI group. B. The y-axis shows the parameter estimates for each group using the contrast with which each ROI was defined in the adults (MPFC-dACC, Self – Friend; MPFC - Cing, Self-Agree - Self-Disagree; LIFG, RIFG, dACC, Reflected – Self). No significant group differences were observed, but there were trending differences in the MPFC-dACC and MPFC-Cing clusters.
Discussion
We compared neural activations using a self-evaluation task in nutritionally rehabilitated adolescents recently treated for AN to healthy adolescents without any pre-existing psychiatric illnesses. Using direct, whole-brain comparisons, these groups did not show any neural differences. Using an ROI analysis based on regions associated with AN in the same task in adult women, we found that clinical symptoms correlated with activations of MPFC. Specifically, activation of the MPFC-dACC cluster correlated with concerns about body shape, and activation of the MPFC-Cing correlated with anxiety levels. These data suggest that differences in the utilization of frontal brain regions during social evaluations may contribute to both anxiety and body shape concerns in AN. Clinical outcomes were associated with differences in activation of the posterior cingulate and precuneus, providing pilot data that neural differences related to social evaluations may have clinical predictive value. In sum, these data suggest neurodevelopmental changes in social perception may be important in the pathology of AN.
Subjects that scored high on the BSQ utilized MPFC-dACC more when completing Friend evaluations than Self evaluations for both the AN-A and HC-A groups. This suggests that high concerns about body shape are associated with neural differences during social evaluations. Interestingly, these neural differences reflect elevated use of frontal brain regions when thinking about other people than when thinking directly about oneself, potentially a factor that underlies the high sensitivity to social interactions observed in AN (Ambwani et al., 2016). Consistent with this data, recovery-related changes in the neural responses of MPFC to negative body images have been reported in adults with AN (Pruis et al., 2012).
The relationship between anxiety and activation of the MPFC-Cing cluster when conducting self rather than friend evaluations was strongest for the AN-A group; this area also showed a trend towards more activation for self-agreement than self-disagreement in the AN-AR group relative to the AN-AI group. Anxiety is commonly comorbid in AN (Kaye et al., 2004) and serves as a negative prognostic factor for recovery from AN (Zerwas et al., 2013). Jarcho (Jarcho et al., 2016) recently reported that preadolescents (11) who as children showed high levels of social reticence (anxious and avoidant behavior) also showed increased neural activation of the dACC and insula when anticipating social interactions compared to children with low social reticence. In adults with anxiety disorders, Alvarez et al. (2015) found that activations of the cingulate and insula during anticipation of unpredictable, physical threat was also increased for those with elevated trait-levels of anxiety. Our data extend this work by suggesting that in adolescent AN, biological differences in processing anxiety may contribute to altered self-perception.
Importantly, the Reflected – Self contrast was designed to examine the cognitive process of mentalization, thinking about oneself from another's perspective compared to direct self-evaluations; the activations observed in these adolescents were different from those commonly reported in healthy adults. Mentalization typically results in engagement of more neural regions than direct self-appraisals in adults (D'Argembeau et al., 2007; McAdams et al., 2016; McAdams and Krawczyk, 2014; Ochsner et al., 2005; Veroude et al., 2014); the opposite was observed in both groups of adolescents. Here, only the precuneus was utilized more for the 3rd person relative to 1st person appraisals in the adolescents, while visual and parietal regions were more engaged for 1st person appraisals. Neurotypical brain development may be characterized by specific changes in the neural regions recruited for mentalization. Differences in the utilization of MPFC for self-relevant, social stimuli have been observed in comparisons of healthy adolescents and adults (Pfeifer et al., 2013; Pfeifer et al., 2007; Somerville et al., 2013). The engagement of ventromedial prefrontal cortex for social, but not academic, judgements has also been associated with age and pubertal development (Pfeifer et al., 2013), and sex differences in the utilization of the precuneus have been observed in late adolescents and young adults. (Veroude et al., 2014) We hypothesize that AN, by interfering with fat stores necessary for the onset of puberty, may disrupt neurotypical brain development, potentially leading to persistent, biological differences in social self-evaluations.
One of the study strengths is that we also explored if any factors, clinical or neural, measured after successful weight-recovery differed amongst the relapsing compared to sustained recovery group. Surprisingly, the AN-AR subjects had higher scores on the EAT than the AN-AI subjects, possibly suggesting improved insight about eating disorder symptoms. From a neural standpoint, the AN-AR group showed elevated activation of the PC-PreC for Friend relative to Self evaluations compared to the AN-AI group. Many studies in adults with AN have observed reduced activation of the posterior cingulate and precuneus using social tasks (McAdams and Krawczyk, 2014; McAdams et al., 2015), body perception tasks (Mohr et al., 2010) and even resting state studies (Lee et al., 2014; McFadden et al., 2014). Here, we found that PC-PreC activation may predict sustained weight-recovery in adolescent AN. In another study examining clinical outcomes in fifteen adolescents with AN, Schulte-Rather (Schulte-Ruther et al., 2012) reported that less activation in MPFC in response to social stimuli at initial hospitalization (when underweight) correlated with worse outcomes one year later. We report a similar effect in the PC-PreC and trends in MPFC, using neural data obtained after acute weight-recovery. In concert, these data suggest that the ability to complete social self-evaluations about both oneself and others may be an important part of recovery from AN, as this cognitive process engages both MPFC and the PC-PreC. During neurotypical development, the connectivity between these two regions increases for social and emotional stimuli (Kelly et al., 2009). Recently, two different studies using dynamic causal modeling identified a relationship between left parietal regions and medial prefrontal cortex important for self-specific processing in healthy adults (Davey et al., 2016; Sui et al., 2013); disruption of this neurocircuitry may be important in the pathology of AN. In a systematic review, Jewell (Jewell et al., 2016) reported that a consistent relationship between eating pathology and mentalization difficulties has been observed in adolescents with eating disorders; here we propose neural regions that may mediate these problems.
There are limitations to this study. First, although the sample size of 42 is typical of many fMRI studies in adolescents with AN (Bischoff-Grethe et al., 2013; Lock et al., 2011; Schulte-Ruther et al., 2012), a larger sample would be helpful. The clinical outcomes data should be considered exploratory, and supports the need for larger, longitudinal studies to determine the predictive value of these brain differences during recovery. Second, measures to directly assess pubertal stage were not obtained. Future studies should consider both hormonal function as well as the duration and timing of underweight periods to determine if neurodevelopmental differences related to social self-evaluation predispose to AN, or result from hormonal or nutritional side-effects from AN. Third, only four subjects in the AN-A group were not on psychotropic medication, preventing examination of the possible effects of these medications on neural responses during self and other evaluation. However, as no whole-brain group differences in neural activations were identified for the two groups despite differences in medication use, and no medication-related neural differences were observed in adults with the same task, sustained use of antidepressant medication appears unlikely to directly alter social evaluations.
In conclusion, this study provides support for the hypothesis that neural differences in social self-evaluation are clinically relevant in adolescent AN, and specifically relates activations of midline cortical regions to body perception, anxiety, and clinical course. Understanding how treatments may change activations of these regions may be particularly important to improve the course of AN. Do elevated body shape concerns cause more MPFC-dACC utilization or is it that subjects with more MPFC-dACC engagement during Friend evaluations are at risk for the development of elevated body shape concerns? Does elevated anxiety result from an insecure sense of self reflected by differences in MPFC-Cing? Or does this neural difference itself contribute to anxiety? Does the increased activation of the posterior cingulate and precuneus for Friend evaluations relative to Self evaluations reflect improved interpersonal relationships or that the ability to perceive others is different for those that recovery? These are important questions that can be answered only through longitudinal imaging studies of social self evaluations in AN. Ideally, within-subject changes in brain function will be correlated with changes in clinical symptoms, and potentially linked to the use of specific interventions. This type of data is needed to facilitate the development of treatments that can target neural regions both directly, such as real-time fMRI, or indirectly by focusing on specific cognitions associated with regional neural differences.
Supplementary Material
Highlights.
Adolescents evaluated the relevance of social adjectives using fMRI.
Adolescents recovered from anorexia nervosa were compared to healthy adolescents.
Medial prefrontal cortex engagement was related to anxiety in both groups.
Body shape concern correlated with frontal brain activity in anorexia nervosa.
Altered social neural function contributes to clinical pathology in anorexia nervosa.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez RP, Kirlic N, Misaki M, Bodurka J, Rhudy JL, Paulus MP, Drevets WC. Increased anterior insula activity in anxious individuals is linked to diminished perceived control. Transl Psychiatry. 2015;5:e591. doi: 10.1038/tp.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambwani S, Berenson KR, Simms L, Li A, Corfield F, Treasure J. Seeing things differently: An experimental investigation of social cognition and interpersonal behavior in anorexia nervosa. Int J Eat Disord. 2016;49(5):499–506. doi: 10.1002/eat.22498. [DOI] [PubMed] [Google Scholar]
- Bernstein IH, Rush AJ, Stegman D, Macleod L, Witte B, Trivedi MH. A comparison of the QIDS-C16, QIDS-SR16, and the MADRS in an adult outpatient clinical sample. CNS Spectr. 2010;15(7):458–468. doi: 10.1017/s1092852900000389. [DOI] [PubMed] [Google Scholar]
- Bischoff-Grethe A, McCurdy D, Grenesko-Stevens E, Irvine LE, Wagner A, Yau WY, Fennema-Notestine C, Wierenga CE, Fudge JL, Delgado MR, Kaye WH. Altered brain response to reward and punishment in adolescents with anorexia nervosa. Psychiatry Res. 2013;214(3):331–340. doi: 10.1016/j.pscychresns.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A, Maquet P, Salmon E. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. J Cogn Neurosci. 2007;19(6):935–944. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- Davey CG, Pujol J, Harrison BJ. Mapping the self in the brain's default mode network. Neuroimage. 2016;132:390–397. doi: 10.1016/j.neuroimage.2016.02.022. [DOI] [PubMed] [Google Scholar]
- Fairchild H, Cooper M. A multidimensional measure of core beliefs relevant to eating disorders: preliminary development and validation. Eat Behav. 2010;11(4):239–246. doi: 10.1016/j.eatbeh.2010.05.004. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer Robert L, Miriam Gibbon, Williams Janet BW. (SCID-I/P) Biometrics Research. New York State Psychiatric Institute; New York: 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. [Google Scholar]
- Garner DM, Olmsted MP, Bohr Y, Garfinkel PE. The eating attitudes test: psychometric features and clinical correlates. Psychol Med. 1982;12(4):871–878. doi: 10.1017/s0033291700049163. [DOI] [PubMed] [Google Scholar]
- Jarcho JM, Davis MM, Shechner T, Degnan KA, Henderson HA, Stoddard J, Fox NA, Leibenluft E, Pine DS, Nelson EE. Early-childhood social reticence predicts brain function in preadolescent youths during distinct forms of peer evaluation. Psychol Sci. 2016;27(6):821–835. doi: 10.1177/0956797616638319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell T, Collyer H, Gardner T, Tchanturia K, Simic M, Fonagy P, Eisler I. Attachment and mentalization and their association with child and adolescent eating pathology: A systematic review. Int J Eat Disord. 2016;49(4):354–373. doi: 10.1002/eat.22473. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Bulik CM, Thornton L, Barbarich N, Masters K. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am J Psychiatry. 2004;161(12):2215–2221. doi: 10.1176/appi.ajp.161.12.2215. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, Margulies DS, Castellanos FX, Milham MP. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex. 2009;19(3):640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Lee S, Ran Kim K, Ku J, Lee JH, Namkoong K, Jung YC. Resting-state synchrony between anterior cingulate cortex and precuneus relates to body shape concern in anorexia nervosa and bulimia nervosa. Psychiatry Res. 2014;221(1):43–48. doi: 10.1016/j.pscychresns.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Lock J, Garrett A, Beenhakker J, Reiss AL. Aberrant brain activation during a response inhibition task in adolescent eating disorder subtypes. Am J Psychiatry. 2011;168(1):55–64. doi: 10.1176/appi.ajp.2010.10010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams CJ, Jeon-Slaughter H, Evans S, Lohrenz T, Montague PR, Krawczyk DC. Neural differences in self-perception during illness and after weight-recovery in anorexia nervosa. Soc Cogn Affect Neurosci. 2016 doi: 10.1093/scan/nsw092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams CJ, Krawczyk DC. Who am I? How do I look? Neural differences in self-identity in anorexia nervosa. Soc Cogn Affect Neurosci. 2014;9(1):12–21. doi: 10.1093/scan/nss093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams CJ, Lohrenz T, Montague PR. Neural responses to kindness and malevolence differ in illness and recovery in women with anorexia nervosa. Hum Brain Mapp. 2015;36(12):5207–5219. doi: 10.1002/hbm.23005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden KL, Tregellas JR, Shott ME, Frank GK. Reduced salience and default mode network activity in women with anorexia nervosa. J Psychiatry Neurosci. 2014;39(3):178–188. doi: 10.1503/jpn.130046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr HM, Zimmermann J, Roder C, Lenz C, Overbeck G, Grabhorn R. Separating two components of body image in anorexia nervosa using fMRI. Psychol Med. 2010;40(9):1519–1529. doi: 10.1017/S0033291709991826. [DOI] [PubMed] [Google Scholar]
- Nagl M, Jacobi C, Paul M, Beesdo-Baum K, Hofler M, Lieb R, Wittchen HU. Prevalence, incidence, and natural course of anorexia and bulimia nervosa among adolescents and young adults. Eur Child Adolesc Psychiatry. 2016 doi: 10.1007/s00787-015-0808-z. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, Cooper JC, Gabrieli JD, Kihsltrom JF, D'Esposito M. The neural correlates of direct and reflected self-knowledge. Neuroimage. 2005;28(4):797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Kahn LE, Merchant JS, Peake SJ, Veroude K, Masten CL, Lieberman MD, Mazziotta JC, Dapretto M. Longitudinal change in the neural bases of adolescent social self-evaluations: effects of age and pubertal development. J Neurosci. 2013;33(17):7415–7419. doi: 10.1523/JNEUROSCI.4074-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Lieberman MD, Dapretto M. “I know you are but what am I?!”: neural bases of self- and social knowledge retrieval in children and adults. J Cog Neurosci. 2007;19(8):1323–1337. doi: 10.1162/jocn.2007.19.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Masten CL, Borofsky LA, Dapretto M, Fuligni AJ, Lieberman MD. Neural correlates of direct and reflected self-appraisals in adolescents and adults: when social perspective-taking informs self-perception. Child Dev. 2009;80(4):1016–1038. doi: 10.1111/j.1467-8624.2009.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruis TA, Keel PK, Janowsky JS. Recovery from anorexia nervosa includes neural compensation for negative body image. Int J Eat Disord. 2012;45(8):919–931. doi: 10.1002/eat.22034. [DOI] [PubMed] [Google Scholar]
- Rosen JC, Jones A, Ramirez E, Waxman S. Body Shape Questionnaire: studies of validity and reliability. Int J Eat Disord. 1996;20(3):315–319. doi: 10.1002/(SICI)1098-108X(199611)20:3<315::AID-EAT11>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Schulte-Ruther M, Mainz V, Fink GR, Herpertz-Dahlmann B, Konrad K. Theory of mind and the brain in anorexia nervosa: relation to treatment outcome. J Am Acad Child Adolesc Psychiatry. 2012;51(8):832–841 e811. doi: 10.1016/j.jaac.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Shear MK, Vander Bilt J, Rucci P, Endicott J, Lydiard B, Otto MW, Pollack MH, Chandler L, Williams J, Ali A, Frank DM. Reliability and validity of a structured interview guide for the Hamilton Anxiety Rating Scale (SIGH-A) Depress Anxiety. 2001;13(4):166–178. [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Ruberry EJ, Dyke JP, Glover G, Casey BJ. The medial prefrontal cortex and the emergence of self-conscious emotion in adolescence. Psychol Sci. 2013;24(8):1554–1562. doi: 10.1177/0956797613475633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein KF, Corte C. Identity impairment and the eating disorders: content and organization of the self-concept in women with anorexia nervosa and bulimia nervosa. Eur Eat Disord Rev. 2007;15(1):58–69. doi: 10.1002/erv.726. [DOI] [PubMed] [Google Scholar]
- Sui J, Rotshtein P, Humphreys GW. Coupling social attention to the self forms a network for personal significance. Proc Natl Acad Sci USA. 2013;110(19):7607–7612. doi: 10.1073/pnas.1221862110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veroude K, Jolles J, Croiset G, Krabbendam L. Sex differences in the neural bases of social appraisals. Soc Cogn Affect Neurosci. 2014;9(4):513–519. doi: 10.1093/scan/nst015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) 1999 [Google Scholar]
- Zerwas S, Lund BC, Von Holle A, Thornton LM, Berrettini WH, Brandt H, Crawford S, Fichter MM, Halmi KA, Johnson C, Kaplan AS, La Via M, Mitchell J, Rotondo A, Strober M, Woodside DB, Kaye WH, Bulik CM. Factors associated with recovery from anorexia nervosa. J Psychiatr Res. 2013;47(7):972–979. doi: 10.1016/j.jpsychires.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


