Abstract
Background:
Limonium bicolor, a halophytic species, can grow in saline or saline-alkali soil, is well known as a traditional Chinese medicine. Recently it attracted much attention for its treatment for cancer.
Objective:
The present study was performed to evaluate this species from the phytochemical standpoint and the possible relationship between the antitumor activity and its natural products.
Materials and methods:
The chemical constituents from the flowers of L. bicolor were investigated through bioassay-guided fractionation and isolation. All the individual compounds were characterized by spectroscopic analysis and their potential antitumor activity was tested against three different human tumor cell lines by MTT assays.
Results:
The EtOAc extract was proven as the most potent fraction and further fractionation led to the isolation of 15 natural flavonoids, which were characterized as luteolin (1), acacetin (2), quercetin (3), isorhamnetin (4), kaempferol (5), eriodictyol (6), kaempferol-3-O-α-L-rhamnoside (7), kaempferol-3-O-β-D-glucoside (8), quercetin-3-O-α-L-rhamnoside (9), quercetin-3-O-β-D-glucoside (10), quercetin-3-O-β-D-galactoside (11), myricetin-3-O-α-L-rhamnoside (12), kaempferol-3-O-(6″-O-galloyl)-β-D-glucoside (13), hesperidin (14) and rutin (15). The biotesting results demonstrated that both compounds 1 and 3 showed good cytotoxicity against human colon cancer cells (LOVO). Compound 5 exhibited relative greater growth inhibition against both human breast cancer cells (MCF-7) and osteosarcoma cell lines (U2-OS) at the concentration of 100 μg/mL.
Conclusion:
On the basis of these findings, the flavonoids were deduced to be potentially responsible for the antitumor activity of L. bicolor. The preliminary structure–activity relationship analysis suggests that the 3-O-glycosylation moiety in natural flavonoids was not essential for the antiproliferative activity on LOVO and U2-OS cells.
SUMMARY
The phytochemical investigation of Limonium bicolor led to the isolation of 15 flavonoids.
The biotesting of the isolates against three different human tumor cell lines was evaluated.
The structure-antitumor activity relationship between the isolated flavonoids was discussed.
Abbreviation used: MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, EtOAc: Ethyl acetate; LOVO: human colon cancer; MCF-7: human breast, cancer; U2-OS: human osteosarcoma; 5-FU: 5-Fluorouracil; DMSO: dimethyl sulfoxide, NMR: nuclear magnetic resonance; HR-ESI-MS: high resolution electrospray ionization mass chromatography, HPLC: high performance liquid chromatography, EtOH: ethanol; n-BuOH: n-butanol; CC: column chromatography, TLC: thin layer chromatography; PBS: phosphate-buffered saline.
Keywords: Limonium bicolor, flavonoids, antitumor activity
INTRODUCTION
Among the hundreds of salt-tolerant halophytes, many Limonium species of the family Plumbaginaceae have been used to treat a wide variety of therapeutic purposes. Limonium bicolor (Bunge) O. Kuntze, a halophytic species can grow in saline or saline-alkali soil, is well known as a traditional Chinese medicine to be used for the treatment of anemia, hemostasis, emmeniopathy and carcinoma uteri.[1] In literatures, the presence of polysaccharides,[2] flavonoids,[3,4,5,6] steroids,[7] and sulfated phenolics[8] in Limonium species have been reported. However, a comprehensive evaluation of L. bicolor from the phytochemical standpoint was scarcely performed, let alone the possible relationship between the antitumor activity and the natural products from this species. The present research focuses on the isolation and identification of the active chemical constituents from L. bicolor. Furthermore, the evaluation of the isolated compounds on potential antitumor activity, is also the purpose of this paper.
MATERIALS AND METHODS
Plant material
The aerial parts of L. bicolor (Bunge.) O. Kuntz were collected in June 2011 in the south of Taibai Mountain, Shaanxi (China). The plant was identified by Professor Liu Qi-Xin at the Institute of Botany, Jiangsu Province and Chinese Academy of Sciences, Nanjing (China). A voucher specimen (No. 11808-1) was deposited in the herbarium, Institute of Botany, Jiangsu Province and Chinese Academy of Sciences.
Chemicals and instrumentations
Silica gel 60 (0.015–0.040 mm; Merck) was used as normal phase, whereas LiChroprep RP-18 (40-63 μm; Merck) was used as reversed phase column material. MCI gel CHP20P (75-150 μm; Mitsubishi Chemical Corp.) and Sephadex LH-20 (GE healthcare) were used for column chromatography as well. MCF-7, a breast cancer line in women, and LOVO, a human colon carcinoma cell line, together with U2-OS, an osteosarcoma cell were all obtained from Chinese Academy of Sciences Center for Type Culture Collection (Shanghai). 5-Fluorouracil (5-FU) was used as a positive control. Dimethyl sulfoxide (DMSO) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from BioSHARP and Solarbio Company, respectively. All the other cell-culture reagents were purchased from Gibco Company and all the other chemicals were of the highest-grade available.
TLC analyses were carried out on silica gel plates (KG60-F254, Merck). The melting point was measured with an X-6 micromelting point apparatus (Beijing Tech). 1H and 13C NMR spectra were obtained on a Bruker Avance III 400 spectrometer in DMSO-d6 (Sigma-Aldrich). HR–ESI–MS spectrum was measured on an Agilent 6530 UPLC-Q-TOF mass spectrometer. Preparative HPLC was performed on Shimadzu model LC-6AD pump equipped with a SPD-20A detector. The absorbance of 96-well microtiter plate in inhibitory assay was measured with a microplate spectrophotometer Molecular Devices Plus 384.
Extraction of the flavonoids
The flower parts of L. bicolor (7.5 kg) were dried and cut into small pieces and extracted with 80% aqueous EtOH at room temperature for 30 days twice to afford crude extract (450 g) after evaporation in vacuo of the solvent. The crude extract was suspended in water and partitioned successively with n-hexane, EtOAc, and n-BuOH. The fractionations were filtered and concentrated by evaporation under reduced pressure with a rotavapor at 40°C to afford a dark green n-hexane residue, a dark brown EtOAc residue (120 g, 1.6% yield), and a dark-brown n-BuOH residue, respectively.
Purification and isolation of the flavonoids
All the aforementioned residues were applied to evaluate their cytotoxicity against three different human tumor cell lines. At a concentration of 100 μg/mL, the EtOAc extract showed the highest inhibitory activity of 87.89%, 99.04%, and 80.78% against LOVO, U2-OS, and MCF-7 cell lines, respectively.
The EtOAc extract (120 g) was further subjected to macroporous resin D101 column chromatography (CC) eluting with a gradient solvent system (H2O/EtOH). Fractions E1~5 were obtained after being pooled according to their TLC profiles. Fraction E2 (12 g) was further subjected to repeated CC over Polyamide and Sephadex LH-20 to furnish fractions. The combined fractions were finally purified by prep-HPLC to afford compounds 1 and 2. Fractions E3 (25 g) and E4 (10 g) were purified through repeated CC followed by prep-HPLC method to yield compounds 3-11 and 12-15, respectively.
Cytotoxic assay against LOVO, U2-OS, MCF-7 cell lines
According to the documented method with some modification,[9,10,11] the antitumor activities of the isolated fifteen compounds were evaluated against human tumor cell lines using the MTT assay, which is based on the ability of glycolytic pathway enzymes to cleave MTT to the blue compound formazan. Briefly, cells were seeded in 96-well microplates (1 × 104 cells/well in 200 μL of medium). After 12 h, the cells were treated with serial concentrations (4, 20, and 100 μg/mL) of individual flavonoid for 48 h, and 5-FU was used as a positive control. The final concentration of DMSO in culture medium was maintained at 0.5%. After the exposure period, 10 μL of MTT (5 mg/mL) in PBS solution was added to each well and the plate was further incubated for 4 h. The supernatant was removed carefully by pipetting from wells without disturbing the attached cells and formazan crystals were solubilized by adding 150 μL of DMSO to each well. The absorbance at 570 nm was measured with a microplate reader using wells without cells as control. The cytotoxicity (%) of samples against the proliferation of cancer cell lines was calculated from the following formula: (A570 of control cells-A570 of treated cells)/A570 of control cells × 100%. All the experiments were performed in triplicate.
RESULTS AND DISCUSSION
Identification of the isolated compounds
Fifteen flavonoids [Figure 1] including types of flavone, dihydroflavone, flavonol, and their glycosides were isolated from the flowers of L. bicolor. The structures of all the isolated compounds were characterized by analysis of their spectral data (ESI-MS, 1H and 13C NMR) and by comparison with literature data. They were identified as luteolin 1,[12] acacetin 2,[13] quercetin 3,[14] isorhamnetin 4,[12] kaempferol 5,[14] eriodictyol 6,[14] kaempferol-3-O-α-L-rhamnoside 7,[13] kaempferol-3-O-β-D-glucoside 8,[15] quercetin-3- O-α-L-rhamnoside 9,[14] quercetin-3-O-β-D-glucoside 10,[15] quercetin-3-O-β-D- galactoside 11,[14] myricetin-3-O-α-L-rhamnoside 12,[14] kaempferol-3-O-(6″-O-galloyl)-β-D-glucoside 13,[15] hesperidin 14,[16] and rutin 15.[15] Among them, the compounds 4, 5, 7, 9, 10, 11, and 15 were isolated for the first time from this plant, whereas the compounds 2, 8, 13, and 14 have not been obtained from the genus Limonium.
Figure 1.
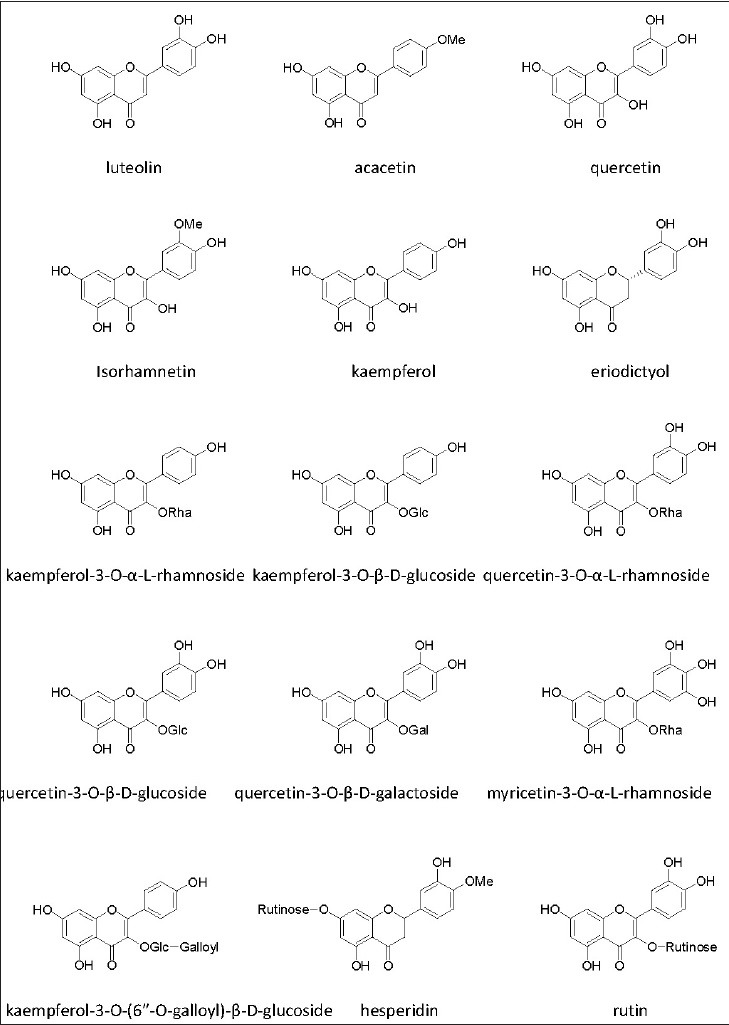
Structures of natural flavonoids isolated from the flowers of L. bicolor
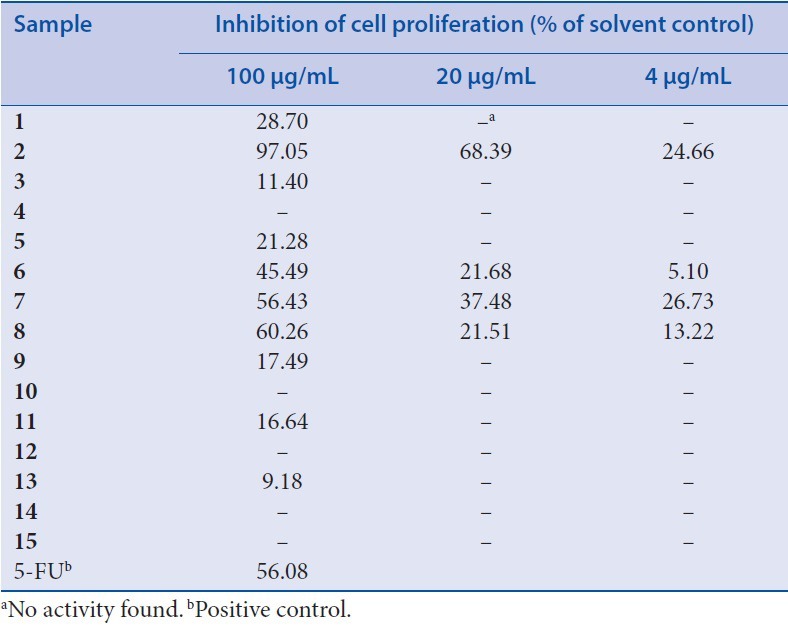
Inhibitory activities of isolates against LOVO cells
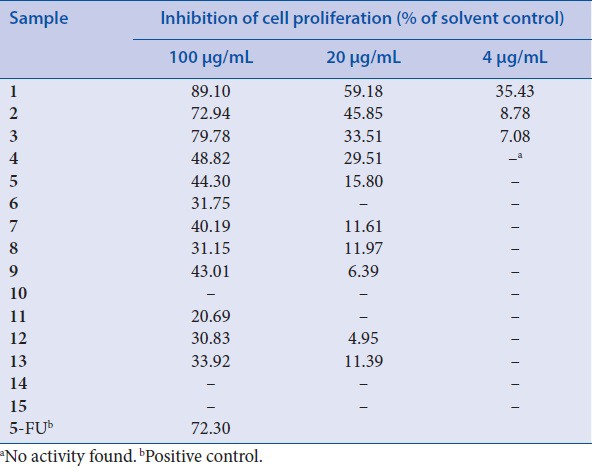
The potential anticancer activity of the isolated compounds was evaluated in terms of LOVO proliferation. As shown in [Table 1], luteolin,[1] quercetin,[3] and acacetin[2] exhibited excellent inhibitory activities against LOVO cells when 100 μg/mL were used, with values higher than that of positive 5-FU (72.30%). At same concentration, isorhamnetin[4] and kaempferol[5] both displayed a moderate cytotoxicity against LOVO cells. In particular, the inhibition effect was reduced with the decreased concentration and a dose-dependent effect was observed on cell viability and proliferation in the tested range. It is noteworthy that both kaempferol-3-O-α-L-rhamnoside[7] and quercetin-3-O-α-L-rhamnoside[9] exhibited a reduced effect to the same concentration of their aglycones, suggesting that 3-O-glycosylation moiety in these flavonoids was not essential for the inhibitory activity. This conclusion could be proved by the fact that no clear inhibition of cell proliferation was found when quercetin-3-O-β-d-glucoside,[10] hesperidin[14] and rutin[15] were used.
Table 1.
Inhibitory effect of isolates from L. bicolor on proliferation in LOVO cells
Inhibitory activities of isolates against U2-OS cells
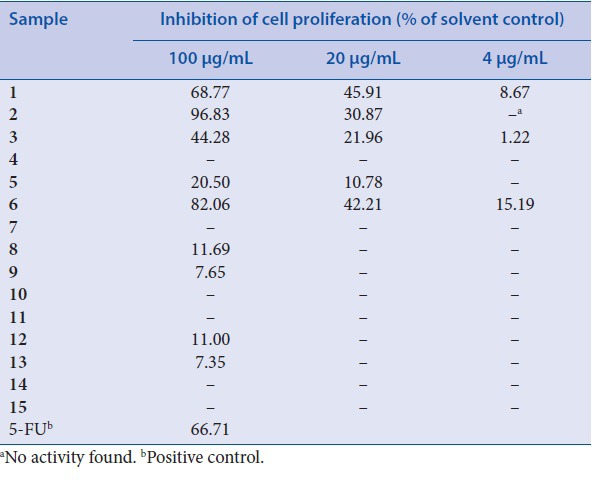
As shown in Table 2, luteolin[1] and acacetin[2] all showed a remarkable cytotoxicity on U2-OS cells with serial concentrations. The dose-dependent effects were observed in U2-OS cells as well. Interestingly, eriodictyol[6] resulted in a considerable inhibition of proliferation against U2-OS cells, which was relative slight in response to cytotoxicity against LOVO cells. On the contrary, kaempferol[5] displayed almost no inhibition activity against U2-OS cells at serial concentrations. In addition, only kaempferol-3-O-β-D-glucoside[8] and myricetin-3-O-α-L-rhamnoside[12] showed very limited cytotoxicity (inhibition >10%) against U2-OS cells, suggesting that glycosylation of flavonoid aglycones will decrease their abilities on antiproliferative property.
Table 2.
Inhibitory effect of isolates from L. bicolor on proliferation in U2-OS cells
Inhibitory activities of isolates against MCF-7 cells
As shown in Table 3, only acacetin[2] resulted in a considerable inhibition of proliferation against MCF-7 cells, suggesting a particular effect regarding the cytotoxicity of this compound. On the contrary to the effect of flavonoids against LOVO and U2-OS cells, kaempferol-3-O-α-L-rhamnoside[7] and kaempferol-3-O-β-D-glucoside[8] displayed relative higher inhibition activity against MCF-7 cells at serial concentrations. The rest of isolated flavonoids showed very limited cytotoxicity even with highest concentration, whereas both hesperidin[14] and rutin[15] with two sugars moiety in their structures displayed no clear inhibition of cell proliferation, suggesting that glycosylation by only one single sugar on aglycones could slightly increase their abilities on antiproliferative property.
Table 3.
Inhibitory effect of isolates from L. bicolor on proliferation in MCF-7 cells
CONCLUSION
Although dozens of natural products were reported in genus Limonium, it is still important to point out that it is rare for such abundant flavonoids isolated from one single species. Natural flavonoids, triterpenoids, and alkaloids were reported to be related with cytotoxicity against cancer cells.[17,18,19,20] However, the potential anticancer activity of the isolates from L. bicolor was far less explored. In the present study, many of the flavonoids isolated from the flowers of L. bicolor seem active for the inhibition of the cancer cell lines studied. The influence of the type of flavonoid itself, the glycosylation, and the number of sugar moieties in their structure were evaluated. By comparison of the inhibitory activity of proliferation in LOVO cell lines, luteolin,[1] acacetin[2] and quercetin[3] showed relative higher cytotoxicity, suggesting that one unsubstituted allylic hydrogen on C3-position and two adjacent hydroxyl groups in B-ring seem much more important for the inhibitory potency. These results were in agreement with the previous report on unglycosylated flavonoids.[21] In addition, the present study demonstrated that the flavonoid glycosides with sugar moiety in their structure displayed less significant effect on the inhibition of LOVO cells, indicating that 3-O-glycosylation in flavonoid was not essential for the antiproliferative activity. Regarding the inhibition of U2-OS cell lines by the isolated flavonoids, both acacetin[2] as an O-methylated flavone and eriodictyol[6] as a flavanone have proven to show strong cytotoxicity, implying that they could play important roles of action with specific mechanism. Among the rest compounds tested, such as quercetin-3-O-α-L-rhamnoside,[9] quercetin-3-O-β-D-glucoside,[10] quercetin-3-O-β-D- galactoside,[11] and rutin,[15] bearing different types of sugar core, were inactive against the U2-OS cell lines, indicating that addition of sugar moiety at position C3 was meaningless for the increase of the inhibitory activity in vitro. The present study also demonstrated that acacetin[2] displayed the most significant effect on the inhibition of proliferation in MCF-7 cell lines, suggesting that the methyl ester of the isolates influence their activities a lot.
As a conclusion, the firstly anticancer screening of fractionations characterized the EtOAc extract as the most potent fraction. Following a bioassay-guided chemical investigation on its antitumor constituents of the flowers of L. bicolor, led to the trace of 15 natural flavonoids. The result from the present study would be a complementary evidence for the use of L. species in the treatment of cancer. The biological screening suggested that the flavonoids in L. bicolor are among the major constituents responsible for its anticancer activity.
Financial support and sponsorship
This research was supported by the National Natural Science Fund of China (No. 31400287), and the Key Laboratory Fund of Jiangsu Center forResearch Development of Medicinal Plants (No. Yaoyan 201102).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Su Jiang. Encyclopedia of Chinese material medica. Shanghai: New Medicine College; 1977. p. 2212. [Google Scholar]
- 2.Zhang LR, Yan XF, Zheng ZH, Zou GL. Interaction of water-soluble polysaccharides of Limonium bicolor with BSA. Chem Nat Compd. 2006;42:389–90. [Google Scholar]
- 3.Wang JX, Wei YX. Studies on the chemical constituents of hypogeal part from Limonium bicolor. J Chin Med Mater. 2006;29:1182–4. [PubMed] [Google Scholar]
- 4.Asen S, Plimmer JR. 4,6,4’-Trihydroxyaurone and other flavonoids from Limonium. Phytochemistry. 1972;11:2601–3. [Google Scholar]
- 5.Zhang LR, Zou GL. Flavanol of Limonium bicolor. Chem Nat Compd. 2004;40:602–3. [Google Scholar]
- 6.Barron D, Varin L, Ibrahim RK, Harborne JB, Williams CA. Sulphated flavonoids an update. Phytochemistry. 1988;27:2375–95. [Google Scholar]
- 7.Whitinga P, Savchenkoa T, Sarkera SD, Rees HH, Dinan L. Phytoecdysteroids in the genus Limonium (Plumbaginaceae) Biochem Syst Ecol. 1998;26:695–8. [Google Scholar]
- 8.Gadetskaya AV, Tarawneh AH, Zhusupova GE, Gemejiyeva NG, Cantrell CL, Cutler SJ, et al. Sulfated phenolic compounds from Limonium caspium: Isolation, structural elucidation, and biological evaluation. Fitoterapia. 2015;104:80–5. doi: 10.1016/j.fitote.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meng DL, Li X, Han LF, Zhang LL, An WW, Li X. Four new quassinoids from the roots of Eurycoma longifolia Jack. Fitoterapia. 2014;92:105–10. doi: 10.1016/j.fitote.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Yu LM, Zhao MM, Yang B, Bai WD. Immunomodulatory and anticancer activities of phenolics from Garcinia mangostana fruit pericarp. Food Chem. 2009;116:969–73. [Google Scholar]
- 11.Liu XK, Ye BJ, Wu Y, Lin ZH, Zhao YQ, Piao HR. Synthesis and anti-tumor evaluation of panaxadiol derivatives. Eur J Med Chem. 2011;46:1997–2002. doi: 10.1016/j.ejmech.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 12.Xi ZX, Chen WS, Wu ZJ, Wang Y, Zeng PY, Zhao GJ, et al. Anti-complementary activity of flavonoids from Gnaphalium affine D. Don. Food Chem. 2012;130:165–70. [Google Scholar]
- 13.Shen J, Liang J, Peng SL, Ding LS. Chemical constituents from Saussurea stella. Nat Prod Res Dev. 2004;16:391–4. [Google Scholar]
- 14.Ye G, Huang CG. Flavonoids of Limonium aureum. Chem Nat Compd. 2006;42:232–4. [Google Scholar]
- 15.Peng ZF, Strack D, Baumert A, Subramaniam R, Goh NK, Chia TF. Antioxidant flavonoids from leaves of Polygonum hydropiper L. Phytochemistry. 2003;62:219–28. doi: 10.1016/s0031-9422(02)00504-6. [DOI] [PubMed] [Google Scholar]
- 16.Zhang XT, Yin ZQ, Ye WC, Ni L, Zhao SX. Chemical Constituents from Lithospermum zollingeri. Chin J Nat Med. 2005;3:357–8. [Google Scholar]
- 17.Yu LM, Zhao MM, Yang B, Bai WD. Immunomodulatory and anticancer activities of phenolics from Garcinia mangostana fruit pericarp. Food Chem. 2009;116:969–73. [Google Scholar]
- 18.Chen XB, Chen GY, Liu JH, Lei M, Meng YH, Guo DA, et al. Cytotoxic cucurbitane triterpenoids isolated from the rhizomes of Hemsleya amabilis. Fitoterapia. 2014;94:88–93. doi: 10.1016/j.fitote.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Kurimoto S, Takaishi Y, Ahmed FA, Kashiwada Y. Triterpenoids from the fruits of Azadirachta indica (Meliaceae) Fitoterapia. 2014;92:200–5. doi: 10.1016/j.fitote.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Nair JJ, Van Staden J. Cytotoxicity studies of lycorine alkaloids of the Amaryllidaceae. Nat Prod Commun. 2014;9:1193–210. [PubMed] [Google Scholar]
- 21.Chang H, Mi MT, Ling WH, Zhu JD, Zhang QY, Wei N, et al. Structurally related cytotoxic effects of flavonoids on human cancer cells in vitro. Arch Pharm Res. 2008;31:1137–44. doi: 10.1007/s12272-001-1280-8. [DOI] [PubMed] [Google Scholar]


