Abstract
Background:
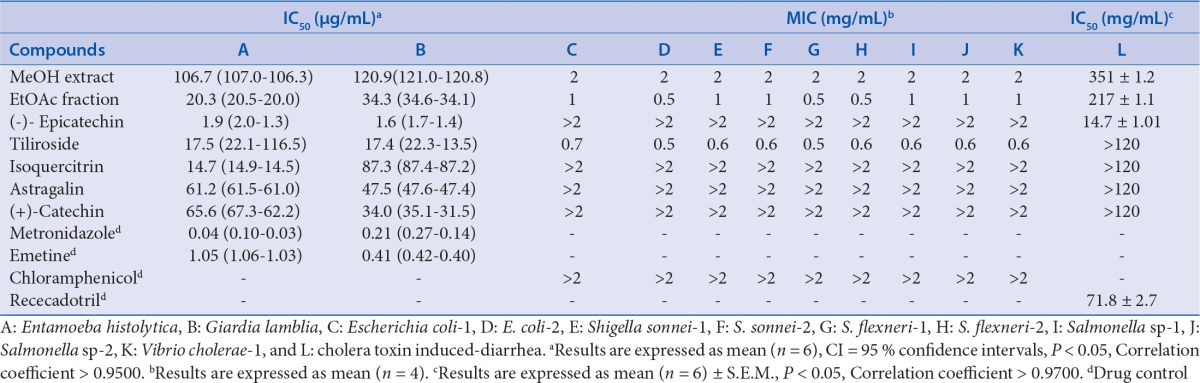
Chiranthodendron pentadactylon Larreat. (Sterculiaceae) is a Mexican plant used in traditional medicine for the treatment of heart disease symptoms and infectious diarrhea.
Objective:
To evaluate in vitro antiprotozoal and antibacterial activities and in vivo antidiarrheal activity from the flowers of C. pentadactylon using the extract, fractions, and major isolated flavonoids.
Materials and methods:
Bioassay-guided fractionation of the methanol extract of C. pentadactylon (MECP) led to the isolation of five flavonoids, tiliroside, astragalin, isoquercitrin, (+)-catechin, and (-)-epicatechin. Antimicrobial activities were tested on two protozoa (Entamoeba histolytica and Giardia lamblia) and nine bacterial enteropathogens (two Escherichia coli strains, two Shigella sonnei strains, two Shigella flexneri strains, two Salmonella sp. strains, and Vibrio cholerae) isolated from feces of children with acute diarrhea or dysentery and resistant to chloramphenicol. Also, antidiarrheal activity was tested on cholera toxin-induced diarrhea in male Balb-c mice.
Results:
Epicatechin was the most potent antiamoebic and antigiardial compound with IC50 values of 1.9 μg/mL for E. histolytica and 1.6 μg/mL for G. lamblia; tiliroside showed moderate antiprotozoal activity against both protozoan. In contrast, in the antibacterial activity, tiliroside was the most potent compound on all microorganisms with minimum inhibitory concentration values less than 0.7 mg/mL. In the case of cholera toxin-induced diarrhea, epicatechin was the most potent flavonoid with IC50 of 14.7 mg/kg.
Conclusion:
Epicatechin and tiliroside were the flavonoids responsible for antimicrobial andantidiarrheal activities of C. pentadactylon. Its antiprotozoal, antibacterial, and antidiarrheal properties are in good agreement with the traditional medicinal use of C. pentadactylon for the treatment of infectious diarrhea.
SUMMARY
Epicatechin was the most potent antiamoebic and antigiardial compound with IC50 values of 1.9 μg/mL for E. histolytica and 1.6 μg/mL for G. lamblia.
Tiliroside showed antibacterial activity against all microorganisms tested with MIC values less than 0.7 mg/mL.
Epicatechin was the most potent flavonoid on cholera toxin-induced diarrhea with IC50 of 14.7 mg/kg.
Abbreviations used: MECP: Methanol extract of C. pentadactylon
Keywords: Chiranthodendron pentadactylon Larreat, (-)-epicatechin, flavonoids, infectious diarrhea, Sterculiaceae, Tiliroside
INTRODUCTION
Worldwide gastrointestinal infections are the most common causes of diarrhea and are responsible for more deaths than gastrointestinal cancers, peptic ulcer, or inflammatory bowel disease. Diarrheal diseases are responsible for 1.5 million deaths every year in low- and middle-income countries, affecting principally children younger than 5 years.[1,2,3] There are an estimated 2.5 billion episodes of childhood diarrhea each year.[1,2] In México, during the past 10 years gastrointestinal infections have been a serious health problem and were the second common cause of morbidity among all age groups. There are vast numbers of bacteria, viruses, and parasites that can cause diarrheal disease, including Escherichia coli, Salmonella spp., Shigella spp., Clostridium difficile, Aeromonas hydrophila, Yersinia enterocolitica, Campylobacter jejuni, Vibrio cholerae, rotavirus, calicivirus, astrovirus, Giardia lamblia, and Entamoeba histolytica.[4,5]
Chiranthodendron pentadactylon is a medicinal plant that grows in Mexico. It is known with the name “flor de manita” in the States of Guerrero, Morelos, Puebla, and Veracruz; its flowers are used to treat mainly heart illness, epilepsy, and as anecdotic remedy for infectious diarrhea.[6,7,8] Because of its popularity, pharmacologic and phytochemical studies have been conducted by Calzada and collaborators since 2005 on C. pentadactylon. Briefly, MeOH extracts from the flowers of “flor de manita” had antibacterial, antiprotozoal, vasorelaxant, antisecretory, and antipropulsive properties.[9,10,11,12,13] Also, MeOH extract does not cause toxicity signs and death a dose of 4.8 g/kg p.o.[14] Phytochemical studies on C. pentadactylon have revealed the presence of hydrocarbons, steroids, sugars, and flavonoids.[6,14,15] Previous studies on antisecretory activity revealed that (-)-epicatechin exerted antisecretory activity through Vibrio cholera toxin inhibition.[14,16] Although antimicrobial and antipropulsive properties of MeOH extract of C. pentadactylon have been described, bioassay-guided studies to isolate its antiprotozoal, antibacterial, and antidiarrheal constituents have not been experimentally explored. The aim of this work was to evaluate antimicrobial and antidiarrheal activities from the flowers of C. pentadactylon using the major isolated flavonoids to obtain additional information that support the anecdotic use of “flor de manita” as an agent for the treatment of infectious diarrhea in Mexican traditional medicine.
MATERIALS AND METHODS
General experimental procedures
IR spectra were recorded as KBr pellets on a Perkin-Elmer 599 B spectrophotometer. 1H-NMR (300 MHz) and 13C-NMR (75 MHz) spectra were registered on a Varian VXR-300S spectrometer in CDCl3 with TMS as internal standard. The chemical shifts are reported in δ units (ppm). EI-MS were taken on a Hewlett-Packard 5985 apparatus, at ionization energy of 70 eV. Melting points were determined in a Fischer Johns apparatus and are uncorrected. Optical rotations were taken on a digital polarimeter JASCO Dip 360. CC: silica gel 60 (Merck, 30-70 mesh). TLC: 0.25 mm or 1 mm precoated silica gel60 F254 (Merck).
Plant material
The flowers from C. pentadactylon (Sterculiaceae) were collected by Dr. Fernando Calzada in October 2014 in Ozumba, State of Mexico, Mexico. The plant material was authenticated by MS Abigail Aguilar-Contreras, of the Herbarium IMSSM of Instituto Mexicano del Seguro Social (IMSS) where the voucher specimen (No. 14404) was deposited.
Extraction from C. pentadactylon
The air-dried flowers (1.0 kg) were ground and extracted by maceration at room temperature with MeOH (2 L × two times). After filtration, the solvent was evaporated in vacuo to yield 142 g of red extract (13.8%).
Isolation of flavonoids from the flowers of C. pentadactylon
Flavonoids were isolated from the MeOH extract from the flowers of C. pentadactylon according to the method of Velázquez.[14] Briefly, the MeOH active extract (40 g) was suspended in 10% MeOH–water (70 mL) and successively partitioned with CH2Cl2 (70 mL × three times, 3.0 g) and EtOAc (70 mL × three times, 1.5 g). The aqueous residual layer (ARL, 1.9 g) was lyophilized. A portion of the EtOAc fraction (500 mg) was purified by preparative TLC (Merck, TLC silica gel 60 F254, EtOAc-MeOH–H2O, 100:16.5:13.5) to give astragalin (yellow powder, MP 204-205°C, 20.0 mg), tiliroside (yellow powder, MP 207-208°C, 25.0 mg), (+)-catechin (brown light powder, MP 182-185°C, 18.3 mg), (-)-catechin (brown light powder, MP 242-244°C, 21.7 mg) and isoquercitrin (yellow powder, MP 187–188°C, 23.3 mg). The compounds were characterized by the reported 13C and 1H NMR data, as well as by direct comparison of TLC and MP with authentic samples available in our laboratory.[14]
Antiprotozoal assays
E. histolytica strain HM1-IMSS used in all experiments was grown axenically at 37°C in TYI-S-33 medium supplemented with 10% heat inactivated bovine serum. In the case of G. lamblia, strain IMSS: 8909:1 was grown in TYI-S-33 modified medium supplemented with 10% calf serum and bovine bile. The trophozoites were axenically maintained and assays were employed in the log phase of growth. In vitro susceptibility tests were performed using a subculture method previously described.[17] Briefly, E. histolytica (6 × 103) or G. lamblia (5 × 104) trophozoites were incubated for 48 h at 37°C in the presence of different concentrations (2.5-200 μg/mL) of the crude extract or pure compounds in dimethyl sulfoxide (DMSO). Each test included a control (culture medium plus trophozoites and DMSO), a blank (culture medium), metronidazole (Sigma) and emetine (Sigma) as amoebicidal and giardicidal drugs. After incubation, the trophozoites were detached by chilling and 50 μL samples of each tube were subcultured in fresh medium for another 48 h, without antiprotozoal samples. The final number of parasites was determined with a hemocytometer and the percentages of trophozoites growth inhibition were calculated by comparison with the control culture. The results were confirmed by a colorimetric method: the trophozoites were washed and incubated for 45 min at 37°C in phosphate buffer saline with MTT (3-[4,5-dimethylhiazol-2-il]-2,5-diphenyl tetrazolium bromide) and phenazine methosulfate. The dye produced (formazan) was extracted and the absorbance was determined at 570 nm. The experiments were performed in duplicate for each protozoan and repeated at least three times. Data were analyzed using probit analysis.[18] The percentage of trophozoites surviving was calculated by comparison with the growth in the control group. The plot of probit against log concentration was made; the best straight line was determined by regression analysis and the 50% inhibitory concentration (IC50) values were calculated. The regression coefficient, its level of significance (P), and correlation coefficient were calculated and 95% CI values determined.
Antibacterial testing
The samples were tested against nine microorganisms, two Escherichia coli species, two Shigella sonnei species, and two Shigella flexneri species, two Salmonella sp. species, and Vibrio cholerae. The bacterial inoculum of each strain was obtained from fresh colonies grown on Müller-Hinton agar plates (MHA, Sigma). All bacterial strains used in this study were isolated from the feces of children with acute diarrhea or bloody diarrhea.[19] Also, all strains tested are resistant to chloramphenicol. The minimum inhibitory concentration (MIC) of samples was accurately determined by agar dilution technique.[10] Briefly, the extract, fractions, and pure compounds for testing were dissolved in DMSO and serially diluted in melted MHA plates (100 mm × 15 mm) to obtain the final concentrations: 0.5, 1, 2, 4, and 8 mg/mL (extract and fractions) or 100-2000 μg/mL (pure compounds). The solvent did not exceed 1% concentration and did not affect the growth of any of the microorganisms. The cultures were diluted in Müller–Hinton broth (MHB, sigma) at a density adjusted to a 0.5 McFarland turbity standard (1.5 × 108 colony-forming units [CFU]/mL). The inoculum was added to a Steer's replicator calibrated to deliver 104 CFU. Then Petri dishes were inoculated and incubated at 37°C, examined after 24 h, and further incubated for 72 h. Chloramphenicol (Sigma) was used as reference standard and for comparative purpose. The lowest concentration of the sample in a plate that failed to show any visible macroscopic bacterial growth was considered as the MIC. The MIC determination was performed in duplicate for each organism, and the experiment was repeated two times.
Animals
Male Balb-c mice were obtained from animal house of the National Medical Center “Siglo XXI” from Mexican Institute of Social Security (IMSS). Investigations using experimental animals were conducted in accordance by the Official Mexican Rule.[20] They were maintained in a temperature room (22 ± 2°C) on a 12-h light–dark natural cycle. Mice were fed with standard diet and water ad libitum. These studies were conducted with the approval of the Speciality Hospital Bio-Ethical Committee of the National Medical Center “Siglo XXI” from IMSS (Approval No.: R-2012-3601-182).
Cholera toxin
Lyophilized powder (1 mg) of cholera toxin (Sigma) containing approximately 220,000 units/mg of protein was suspended in 5 mL of sterile water. For the study, aliquots of the toxin solutions were dissolved in sterile water (0.5 mL) to obtain a concentration of 10 μg/mL.
Cholera toxin-induced diarrhea
Male Balb-c mice (20-22 g, 8 weeks old) were randomly allocated in six groups (n = 6), with free access to water. Diarrhea was induced in the experimental groups by cholera toxin (0.5 mL of cholera toxin a concentration of 10 μg/mL × mouse) except of blank. After 30 min, materials were administrated as follows: blank (0.5 mL of 2% DMSO solution in water), positive control (0.5 mL of 2% DMSO solution in water), MECP (200, 300, 350, and 700 mg/kg in 0.5 mL of a 2% DMSO in water), fractions (200, 300, 350, and 700 mg/kg in 0.5 mL of a 2% DMSO in water), racecadotril (Ferrer Internacional, Hidrasec, 100 mg) was used as antidiarrheal agent (10, 20, 40, 80, and 120 mg/kg in 0.5 mL of a 2% DMSO in water), and flavonoids (10, 20, 40, 80, and 120 mg/kg in 0.5 mL of a 2% DMSO in water). All test materials and cholera toxin were administered intragastrically. Immediately after administration, the animals were placed in cages lined with adsorbent paper and were observed for 4 h then the total mass of fecal output (mg) was measured and expressed in percentage of inhibition.
Statistical analysis
After the plot of percentage of inhibition against concentration was made, the best straight line was determined by regression analysis and the 50% inhibitory concentration (IC50) values were calculated. The regression coefficient, its level of significance (P), and correlation coefficient were calculated. The experiments were performed two times (six animals each times) for each concentration. IC50 values are mean ± S.E.M. P was less than 0.05 (differences between groups were analyzed by one-way ANOVA followed by Dunnett and Bonferroni posthoc test). GraphPad Prism Version 5.03 was used.
RESULTS
Active compounds of C. penthadatylon
In the present work, the flowers C. pentadactylon were extracted with MeOH. The MeOH extract was fractionated into fractions of different polarity by organic solvent extractions with CH2Cl2 and EtOAc. All fractions (CH2Cl2, EtOAc, and ARL) were tested for in vitro antiprotozoal and antibacterial activities and the in vivo antidiarrheal activity in mice [Table 1]. As the EtOAc-soluble fraction showed the best antimicrobial and antidiarrheal inhibitory activities, it was purified by preparative TLC to yield three flavonol glycosides and two flavan-3-ols. These compounds were identified as astragalin, tiliroside, isoquercitrin, (–)-epicatechin, and (+)-catechin [Figure 1] The CH2Cl2 and ARL fractions were discarded because none of them showed activity in all the tests.
Table 1.
Antiprotozoal, antibacterial, and antidiarrheal activities of the MeOH extract, active fraction, and flavonoids from C. pentadactylon
Figure 1.
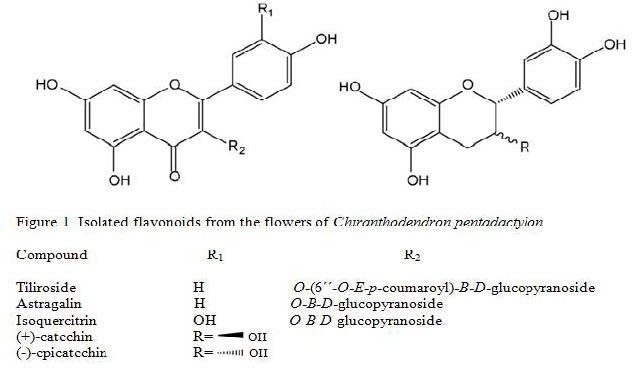
Isolated flavonoids from the flowers of C. pentadactylon
Antiprotozoal, antibacterial and antidiarrheal activities
The results obtained confirm that the methanol extract is effective on all enteropathogens tested and cholera toxin-induced diarrhea [Table 1]. The MeOH extract exhibited antiprotozoal activity against both protozoa with IC50 values of 106.7 μg/mL for E. histolytica and 120.9 μg/mL for G. lamblia. In the case of antibacterial activity, it showed effect on all bacterial strains with MIC values 2 mg/mL. Thus, the activity of the Me OH extract from C. pentadactylon was superior to that chloramphenicol (MIC values > 2 mg/mL). In addition, antidiarrheal activity of MeOH extract was tested on cholera toxin-induced diarrhea in mice model and showed inhibitory effect with an IC50 of 351 mg/kg. Its antidiarrheal property is in agreement with the antisecretory activity previously reported to the MeOH extract of C. pentadactylon.[14] Further chemical fractionation indicated that antimicrobial and antidiarrheal properties were associated with the EtOAc-soluble fractions. Then (-)-epicatechin was isolated as the most potent antiamoebic and antigiardial compound with IC50 values of 1.9 μg/mL for E. histolytica and 1.6 μg/mL for G. lamblia; tiliroside showed moderate antiprotozoal activity against both protozoan. The antiprotozoal activities of epicatechin and tiliroside are in agreement with the results published the author's group and confirm that both flavonoids may be a leading compounds in the development of antiamoebic and antigiardial drugs.[21,22] (In contrast, the antibacterial activity, tiliroside was the most potent compound on all microorganisms with MIC values < 0.7 mg/mL.) The antibacterial activity of tiliroside was superior to that of chloramphenicol. In the case of cholera toxin-induced diarrhea, (-)-epicatechin was the most potent flavonoid with IC50 of 14.7 mg/kg. Its effect was superior to that of racecadotril (IC50 of 71.8 mg/kg) used as antidiarrheal agent. Of the remaining flavonoids, none of them exhibited antidiarrheal activity in the model used at concentrations lower than 120 mg/kg. (-)-Epicatechin and tiliroside were the flavonoids responsible for in vitro and in vivo activities of C. pentadactylon. Its antidiarrheal properties are in good agreement with the traditional medicinal use of C. pentadactylon for the treatment of infectious diarrhea.
DISCUSSION
In developing countries, infectious diarrhea is a major cause of mortality and morbidity. In this sense, medicinal plants with traditional use have been an important source of new biologically active molecules. In spite of the use of C. pentadactylon in Mexican traditional medicine for the treatment of infectious diarrhea and that antimicrobial and antipropulsive properties of MeOH extract of C. pentadactylon have been described, bioassay-guided studies to isolate its antiprotozoal, antibacterial, and antidiarrheal constituents have not been experimentally explored. In the present study, we demonstrate that MeOH extract and its flavonoids have antimicrobial activity and that this activity is important on enteropathogens associated with severe bloody diarrhea (Shigella spp.) and with severe watery diarrhea (Vibrio cholerae). The spectrum of antimicrobial activity of this plant is very important considering the episodes associated with Shigella, V. cholera, and E. histolytica in Mexico.[5] In Mexico, people use one or two handfuls of flowers and drink a cup of tea for 4 days without side effects, which is in agreement with the results reported by Velázquez et al.[14] Extract, EtOAc fraction, and tiliroside were effective on bacterial isolates with low sensitivity to chloramphenicol. The use of chloramphenicol is no longer recommended because of the widespread resistance to it. It is important to note that the effect of tiliroside was superior to chloramphenicol. In summary, the results of the present study along with the properties previously described from the extract and flavonoids obtained from C. pentadactylon could suggest that the mechanism by which the flowers inhibit the infectious diarrhea such as secretory diarrhea and dysentery involves antiamoebic, antigiardial, antibacterial, antisecretory, spasmolytic, antipropulsive, and anti-cholera toxin-induced diarrhea. Finally, the results open the possibility of investigating the use of the flowers from C. pentadactylon as a therapeutic source for the treatment of infectious diarrheal diseases.
CONCLUSION
(-)-Epicatechin and tiliroside were the flavonoids responsible for antimicrobial and antidiarrheal activities of C. pentadactylon. Its antiprotozoal, antibacterial, and antidiarrheal properties are in good agreement with the traditional medicinal use of C. pentadactylon for the treatment of infectious diarrhea.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest
Acknowledgement
The author is grateful to M. Sc Abigail Aguilar for identifying the plant material and MVZ Jaime Herrera and Enrique Formenty of animal house from IMSS. Supported by Grant of PRODEP-México. We are grateful the research scholarship of IMSS Foundation A.C. given to Dr-Normand Garcia Hernandez
REFERENCES
- 1.Casburn-Jones AC, Farthing MJ. Management of infectious diarrhoea. Gut. 2004;53:296–305. doi: 10.1136/gut.2003.022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNICEF/WHO 2009. Diarrhoea: why children are still dying and what can be done. WHO. 6. [Last accessed on 2016 Jan 4]. Available from: http://apps.who.int/iris/bitstream/10665/44174/1/9789241598415_eng.pdf .
- 3.Liu L, Johnsin H, Causens S, Perin J, Scott S, Lawmn J, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–61. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 4.Paniagua GL, Monroy E, Garcia-Gonzalez O, Alonso J, Negrete E, Vaca S. Two or more enteropathogens are associated with diarrhoea in Mexican children. Ann Clin Microbiol Antimicrob. 2007;6:17. doi: 10.1186/1476-0711-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dirección General de Epidemiología (DGE). Anuarios de morbilidad 2008–2013. [Last accessed on 2015 Jul 15]. Available from: www.epidemiologia.salud.gob.mx/anuario/html/anuarios.html .
- 6.Linares E, Flores B, Bye R. Selección de plantas medicinales de México. Mexico: Limusa; 1988. [Google Scholar]
- 7.Argueta A, Cano L, Rodarte M. Atlas de las plantas de la medicina tradicional Mexicana, vols I-III. Mexico. Instituto Nacional Indigenista. 1994 [Google Scholar]
- 8.Cáceres A. Plantas de uso medicinal en Guatemala. Universidad de San Carlos de Guatemala. Editorial Universitaria. 1996 [Google Scholar]
- 9.Perusquía M, Mendoza S, Bye R, Linares E, Mata R. Vasoactive effects of aqueous extracts from five Mexican medicinal plants on isolated rat aorta. J Ethnopharmacol. 1995;46:63–9. doi: 10.1016/0378-8741(95)01230-b. [DOI] [PubMed] [Google Scholar]
- 10.Alanís AD, Calzada F, Cervantes JA, Torres J, Ceballos GM. Antibacterial properties of some plants used in Mexican traditional medicine for the treatment of gastrointestinal disorders. J Ethnopharmacol. 2005;100:153–7. doi: 10.1016/j.jep.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Velázquez C, Calzada F, Torres J, González F, Ceballos G. Antisecretory activity of plants used to treat gastrointestinal disorders in Mexico. J Ethnopharmacol. 2006;103:66–70. doi: 10.1016/j.jep.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 12.Calzada F, Yépez-Mulia L, Aguilar A. In vitro susceptibility of Entamoeba histolytica and Giardia lamblia to plants used in Mexican traditional medicine for the treatment of gastrointestinal disorders. J Ethnopharmacol. 2006;108:367–70. doi: 10.1016/j.jep.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 13.Calzada F, Arista R, Pérez H. Effect of plants used in Mexico to treat gastrointestinal disorders on charcoal-gum acacia-induced hyperperistalsis in rats. J Ethnopharmacol. 2010;128:49–51. doi: 10.1016/j.jep.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 14.Velázquez C, Calzada F, Esquivel B, Barbosa E, Calzada S. Antisecretory activity from the flowers of Chiranthodendron pentadactylon and its flavonoids on intestinal fluids accumulation induced by Vibrio cholera toxin in rats. J Ethnopharmacol. 2009;126:455–8. doi: 10.1016/j.jep.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Lara F, Márquez C. Plantas Medicinales de México: Composición usos y actividad biológica. México: UNAM; 1996. [Google Scholar]
- 16.Velázquez C, Correa-Basurto J, García-Hernández N, Barbosa E, Tesoro-Cruz E, Calzada S, et al. Anti-diarrheal activity of (–)-epicatechin from Chiranthodendron pentadactylon Larreat: experimental and computational studies. J Ethnopharmacol. 2012;143:716–9. doi: 10.1016/j.jep.2012.07.039. [DOI] [PubMed] [Google Scholar]
- 17.Calzada F, Meckes M, Cedillo-Rivera R, Tapia-Contreras A, Mata R. Screening of Mexican medicinal plants for antiprotozoal activity. Pharmaceut Biol. 1998;36:305–30. [Google Scholar]
- 18.Finney DL. Probit analysis. New York: Cambridge University Press; 1970. [Google Scholar]
- 19.Torres J, González-Arroyo S, Pérez R, Muñoz O. Inappropriate treatment in children with bloody diarrhea: clinical and microbiological studies. Arch Med Res. 1995;26:23–9. [PubMed] [Google Scholar]
- 20.Norma Oficial Mexicana, NOM-062-ZOO-1999 (revised in 2001), Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio. Diario Oficial de la Federación. de diciembre de 1999. 2001 [Google Scholar]
- 21.Calzada F, Meckes M, Cedillo-Rivera R. Antiamoebic and antigiardial activity of plant flavonoids. Planta Med. 1999;65:78–9. doi: 10.1055/s-2006-960445. [DOI] [PubMed] [Google Scholar]
- 22.Barbosa E, Calzada F, Campos R. In vivo antigiardial activity of three flavonoids isolated of some medicinal plants used in Mexican traditional medicine for the treatment of diarrhea. J Ethnopharmacol. 2007;109:552–4. doi: 10.1016/j.jep.2006.09.009. [DOI] [PubMed] [Google Scholar]