Abstract
Background:
Ebenus boissieri Barbey is an Antalya, Turkey-endemic plant belonging to Fabaceae family. The aerial parts and the roots of E. boissieri Barbey were used in this study.
Objective:
In the present study, we have examined the apoptotic effects of hydroalcoholic extracts of E. boissieri Barbey in human cervical cancer cell line HeLa.
Materials and Methods:
To determine the cytotoxic effect, cells were treated with various concentrations of extracts for 24, 48, and 72 h incubation periods. Cytotoxic effects were examined by Cell Titer 96 aqueous nonradioactive cell proliferation assay and the results were corrected by live/dead viability/cytotoxicity assay and trypan blue exclusion assay. Apoptotic effects were studied with multicaspase kit. Tumor necrosis factor-alpha (TNF-α) and interferon gamma (IFN-γ) release were also measured by enzyme-linked immunosorbent assay.
Results:
According to the results, E. boissieri Barbey extract caused significant increase in caspase levels. Thus, we suggest that the extract induces cells to undergo apoptosis. Especially, there was a sharp induction in caspase-3 activity. Levels of both TNF-α and IFN-γ in extract-treated groups were significantly and dose dependently exalted as compared to their relative controls.
Conclusion:
The effects of the extract on caspase-3, TNF-α, and IFN-γ levels mediate the plausible mechanism of apoptosis induction in HeLa. To the best of our knowledge, this is the first report indicating any pharmacological properties of E. Boissieri on HeLa cells.
SUMMARY
HeLa cell viability was reduced in dose-dependent manner for 72 h with an IC50 of approximate 28.03 μg/mL for aerial and 41.02 μg/mL for root
HeLa cells, exposure to the aerial extract led to 1.9, 3.8, 1.2, 2.4, and 3.45 fold induction of all caspases activities (-2, -3, -6, -8, and -9, respectively)
Both 30 μg/mL of aerial and 45 μg/mL of root extracts allowed the production of anticancer cytokines (TNFalpha; IFNgamma) in HeLa cell culture supernatants.
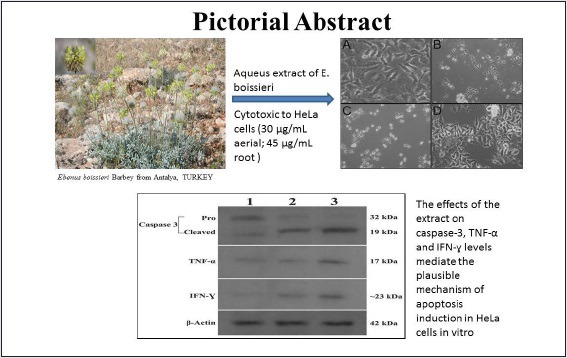
Abbreviations used: Tumor necrosis factor-alpha (TNF-α); Interferon gamma (IFN-γ); 3-(4, 5 dimethylthiazol-2-yl)-5-(3- carboxymethoxy-phenyl)-2-(4-sulfonyl)-2H-tetrazolium (MTS); Phosphate-Buffered Saline (PBS); Fetal Bovine Serum (FBS); para-Nitroanilin pNA; Enzyme-Linked ImmunoSorbent Assay (ELISA); Sodium Dodesyl sulphate –Polyacrilamide gel electrophoresis (SDS-PAGE); Tris-Buffered Saline (TBS); Hydocloric acid (HCl); Standart Error of Mean (SEM); National Cancer Institute (NCI); half maximal inhibitory concentration (IC50)
Keywords: Apoptosis, cervical cancer, caspase, Ebenus, interferon-gamma, tumor necrosis factor
INTRODUCTION
Worldwide, cervical cancer is the second most common cancer among women.[1] Although the mortality rate of cervical cancer is high, it is avoidable through screening and treatment of precancerous lesion and the use of vaccines.[2,3] Conventional cancer therapies such as chemotherapy and radiotherapy will eradicate cancer cells and also affect some healthy cells and cause unacceptable adverse effects.[2,4] Thence, researchers have focused on natural products, which led to the finding of new nontoxic bioactive compounds.
Killing of tumors through the induction of apoptosis has been recognized as a novel strategy for the identification of anticancer drugs.[5,6,7] Apoptosis is an energy-dependent type of programmed cell death and contributes to tumorigenesis.[8] Generally, apoptotic pathways can be subdivided into two categories named as extrinsic and intrinsic pathways.[9,10]
Plants and plant products are known to be effective and versatile chemopreventive agents against various types of cancer.[11] Since traditional background of Anatolian medicine shows an extensive use of plants as useful pharmaceuticals, Turkey is, therefore, considered as a promising region for discovery of new plant products. The genus Ebenus L. belongs to the family of Fabaceae. Ebenus was revised by Huber-Morath in the flora of Turkey.[12] Due to their close resemblance to Astragalus species, they are often called with similar vernacular names by the inhabitants. Experimental (in vitro and in vivo) and clinical investigations on Astragalus membranaceus roots, a components of traditional Chinese medicine, have revealed the extracts to possess significant effects against various types of cancers.[13] In the present study, in vitro cytotoxic, antiproliferative, and immunmodulatory effects of aqueous extracts of E. boissieri Barbey were investigated in HeLa cervical carcinoma cell line. To the best of our knowledge, this is the first report indicating any pharmacological properties of E. boissieri on HeLa cells.
MATERIALS AND METHODS
Plant material
E. boissieri Barbey is a 50–60 cm long perennial herb. It has yellow flowers. It is endemic to Turkey, where it only grows in Antalya province in Turkey. The roots and flowering aerial parts of E. boissieri were collected in Turkey, C3 Antalya, Korkuteli district (36ºc 56’ 51’’N, 030ºc 09’ 41’’E), stony hillsides and steppe about 1290 m above sea level at the middle of June 2008. A voucher specimen is deposited at AKDU (Herbarium of the Biology Department of Akdeniz University) as Göktürk 7201.
Preparation of plant extracts
Either dried roots or aerial parts of E. boissieri were powdered and individually macerated in 80% ethanol for 2 days at room temperature. The extracts were then filtered and evaporated to dryness under reduced pressure to yield root extract (20.2% w/w) and aerial part extract (32.7% w/w).
Cell lines and culture conditions
HeLa cells (ATCC®CCL-2™) were cultured in DMEM (Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS), 10 μg/mL gentamicin, and 5% sodium pyruvate. A total of 293 T cells were maintained in RPMI 1640 supplemented with 10% FBS, 100 mg/L streptomycin, and 100 mU/L penicilin. The cells were incubated in 5% CO2 with 95% humidity at 37°C.
Cell proliferation assay
Cell proliferation was estimated using a Cell Titer 96 aqueous nonradioactive cell proliferation assay (Promega, Madison, WI, USA), which is based on the cleavage of 3-(4, 5 dimethylthiazol-2-yl)-5-(3-carboxymethoxy-phenyl)-2-(4-sulfonyl)-2H-tetrazolium (MTS) into a formazan product soluble in tissue culture medium. The cells were seeded at 1 × 104 cells per well in 200 μL complete medium onto 96-well plates to ensure the exponential growth throughout the experimental period and to ensure a linear relationship between optic density and cell number when analyzed by MTT assay. Cells were allowed to attach for 24 h. After the cells reached 80–90% confluency, the medium was removed, washed with phosphate-buffered saline (PBS) and replaced with medium containing only 1% (v/v) FBS and the cells were further incubated for 4 h and then washed one more time with PBS. Subsequently, the cells were treated with various concentrations (0.01-1000 μg/mL) of aqueous aerial and root extracts prepared in 1% FBS containing complete medium. A cytotoxic drug, doxorubicin-HCl, was used as positive control. The cells were grown at 37°C for three different incubation periods (24, 48, and 72 h). The medium was gently aspirated to terminate the experiment and 180 μL serum-free complete medium and 20 μL of MTT was added to each well and incubated for 4 h. The absorbances at 490 nm were measured in a microplate reader (Thermo Labsystem Multiscan Spectrum, Thermolabsystem, Chantilly, VA, USA), using wells without cells as background. The sample readings calculated by subtracting the average of background absorbances. All experiments were performed at least four times. The half-maximal inhibitory concentration (IC50) of each extract was derived by a nonlinear regression model (curve-fit) based on sigmoidal dose response curve (variable slope) and computed using Graph-Pad Prism, version 4.00 (Graph-Pad Software, San Diego, CA, USA). The growth inhibition was determined using: Growth inhibitory activity (%) = [1- (OD value of sample/OD value of control group)] ×100%.
Cell viability
The Live/Dead Viability/Cytotoxicity kit for Mammalian cells (Invitrogen, Eugene, OR, USA) was used to determine the cell viability. Briefly, the cells were seeded at 1 × 104 cells per well in 200 μL complete medium on to 96-well plates and allowed to attach for 24 h. The same protocol described above was used for treatment of the cells with the extracts. According to the manufacturer's protocol, cells were dually stained with two probes that enable the simultaneous determination of live and dead cells in a sample. The polyanionic dye calcein-AM is converted to the intensely fluorescent calcein by intracellular esterase activity within live cells. EthD-1 was used to identify dead and/or dying cells as it exclusively enters cells through disrupted cell membrane and binds to nucleic acids. Fluorescent intensity was measured on a LS55 Luminescence Plate Reader (Pelkin Elmer Inc., Waltham, MA, USA) at 485-530 nm excitation and 630–645 nm emission wavelengths, respective to each reagent dye. The experimental data were pooled and used for statistical comparisons using Student's t-test.
A second viability assay, trypan blue exclusion, was used to verify results. Cells were plated on the 12-well plates at a cell density of 50,000 cells/well in 1 mL of complete medium and allowed to attach in a CO2 incubator for 24 h. At the end of each experiment, the cells were collected by trypsinization and cell viability was assessed by counting live versus dead cells on a hemocytometer using standard trypan blue (0.4% in PBS) exclusion staining. The percentage of viable cells (%) was calculated as; [(the number of live plus dead cells-the number of dead cells)/the number of live plus dead cells] × 100%.[14]
Multicaspase assay
Apo Target caspase colorimetric protease assay sample kit (Catalog No. KHZ1001, Invitrogen Corp., Camarillo, CA, USA) was used to determine the activity of caspase-2, -3, -6, -8 and -9. Cells (5 × 105 cells/well in 6-well plate) (treated and untreated) were collected and transferred into sterile test tube and lysed using the cell lysis buffer supplied. Samples (50 μL) of the lysate were aliquoted into wells of a standard black 96 well-microplate, to which 50 μL of reaction buffer containing 10 mM DTT were then added to the sample wells. Substrates selective for each of the caspase forms (5 μL of 4 mM; final concentration 200 μM) were added to the appropriate wells and the plate was then incubated at 37°C for 2 h. Absorbance at 405 nm was then read in a microplate reader.
The absorbance from treated samples was compared with untreated control to allow determination of the change in caspase activity. The selective substrates VDVAD-pNA, DEVD-pNA, VEID-pNA, IETD-pNA, and LEHDpNA were used for caspase-2, caspase-3, caspase-6, caspase-8, and caspase-9, respectively.
Estimation of TNF-α and IFN-γ
Release of tumor necrosis factor-alpha (TNF-α) and interferon gamma (IFN-γ) was assayed by enzyme-linked immunosorbent assay (ELISA) as described by the manufacturer (Cat. No: KHC3011and Cat. No. KHC4021, In vitrogen Corp., Camarillo, CA, USA, respectively). Human TNF-α and human IFN-γ were diluted and used as standards. Serial dilutions starting 1000 pg/mL to 15. 6 pg/mL were used to establish the standard curve. Average results from four independent experiments were used to compare nontreated and treated cells.
Western blot
To find out whether changes in caspase-3 activity and concentrations of TNF-α and IFN-γ were due to changes in protein content, cell homogenates were studied by standard western blot techniques. Briefly, 25 μg of homogenate protein were separated on a 10% acrylamide gel by SDS-PAGE and then transferred to poly vinylidene difluoride membranes (Hybond-P, Amersham Pharmacia Biotech, Piscataway, NJ, USA) with a semidry transfer apparatus. The membranes were blocked with TBS-5% milk and then probed with anti caspase-3, anti -TNF-α, and anti-IFN-γ (sc-2710281, sc-1350, and sc-59993; 1:200, 1:100 and 1:100, respectively, Santa Cruz Biotechnology, Santa Cruz, CA, USA). The primary antibody was detected with horseradish peroxidase-conjugated antimouse secondary antibody (sc-2005, diluted 1:5000, 1:2000 and 1:1000 for caspase-3, TNF-α and IFN-γ, respectively, Santa Cruz Biotechnology, Santa Cruz, CA, USA). The membranes were washed and blots were developed using an enhanced chemiluminescence western blotting detection kit (ECL Plus kit, Amersham Biosciences RPN2132, USA) and exposed to x-ray film (Sigma C4729-1EA, Sigma Z363006-50) for 2-30 s. Molecular weight standarts (See Blue® Plus2, LC 5925, Life Technologies, USA) were used to determine molecular weights of the visualized bands.
Statistical analysis
All values are expressed as the mean ± standard error of the mean (SEM). Data were analyzed using one-way analysis of variance followed by Dunnett's multiple comparisons test for comparison of group means to control or by the Student's t-test. (Graph Pad InStat., USA). A P value of < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
In vitro cytotoxicity against HeLa and 293T cells
Worldwide, cervical cancer is one of the major problem, reporting about 15% of neoplasms.[1] Conventional treatment procedures include chemotherapy, radiotherapy, and surgical ablation which show insufficient results; therefore, safer and more effective alternative treatments for different types of cancers are needed. Recently, a number of studies have published both in vitro and in vivo beneficial effects of herbal medicines in cancer treatments.[14,15,16] The members of Fabaceae family have been shown to inhibit different types of cancer cells.[14,17,18] Ebenus is also belonging to Fabaceae family; however, there is no report of Ebenus spp. displaying an antitumor effect and having apoptosis inducing activity on cancer cells.
To carry out to screen the cytotoxic potential of the extracts of E. boissieri was determined by MTT dye uptake method. The dose range (0.01-1000 μg/mL) was determined for both plant extracts and Doxorubicin-HCl in our previous study.[19] As shown in Figure 1A and 1B, root extract had more inhibitory activity on proliferation of 293T than HeLa cells. In HeLa cells, the IC50 of aerial and root extract were approximately 92.35 ± 0.39, 49.82 ± 0.27, 28.03 ± 0.12 μg/mL and 250 ± 5.83, 100.16 ± 4.28, 41.04 ± 1.09 for 24, 48, and 72 h, respectively. The IC50 values of Doxorubicin-HCl on HeLa cells were 0.37 ± 0.08, 0.25 ± 0.12, 0.09 ± 0.47 μg/mL for 24, 48, and 72 h, respectively. According to our results, aerial and root extracts had no significant antiproliferative effect on human embryo kidney 293T cells, this result indicated that these extracts had no direct cytotoxicity to noncancerous cells (P > 0.05). Hence, we concluded that the effect of both aerial and root extracts on proliferation of HeLa cells was availed to examine in subsequent experiments.
Figure 1.
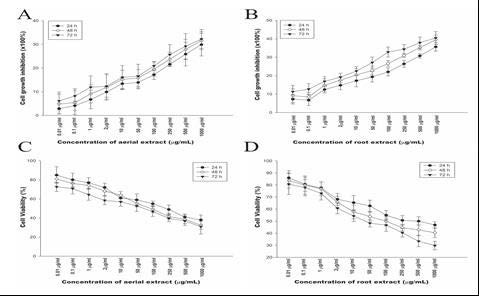
The in vitro inhibition of cell growth. Dose-response analysis of 293 T cells to hydroalcoholic aerial and root extracts. 293 T cells were incubated with increasing concentrations (0.01-1000 μg/mL) of (A) hydroalcoholic aerial (B) and root extracts for 24, 48, and 72 h. Cytotoxic effects of (C) hydroalcoholic aerial and (D) root extracts against HeLa cervical cancer cell line for 24, 48, and 72 h. Cell proliferation was determined by MTT assay. The graphs are plotted as percent inhibition when compared with control cells. Results are representative of four independent experiments with eight replicates and are presented as mean ± SEM.
Many herbals and phytochemicals have been reported for their cytoprotective effect using MTT assay.[20] The criteria of cytotoxic activity for the extracts having potential anticancer activity as established by the American National Cancer Institute (NCI) is an IC50 less than 30 μg/ml in the preliminary assay.[21] Since the IC50 concentration of aerial extract in HeLa cell line was 28.03 μg/mL; therefore, it might be a potent anticancer therapeutic agent.
We examined the antiproliferative effects of aqueous extracts of aerial and root parts on HeLa and 293T cell growth. Cell viability was determined 24, 48, and 72 h after extract treatment. HeLa cell viability was reduced in dose-dependent manner for 72 h with an IC50 of approximate 28.03 μg/mL for aerial and 41.02 μg/mL for root [Figure 1C and 1D]. These findings indicated that both aerial and root extracts significantly decreased proliferation of cervical cancer cells in dose- and time-dependent manner. Based on the viability trend obtained; 30 μg/mL aerial and 45 μg/mL root extracts were chosen for subsequent experiments.
According to the MTT test results, at the end of 72 h, 30 μg/mL aerial and 45 μg/mL root extracts caused 78% and 56.25 % inhibition in growth of HeLa cells, respectively [Figure 2A and 2B]. As can be seen from the percentage of the growth inhibition rates, aerial extract was found to be more effective on HeLa cells than root extract. In terms of evaluating the success of antiproliferative effects of this plant, this is quite important result of this study. We should mention that no decrease in the viability of cells treated with low-doses of aerial or root extracts was observed (data not shown). The results obviously demonstrate that aqueous aerial and root extracts of E. boissieri inhibit the growth of cervical cancer cells (P < 0.001). In contrast, neither aerial nor root extract of this plant inhibit the proliferation of 293T cells for doses up to 250 μg/mL (P > 0.05). Given the fact that no cytotoxic effects were observed against normal cells, our data clearly indicate that E. boissieri exerts a selective antiproliferative activity against cervical cancer cells.
Figure 2.
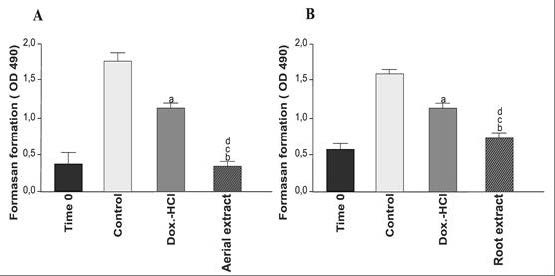
The effect of hydroalcoholic aerial and root extracts on cell growth of HeLa. Cells were treated with (A) 30 μg/mL aerial extract and (B) 45 μg/mL root extract. Cell growth was determined after 72 h using MTS solution. Time 0 demonstrates the number of cells before treatment. Viability of the cells was measured by MTT assay. Each bar represents time-dependent changes in viability. Results are representative of four independent experiments with eight replicates and are presented as mean ± SEM. (A and B) significantly different from the control group; P < 0.01and P < 0.001, respectively. (C and D) significantly different from doxorubicin-HCl-treated group in same incubation time, P < 0.05 and P < 0.01.
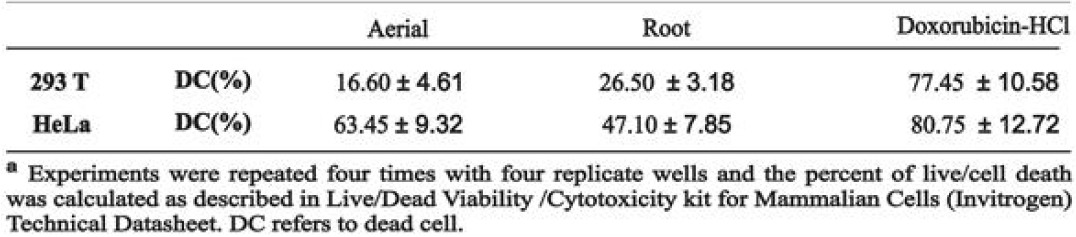
To clarify the effects of aqueous aerial and root extracts of E. boissieri on cell growth, 293T and HeLa cells were plated on 12-well plates (50,000 cells/well) and 36 h after plating, treated with 30 μg/mL aerial and 45 μg/mL root extracts (in medium supplemented with 1% FBS, with eight repeats of each treatment). The number of dead cells was determined 72 h after the treatment using the trypan blue exclusion assay. The number of live cells decreased in both extract treated group [Figures 3 and 4]. Comparing the microscopic images cell death was observable with the abundance of seemingly condensed apoptotic cells and cell fragments in aerial extract-treated group as well as in the group treated with root extract. The total cell number was also changed by these treatments which indicate that extracts not only induce cell death but also inhibit cell proliferation. The effects of aqueous aerial and root extracts of E. boissieri were compared. The percent decrease in cell survival was calculated for each independent experiment and the average of five experiments is shown in Table 1. Using live/dead viability assay as a marker of both viable and nonviable cells, similar results were obtained [Table 2].
Figure 3.
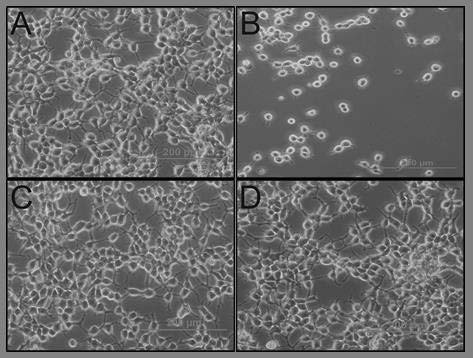
293T cells were seeded in 12-well plates (50,000 cells/well) and the number of live and dead cells was determined by the tryphan blue exclusion test and the cells were examined at the end of 72 h. Appearances of the cells untreated (A: Control) and treated (B: Doxorubicin-HCl, C: Aerial extract, D: Root extract) were photographed under a contrast phase microscope (Olympus-IX71).
Figure 4.
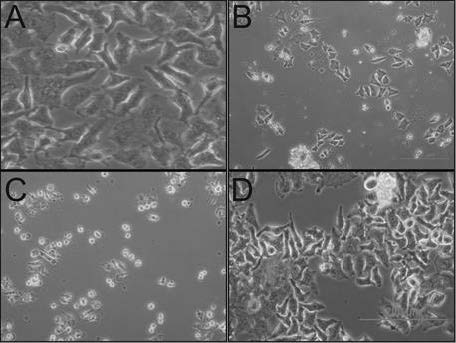
HeLa cells were seeded in 12-well plates (50,000 cells/well) and the number of live and dead cells was determined by the tryphan blue exclusion test. Appearances of the cells untreated (A: Control) and treated (B: Doxorubicin-HCl, C: Aerial extract, D: Root extract) were photographed under a contrast phase microscope (Olympus-IX71) at the end of 72 h.
Table 1.
Trypan blue test results
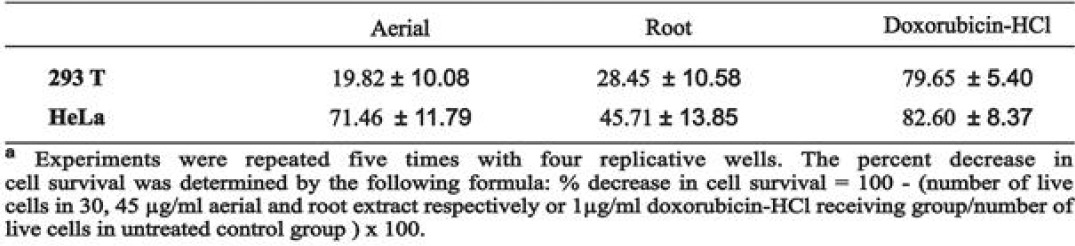
Table 2.
Live-dead test results
Effects of aerial and root extracts on expression of apoptosis-related proteins
Apoptosis plays a significant role in the progression of tumorigenesis and is believed to be deregulated in cancer. Recently, the most popular therapeutic goal for cancer is to trigger apoptotic death selectively in tumor cells. Most of the chemotherapeutics kill the cancer cells by initiating the apoptotic pathway.[22] The activation of caspase-3 which is mediated through proteolytic cleavage of procaspase-3 via upstream caspases (caspase 7/9 or caspase-8) is the key biochemical event involved in the induction of apoptosis. The caspase-3 activity, involved in both mitochondrial (extrinsic) as well as death receptor (intrinsic) pathways of apoptosis, was expected in drug induced cytotoxicity of cancer cells.[23] In the present study, for HeLa cells, exposure to aerial and root extracts of E. boissieri led to induction of activity of all the caspases studied (-2, -3, -6, -8 and -9), while significant increase was observed in the activity of caspase-3. There was a time-dependent increase in all caspase activity, suggesting that extracts trigger cells to undergo apoptosis. The effect of both aerial and root extracts on activity of the initiator caspase-2, -8, and -9 as well as the executioner caspase-3 and -6 is shown in Figure 5A and B. For HeLa cells, exposure to the aerial extract led to 1.9, 3.8, 1.2, 2.4, and 3.45 fold induction of all caspases activities (-2, -3, -6, -8, and -9, respectively) [Figure 5A]. Similarly, exposure to the root extract caused 1.3, 2.9, 1.09, 1.73, and 2.6 fold increase in all caspase activities (-2, -3, -6, -8, and -9, respectively) [Figure 5B].
Figure 5.
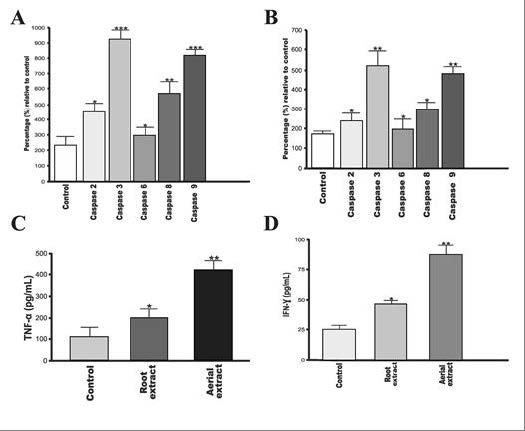
Effects of aerial and root extracts on expression of apoptosis-related proteins. Both (A) aerial (B) and root extracts induced all caspases activation in cervical cancer cells. 5 μmol/mL of reversible aldehyde inhibitors, were used to confirm that the observed fluorescence signal in cell population is due to the activity of caspase-like proteases. (C) TNF-α (D) and IFN-γ protein expression is significantly induced in the cells. The expression of TNF-α and IFN-γ in HeLa cells following treatment for 72 h with aqueous aerial or root extracts was determined by ELISA. Each data value indicates the mean ± SEM of four independent experiments. ** P < 0.01 and *** P < 0.001, significantly different as compared with the control group; Student's t-test.
Moreover, lysates of treated cells were subjected to western blot analysis and the results showed that the protein expression of the active procaspase-3 was significantly decreased due to transform to active form, associated with the disappearance of inactive procaspase-3. To confirm whether induction of apoptosis is actually due to caspase-3 activation, studies using specific inhibitor of caspase-3 were conducted and revealed a significant reduction in the induction of extract-mediated apoptosis. Given the fact that the ability of extracts of E. boissieri to induce apoptosis in HeLa cells. According to these results the extracts of E. boissieri will be a promising candidate for cervical cancer treatment.
The multifunctional cytokines TNF-α and IFN-γ were found to play important role in apoptosis and cancer as well as in inflammation and immunity.[24,25] IFN-γ potentially activates caspase and therefore results in subsequent cell death.[26] We examined the potential effects of aerial and root extracts on TNF-α and IFN-γ concentrations on account of their roles in activation of apoptosis by an ELISA according to the manufacturers’ protocol. Both 30 μg/mL of aerial and 45 μg/mL of root extracts allowed the production of anticancer cytokines in HeLa cell culture supernatants. The levels of TNF-α and IFN-γ showed considerable increase (P < 0.05) in HeLa culture supernatants treated with either aerial or root extract in comparison with the control group (untreated cells) [Figure 5C and 5D]. Similarly, western blot analysis clearly shows that incubation of HeLa cells with extracts resulted as a significant increase in the expression of TNF-α and IFN-γ at protein levels [Figure 6].
Figure 6.
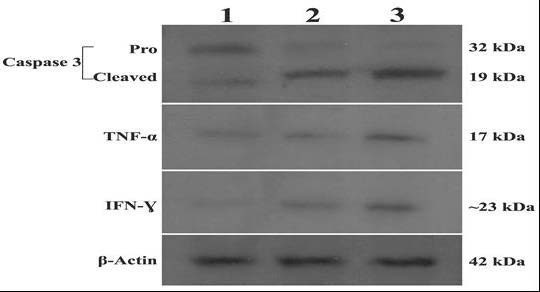
Lysates from HeLa cells were incubated with 30 μg/mL aerial (Lane 2) or 45 μg/mL root (Lane 3) extracts were analyzed by Western blotting for caspase-3, TNF-α and IFN-γ protein expression levels. Aerial- or root-treated cells show cleaved (activated) caspase-3, whereas untreated (Lane 1) cells have no cleaved caspase-3. β-actin was used as internal control.
The data presented here demonstrate for the first time that E. boissieri aerial extract exhibited a high antiproliferative activity and was a potent inducer for the immunoregulatory cytokines TNF-α and IFN-γ in HeLa cervical cancer cells. The activation of TNF-α expression by the aerial extract in cervical cancer cells most likely occurs with the involvement of increased expression of IFN-γ that directly binds to caspases. Our results showed both aerial and root extract appear to be nontoxic to normal 293T cells as compared to their effects on cervical cancer cells;hence, we did not record any induction in secretion of TNF-α and IFN-γ in 293T cells.
CONCLUSIONS
The potential cytotoxic activity of E. boissieri extracts against cervical cancer cells was investigated in this in vitro experimental study. For the first time, the results in this study revealed that aqueous extracts of E. boissieri induce apoptosis in cervical cancer cells by altering the amount of caspase-3, TNF-α, and IFN-γ. Though the active ingredients and precise mode of action of this plant are not yet clarified, its potential antitumor and immunomodulatory activity, along with the selectivity against cancer cells observed in vitro, suggest that aqueous extracts of this plant are avail additional studies. Further chemical and pharmacological investigations to identify the active constituents of this plant and also to screen their mechanisms of action are recommended for anticancer drug discovery.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest
Acknowledgement
This work was supported by The Scientific Research Project Coordination Unit of Akdeniz University (Project no: 2012.06.0115.032) and Akdeniz University, Science Faculty, Department of Biology. We thank to Akdeniz University, Medicine Faculty, Department of Histology and Embryology for their technical support in photographing the cell images.
REFERENCES
- 1.Ganesh G, Abhishek T, Saurabh N, Sarada NC. Cytotoxic and apoptosis induction potential of Mimusops elengi L. in human cervical cancer (SiHa) cell line. J King Saud University-Science. 2014;26:333–337. [Google Scholar]
- 2.Ng WK, Yazan LS, Ismail M. Thymoquinone from Nigella sativa was more potent than cisplatin in eliminating of SiHa cells via apoptosis with down-regulation of Bcl-2 protein. Toxicol In vitro. 2011;25:1392–8. doi: 10.1016/j.tiv.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 3.Berrington DL, Lall N. Anticancer activity of certain herbs and spices on the cervical epithelial carcinoma (HeLa) cell line. Evid Based Complement Alternat Med 2012. 2012 doi: 10.1155/2012/564927. 564927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flay LD, Matthews JH. The effects of radiotherapy and surgery on the sectual function of women treated for cervical cancer. Int J Radiat Oncol Biol Phys. 1995;31:399–404. doi: 10.1016/0360-3016(94)E0139-B. [DOI] [PubMed] [Google Scholar]
- 5.Panchal RG. Novel therapeutics strategies to selectively kill cancer cells. Biochem Pharmacol. 1998;55:247–52. doi: 10.1016/s0006-2952(97)00240-2. [DOI] [PubMed] [Google Scholar]
- 6.Watson AJ. Manipulation of cell death-the development of novel strategies for the treatment of gastrointestinal disease. Aliment Pharm Ther. 1995;9:215–26. doi: 10.1111/j.1365-2036.1995.tb00376.x. [DOI] [PubMed] [Google Scholar]
- 7.Fisher DE. Apoptosis in cancer therapy: crossing the threshold. Cell. 1994;78:539–42. doi: 10.1016/0092-8674(94)90518-5. [DOI] [PubMed] [Google Scholar]
- 8.Cao W, Li XQ, Wang X, Fan HT, Zhang XN, Hou Y, et al. A novel polysaccharide isolated from Angelica sinensis (Oliv) Diels induces the apoptosis of cervical cancer HeLa cells through an intrinsic apoptotic pathway. Phytomedicine. 2010;17:598–605. doi: 10.1016/j.phymed.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Wajant H. The Fas signaling pathway: more than a paradigm. Science. 2002;296:1635–6. doi: 10.1126/science.1071553. [DOI] [PubMed] [Google Scholar]
- 10.Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369–77. doi: 10.1016/s0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]
- 11.Esakkirajan M, Prabhu NM, Arulvasu C, Beulaja M, Manikandan R, Thiagarajan R, et al. Anti-proliferative effect of a compound isolated from Cassia auriculata against human colon cancer cell line HCT 15. Spectrochim Acta A. 2014;120:462–6. doi: 10.1016/j.saa.2013.09.102. [DOI] [PubMed] [Google Scholar]
- 12.Huber-Morath A, Ebenus L. Flora of Turkey and the East Aegean Island. Vol. 3. Edinburgh: Edinburgh University Press; 1970. pp. 590–6. [Google Scholar]
- 13.Ren S, Zhang H, Mu Y, Sun M, Liu P. Pharmacological effects of Astragaloside IV: a literature review. J Trad Chin Med. 2013;33:413–6. doi: 10.1016/s0254-6272(13)60189-2. [DOI] [PubMed] [Google Scholar]
- 14.Aydemir EA, Oz ES, Gokturk RS, Ozkan G, Fiskin K. Glycyrrhiza flavescens subssp. Antalyensis exerts antiproliferative effects on melanoma cells via altering TNF-α and IFN-α. Food Chem Toxicol. 2011;49:820–8. doi: 10.1016/j.fct.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Tsai YL, Chiu CC, Yi-Fu Chen J, Chan KC, Lin SD. Cytotoxic effects of Echinacea purpurea flower extracts and cichoric acid on human coclon cancer cells through induction of apoptosis. J Ethnopharmacol. 2012;143:914–919. doi: 10.1016/j.jep.2012.08.032. [DOI] [PubMed] [Google Scholar]
- 16.Machana S, Weerapreeyakul N, Barusrux S. Anticancer effect of the extracts from Polyalthia evecta against human hepatoma cell line (HepG2) Asian Pac J Trop Biomed. 2012;2:368–74. doi: 10.1016/S2221-1691(12)60058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung WT, Lee SH, Kim JD, Sung NS, Hwan B, Young L, et al. Effect of the extracts from Glycyrrhiza uralensis fisch on the growth characteristics of human cell lines: Anti-tumor and immune activation activities. Cytotechnology. 2001;37:55–64. doi: 10.1023/A:1016111713056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jo EH, Kim SH, Ra JC, Kim SR, Cho SD, Jung JW, et al. Chemo preventive properties of the ethanol extract of chinese licorice (Glycyrrhiza uralensis) root: induction of apoptosis and G1 cell cycle arrest in MCF-7 human breast cancer cells. Cancer Lett. 2005;230:239–47. doi: 10.1016/j.canlet.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 19.Aydemir E, Simsek E, Imir N, Göktürk RS, Yesilada E, Fiskin K. Cytotoxic and apoptotic effects of Ebenus boissieri Barbey on human lung cancer cell line A549. Pharmacogn Mag. 2015;11:S37–45. doi: 10.4103/0973-1296.157679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sreelatha S, Jeyachitra A, Padma PR. Antiproliferation and induction of apoptosis by Moringa oleifera leaf extract on human cancer cells. Food Chem Toxicol. 2011;49:1270–5. doi: 10.1016/j.fct.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Kang SC, Lee CM, Lee JH, Oh JS, Kwak JH, Zee OP. Evaluation of oriental medicinal herbs for estrogenic and antiproliferative activities. Phtother Res. 2006;20:1017–9. doi: 10.1002/ptr.1987. [DOI] [PubMed] [Google Scholar]
- 22.Kim SC, Park SJ, Lee JR, Seo JC, Yang HY, Byun SH. Cytoprotective activity of Glycyrrhiza radix extract against arsenite-induced cytotoxicity. Evid Based Complement Alternat Med. 2008;5:165–71. doi: 10.1093/ecam/nem014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–8. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 24.Jerlinek D, Lipsky P. Enhancement of human B cell proliferation and differentiation by tumor necrosis factor-A and interleukin 1. J Immunol. 1987;139:2970–6. [PubMed] [Google Scholar]
- 25.Jourdan M, Tarte K, Legouffe E, Brochier J, Rossi JF, Klein B. Tumor necrosis factor is a survival and proliferation factor for human myeloma cells. Eur Cytokine Netw. 1999;10:65–70. [PMC free article] [PubMed] [Google Scholar]
- 26.Guan YQ, Li Z, Liu JM. Death signal transduction induced by co-immobilized TNF- α plus IFN- γ and the development of polymeric anti-cancer drugs. Biomaterials. 2010;31:9074–85. doi: 10.1016/j.biomaterials.2010.08.044. [DOI] [PubMed] [Google Scholar]


