Abstract
Background:
The garden snail, Helix aspersa, is a big land snail widely found in the Mediterranean countries. It is one of the most consumed species and widely used in zootherapy.
Objective:
The present study was carried out to investigate for the first time the first time the antitumor activity of an aqueous extract from Helix aspersa.
Materials and Methods:
The effect of H. aspersa extract was studied on a triple negative breast cancer cell line Hs578T. Firstly, the morphological changes and the mode of cell death induced by the extract have been evaluated by microscopy and acridine orange/ethidium bromide staining. The effect of the extract at dilution 0.1% and 1% was then tested on some genes, regulators of cell death and proliferation like tumor necrosis factor α (TNFα), NF- κB, and the tumor suppressor genes P53 and PTEN.
Results:
Data demonstrate that the extract induces necrosis in tumor cells. It enhances significantly the expression of TNFα; mRNA levels were 20 and 10 times more important in treated cells compared to nontreated cells. NF-κB and PTEN were inhibited with the dilution 1% after 8 and 24 hours of treatment. P53 expression was further inhibited but only with the highest dose, after 4, 8, and 24 hours.
Conclusion:
Our results show that H. aspersa extract has an antitumor activity against Hs578T cells; it is a potent stimulator for TNFα and a good inhibitor for NF-κB.
Abbreviations used: AO: acridine orange; Bcl-2: B cell lymphoma 2. cDNA: complementary DNA; ELISA: enzyme linked immunosorbent assay; EB: ethidium bromide; IC50: the half maximal inhibitory concentration; mRNA: messenger RNA. MAPK: mitogen-activated protein kinase; NF-κB: nuclearfactorkappa B; PBS: phosphate buffered saline. PI3K: phospho-inositol 3 kinase; PTEN: phosphatase and tensin homolog; ROS: reactive oxygen species. RT-PCR: reverse transcription polymerase chain reaction; TNFα: tumor necrosis factor alpha. TNFR1: TNF receptor-1; TP53: tumor protein 53
Keywords: Breast cancer, Helix aspersa, necrosis, NF-Kβ, TNFα
INTRODUCTION
After cardiovascular disease, cancer is the second leading cause of death in the world.[1] The treatment is basically on synthetics and chemotherapeutics; these kinds of treatments cause various harmful side effects to human beings.[2] For this reason, the search for new natural and safe drugs is the aim of the different laboratories in the world. Plant and marine species are the major source of natural drugs; few researches have been interested in purifying bioactive molecules from other terrestrial consumed species like snails.
Snails are a member of mollusk, the second largest phylum in the animal kingdom with about 100,000 living species. One of the most consumed species is the garden common snail, Helix aspersa; it is a small nutrient with high contents of proteins and minerals and low contents of fat and cholesterol.[3,4]
Snails have been used in medicine since antiquity and are prepared by several methods; they are recommended for stomach pain, vertigo, nephritis, and respiratory and cardiovascular diseases.[5] Nowadays, lectins from snails are used as a marker for metastatic tissues in breast and colon cancers.[6,7]
Initially, we have observed that the Helix aspersa extract had an antitumor effect on breast cancer cell line Hs578T.[8] This finding led us to investigate its mode of action and understand the mechanism involved. In particular, we focused on the expression of TNFα, a stimulator of the extrinsic pathway of apoptosis;[9] NF-κB, a cell proliferation regulator[10] and the tumor suppressor genes P53 and PTEN.[11]
TNF α is a key cytokine that plays an important role in inflammation, immunity, and diverse cellular events. It modulates cell proliferation, necrosis, and apoptosis.[12] The major source of TNFα is activated macrophages; it can also be produced by other cells including fibroblasts, astrocytes, kuppfer cells, smooth muscles, keratinocytes, and tumor cells. It contributes to the metastatic process, invading the host tissues, penetrating to blood vessel's endothelium and establishing tumor cell growth at secondary sites.[13]
NF-KB, a transcription factor that plays important roles in cancer development, appears to be involved in the regulation of cell proliferation, apoptosis control, angiogenesis promotion, and invasion/metastasis stimulation.[14]
P53 is encoded by the TP53 gene, located at 17p13; it plays an important role in mediating cell response to various stresses,[15] mainly inducing or repressing a number of genes involved in cell cycle arrest, senescence, apoptosis, DNA repair, and angiogenesis.[16] Indeed, P53 mutation is associated with more aggressive disease and worse overall survival, like breast and prostate cancers.[17]
Next to P53, PTEN is the most common tumor suppressor to be lost or inactivated in human cancers like glioblastoma, prostate, and breast cancers.[18] The PTEN gene encodes a dual specificity lipid and protein phosphatase. PTEN modulates cell growth, migration, and survival by antagonizing the phospho-inositol 3 kinase (PI3K)/AKT signaling.[19]
MATERIALS AND METHODS
Cell culture: Human breast cancer Hs578T cell line was obtained from the laboratory of functional genomics and experimental pathology of the oncologic institute “Ion Chiricuta” (Cluj Napoca- Roumania). The cells were cultured at 37°C and humidified atmosphere of 5% CO2, in Dulbecco's Modified Eagle's Medium high glucose supplemented with 1% nonessential amino acids, 1% L-glutamine (200 mM), 1% gentamicin (10 mg/ml), 1% insulin, and 10% fetal bovine serum. All reagents were purchased from Sigma Aldrich, Germany.
Preparation of H. aspersa extract: The H. aspersa snails were maintained fasting during at least 2 weeks in order to empty their gut. Before use the snails were washed in 3 or 4 bath of 10% Nacl solution (to completely eliminate their mucus). After removing the shell, the snails were homogenized. Three volumes of water per one volume of wet tissues were then added to the homogenate. The crude extract was filtered and centrifuged for 10 minutes at 5000 g. The supernatants were collected and sterilized using 22 μm Millipore filter and served as extract to treat cells. The final concentration of the extract was 0,33 g/l.
Acridine orange/Ethidium bromide staining: Cells were seeded for 24 hours in the presence of H. aspersa extract; at 0.1% and 1% dilutions of crude extract. Cells were stained by Acridine orange (AO)/ethidium bromide (EB), the dye mix for staining was 100 μg/ml AO and 100 μg/ml EB in PBS (pH = 7).[20]
RT-PCR
To study gene expression, cells were treated with the extract at different times 4, 8 and 24 hours.
Total cellular RNA was isolated using TRiagen (Sigma, Germany) and converted to cDNA with The Random Hexamer Transcriptor First Strand cDNA Synthesis Kit (Roche Diagnosis, Germany) following manufacturer's instruction.
Quantitative RT-PCR was performed using TaqMan Master Kit and light cycler. All samples samples were run in triplicates and the β actin was amplified as an internal control. Primers used are presented in Table 1.
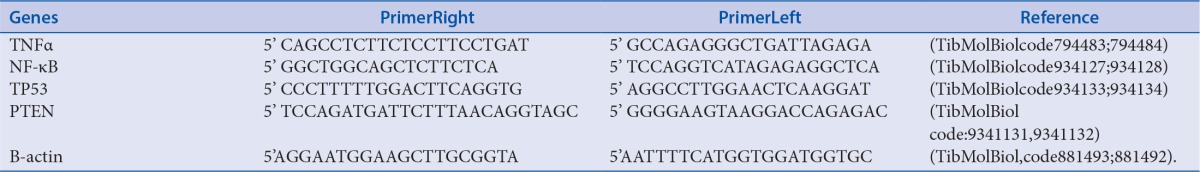
Table 1.
Primers used in RT-PCR
Quantification of relative gene expression was done using the competitive threshold cycle CT method. CT values were averaged for triplicate wells and subtracted from corresponding CT of internal reference RNA to obtain ΔCT values. The averaged control ΔCT was subtracted from the experimental ΔCT to yield ΔΔCT. The fold change was calculated as 2-ΔΔCT for experimental versus control.[21]
ELISA Assay: The concentration of TNFα in culture medium was determined by ELISA assay and using TNFα human Boster immunoleader kit (Cliniscience France).
STATISTICS Data were expressed as mean ± SD from at least three separate experiments performed on triplicate samples. The differences between experimental conditions and controls were analyzed using test t (p.<.0.05 is considered statistically significant). Statistical analyses were carried out using Graph Pad Prism software (free trial).
RESULTS
Morphological identification of cell death
Cells were treated with two doses of H. aspersa extract, the first dose corresponded to the 1% dilution (1:100 dilution of filtered extract) which is approximately equal to IC50.[8] The second dose was 10 times less than the first one.
To determine if H. aspersa extract induces necrosis or apoptosis in breast cancer cell line Hs578T, cells were stained by AO/EB.
AO/EB staining provides a reliable method to measure cells in different compartments of cell death; live, apoptotic, and necrotic cells were differentiated using fluorescence microscopy. AO (excitation: 502 nm; emission: 525 nm) permeates all cells and makes the nuclei appear green. It will also enter acidic compartments such as lysosomes and become protonated and sequestered. In low pH conditions such as this, AO will emit orange fluorescence (emission ~590 nm) when excited by blue light (475 nm). EB (exitation: 360 nm, emission: 590 nm) is only taken up by cells when cytoplasm membrane integrity is lost and stains nuclei in red. Therefore, live cells (showed in Figure 1A by a green arrow) have normal green nuclei. Apoptotic cells (showed in Figure 1C by a yellow arrow) have a bright green nucleus with condensed or fragmented chromatin and cells that have died from necrosis have an orange/yellow nucleus (showed in Figure 1B and 1C by a red arrow).
Figure 1.
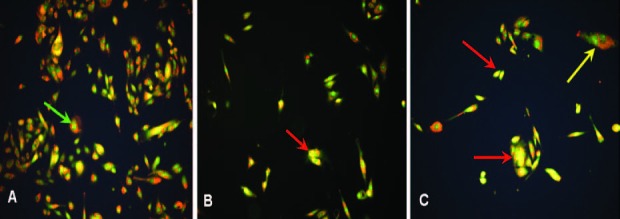
Microscope fluorescence image of Hs578T cells treated with H. aspersa extract and stained by AO/EB observed at 100X magnification: (A) Control. (B) Cells treated by the dilution 0.1%. (C) Cells treated by the dilution 1%. Live cell showed by a green arrow, apoptotic cell showed by a yellow arrow, necrotic cell showed by a red arrow.
The analysis of the fluorescence and comparison between the two doses [Figure 1B and 1C] indicates that the extract induces necrosis. At 1%, the presence of some apoptotic cells has been also noted. Thus, the extract induces necrosis rather than apoptosis in Hs578T cell line.
Effect of H. aspersa extract on TNFα expression
TNFα mRNA (messenger RNA) levels were clearly elevated, after 4 and 8 hours, in Hs578T treated samples as indicated in Figure 2.
Figure 2.
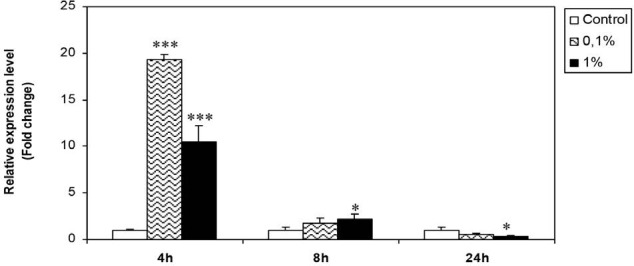
Effect of H. aspersa extract on the expression of TNFα mRNA in breast cancer cell line Hs578T (*significant p < 0.05, *** significant p < 0.001, compared to control)
After 4 hours, TNFα expression was 20 and 10 times more important with dilutions 0.1% and 1%, respectively, after treatment. Statistically and compared to controls, the increase of TNFα mRNA was highly significant (p < 0.001).
After 8 hours, mRNA levels were decreased, but they remained significantly higher than those registered in control cells, especially with the 1%. dilution.
After 24 hours of treatment with the two doses, TNFα expression has been significantly inhibited (p < 0.05).
Results from ELISA assay [Figure 3] showed that TNFα concentrations rise after 8 hours of exposition to H. aspersa extract, and still higher after 24 hours. This data confirms that the extract stimulates TNFα production. In addition, the dose 0.1% has a more stimulatory effect.
Figure 3.
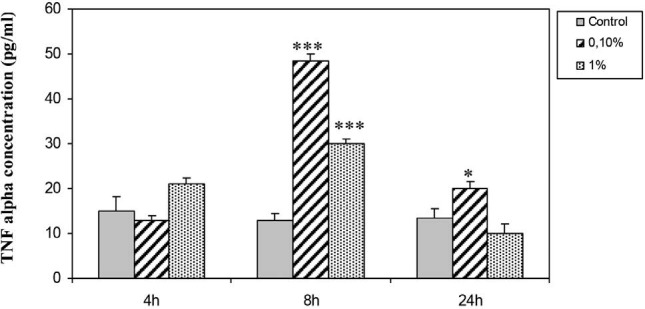
Effect of H. aspersa extract on TNFα concentration in culture supernatant of breast cancer cell line Hs578T (* significant p < 0.05, *** significant p < 0.001, compared to control)
Effect of H. aspersa extract on NF-κB expression
Treatment of Hs578T cells by H. aspersa extract at 0.1% decreases NK-κB expression after 4, 8, and 24 hours compared to control. At 1%, the effect of the extract was observed after 8 and 24 hours, we noted a significant decrease of NF-κB expression compared to nontreated cells [Figure 4]. The highest dose (1%) had more inhibitory effect on NK-κB.
Figure 4.
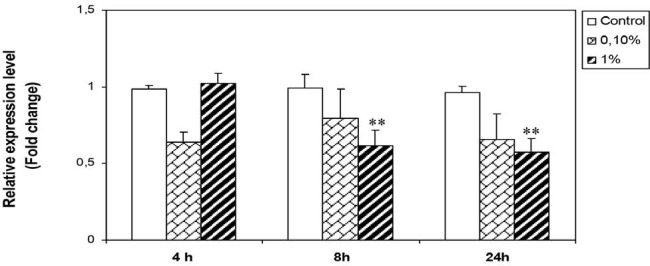
Effect of H. aspersa extract on the expression of NF-κB mRNA in breast cancer cell line Hs578T (**significant p < 0.01, compared to control).
Effect of H. aspersa extract on PTEN expression
Treatment of Hs578T cells with H. aspersa extract resulted in the diminution of PTEN expression levels with the two doses 0.1 and 1%. A modest increase in PTEN expression was observed after 4 hours of treatment with the lowest dose (0.1%). The highest dose (1%), however, had more inhibitory effect (p < 0.05) [Figure 5].
Figure 5.
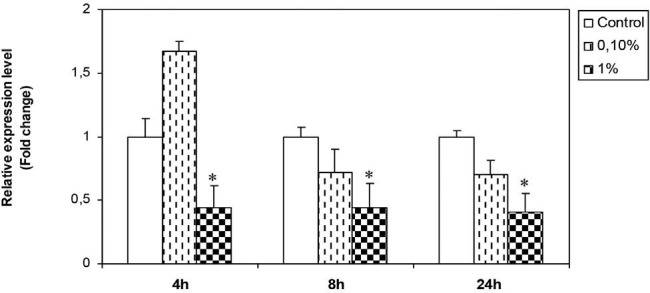
Effect of H. aspersa extract on the expression of PTEN mRNA in breast cancer cell line Hs578T (*significant p < 0.05, compared to control)
Effect of H. aspersa extract on P53 expression
From Figure 6, we note that, no significant change has been registered with the 0.1% dilution. At 1% the extract significantly decreases p53 mRNA levels after 8 and 24 hours of treatment.
Figure 6.
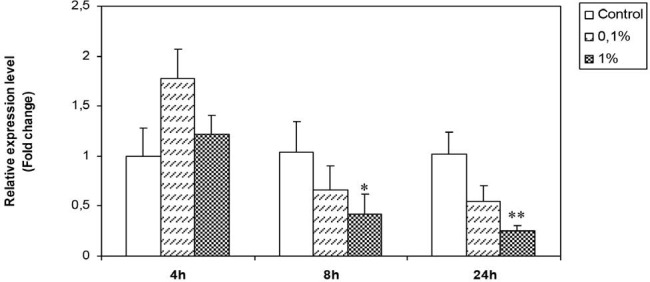
Effect of H. aspersa extract on the expression of p53 mRNA in breast cancer cell line Hs578T (*significant p < 0.05, ** significant p < 0.01, compared to control).
DISCUSSION
The main aim of this study was to determine the mode of action of the aqueous extract from H. aspersa on breast cancer cells. The fluorescence staining shows that H. aspersa induces necrosis in Hs578T cells; at the same time it enhances the TNFα expression significantly. This cytokine had been demonstrated to stimulate both apoptosis and necrotic cell death, depending on cell type and treatment conditions.[22] The necrosis induced by our extract seemed to be mediated by high levels of TNFα. The extract has stimulated the expression and secretion of TNFα, in culture medium. Therefore, the cytokine stimulates its specific receptor and act by autocrine mode.
The TNFα induced necrosis is a form of cell death called “necroptosis” that resulted from an incomplete execution of apoptosis program, in which caspases and NF-κB are inhibited. These characteristics are in accordance with our results because H. aspersa extract blocked the apoptotic process and the complete apoptosis has been revealed only in some cases.
TNFα enhancement in our experiments may have resulted from the effect of H. aspersa lectins, and change of glycozylation at the surface of tumor cells. A lectin from Helix pomatia, a similar species of snails, has been shown to recognize diverse epitopes on tumor cells like O-linked α-N-acetylgalactosamine on Tn epitopes [6–7], integrin α6, transcription factors heterogeneous nuclear ribo-nuclear protein (HnRNPs), heat shock protein 27 (Hsp27) and enolase 1 (ENO1) in breast and colon tumor cells. H. pomatia lectin binding was also observed in the golgi apparatus in the T47D and MCF7 cells.[23]
Indeed, a similar augmentation of TNFα expression had been registered with lectin extracted from mushroom.[24]
Another explanation is that H. aspersa extract has a pro-oxidant activity and may act by generation of reactive oxygen species (ROS). A high level of ROS can lead to necrotic cell death while, a low level leads to apoptotic cell death.[25] Moreover, Sakon et al., 2003 have reported that TNF stimulation leads to accumulation of ROS, which is essential for prolonged mitogen-activated protein kinase (MAPK) activation and cell death.[26] TNF receptor-1 (TNFR1) has been shown to initiate necrotic cell death[27] and leads to the generation of ROS, which functions as second messengers in the necrotic cell death pathway.[28]
Furthermore, our results show that H. aspersa extract is a good inhibitor of NF-KB. Recent studies have reported that inhibition of NF-KB alone or in combination with cancer therapies leads to tumor cell death or growth inhibition.[14] Li et al., 2003 have reported that the increased ROS generation might contribute to the induction of apoptosis and revealed that NF-KB activity was almost completely inhibited by preventing the degradation of IkBα. Additionally, the level of bcl-2 was decreased.[29]
The P53 and PTEN downregulation induced by H. aspersa extract is the result of TNFα overexpression. Different contradictory effects of TNFα have been reported on P53 expression. It was shown to increase P53 expression with HT29 cells and to decrease or minimally change its expression in HCT116 cells.[30]
Treatment of cancer cell lines with TNFα decreases PTEN expression and the overexpression of TNFα lowers PTEN expression via TNFα/NIK/NF-kB pathway.[31]
Currently, anticancer drugs are developed with the aim to maximize apoptosis. However, cancer cells eventually become resistant to these therapeutics and multiple mechanisms may contribute to resistance to apoptosis. So, necrosis is an alternative pathway with fewer mechanisms of resistance compared to apoptosis. Targeted necrosis has potential clinical utility because this cell death mechanism retains the cancer cell specificity of apoptosis and bypasses the apoptotic resistance by redirection into necrosis.[32]
In addition, Olofsson et al., 2007 have shown that chemotherapy induces more necrotic than apoptotic cell death in breast cancer patients, and this necrotic response is associated with a better survival.[33] So the induction of necrosis by H. aspersa extract may be an interesting characteristic and can open a new perspective in cancer therapy.
CONCLUSION
Our results demonstrate for the first time that H. aspersa extract has a high cytotoxicity against breast cancer cells. The extract can be used as an associated treatment, in chemotherapy that enhances tumor sensitivity by downregulating BcL2[8] and NF-κB; because tumor resistance is usually correlated with overexpression of these factors. Although the propriety of H. aspersa extract as an inducer of necroptosis has to be further clarified, the present study opens new perspectives in the search for new natural anticancer drugs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors are thankful to Dr Bensouyad Abdelhalim from Constantine University for language revision.
REFERENCES
- 1.Amin A, Moussa M. Merits of anti cancer plants from the Arabian Gulf region. Cancer therapy. 2007;5:55–66. [Google Scholar]
- 2.Boopathly NS, Kathiresan K. Anti cancer drugs from marine flora: An overview. J. Oncol. 2010 doi: 10.1155/2010/214186. article ID 214186, 18 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cagiltay F, Erkan N, Tosun D, Selcuk A. Amino acid, Fatty acid, vitamin and mineral contents of Edible garden snail (Helix aspersa) Journal of Fisheries Sciences. 2011;5:354–63. [Google Scholar]
- 4.Ozogul y, Ozygul F, Olgunoglu A. Fatty acid profile and mineral content of wild snail Helix pomatia from the region of south of the turkey. Eur. Food. Res. Technol. 2004;221:547–49. [Google Scholar]
- 5.Bonnemain B. Helix and drugs: Snails for western health care from antiquity to the present. eCAM. 2005;2:25–28. doi: 10.1093/ecam/neh057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker RA. Helix pomatia and prognosis and prognosis of breast cancer. Br J Cancer. 1993;68:453–54. doi: 10.1038/bjc.1993.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lescar J, Sanchez JF, Audfay A, Coll JL, Breton C, Mitchell EP, et al. Structural basis for recognition of breast and colon cancer epitopes Tn antigen and forssman disaccharide by helix pomatia lectin. Glycobiology. 2007;17:1077–083. doi: 10.1093/glycob/cwm077. [DOI] [PubMed] [Google Scholar]
- 8.El Ouar I, Braicu C, Naimi D, Irimie A, Berindan-Neagoe I. Anti tumour effect of aqueous extract from Helix aspersa. Int J Pharm Bio Sci. 2013;4:1325–1332. [Google Scholar]
- 9.Elmore S. Apoptosis: A Review of Programmed Cell Death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishikori M. Their Roles in Lymphoid Malignancies. J. Clin. Exp. Hematopathol. 2005;45:15–24. [Google Scholar]
- 11.Liu J, Voisin V, Wang S, Wang DY, Jones RA, Datti A, et al. Combined deletion of PTEN and p53 in mammary epithelium accelerates triple-negative breast cancer with dependency on eEF2K. EMBOMol Med. 2014;6:1542–1560. doi: 10.15252/emmm.201404402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu ZG. Molecular mechanism of TNF signaling and beyond. Cell Research. 2005;15:24–27. doi: 10.1038/sj.cr.7290259. [DOI] [PubMed] [Google Scholar]
- 13.Szlosarek P, Charles KA, Balkwill FR. Tumor necrosis factor α as tumor promoter. Eur j cancer. 2006;42:745–50. doi: 10.1016/j.ejca.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Madonna G, Ullman D, Gentilcore G, Palmieri G, Ascierto PA. NF-κB as potencial target in the treatmnt of melanoma. J Transl Med. 2012;10:53. doi: 10.1186/1479-5876-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lacroix M, Toillon RA, Leclerq G. P53 and breast cancer, an update. Endocrine Related Cancer. 2006;13:293–325. doi: 10.1677/erc.1.01172. [DOI] [PubMed] [Google Scholar]
- 16.Turner N, Moretti E, Siclari O, Migliaccio I, Santarpia L, D’Incalci M, et al. Targeting triple negative breast cancer: Isp53 the answer? Cancer Treat Rev. 2013;39:541–50. doi: 10.1016/j.ctrv.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Gasco M, Shami S, Crook T. The P53 pathway in breast cancer. Breast Cancer Research. 2002;4:70–76. doi: 10.1186/bcr426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okumura N, Yoshida H, Kitagishi Y, Nishimura Y, Matsuda S. Alternative splicings on p53, BRCA1 and PTEN genes involved in breast cancer. Biochemical and Biophysical Research Communications. 2011;413:395–99. doi: 10.1016/j.bbrc.2011.08.098. [DOI] [PubMed] [Google Scholar]
- 19.Saal L H, Gruvberger-Saal S K, Persson C, Lövgren K, Jumppanen M, Staaf J, et al. Recurrent gross mutations of the PTEN tumor suppressor gene in breast cancers with deficient DSB repair. Nat Genet. 2008;40:102–07. doi: 10.1038/ng.2007.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribble D, Goldstein NB, Norris DA, Shellman YG. A simple technique for quantifying apoptosis in 96-well plates. BMC Biotechnology. 2005;5:12. doi: 10.1186/1472-6750-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real time PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–08. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Galluzi L, Kepp O, Kromer G. RIP kinases initiate programmed necrosis. J. Mol. Cell. Biol. 2002;1:8–10. doi: 10.1093/jmcb/mjp007. [DOI] [PubMed] [Google Scholar]
- 23.Rambaruth NDS, Greenwell P, Dwek MV. The lectin Helix pomatia agglutinin recognizes O-GlcNAc containing glycoproteins in human breast cancer. Glycobiology. 2012;22:839–48. doi: 10.1093/glycob/cws051. [DOI] [PubMed] [Google Scholar]
- 24.Mannel DN, Becker H, Gundlt A, Kist A, Franz H, et al. Induction of tumor necrosis factor expression by a lectin from Viscum album. Cancer Immunol Immunother. 1991;33:177–82. doi: 10.1007/BF01756139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kannan K, Jain SK. Oxidative stress and apoptosis. Pathophysiology. 2000;7:153–63. doi: 10.1016/s0928-4680(00)00053-5. [DOI] [PubMed] [Google Scholar]
- 26.Sakon S, Xue X, Takekawa M, Sasazui T, Okazaki Kajma Y, Piao JH. NF-kB inhibits TNF induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO journal. 2003;22:3838–909. doi: 10.1093/emboj/cdg379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Organ MJ, Kim YS, Liu ZG. TNF alpha and reactive oxygen species in necrotic cell death. Cell Res. 2008;18:343–49. doi: 10.1038/cr.2008.31. [DOI] [PubMed] [Google Scholar]
- 28.Shen HM, Pervaiz S. TNF receptor superfamily-induced cell death: redox-dependent execution. FASEBJ. 2006;20:1589–598. doi: 10.1096/fj.05-5603rev. [DOI] [PubMed] [Google Scholar]
- 29.Li HL, Chen DD, Li XH, Zhang HW, Lü YQ, Ye CL, et al. Changes of NF-kB, p53, Bcl-2 and caspase in apoptosis induced by JTE-522 in human gastric adenocarcinoma cell line AGS cells: role of reactive oxygen species. Word j of Gastroentrolo. 2003;8:431–5. doi: 10.3748/wjg.v8.i3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pastor DM, Irby RB, Poritz LS. Tumor mecrosis factor alpha induces p53 up regulated of apoptosis expression in colorectal cancer cell lines. Dis Colon Rectum. 2010;53:257–63. doi: 10.1007/DCR.0b013e3181c522c7. [DOI] [PubMed] [Google Scholar]
- 31.Kim S, Domon-Dekk C, Kang J, Chung DH, Freund JN, Mark Ever B, et al. Down-regulation of the tumor suppressor PTEN by the tumor necrosis factor-alpha/nuclear factor-kappaB (NF-kappaB)-inducing kinase/NF-kappaB pathway is linked to a default IkappaB-alpha autoregulatory loop. J Biol Chem. 2004;279:4285–291. doi: 10.1074/jbc.M308383200. [DOI] [PubMed] [Google Scholar]
- 32.Chaabane W, User SD, El-Gazzah M, Jaksik R, Sajjadi E, Rzeszowska-Wolny J, et al. Autophagy, Apoptosis, mitoptosis and Necrosis: Interdependence between Those Pathways and Effects on Cancer. Arch Immunol Ther Exp. 2013;61:43–58. doi: 10.1007/s00005-012-0205-y. [DOI] [PubMed] [Google Scholar]
- 33.Olofsson M. H, Ueno T, Pan Y. Cytokeratin-18 is a useful serum biomarker for early determination of response of breast carcinomas to chemotherapy. Clin Cancer Res. 2007;13:3198–206. doi: 10.1158/1078-0432.CCR-07-0009. [DOI] [PubMed] [Google Scholar]