Abstract
Background:
Corylopsis coreana Uyeki (Hamamelidaceae) is a medicinal plant cultivated in Northeast Asia. Previously, we have reported that an ethanol extract of Corylopsis coreana Uyeki flos (ECCF) contains four active compounds with antioxidant activity.
Objective:
The aim of this study was to investigate the antimicrobial spectrum against infectious bacteria and anti-inflammatory effect of ECCF in a mouse model of acute local inflammation.
Materials and Methods:
In vitro antimicrobial susceptibility was evaluated using standard plate assay technique. Antimicrobial activities (minimum inhibitory concentration, MIC; μg/mL) were determined with the serial dilution method. In vivo anti-inflammatory activity was studied using a mouse model of carrageenan-induced air pouch inflammation.
Results:
The ECCF showed antimicrobial activities against general bacteria and drug-resistant bacteria including Staphylococcus aureus, Micrococcus luteus ATCC 9341, Mycrobacterium smegmatis ATCC 9341, Mycrobacterium smegmatis ATCC 9341, Salmonella typhimrium KCTC 1925, and nine methicillin-resistant Staphylococcus aureus strains, with MIC values ranging from 250 to 1000 μg/mL. In in vivo mouse model, inflammatory morphologic changes observed in the air pouch membrane were restored to its normal condition by the ECCF treatment. Moreover, the ECCF significantly reduced exudate volumes, protein contents, inflammatory cell counts, and pro-inflammatory cytokine levels in the exudates recovered from air pouches of the mouse model. Flavonoids in the ECCF were found to contain bergenin, quercitrin, and quercetin with reported anti-inflammatory activity via suppressing tumor necrosis factor-α production.
Conclusion:
To the best of our knowledge, this is the first report to demonstrate the antimicrobial and anti-inflammatory activities of ECCF. Our results suggest that the ECCF might potentially serve as an alternative or complementary medicine for treating inflammatory diseases caused by microbial infection.
SUMMARY
ECCF showed antimicrobial activity against infectious bacteria and multidrug-resistant strains.
ECCF exhibited anti-inflammatory activity in a carrageenan-induced air pouch mouse model.
ECCF contained several active constituents such as bergenin, quercitrin, and quercetin.
Abbreviations used: Corylopsis coreana CCF: Uyeki flos, ECCF: ethanol extract of CCF
Keywords: Antimicrobial activity, Corylopsis coreana Uyeki flos, inflammation, TNF-α
INTRODUCTION
Inflammation is an immune response of damaged tissues to injury induced by physical, chemical, or biological attack. During an inflammatory process, neutrophils are the first cells that are attracted toward the injured area to cope with bacteria or antigens to remove them or release inflammatory mediators to attract macrophages to the site of inflammation.[1] Specific immune responses against foreign antigens are generated by macrophages. Together with the release of pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, interferon (INF)-γ, interleukin(IL)-1, and IL-6, they can cause an influx of monocytes, granulocytes, lymphocytes, and mast cells.[2] Pro-inflammatory cytokines and mediators can regulate the functional activities of immune cells. They are also involved in the pathogenesis of inflammatory diseases such as shock, hemorrhagic shock, multiple sclerosis, rheumatoid arthritis, ulcerative colitis, and atherosclerosis.[3] These processes can initiate a multitude of immune pathologic responses to remove infectious bacterium or agents, leading to the restoration of homeostasis in acute and chronic inflammatory disorders.[4]
Recently, a number of plant extracts and phytochemicals therein have attracted increasing attention for the treatment of various inflammatory diseases.[5,6,7,8] Corylopsis coreana Uyeki (Hamamelidaceae) is a medicinal plant cultivated in Northeast Asia.[9] Water extract, decoction, and infusion preparations of flowers of C. coreana Uyeki (CCF) has been used as a folk medicine in South Korea because of its antipyretic and antivomiting properties.[10] Moreover, some species in the genus of Corylopsis such as witch hazel have been widely used as a traditional medicine for treating skin irritation and inflammatory disease.[11] Previously, we have reported that the ethanol extract of the flower of C. coreana Uyeki (ECCF) contained four active components (i.e., quercetin, quercitrin, bergenin, and isosalipurposide) with antioxidant activity.[10] Bergenin, quercetin, and quercitrin have been reported to possess pharmacologic activity against inflammation and microbial infection.[12,13,14] However, to the best of our knowledge, the antimicrobial and anti-inflammatory effects of ECCF have not been reported.
Therefore, we herein investigated the antimicrobial activity against infectious microbes and multidrug-resistant (MDR) strains and anti-inflammatory activity of ECCF. A carrageenan-induced air pouch model was used as an in vivo mouse model of acute local inflammation. Various inflammatory cells and pro-inflammatory cytokines were monitored to evaluate the anti-inflammatory activity of ECCF.
MATERIALS AND METHODS
Plant materials and preparation of ECCF
CCF was collected in May 2013 near Jogye Mountain (Jeonnam, Korea). A voucher specimen (MNUCSS-CC-01) was deposited in the College of Pharmacy, Mokpo National University. The flower was separated for this study. The air-dried and powdered CCF (200 g) was extracted twice with 80% ethanol (1000 mL) at room temperature for 3 days. After filtration, the resultant ethanol extract was evaporated, freeze dried, and stored at -50°C. Crude extract was resuspended in distilled water and filtered using a 0.4 μm membrane.
Antimicrobial activity analysis
In order to find the inhibitory effect of ECCF against infectious and multi-antibiotics resistant microbes, methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Staphylococcus aureus (VRSA), carbapenemase-producing strains (IMP), and extended spectrum β-lactamase-producing strains (ESBL) were used in this study. These strains were kindly provided by professor Jin-Cheol Yoo of Chosun University (Gwangju, Republic of Korea). All strains were stored in Mueller-Hinton broth (Difco, Becton, Dickinson and Company, Franklin Lakes, NJ, USA) with 15% glycerol at -80°C. The standard plate assay technique described by Iwasa et al.[15] was employed to determine the effect of CCF on test strains with slight modifications. Briefly, ECCF (1 mg/40 μL) were loaded onto paper disks (sterilized, Adventec, Toyo, Kaisha Ltd., Tokyo, Japan) and transferred to Mueller–Hinton agar plate containing test strains of 106 colony-forming units.[15] The susceptibilities of the microbes to ECCF were expressed as plus or a minus. If the width of the transparent zone was 1.2 mm or greater, the susceptibility was a plus. However, if the width of the transparent zone was less than 1.2 mm, the susceptibility was a minus. The in-vitro antimicrobial activities expressed with minimum inhibitory concentration (MIC, μg/mL) were determined with the serial dilution method.[16]
Carrageenan-induced air pouch inflammation model in mice
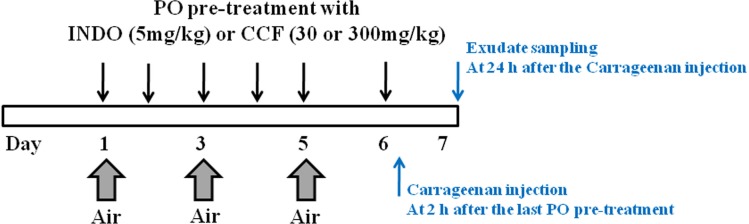
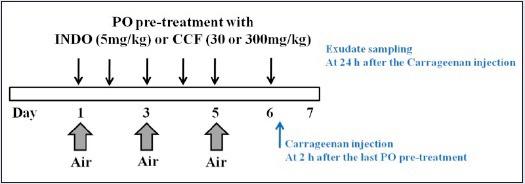
Male ICR mice (20-25 g) were purchased from Orient Bio Inc. (Seongnam, Korea). Animal study was scheduled as shown in Figure 1. To make space (or air pouch) under skin, 2-mL air was inserted three times into the intrascapular area of mice's back for 6 days. Animals were divided into two groups. Animals (n = 6) in the first group were not treated with carrageenan. Animals (n = 24) in the other group were treated with carrageenan. The animal study was repeated once with the same protocol. Carrageenan-treated group consisted of four subgroups: (1) inflammation-induced group was injected with carrageenan after normal saline oral administration, (2) 5 mg/kg indomethacin-treated group (indomethacin was used as an anti-inflammatory drug), (3) 30 mg/kg ECCF-treated group, and (4) 300 mg/kg ECCF-treated group. Indomethacin and ECCF were orally administered for 6 days. At 2 h after treatment, carrageenan solution (1 mL, 2 w/v% dissolved in saline) was injected into the air pouch.
Figure 1.
Schematic diagram illustrating the schedule for the preparation of carrageenan-induced air pouch inflammation mouse model and oral pretreatment with indomethacin (5 mg/kg) or ECCF (30 or 300 mg/kg)
Pro-inflammatory cytokine measurement and histopathological analysis
At 24 h after carrageenan insertion, all mice were sacrificed, and exudate was collected. The numbers of total and differential cells of the exudate in the pouch were counted using Hemavet Multispecies Hematology System (Drew Scientific Inc., Waterbury, CT, USA). The exudate was centrifuged at 10,000 rpm for 20 min at 4°C. The supernatant was used as test sample. Protein concentration was determined using Bradford method.[17] Supernatant was analyzed for TNF-α and IL-6 levels using enzyme-linked immunosorbent assay (ELISA) with commercial kits (BD Biosciences, Franklin Lakes, NJ, USA) according to the manufacturer's instructions. The lower limit of detection of TNF-α and IL-6 were determined to be 1.07 and 3.8 pg/mL, respectively. After harvesting the exudate, the skin tissues were collected, fixed in 10% (v/v) formaldehyde solution, dehydrated in a graded ethanol series (99.9, 90, 80, and 70%), and embedded in paraffin. Paraffin-embedded skin tissues were then sectioned (4 µm) and stained with hematoxylin and eosin.
Ethics statement
All animals were maintained according to the guidelines of the Institutional Animal Care and Use Committee of Dongshin University. Experiments were performed after obtaining approval for the study protocol (Approval No. 2014-08-03).
HPLC conditions
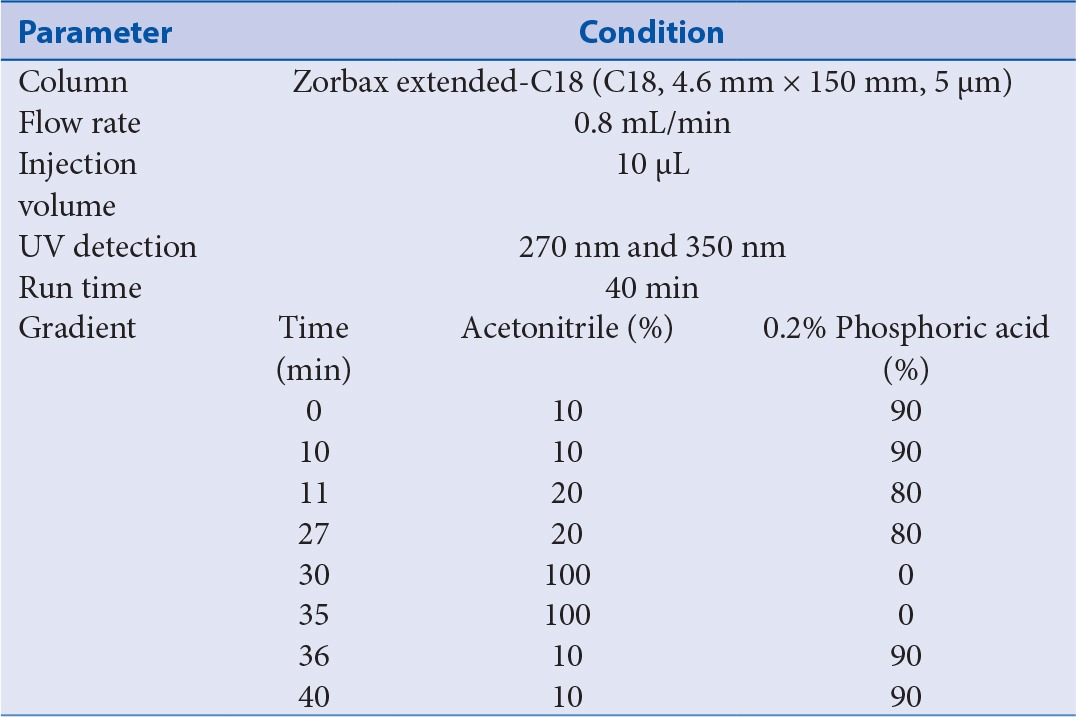
All HPLC analyses were performed on a Waters Alliance 2695 HPLC system with photodiode array detector. An Agilent Zorbax extended C18 (5 µm, 150 mm × 4.6 mm) column was used as the analytical column. Mobile phase was a mixture of solvent A (methanol) and B (water containing 0.1% acetic acid) employing gradient elution (from 10:90 to 100:0, v/v) at a flow rate of 1 mL/min. HPLC conditions are summarized in Table 1. Column temperature was maintained at 25°C. Detection wavelength was set at 270 nm for bergenin and 350 nm for quercetin and quercitrin. Solvent was filtered through a 0.22 µm filter and degassed. Sample injection volume was 10 µL.
Table 1.
Chromatographic conditions of HPLC for the determination of four phytochemicals
Statistical analysis
All data were expressed as mean ± standard deviation. Student's t-test was used for statistical analysis. A P value of less than 0.01 or 0.05 was considered as statistically significant.
RESULTS
Antimicrobial effects of ECCF
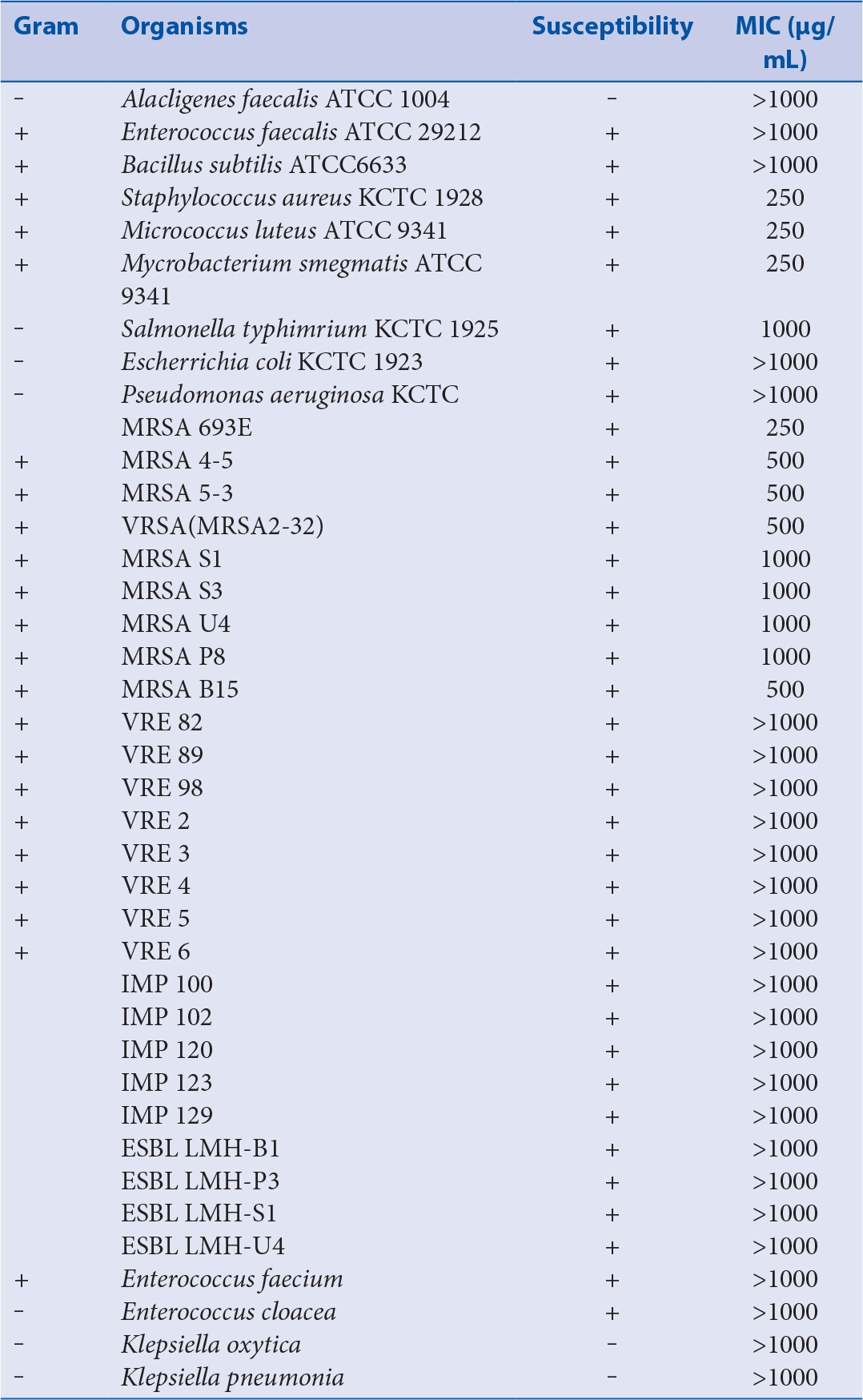
The susceptibilities of various microorganisms to ECCF and their corresponding MIC values are shown in Table 2. The ECCF was found to exhibit significant antimicrobial activities against Staphylococcus aureus, Micrococcus luteus ATCC 9341, Mycrobacterium smegmatis ATCC 9341, Mycrobacterium smegmatis ATCC 9341, Salmonella typhimrium KCTC 1925, and nine MRSA strains, with MIC values ranging from 250 to 1000 μg/mL.
Table 2.
Susceptibilities of various microorganisms to ECCF and their corresponding MIC values
Exudate volumes and protein contents
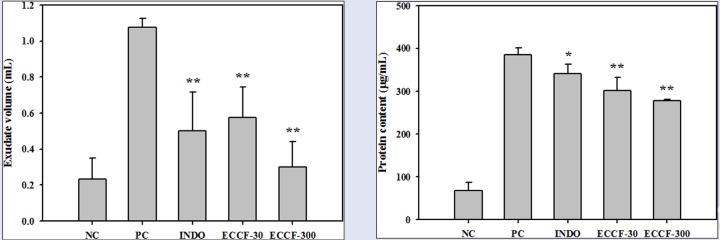
The effects of ECCF on the volumes and protein contents of the exudates recovered from air pouches of normal mice and carrageenan-induced air pouch inflammation mouse model are shown in Figure 2. Subcutaneous injection of carrageenan into air pouches without pretreatment (PC) significantly increased the volumes and protein levels of exudate (by about 4.7- and 5.6-fold, respectively) compared with those in normal mice (NC). Pretreatment with ECCF at doses of 30 and 300 mg/kg (ECCF-30 and ECCF-300, respectively) significantly reduced the volumes of exudates recovered from air pouches and carrageenan-induced protein leakage in a dose-dependent manner.
Figure 2.
The volumes (A) and protein contents (B) of the exudates recovered from air pouches of normal mice (NC) or carrageenan-induced air pouch inflammation mouse model after oral administration of saline (PC), 5-mg/kg indomethacin (IND-5), 30-mg/kg ECCF (ECCF-30), or 300-mg/kg ECCF (ECCF-300) to mice (n = 6)
Inflammatory cell infiltration
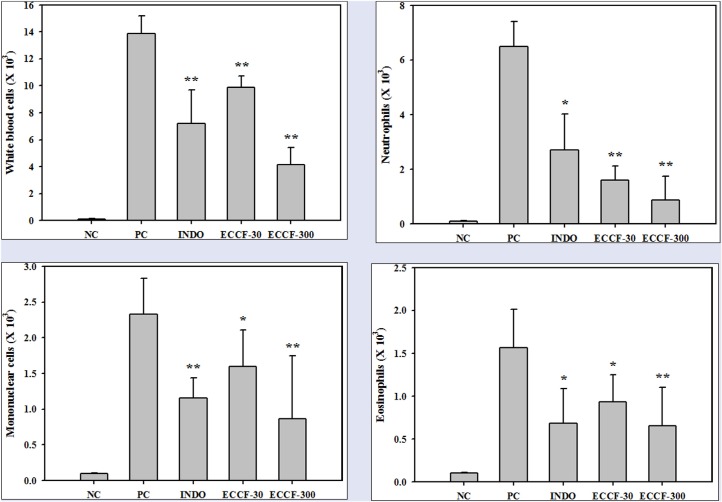
The effects of ECCF on the numbers of white blood cells, neutrophils, mononuclear cells, and eosinophils in the exudates recovered from air pouches of normal mice and carrageenan-induced air pouch inflammation mouse model are shown in Figure 3. The numbers of white blood cells, neutrophils, monocytes, and eosinophils were markedly increased in the carrageenan-injected air pouch exudates (PCs) compared with those in normal mice (NC). Pretreatment with ECCF at doses of 30 and 300 mg/kg (ECCF-30 and ECCF-300, respectively) significantly reduced the numbers of white blood cells (by about 29 and 70%, respectively Figure 3a) and the numbers of neutrophils (by about 58.2 and 75.4%, respectively Figure 3). Additionally, the numbers of monocytes and eosinophils were significantly reduced (by about 62.3 and 59.1%, respectively) by pretreatment with ECCF at dose of 300 mg/kg [Figure 3C and 3D].
Figure 3.
The numbers of white blood cells (A), neutrophils (B), mononuclear cells (C), and eosinophils (D) in the exudates recovered from air pouches of normal mice (NC) or carrageenan-induced air pouch inflammation mouse model after oral administration of saline (PC), 5-mg/kg indomethacin (IND-5), 30-mg/kg ECCF (ECCF-30), or 300-mg/kg ECCF (ECCF-300) to mice (n = 6)
Pro-inflammatory cytokines
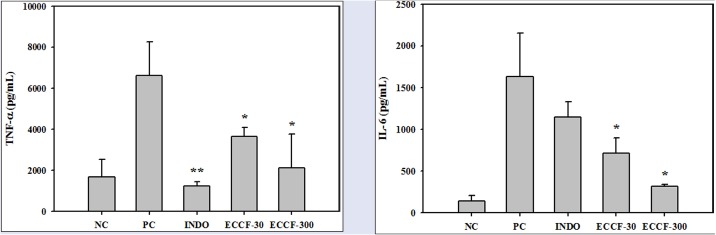
The effects of CCF extract on the levels of TNF-α and IL-6 in the exudate recovered from air pouches of normal mice and carrageenan-induced air pouch inflammation mouse model are shown in Figure 4. Subcutaneous injection of carrageenan into air pouches without pretreatment (PC) significantly increased concentrations of TNF-α and IL-6 in the exudates compared with normal mice (NC). Pretreatment with ECCF at doses of 30 and 300 mg/kg significantly reduced the exudate levels of TNF-α and IL-6. Pretreatment with indomethacin at a dose of 5 mg/kg significantly reduced the exudate levels of TNF-α by 81.3% [Figure 4A]. However, it did not significantly change the exudate levels of IL-6 [Figure 4B].
Figure 4.
Levels of TNF-α (A) and IL-6 (B) in the exudates recovered from air pouches of normal mice (NC) or carrageenan-induced air pouch inflammation mouse model after oral administration of saline (PC), 5-mg/kg indomethacin (IND-5), 30-mg/kg ECCF (ECCF-30), or 300-mg/kg ECCF (ECCF-300) to mice (n = 6)
Histopathological analysis
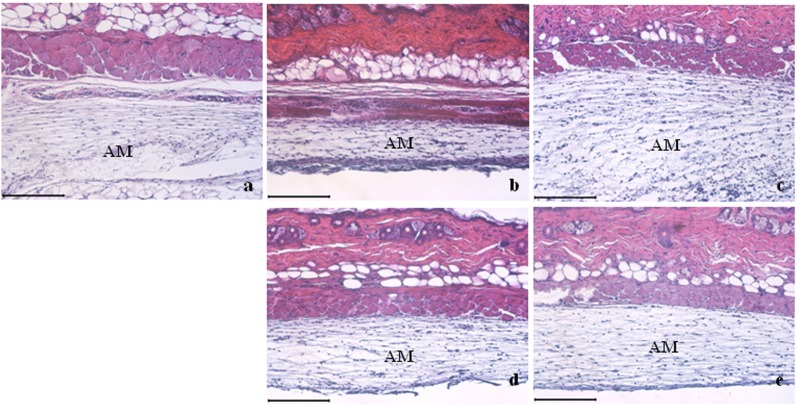
The thickness of skin in the carrageenan treatment group was greater than those in the control and indomethacin-treated group as the size of the air pouch membrane (AM) increased [Figure 5]. The thickness of skin in the control group was similar to that in the indomethacin-treated group. This could have been due to that indomethacin treatment blocked carrageenan-induced inflammatory responses in the air pouch membrane [Figure 5A and 5C]. The thickness of AM in the carrageenan-treated group was smaller than that in all the other groups, indicating that the size of AM was restored to its control level by ECCF treatment in a dose-dependent manner [Figure 5B].
HPLC analysis of ECCF
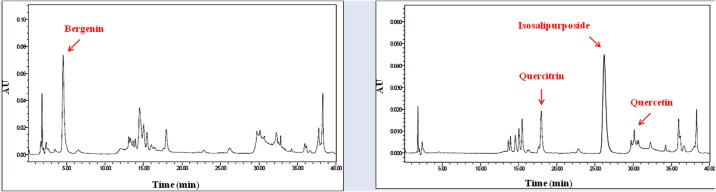
Representative HPLC chromatograms of crude ethanol extract of CCF monitored at UV wavelength of 270 or 380 nm are shown in Figure 6. From the ECCF, we identified three flavonoids (bergenin, quercetin, and quercitrin) and one isocoumarine (isosalipurposide). The contents of bergenin, isopurposide, quercitrin, and quercetin in the ECCF were determined to be 17.01, 6.10, 1.46, and 0.04% (w/w), respectively.
Figure 5.
Representative histologic sections of air pouch membranes (AM). The scale bar represents 200 μm. (A) Control; (b) Carrageenan-treated group; (c) 5-mg/kg indomethacin-treated group; (d) 30-mg/kg ECCF-treated group; (e) 300-mg/kg ECCF-treated group
Figure 6.
Representative HPLC chromatograms of ECCF monitored at UV wavelength of 270 nm (A) or 380 nm (B)
DISCUSSION
Infections caused by drug-resistant bacteria remain a major problem.[18] Some herbal sources are reported to have the potential to modify resistance of S. aureus and enhance the antibacterial action of antibiotics.[19,20] Herein, we evaluated the antimicrobial activity of ECCF against general infectious and MDR microbes through antimicrobial susceptibility test and MIC method. The results of this study showed that the ECCF inhibited the growth of MRSA, VRSA, IMP, and ESBL. This is the first report on the antimicrobial activity of ECCF against drug-resistant strains.
Subcutaneous carrageenan-air pouch model was employed as an acute local inflammation model in this study. The use of air pouch model in mice can mimic inflamed synovial membrane of patients with rheumatoid arthritis. In this model, subcutaneous injections of air into the back resulted in formation of a cavity similar to synovium. In addition, the injection of carrageenan in this cavity induced inflammation. This site can serve as a reservoir of cells and mediators such as TNF-α and IL-6 that can be measured in locally accumulating fluid.[21] As shown in Figure 2, carrageenan markedly increased both the exudate volumes and protein contents, suggesting a vascular leakage of serum contents as reported previously. Such carrageenan-induced exudate production and protein leakage were significantly reduced by pretreatment with indomethacin (5 mg/kg) and ECCF (at doses of 30 and 300 mg/kg). These results could be attributed to disruption to inflammatory mediators’ formation or to inhibition of receptor function.
The results shown in Figure 3 and Figure 4 clearly indicate that the ECCF significantly reduces inflammatory cell counts (white blood cells, neutrophils, mononuclear cells, and eosinophils) and pro-inflammatory cytokine levels (TNF-α and IL-6) in the carrageenan-induced air pouch inflammation mouse model. TNF-α is known to play an important role in the inflammatory process. TNF-α is released from activated monocytes and macrophages. It can increase vascular endothelial permeability,[22] which might increase the exudate volumes and protein contents. Neutrophils are the main cellular components recruited to acute inflammatory sites induced by carrageenan. They also play central roles in the inflammatory process by secreting cytokines including TNF-α.[4,23] Therefore, our results suggest that the anti-inflammatory effects of ECCF might be mediated by the inhibition of pro-inflammatory cytokine TNF-α. Among diverse inflammatory mediators that can induce vascular permeability, nitric oxide (NO) is well known as a major factor involved in various inflammatory diseases and pain induction/perception.[24,25] Therefore, it is speculated that TNF-α/NO pathway might be involved in the induced inflammatory processes in this study, which merits further study.
We identified and quantified bergenin, quercitrin, quercetin, and isosalipurposide from the ECCF. The three flavonoids (i.e., bergenin, quercitrin, and quercetin) are widely found in food products derived from plant sources. It has been reported that they possess antioxidant, antimicrobial, and anti-inflammatory activities.[26,27,28,29] Gao et al.[30] have reported that bergenin can attenuate inflammatory cell infiltration and decrease the concentration of NO, TNF-α, IL-1β, and IL-6 in a mouse model of lipopolysaccharide-induced mastitis. Moreover, bergenin can downregulate the phosphorylation of nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinases (MAPK) signaling pathway proteins in mammary glands with mastitis.[30] Garcia-Mediavilla et al.[31] have reported that quercetin can significantly inhibit the mRNA levels of iNOS, COX-2, and CRP (reactive C-protein). Quercitrin can specifically suppress the nuclear localization step of NF-κB p65 in lipopolysaccharide-mediated NF-κB activation, which can downregulate lipopolysaccharide-induced NO production and iNOS expression.[32] Taken together, these flavonoids might have contributed to the anti-inflammatory effects of ECCF in the mouse model of carrageenan-induced air pouch inflammation.
CONCLUSION
In this study, the antimicrobial and anti-inflammatory effects of ECCF were evaluated. Antimicrobial assay demonstrated that the ECCF showed broad antimicrobial susceptibilities against infectious microbes including drug-resistant strains. The in vivo mouse study demonstrated that the ECCF suppressed the exudate volume, protein leakage, inflammatory cell counts, and pro-inflammatory cytokine levels in a dose-dependent manner. In addition, the ECCF was found to contain several well-known anti-inflammatory phytochemicals such as bergenin, quercetin, and quercitrin. To the best of our knowledge, this is the first report to demonstrate the antimicrobial and anti-inflammatory activities of ECCF. Our results suggest that the ECCF might potentially serve as an alternative or complementary medicine for treating inflammatory diseases caused by microbial infection.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest
REFERENCES
- 1.Jan S, Khan MR. Antipyretic, analgesic and anti-inflammatory effects of Kickxia ramosissima. J Ethnopharmacol. 2016;182:90–100. doi: 10.1016/j.jep.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 2.White M. Mediators of inflammation and the inflammatory process. J Allergy Clin Immunol. 1999;103:S378–81. doi: 10.1016/s0091-6749(99)70215-0. [DOI] [PubMed] [Google Scholar]
- 3.Kim WS, Choi WJ, Lee S, Kim WJ, Lee DC, Sohn UD, et al. Anti-inflammatory, antioxidant and antimicrobial effects of artemisinin extracts from Artemisia annua L. Korean J Physiol Pharmacol. 2015;19:21–7. doi: 10.4196/kjpp.2015.19.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin S, Jeon JH, Park D, Jang JY, Joo SS, Hwang BY, et al. Anti-inflammatory effects of an ethanol extract of Angelica gigas in a Carrageenan-air pouch inflammation model. Exp Anim. 2009;58:431–6. doi: 10.1538/expanim.58.431. [DOI] [PubMed] [Google Scholar]
- 5.Kloppenburg M, Dijkmans BA, Breedveld FC. Antimicrobial therapy for rheumatoid arthritis. Baillieres Clin Rheumatol. 1995;9:759–69. doi: 10.1016/s0950-3579(05)80312-7. [DOI] [PubMed] [Google Scholar]
- 6.Ianaro A, Ialenti A, Maffia P, Sautebin L, Rombola L, Carnuccio R, et al. Anti-inflammatory activity of macrolide antibiotics. J Pharmacol Exp Ther. 2000;292:156–63. [PubMed] [Google Scholar]
- 7.Sandoval M, Okuhama NN, Zhang XJ, Condezo LA, Lao J, Angeles FM, et al. Anti-inflammatory and antioxidant activities of cat's claw (Uncaria tomentosa and Uncaria guianensis) are independent of their alkaloid content. Phytomedicine. 2002;9:325–37. doi: 10.1078/0944-7113-00117. [DOI] [PubMed] [Google Scholar]
- 8.Chen BQ, Cui XY, Zhao X, Zhang YH, Piao HS, Kim JH, et al. Antioxidative and acute antiinflammatory effects of Torreya grandis. Fitoterapia. 2006;77:262–7. doi: 10.1016/j.fitote.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Kim MH, Ha SY, Oh MH, Kim HH, Kim SR, Lee MW. Anti-oxidative and anti-proliferative activity on human prostate cancer cells lines of the phenolic compounds from Corylopsis coreana Uyeki. Molecules. 2013;18:4876–86. doi: 10.3390/molecules18054876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seo JH, Kim JE, Shim JH, Yoon G, Bang MA, Bae CS, et al. HPLC analysis, optimization of extraction conditions and biological evaluation of Corylopsis coreana Uyeki Flos. Molecules. 2016;21:E94. doi: 10.3390/molecules21010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, Provan GJ, Helliwell K. Determination of hamamelitannin, catechins and gallic acid in witch hazel bark, twig and leaf by HPLC. J Pharm Biomed Anal. 2003;33:539–44. doi: 10.1016/s0731-7085(03)00303-0. [DOI] [PubMed] [Google Scholar]
- 12.Hollman PC, Katan MB. Dietary flavonoids: intake, health effects and bioavailability. Food Chem Toxicol. 1999;37:937–42. doi: 10.1016/s0278-6915(99)00079-4. [DOI] [PubMed] [Google Scholar]
- 13.Shah MR, Arfan M, Amin H, Hussain Z, Qadir MI, Choudhary MI, et al. Synthesis of new bergenin derivatives as potent inhibitors of inflammatory mediators NO and TNF-alpha. Bioorg Med Chem Lett. 2012;22:2744–7. doi: 10.1016/j.bmcl.2012.02.096. [DOI] [PubMed] [Google Scholar]
- 14.Silva LR, Teixeira R. Phenolic profile and biological potential of Endopleura uchi extracts. Asian Pac J Trop Med. 2015;8:889–97. doi: 10.1016/j.apjtm.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Iwasa T, Higashide E, Shibata M. Studies of validamycins, new antibiotics 3 Bioassay methods for the determination of validamycin. J Antibiot (Tokyo) 1971;24:114–8. [PubMed] [Google Scholar]
- 16.Sohng JK, Yamaguchi T, Seong CN, Baik KS, Park SC, Lee HJ, et al. Production, isolation and biological activity of nargenicin from Nocardia sp CS682. Arch Pharm Res. 2008;31:1339–45. doi: 10.1007/s12272-001-2115-0. [DOI] [PubMed] [Google Scholar]
- 17.Yoon IS, Son JH, Kim SB, Choi MK, Maeng HJ. Effects of 1alpha, 25-Dihydroxyvitamin D3 on intestinal absorption and disposition of adefovir dipivoxil and its metabolite, Adefovir, in Rats. Biol Pharm Bull. 2015;38:1732–7. doi: 10.1248/bpb.b15-00356. [DOI] [PubMed] [Google Scholar]
- 18.Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010;74:417–33. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibbons S. Anti-staphylococcal plant natural products. Nat Prod Rep. 2004;21:263–77. doi: 10.1039/b212695h. [DOI] [PubMed] [Google Scholar]
- 20.Musumeci R, Speciale A, Costanzo R, Annino A, Ragusa S, Rapisarda A, et al. Berberis aetnensis C Presl extracts: antimicrobial properties and interaction with ciprofloxacin. Int J Antimicrob Agents. 2003;22:48–53. doi: 10.1016/s0924-8579(03)00085-2. [DOI] [PubMed] [Google Scholar]
- 21.Romano M, Faggioni R, Sironi M, Sacco S, Echtenacher B, Di Santo E, et al. Carrageenan-induced acute inflammation in the mouse air pouch synovial model Role of tumour necrosis factor. Mediators Inflamm. 1997;6:32–8. doi: 10.1080/09629359791901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva RR, Oliveira e Silva D, Fontes HR, Alviano CS, Fernandes PD, Alviano DS. Anti-inflammatory, antioxidant, and antimicrobial activities of Cocos nucifera var typica. BMC Complement Altern Med. 2013;13:107. doi: 10.1186/1472-6882-13-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:2118–20. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- 24.Ritchlin CT, Haas-Smith SA, Li P, Hicks DG, Schwarz EM. Mechanisms of TNF-alpha-and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J Clin Invest. 2003;111:821–31. doi: 10.1172/JCI16069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pang L, Hoult JR. Cytotoxicity to macrophages of tetrandrine, an antisilicosis alkaloid, accompanied by an overproduction of prostaglandins. Biochem Pharmacol. 1997;53:773–82. doi: 10.1016/s0006-2952(96)00817-9. [DOI] [PubMed] [Google Scholar]
- 26.Guardia T, Rotelli AE, Juarez AO, Pelzer LE. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco. 2001;56:683–7. doi: 10.1016/s0014-827x(01)01111-9. [DOI] [PubMed] [Google Scholar]
- 27.Jo HY, Kim Y, Nam SY, Lee BJ, Kim YB, Yun YW, et al. The inhibitory effect of quercitrin gallate on iNOS expression induced by lipopolysaccharide in Balb/c mice. J Vet Sci. 2008;9:267–72. doi: 10.4142/jvs.2008.9.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nazir N, Koul S, Qurishi MA, Taneja SC, Ahmad SF, Bani S, et al. Immunomodulatory effect of bergenin and norbergenin against adjuvant-induced arthritis-a flow cytometric study. J Ethnopharmacol. 2007;112:401–5. doi: 10.1016/j.jep.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 29.Nazir N, Koul S, Qurishi MA, Najar MH, Zargar MI. Evaluation of antioxidant and antimicrobial activities of bergenin and its derivatives obtained by chemoenzymatic synthesis. Eur J Med Chem. 2011;46:2415–20. doi: 10.1016/j.ejmech.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 30.Gao XJ, Guo MY, Zhang ZC, Wang TC, Cao YG, Zhang NS. Bergenin plays an anti-inflammatory role via the modulation of MAPK and NF-kappaB signaling pathways in a mouse model of LPS-induced mastitis. Inflammation. 2015;38:1142–50. doi: 10.1007/s10753-014-0079-8. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Mediavilla V, Crespo I, Collado PS, Esteller A, Sanchez-Campos S, Tunon MJ, et al. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur J Pharmacol. 2007;557:221–9. doi: 10.1016/j.ejphar.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 32.Kim BH, Cho SM, Reddy AM, Kim YS, Min KR, Kim Y. Down-regulatory effect of quercitrin gallate on nuclear factor-kappa B-dependent inducible nitric oxide synthase expression in lipopolysaccharide-stimulated macrophages RAW 264 7. Biochem Pharmacol. 2005;69:1577–83. doi: 10.1016/j.bcp.2005.03.014. [DOI] [PubMed] [Google Scholar]