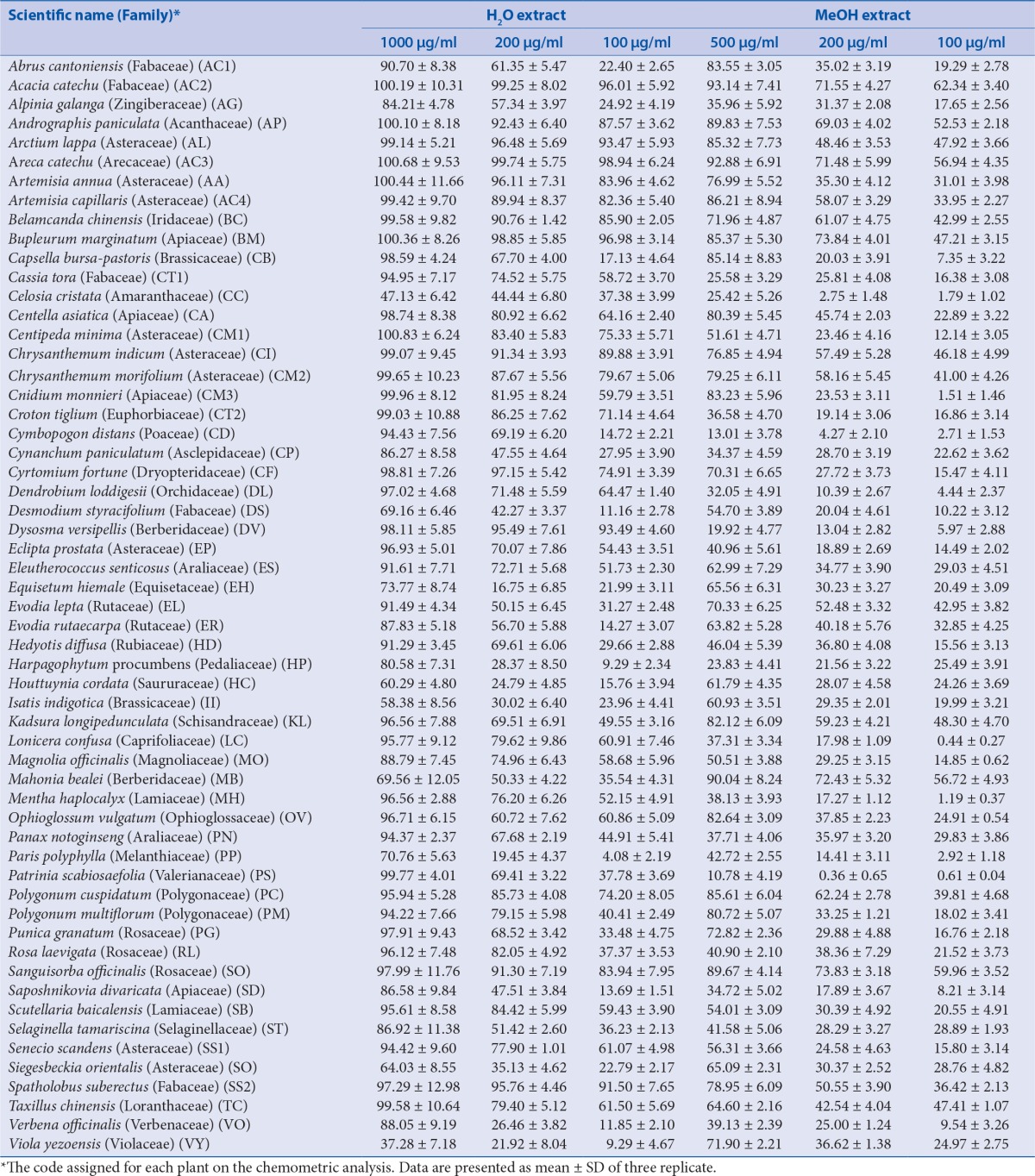
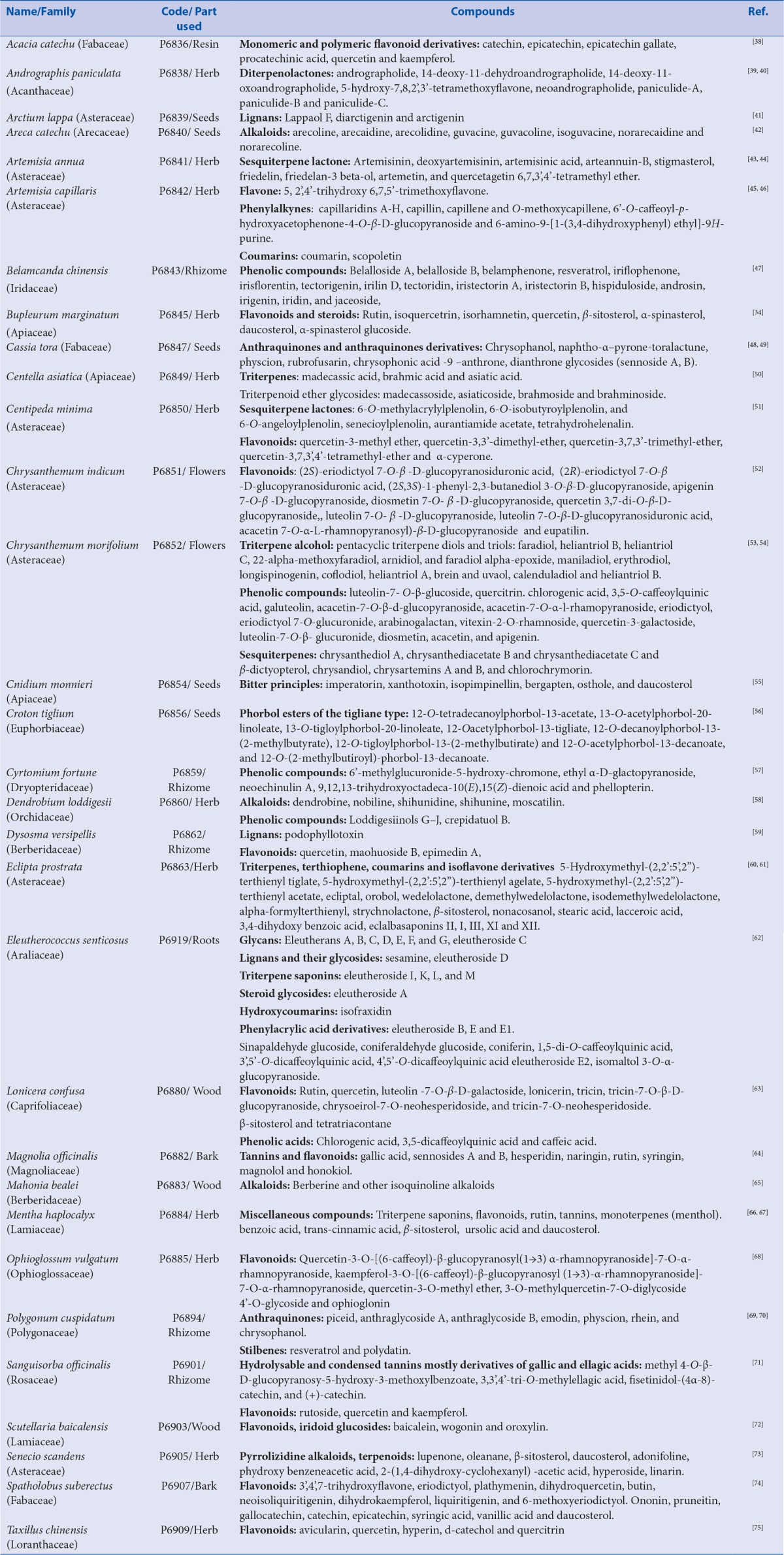
Abstract
Background:
Herbal medicine is widely used all over the world for treating various health disorders. It is employed either alone or in combination with synthetic drugs or plants to be more effective.
Objective:
The assessment of the effect of both water and methanol extracts of 57 widely used plants from Traditional Chinese Medicine (TCM) against the main phase I metabolizing enzyme CYP3A4 in vitro for the first time.
Materials and Methods:
The inhibition of cytochrome P450 activity was evaluated using a luminescence assay. The principal component analysis (PCA) was used to correlate the inhibitory activity with the main secondary metabolites present in the plant extracts. Molecular modeling studies on CYP3A4 (PDB ID 4NY4) were carried out with 38 major compounds present in the most active plant extracts to validate the observed inhibitory effect.
Results:
Aqueous extracts of Acacia catechu, Andrographis paniculata, Arctium lappa, Areca catechu, Bupleurum marginatum, Chrysanthemum indicum, Dysosma versipellis, and Spatholobus suberectus inhibited CYP3A4 is more than 85% (at a dose of 100 μg/mL). The corresponding methanol extracts of A. catechu, A. paniculata, A. catechu, Mahonia bealei, and Sanguisorba officinalis inhibited the enzyme by more than 50%. Molecular modeling studies revealed that two polyphenols, namely hesperidin and rutin, revealed the highest fitting scores in the active sites of the CYP3A4 with binding energies equal to -74.09 and -71.34 kcal/mol, respectively.
Conclusion:
These results provide evidence that many TCM plants can inhibit CYP3A4, which might cause a potential interference with the metabolism of other concomitantly administered herbs or drugs.
SUMMARY
In this study, the inhibitory activity of the aqueous and methanol extracts of 57 widely used plants from Traditional Chinese Medicine (TCM) against the main phase I metabolizing enzyme CYP3A4 was tested in vitro for the first time.
Aqueous extracts of Acacia catechu, Andrographis paniculata, Arctium lappa, Areca catechu, Bupleurum marginatum, Dysosma versipellis, and Spatholobus suberectus inhibited CYP3A4 by more than 85% (at a dose of 100 μg/mL).
The activity could be attributed to the presence of polyphenolics as revealed from the multivariate chemometric analysis and molecular modeling study.
These results provide evidence that many TCM plants can inhibit CYP3A4, which might cause a potential interference with the metabolism of other concomitantly administered herbs or drugs.
Abbreviation used: CHARMm: Chemistry at HARvard Macromolecular Mechanics, CYP: Cytochrome P450, DMSO: Dimethyl Sulfoxide, PCA: Principal Component Analysis, PDB: Protein Data Bank, TCM: Traditional Chinese Medicine
Keywords: Cytochrome P450, herbal–drug interaction, principal component analysis (PCA), Traditional Chinese Medicine (TCM), virtual screening
INTRODUCTION
Phytomedicine is increasingly receiving attention from both the public and healthcare professionals.[1] However, possible interaction(s) between herbal preparations and other concomitantly administered synthetic medications may cause very serious medical problems.[2,3,4,5] Pharmacokinetic interactions between medicinally active plants and other synthetic drugs may cause a change in liberation, absorption, distribution, metabolism, excretion, or toxicity of a respective drug. Apart from that, plant extracts may cause many pharmacodynamic interactions with the receptors and enzymes leading to enhanced or attenuated pharmacological action of therapeutics, which might cause unwanted effects.[6,7] Therefore, understanding the ability of medicinal plants to modulate the metabolizing enzymes is really crucial for a responsible treatment of patients.[8]
The cytochrome P450 (CYP) enzymes are the main players in Phase I metabolism and also involved in the oxidation and elimination of a wide array of xenobiotics (such as drugs and toxins). Drug metabolism involves around 15 different CYP isoforms in which CYP 1A2, 2C9, 2C19, 2D6, 2E1, and 3A4 are the most abundant.[9] CYP3A4 acts on lipophilic substrates and metabolizes about 50% of the drugs in the liver[10] whereas CYP2D6 exhibits a preference for positively charged molecules, usually containing a basic nitrogen. On the other hand, CYP2C9 metabolizes weakly anionic molecules, while CYP1A2 acts on polyaromatic hydrocarbons, and CYP2E1 metabolizes small relatively soluble organic compounds.[11]
CYP3A4 is the most abundant isoform of the human CYP system accounting for approximately 28% of the whole enzyme system.[12,13] The CYP3A4 enzyme usually introduces a hydroxyl or epoxy group to many lipophilic substrates. Then, the hydroxylated xenobiotics become conjugated with glucuronic acid, sulfate, or amino acids. In turn, they become eliminated via the kidney and urine.[14]
A number of therapeutic agents, including natural products, are the main substrates of CYP3A4. Among them, quinidine, vinblastine, ergotamine, berberine, and colchicine are the most known[15] while nifedipine and diazepam figure as common synthetic substrates.[16] Furthermore, CYP3A4 activity can be inhibited by many plant extracts.[17,18] Grapefruit with the furanocoumarin derivatives and flavonoids[19] and kava-kava with its kavalactones[20] are prominent examples for herbal drugs that can cause clinically important modulation in CYP3A4 activity. On the other hand, prolonged use of Hypericum extracts, containing hypericin, results in an induction of the enzyme activity.[21,22,23]
Because the plants used in Traditional Chinese Medicine (TCM) are diverse, our knowledge about the interactions between these herbal drugs and the CYP system is rather limited.[1,24] Therefore, in this communication, we investigated the potential inhibition of CYP3A4 by 57 widely used TCM plants to explore the relevance of this activity with regard to adverse effects. Moreover, statistical analysis using principal component analysis (PCA) was applied to correlate the inhibitory activity with the main compounds present in the plant extracts. Additionally, molecular docking was carried out with 38 major secondary metabolites found in the bioactive plant extracts to validate the inhibition results.
MATERIALS AND METHODS
Plant materials
TCM plants were purchased from Chinese markets. Their identity was ascertained in our laboratory through DNA barcoding technique.[25] Voucher specimens are stored at the Department of Biology, Institute of Pharmacy and Molecular Biotechnology, Heidelberg University under the accession numbers P6835-P6919.
Preparation of the plant extracts
One hundred gram of dried plants were grounded to a fine powder. Plant powders were refluxed with 1 L of either methanol or deionized, distilled water (analytical grade) for 4 h. The methanol (MeOH) extracts were dried over anhydrous Na2SO4 and evaporated till dryness under vacuum at 45°C, whereas the water (H2O) extracts were evaporated directly under the same conditions until dryness. Then, they were lyophilized overnight to ensure optimum dryness. The extracts are kept in tight sealed vials at -20°C away from light until use.
CYP3A4 activity
The CYP3A4 assay kit (P450-GloTM, Promega®, Mannheim, Germany) was used to determine the potential inhibition of recombinant human CYP3A4 enzyme by different plant extracts according to the manufacturer protocol.[26] Briefly, the samples were prepared using dimethyl sulfoxide (DMSO) to give final concentrations of 100, 200, and 500, or 1000 µg/mL where DMSO did not exceed 1% of the solutions. Equal volumes (12.5 µL) of each tested sample and the reaction mixture containing 5 mM luciferin-6’-benzyl ether (CYP3A4 specific substrate) in 100 mM phosphate buffer (pH 7.4) and the enzyme (1 pmol/µL) were incubated at 25°C for 10 min. Then, 25 µL of NADPH regeneration system containing 26 mM NADP+, 66 mM glucose-6-phosphate, 66 mM MgCl2, and 40 U/mL glucose-6-phosphate dehydrogenase in 5 mM citrate buffer (pH 5.5) in 1 M phosphate buffer was added and left for 30 min to activate the enzyme. A luciferin detecting reagent (50 µL) was added to stop the CYP3A4 enzyme activity. The luminescence was detected after 20 min using a TecanTM SafireII Reader (TecanTM, Crailsheim, Germany). The effects of different extracts were evaluated in triplicate relative to blank control containing 1% DMSO. Ketoconazole (10 µM) was used as a positive control.
Molecular modeling studies
In silico molecular modeling of the major compounds present in the bioactive extracts was carried out using Discovery Studio 2.5 (Accelrys® Inc., San Diego, CA, USA) using C-Docker protocol applying both pH-based as well as rule based ionization methods to simulate the physiological conditions and to evaluate the influence of ionization of various ionizable groups on the behavioral interaction of the secondary metabolites at the active site of the enzyme. The x-ray crystal structure of CYP3A4 (PDB ID 4NY4, 2.95 Å) co-crystalized with its lead compound (L), (8R)-3,3-difluoro-8-[4-fluoro-3-(pyridine-3-yl)phenyl]-8-(4-methoxy-3-methylphenyl)-2,3,4,8-tetrahydroimidazo[1,5-a] pyrimidine- 6-amine), was obtained from the protein data bank (www.pdb.org). The standard protein preparation protocol was applied to construct the structure of the enzymes. This was briefly done by the addition of hydrogen atoms to the enzyme and cleansing of all undesirable interactions. Then, the binding site was determined by detection of the binding mode of bioactive conformation of the lead compound (L) with CYP3A4.[27] The structures of the selected compounds were docked inside the binding site after applying CHARMm as the force field and the binding energies and modes for the selected docking poses were determined as described before.[28]
Statistical analysis
Data were presented as means ± mean standard deviation. One-way analysis of variance (ANOVA) with Tukey's post hoc test was used to identify statistically significant (P < 0.05) differences between the groups. Analyses were performed with Prism 5.0 software (Graph Pad®, San Diego, USA). Chemometric analysis of the data was performed using unsupervised pattern recognition techniques applying PCA, where a matrix of the total number of samples (57 samples) multiplied by the different tested doses for both water and alcoholic extracts (six variables) was constructed. PCA was performed by Unscrambler® 9.7 (CAMO, AS, Norway).
RESULTS
All TCM extracts inhibited CYP3A4 activity to some degree [Table 1]. Generally, the H2O extracts were more potent than the MeOH extracts, and their corresponding activities were positively correlated as shown in Figure 1a–c. Therefore, multivariate analysis was applied to statistically evaluate the significance of inhibition of the CYP enzyme. Moreover, clustering the plants samples based on their activity and their chemical profiling was established. The PCA score plot [Figure 2] resulted in two orthogonal PCs, which explained about 89% of the variance in 180-dimensional space using only the first two components (the first PC accounts for 65% of the total variance followed by the second PC with 24%). PCA score plot classified the samples into five main clusters according to their similarity of cytochrome P450 inhibition utilizing all the tested doses as different variables. Cluster I included samples were the strongest CYP inhibitors. This cluster comprises Acacia catechu, Andrographis paniculata, Arctium lappa, Artemisia annua, Artemisia capillaris, Belamcanda chinensis, Bupleurum marginatum, Chrysanthemum indicum, Chrysanthemum morifolium, Polygonum cuspidatum, Sanguisorba officinalis, and Spatholobus suberectus. These samples mostly clustered in the right quadrant. Cluster II was also found in the same right quadrant exhibiting a moderate CYP inhibition (both the water and alcoholic extracts). The left side of the PCA plot contained all samples with an inhibition lower than approximately 50% at the lowest tested dose. They were subdivided into three main clusters (cluster III, IV, and V).
Table 1.
Inhibition of CYP3A4 activity by aqueous and alcoholic extracts from 57 TCM plants
Figure 1(a).
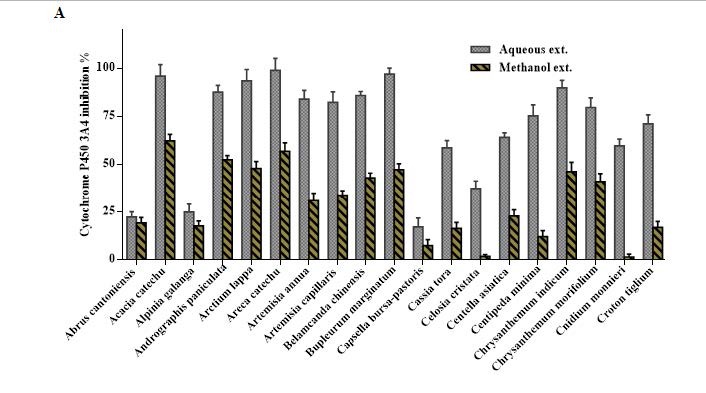
Overview of Cytochrome P450 3A4 inhibition by 100 μg/mL of the aqueous and methanol extracts of 57 TCM plants.
Figure 1 (c).
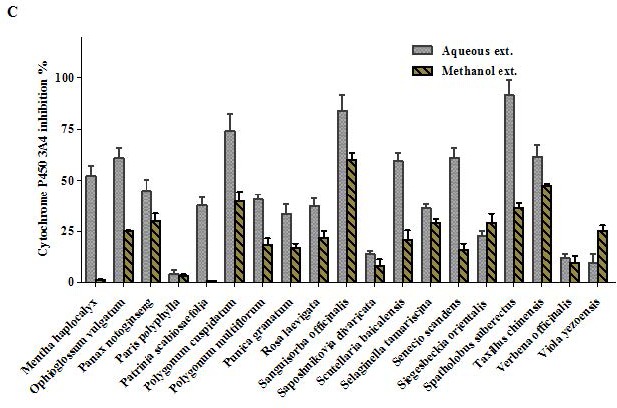
Overview of Cytochrome P450 3A4 inhibition by 100 μg/mL of the aqueous and methanol extracts of 57 TCM plants.
Figure 2.
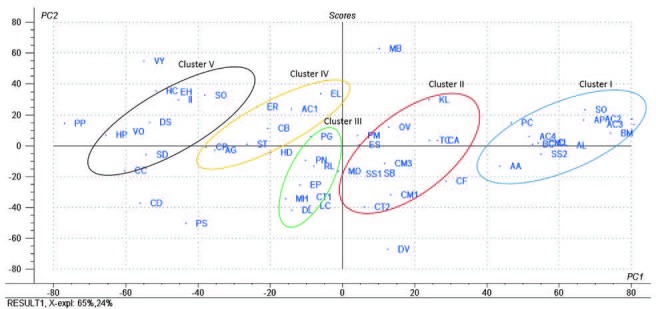
PCA score plot of the aqueous and methanol extracts of 57 tested plants; the codes used are those listed in [Table 1].
Figure 1 (b).
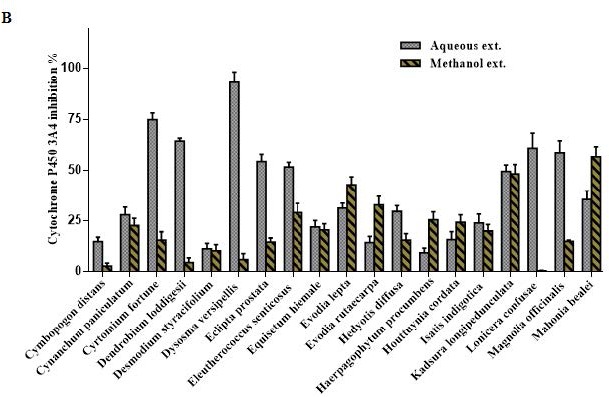
Overview of Cytochrome P450 3A4 inhibition by 100 μg/mL of the aqueous and methanol extracts of 57 TCM plants.
From the results displayed in Table 1 and Table 2, and the multivariate analysis, it is obvious that the majority of secondary metabolites reported in both chemical abstracts and Medline for the corresponding bioactive extracts are phenolic secondary metabolites [Figure 3]. These compounds were docked on CYP3A4 to validate the observed biological activity [Table 3]. The results revealed that two flavonoids namely hesperidin and rutin were the most potent CYP3A4 inhibitors as evidenced from their high fitting scores and consequently, higher stability within the active sites as compared with the lead compound. The binding energies were -74.09, -71.34, and -47.08 kcal/mol for hesperidin, rutin, and original lead compound (L) for the pH-based ionization mode, respectively. We should mention that the binding mode of the lead compound L revealed formation of one hydrogen or ionic bond with the residue Arg 212 of the CYP3A4, in addition to the formation of three π bonds, two of them with the amino acid residue Arg 105 while the third with Arg 212. Whereas for hesperidin six hydrogen or ionic bonds and a π bond were detected, two hydrogen bonds and a π bond are formed with the residue Arg 105, one hydrogen bond with each of Arg 375, Asn 441, Cys 442, and Pro 434. Moreover, rutin forms nine hydrogen or ionic bonds, three of them with the residue Arg 105, two with Glu 374, and a hydrogen bond with each of Arg 375, Asn 441, Gly 481, and Ile 443 in addition to the formation of a π bond with the amino acid residue Arg 105 [Figure 4]. Thus, the formation of extra hydrogen bonds or ionic bonds (if the phenolic OH-groups is dissociated) with the amino acid residues at the active sites of the enzyme is responsible for the comparative firm binding of the two flavonoids with respect to the lead compound. This was revealed from the results of the molecular docking using the rule-based ionization mode [Table 3] that examine the influence of ionization of various functional groups on its interaction at the binding site.
Table 2.
Major secondary metabolites present in the plants producing an inhibition of the CYP 3A4 activity at 100 μg/mL higher than 50%
Figure 3.
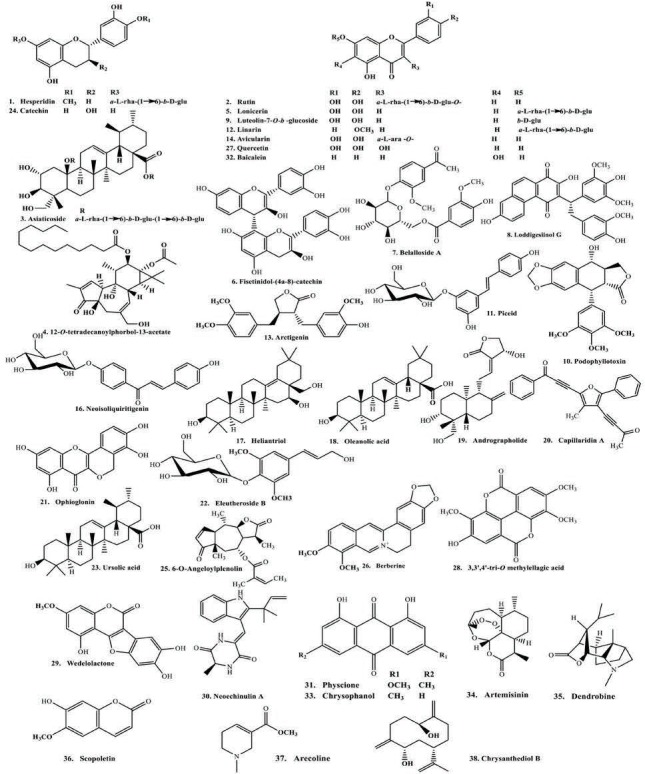
Representative structures of the major CYP 3A4 inhibitors.
Table 3.
In silico molecular modeling of some selected major compounds from our TCM plants on CYP3A4 applying both pH and rule based ionization modes

Figure 4.
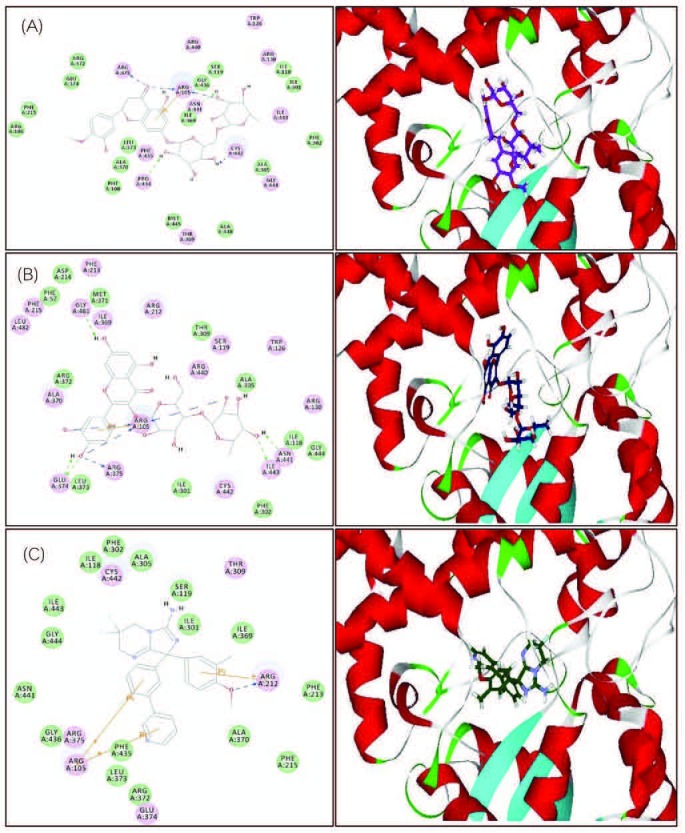
2D and 3D binding modes of hesperidin (A), rutin (B), and the lead compound (C) in the active sites of CYP3A4 using pH-based ionization mode.
DISCUSSION
The notable CYP3A4 inhibitory activity of many of the tested plants could be interpreted in virtue of their secondary metabolites mainly because of polyphenolic class of compounds. One of the underlying causes of this potent efficacy could be attributed to the formation of hydrogen and ionic bonds at the active sites of an enzyme due to the existence of several reactive phenolic OH groups. The phenolic hydroxyl groups can partly dissociate under physiological conditions resulting in O− ions interacting with positively charged amino groups, such as in arginine, lysine, and histidine. The charged and polar polyphenols interact with proteins by forming ionic bonds in addition to hydrogen bonds with several amino acids at the active site which might lead to enzyme inhibition and loss of function.[29]
In the same context, extracts from the heartwood of A. catechu (Fabaceae) exhibited a potent CYP3A4 inhibition that could be attributed to the high contents of catechins and epicatechins (≈50% of the content) that were previously reported to inhibit CYP3A4.[30,31] Additionally, the activity of A. catechu (Arecaceae) could be ascribed to their contents of tannins with their polyphenolic structures especially arecatannin A1–A3 which are abundant in both extracts; as polyphenols they are able to bind the enzyme directly. Moreover, the alkaloid content with mainly arecoline (in the methanol extract) could contribute to the overall activity although this action has not been described for CYP3A4, but arecoline significantly inhibits other forms of cytochrome especially CYP1A1.[32]
Besides, a plethora of secondary metabolites with exocyclic methylene groups as sesquiterpene lactones or reactive double or triple bonds can covalently bind to proteins with SH groups. These protein modification greatly affect the three dimensional structure of proteins with inhibition of their activity and function.[29] A. paniculata and C. morifolium showed notable inhibition to CYP3A4 that could be due to the presence of certain sesquiterpenes with exocyclic methylene groups as andrographolide and chrysanthediol B, respectively that exerted reasonable fitting scores in the docking experiment with binding energy equals to -43.06 for the first and -23.63 kcal/mol for the latter in addition to the presence of several phenolic compounds in the tested extracts.
The inhibitory potency of A. lappa (Asteraceae) is apparently due to its content of lignans, sesquiterpenes, polyacetylenes, and sulfur containing compounds, which are known inhibitors of many cytochromes.[33] The difference in B. marginatum extracts activity could be attributed to the presence of large quantities of flavonoids, mainly rutin, isoquercetrin, quercetin, and isorhamnetin.[34] However, the high solubility of these flavonoids, mainly rutin, in water explains the higher activity of water rather than the alcoholic extracts. Besides, the presence of lignans with a methylenedioxy group in an aromatic ring which are known CYP inhibitors[29] and saikosaponins, which showed a significant inhibition of both CYP1A2 and CYP3A4 may have significantly boosted the efficacy.[35]
An additional representative empathizing on the difference between the potency of water and methanol extracts is M. bealei (Berberidaceae) where the alcoholic extract exerted higher inhibitory activity than the aqueous one. This notable activity could be explained in view of its richness of alkaloids as berberine which carries a methlenedioxy group-a known inhibitor of CYP1A1 and a substrate for the CYP2C9 and CYP2D6.[36,37] However, the presence of few polyhydroxylated flavonoids could explain the moderate activity of the aqueous extract.
CONCLUSIONS
In this study, the effects of 57 widely used TCM plants on the key metabolizing enzyme CYP3A4 were assessed to understand the potential adverse effects that might occur by concomitant administration of many herbal preparations with other drugs. In addition, plant secondary metabolites can also inhibit or stimulate the expression of CYP genes. Since the aqueous extracts of many medicinal plants inhibit the activity of cytochrome p450 enzymes in vitro, as a consequence many adverse interactions can be expected. Furthermore, in vitro and in vivo studies are required on other cytochrome P450 isoforms to help patients who rely on phytomedicine beside other western medicine in treatment avoiding hazards that might be serious.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest
Acknowledgements
The authors would like to thank Dr. Florian Herrmann (formerly Institute of Pharmacy and Molecular Biotechnology, Heidelberg University) for his valuable support in authentication and DNA-profiling of most of the plant samples.
REFERENCES
- 1.Van Wyk B-E, Wink M. Medicinal plants of the world: an illustrated scientific guide to important medicinal plants and their uses. 1st ed. Portland, OR: Timber Press; 2004. [Google Scholar]
- 2.Fricker G. Drug interactions with natural products at the blood brain barrier. Curr Drug Metab. 2008;9:1019–26. doi: 10.2174/138920008786927758. [DOI] [PubMed] [Google Scholar]
- 3.Hu Z, Yang X, Ho PC, Chan SY, Heng PW, Chan E, et al. Herb-drug interactions: a literature review. Drugs. 2005;65:1239–82. doi: 10.2165/00003495-200565090-00005. [DOI] [PubMed] [Google Scholar]
- 4.Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs: a systematic review. Drugs. 2001;61:2163–75. doi: 10.2165/00003495-200161150-00002. [DOI] [PubMed] [Google Scholar]
- 5.Ulbricht C, Chao W, Costa D, Rusie-Seamon E, Weissner W, Woods J. Clinical evidence of herb-drug interactions: a systematic review by the natural standard research collaboration. Curr Drug Metab. 2008;9:1063–120. doi: 10.2174/138920008786927785. [DOI] [PubMed] [Google Scholar]
- 6.Lam YWF, Huang S-M, Hall SD. Herbal supplements-drug interactions: scientific and regulatory perspectives. Drugs and the pharmaceutical sciences. New York: Taylor and Francis; 2006. [Google Scholar]
- 7.Skalli S, Zaid A, Soulaymani R. Drug interactions with herbal medicines. Ther Drug Monit. 2007;29:679–86. doi: 10.1097/FTD.0b013e31815c17f6. [DOI] [PubMed] [Google Scholar]
- 8.Delgoda R, Westlake AC. Herbal interactions involving cytochrome p450 enzymes: a mini review. Toxicol Rev. 2004;23:239–49. doi: 10.2165/00139709-200423040-00004. [DOI] [PubMed] [Google Scholar]
- 9.Rammohan B, Samit K, Chinmoy D, Arup S, Amit K, Ratul S, et al. Human cytochrome P450 enzyme modulation by Gymnema sylvestre: a predictive safety evaluation by LC-MS/MS. Pharmacogn Mag. 2016;12:S389–94. doi: 10.4103/0973-1296.191441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guengerich FP. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol. 1999;39:1–17. doi: 10.1146/annurev.pharmtox.39.1.1. [DOI] [PubMed] [Google Scholar]
- 11.Lewis DFV. Guide to cytochromes P450 : structure and function. London, New York: Taylor and Francis; 2001. [Google Scholar]
- 12.Guengerich FP. Drug metabolism as catalyzed by human cytochrome P450 systems. In: Sigel A, Sigel H, Sigel RKO, editors. Metal ions in life sciences; the ubiquitous roles of cytochrome P450 proteins. Chichester; Hoboken, NJ: John Wiley and Sons, Ltd; 2006. pp. 561–90. [Google Scholar]
- 13.Evans WE, Relling MV. Pharmacogenomics: translating functional genomics into rational therapeutics. Science. 1999;286:487–91. doi: 10.1126/science.286.5439.487. [DOI] [PubMed] [Google Scholar]
- 14.Koolman J, Rohm K-H. Color atlas of biochemistry. 2nd ed. New York: Thieme Medical Publishers, Stuttgart; 2005. [Google Scholar]
- 15.Wink M. Molecular modes of action of cytotoxic alkaloids: From DNA intercalation, spindle poisoning, topoisomerase inhibition to apoptosis and multiple drug resistance. In: Cordell GA, editor. The alkaloids: chemistry and biology. California, London: Academic Press; 2007. pp. 1–47. [DOI] [PubMed] [Google Scholar]
- 16.Ioannides C. Cytochromes P450: role in the metabolism and toxicity of drugs and other xenobiotics, issues in toxicology. Cambridge: RSC Pub; 2008. [Google Scholar]
- 17.Usia T, Watabe T, Kadota S, Tezuka Y. Potent CYP3A4 inhibitory constituents of Piper cubeba. J Nat Prod. 2005;68:64–8. doi: 10.1021/np0401765. [DOI] [PubMed] [Google Scholar]
- 18.Tsukamoto S, Aburatani M, Yoshida T, Yamashita Y, El-Beih AA, Ohta T. CYP3A4 inhibitors isolated from licorice. Biol Pharm Bull. 2005;28:2000–2. doi: 10.1248/bpb.28.2000. [DOI] [PubMed] [Google Scholar]
- 19.Ho PC, Saville DJ, Wanwimolruk S. Inhibition of human CYP3A4 activity by grapefruit flavonoids, furanocoumarins and related compounds. J Pharm Pharm Sci. 2001;4:217–27. [PubMed] [Google Scholar]
- 20.Mathews JM, Etheridge AS, Black SR. Inhibition of human cytochrome P450 activities by kava extract and kavalactones. Drug Metab Dispos. 2002;30:1153–7. doi: 10.1124/dmd.30.11.1153. [DOI] [PubMed] [Google Scholar]
- 21.Markowitz JS, Donovan JL, DeVane CL, Taylor RM, Ruan Y, Wang JS, et al. Effect of St John's wort on drug metabolism by induction of cytochrome P450 3A4 enzyme. JAMA. 2003;290:1500–4. doi: 10.1001/jama.290.11.1500. [DOI] [PubMed] [Google Scholar]
- 22.Bray BJ, Perry NB, Menkes DB, Rosengren RJ. St John's wort extract induces CYP3A and CYP2E1 in the Swiss Webster mouse. Toxicol Sci. 2002;66:27–33. doi: 10.1093/toxsci/66.1.27. [DOI] [PubMed] [Google Scholar]
- 23.Moore LB, Goodwin B, Jones SA, Wisely GB, Serabjit-Singh CJ, Willson TM, et al. St John's wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci USA. 2000;97:7500–2. doi: 10.1073/pnas.130155097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williamson EM, Driver S, Baxter K. Stockley's herbal medicines interactions: a guide to the interactions of herbal medicines, dietary supplements and nutraceuticals with conventional medicines. London, Chicago: Pharmaceutical Press; 2009. [Google Scholar]
- 25.Herrmann F, Romero MR, Blazquez AG, Kaufmann D, Ashour ML, Kahl S, et al. Diversity of pharmacological properties in Chinese and European medicinal plants: cytotoxicity, antiviral and antitrypanosomal screening of 82 herbal drugs. Diversity. 2011;3:547–80. [Google Scholar]
- 26.Cali JJ, Ma D, Sobol M, Simpson DJ, Frackman S, Good TD, et al. Luminogenic cytochrome P450 assays. Expert Opin Drug Metab Toxicol. 2006;2:629–45. doi: 10.1517/17425255.2.4.629. [DOI] [PubMed] [Google Scholar]
- 27.El-Ahmady SH, Ashour ML, Wink M. Chemical composition and anti-inflammatory activity of the essential oils of Psidium guajava fruits and leaves. J Essent Oil Res. 2013;25:475–81. [Google Scholar]
- 28.Mostafa NM, Ashour ML, Eldahshan OA, Singab AN. Cytotoxic activity and molecular docking of a novel biflavonoid isolated from Jacaranda acutifolia (Bignoniaceae) Nat Prod Res. 2016;30:2093–100. doi: 10.1080/14786419.2015.1114938. [DOI] [PubMed] [Google Scholar]
- 29.Wink M. Evolutionary advantage and molecular modes of action of multi-component mixtures used in phytomedicine. Curr Drug Metab. 2008;9:996–1009. doi: 10.2174/138920008786927794. [DOI] [PubMed] [Google Scholar]
- 30.Muto S, Fujita K, Yamazaki Y, Kamataki T. Inhibition by green tea catechins of metabolic activation of procarcinogens by human cytochrome P450. Mutat Res. 2001;479:197–206. doi: 10.1016/s0027-5107(01)00204-4. [DOI] [PubMed] [Google Scholar]
- 31.Chow HH, Hakim IA, Vining DR, Crowell JA, Cordova CA, Chew WM, et al. Effects of repeated green tea catechin administration on human cytochrome P450 activity. Cancer Epidemiol Biomarkers Prev. 2006;15:2473–6. doi: 10.1158/1055-9965.EPI-06-0365. [DOI] [PubMed] [Google Scholar]
- 32.Chang EE, Miao ZF, Lee WJ, Chao HR, Li LA, Wang YF, et al. Arecoline inhibits the 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced cytochrome P450 1A1 activation in human hepatoma cells. J Hazard Mater. 2007;146:356–61. doi: 10.1016/j.jhazmat.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 33.Scott IM, Leduc RI, Burt AJ, Marles RJ, Arnason JT, Foster BC. The inhibition of human cytochrome P450 by ethanol extracts of North American botanicals. Pharm Biol. 2006;44:315–27. [Google Scholar]
- 34.Ashour ML, El-Readi MZ, Tahrani A, Eid SY, Wink M. A novel cytotoxic aryltetraline lactone from Bupleurum marginatum (Apiaceae) Phytochem Lett. 2012;5:387–92. [Google Scholar]
- 35.Saruwatari J, Nakagawa K, Shindo J, Nachi S, Echizen H, Ishizaki T. The in-vivo effects of sho-saiko-to, a traditional Chinese herbal medicine, on two cytochrome P450 enzymes (1A2 and 3A) and xanthine oxidase in man. J Pharm Pharmacol. 2003;55:1553–9. doi: 10.1211/0022357022061. [DOI] [PubMed] [Google Scholar]
- 36.Chatterjee P, Franklin MR. Human cytochrome p450 inhibition and metabolic-intermediate complex formation by goldenseal extract and its methylenedioxyphenyl components. Drug Metab Dispos. 2003;31:1391–7. doi: 10.1124/dmd.31.11.1391. [DOI] [PubMed] [Google Scholar]
- 37.Vrzal R, Zdarilova A, Ulrichova J, Blaha L, Giesy JP, Dvorak Z. Activation of the aryl hydrocarbon receptor by berberine in HepG2 and H4IIE cells: Biphasic effect on CYP1A1. Biochem Pharmacol. 2005;70:925–36. doi: 10.1016/j.bcp.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 38.Stohs SJ, Bagchi D. Antioxidant, anti-inflammatory, and chemoprotective properties of Acacia catechu heartwood extracts. Phytother Res. 2015;29:818–24. doi: 10.1002/ptr.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osman SM, El-Haddad AE, El-Raey MA, El-Khalik SMA, Koheil MA, Wink M. A new octadecenoic acid derivative from Caesalpinia gilliesii flowers with potent hepatoprotective activity. Pharmacogn Mag. 2016;12:S332–6. doi: 10.4103/0973-1296.185752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kleipool RJC. Constituents of Andrographis paniculata Nees. Nature. 1952;169:33–4. [Google Scholar]
- 41.Chan Y-S, Cheng L-N, Wu J-H, Chan E, Kwan Y-W, Lee SM-Y, et al. A review of the pharmacological effects of Arctium lappa (burdock) Inflammopharmacology. 2011;19:245–54. doi: 10.1007/s10787-010-0062-4. [DOI] [PubMed] [Google Scholar]
- 42.Peng W, Liu YJ, Wu N, Sun T, He XY, Gao YX, et al. Areca catechu L (Arecaceae): a review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J Ethnopharmacol. 2015;164:340–56. doi: 10.1016/j.jep.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 43.Zheng G-Q. Cytotoxic terpenoids and flavonoids from Artemisia annua. Planta Med. 1994;60:54–7. doi: 10.1055/s-2006-959408. [DOI] [PubMed] [Google Scholar]
- 44.Ho WE, Peh HY, Chan TK, Wong WS. Artemisinins: pharmacological actions beyond anti-malarial. Pharmacol Ther. 2014;142:126–39. doi: 10.1016/j.pharmthera.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 45.Ma H-Y, Sun Y, Zhou Y-Z, Hong M, Pei Y-H. Two new constituents from Artemisia capillaris Thunb. Molecules. 2008;13:267–71. doi: 10.3390/molecules13020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu T-S, Tsang Z-J, Wu P-L, Liou M-J, Leu Y-L, Chan Y-Y, et al. Phenylalkynes from Artemisia capillaris. Phytochemistry. 1998;47:1645–8. [Google Scholar]
- 47.Monthakantirat O, De-Eknamkul W, Umehara K, Yoshinaga Y, Miyase T, Warashina T, et al. Phenolic constituents of the rhizomes of the Thai medicinal plant Belamcanda chinensis with proliferative activity for two breast cancer cell lines. J Nat Prod. 2005;68:361–4. doi: 10.1021/np040175c. [DOI] [PubMed] [Google Scholar]
- 48.Shibata S, Morishita E, Kaneda M, Kimura Y, Takido M, Takahashi S. Chemical studies on the oriental plant drugs. XX. The constituents of Cassia tora L. Chem Pharm Bull. 1969;17:454–7. doi: 10.1248/cpb.17.454. [DOI] [PubMed] [Google Scholar]
- 49.Lee GY, Kim JH, Choi SK, Kim YH. Constituents of the seeds of Cassia tora with inhibitory activity on soluble expoxide hydrolease. Bioorg Med Chem Lett. 2015;25:5097–101. doi: 10.1016/j.bmcl.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 50.Chong N, Aziz Z, Jhala V, Thaker VS. A systematic review on the chemical constituents of Centella asiatica. J Appl Pharm Sci. 2014;4:043–7. [Google Scholar]
- 51.Taylor RSL, Towers GHN. Antibacterial constituents of the nepalese medicinal herb, Centipeda minima. Phytochemistry. 1998;47:631–4. doi: 10.1016/s0031-9422(97)00534-7. [DOI] [PubMed] [Google Scholar]
- 52.Matsuda H, Morikawa T, Toguchida I, Harima S, Yoshikawa M. Medicinal flowers VI Absolute stereostructures of two new flavanone glycosides and a phenylbutanoid glycoside from the flowers of Chrysanthemum indicum L: their inhibitory activities for rat lens aldose reductase. Chem Pharm Bull. 2002;50:972–5. doi: 10.1248/cpb.50.972. [DOI] [PubMed] [Google Scholar]
- 53.Sun Q-L, Hua S, Ye J-H, Zheng X-Q, Liang Y-R. Flavonoids and volatiles in Chrysanthemum morifolium Ramat flower from Tongxiang County in China. African J Biotechnol. 2010;9:3817–21. [Google Scholar]
- 54.Chen L, Kotani A, Kusu F, Wang Z, Zhu J, Hakamata H. Quantitative comparison of caffeoylquinic acids and flavonoids in Chrysanthemum morifolium flowers and their sulfur-fumigated products by three-channel liquid chromatography with electrochemical detection. Chem Pharm Bull. 2015;63:25–32. doi: 10.1248/cpb.c14-00515. [DOI] [PubMed] [Google Scholar]
- 55.Dien PH, Nhan NT, Le Thuy HT, Quang DN. Main constituents from the seeds of Vietnamese Cnidium monnieri and cytotoxic activity. Nat Prod Res. 2012;26:2107–11. doi: 10.1080/14786419.2011.619186. [DOI] [PubMed] [Google Scholar]
- 56.Salatino A, Salatino MLF, Negri G. Traditional uses, chemistry and pharmacology of Croton species (Euphorbiaceae) J Brazil Chem Soc. 2007;18:11–33. [Google Scholar]
- 57.Yang S-J, Liu M-C, Liang N, Xiang H-M, Yang S. Chemical constituents of Cyrtomium fortumei (J) Smith. Nat Prod Res. 2013;27:2066–8. doi: 10.1080/14786419.2013.824442. [DOI] [PubMed] [Google Scholar]
- 58.Lu Y, Kuang M, Hu G-P, Wu R-B, Wang J, Liu L, et al. Loddigesiinols G-J: α-Glucosidase inhibitors from Dendrobium loddigesii. Molecules. 2014;19:8544–55. doi: 10.3390/molecules19068544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liang M, Liu Y, Ananda S, Zhuo L, Liu L. Toxicology of Dysosma versipallis rhizome: a review. J Med Plant Res. 2010;4:717–21. [Google Scholar]
- 60.Tewtrakul S, Subhadhirasakul S, Cheenpracha S, Karalai C. HIV-1 protease and HIV-1 integrase inhibitory substances from Eclipta prostrata. Phytother Res. 2007;21:1092–5. doi: 10.1002/ptr.2252. [DOI] [PubMed] [Google Scholar]
- 61.Kim HY, Kim HM, Ryu B, Lee JS, Choi JH, Jang DS. Constituents of the aerial parts of Eclipta prostrata and their cytotoxicity on human ovarian cancer cells in vitro. Arch Pharm Res. 2015;38:1963–9. doi: 10.1007/s12272-015-0599-2. [DOI] [PubMed] [Google Scholar]
- 62.Yan-Lin S, Lin-De L, Soon-Kwan H. Eleutherococcus senticosus as a crude medicine: Review of biological and pharmacological effects. J Med Plant Res. 2011;5:5946–52. [Google Scholar]
- 63.Yao X, Gongyu G, Chen G. Determination of active constituents in Lonicera confusa DC by capillary electrophoresis with amperometric detection. Biomed Chromatogr. 2006;20:1192–9. doi: 10.1002/bmc.684. [DOI] [PubMed] [Google Scholar]
- 64.Patocka J, Jakl J, Strunecká A. Expectations of biologically active compounds of the genus Magnolia in biomedicine. J Appl Biomed. 2006;4:171–8. [Google Scholar]
- 65.Hu W, Yu L, Wang M-H. Antioxidant and antiproliferative properties of water extract from Mahonia bealei (Fort) Carr leaves. Food Chem Toxicol. 2011;49:799–806. doi: 10.1016/j.fct.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 66.She GM, Xu C, Liu B, Shi RB. Polyphenolic acids from mint (the aerial of Mentha haplocalyx Briq) with DPPH radical scavenging activity. J Food Sci. 2010;75:C359–62. doi: 10.1111/j.1750-3841.2010.01603.x. [DOI] [PubMed] [Google Scholar]
- 67.She GM, Xu C, Liu B. New monocyclic monoterpenoid glycoside from Mentha haplocalyx. Briq Chem Cent J. 2012;6:37–9. doi: 10.1186/1752-153X-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clericuzio M, Tinello S, Burlando B, Ranzato E, Martinotti S, Cornara L, et al. Flavonoid oligoglycosides from Ophioglossum vulgatum L having wound healing properties. Planta Med. 2012;78:1639–44. doi: 10.1055/s-0032-1315149. [DOI] [PubMed] [Google Scholar]
- 69.Yang F, Zhang T, Ito Y. Large-scale separation of resveratrol, anthraglycoside A and anthraglycoside B from Polygonum cuspidatum Siebet Zucc by high-speed counter-current chromatography. J Chromatogr A. 2001;919:443–8. doi: 10.1016/s0021-9673(01)00846-9. [DOI] [PubMed] [Google Scholar]
- 70.Chu X, Sun A, Liu R. Preparative isolation and purification of five compounds from the Chinese medicinal herb Polygonum cuspidatum Siebet Zucc by high-speed counter-current chromatography. J Chromatogr A. 2005;1097:33–9. doi: 10.1016/j.chroma.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 71.Zhang S, Liu X, Zhang Z-L, He L, Wang Z, Wang G-S. Isolation and identification of the phenolic compounds from the roots of Sanguisorba officinalis L and their antioxidant activities. Molecules. 2012;17:13917–22. doi: 10.3390/molecules171213917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li H-B, Chen F. Isolation and purification of baicalein, wogonin and oroxylin A from the medicinal plant Scutellaria baicalensis by high-speed counter-current chromatography. J Chromatogr A. 2005;1074:107–10. doi: 10.1016/j.chroma.2005.03.088. [DOI] [PubMed] [Google Scholar]
- 73.Wang D, Huang L, Chen S. Senecio scandens Buch-Ham: a review on its ethnopharmacology, phytochemistry, pharmacology, and toxicity. J Ethnopharmacol. 2013;149:1–23. doi: 10.1016/j.jep.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 74.Lee M-H, Lin Y-P, Hsu F-L, Zhan G-R, Yen K-Y. Bioactive constituents of Spatholobus suberectus in regulating tyrosinase-related proteins and mRNA in HEMn cells. Phytochemistry. 2006;67:1262–70. doi: 10.1016/j.phytochem.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 75.Zhu Y-P. Chinese materia medica: chemistry pharmacology applications. New York: CRC Press; 1998. [Google Scholar]