Abstract
Background:
Quorum sensing (QS) plays an important role in the production of virulence factors and pathogenicity in Pseudomonas aeruginosa, and the interruption of QS will be a hopeful pathway to combat bacterial infection.
Objective:
In this study, we selected Forsythia suspense (Thunb.) Vahl from traditional Chinese herbal medicines for its anti-QS activity.
Materials and Methods:
Anti-QS of F. suspense extracts (FSE) was monitored using the Chromobacterium violaceum 12472 bioassay. Standard methods were used to investigate the effects of FSE on QS-controlled virulence factors production, swimming motility, and biofilm establishment in P. aeruginosa PAO1.
Results:
FSE could obviously inhibit the violacein production in C. violaceum 12472 and also could inhibit quorum sensing–regulated virulence factors production and biofilm formation in P. aeruginosa in a concentration-dependent manner. The elastase activity and pyocyanin production were inhibited at a maximum of 40.97 and 47.58% when P. aeruginosa was grown in the presence of 0.25 g/mL FSE, which can also inhibit swimming motility of P. aeruginosa. The biofilm formation ability was decreased about 72.45% when in PAO1 cultured with the 0.25 g/mL FSE. The results suggested that FSE may be used as an alternative drug to control and handle harmful infections caused by bacterial pathogens based on QS inhibition.
SUMMARY
Forsythia suspense water extract could obviously inhibit the purple pigment production in C. violaceum 12472
Forsythia suspense water extract could inhibit QS-regulated virulence factors production and biofilm formation in P. aeruginosa.
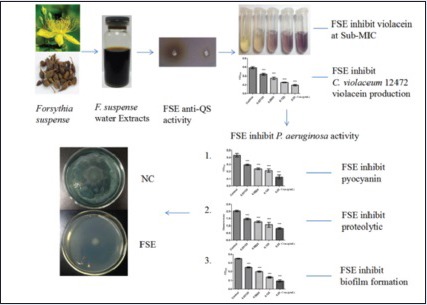
Abbreviations used: QS: Quorum sensing, Pseudomonas aeruginosa P. aeruginosa, Forsythia suspense F. suspense, FSE: F. suspense extracts, Chromobacterium violaceum 12472 C. violaceum 12472, AIs: autoinducers, AHLs: N-acyl-homoserinelactones, LB: Luria-Bertani, MICs: Minimum inhibitory concentrations, CFU: Colony-Forming Units, ATCC: American Type Culture Collection, PBS: phosphate buffered saline
Keywords: Pseudomonas aeruginosa, Forsythia suspense, quorum sensing, virulence factors, biofilm
INTRODUCTION
Antibiotics have been considered to be the most efficacious drug for curing bacterial infectious diseases. As the quantity of antibiotics has increased over the past decades applied in human clinical and animal husbandry, numerous multiple drug-resistant bacterial strains have been isolated. Now researchers are trying their best to find alternative approaches to prevent and treat bacterial infections.[1] Quorum sensing (QS) is a pattern of bacterial communication used to detect the density and control collectively behaviors. This process depends on the production, release, and group-wide detection of signal molecules named autoinducers (AIs), which are typically N-acyl-homoserine-lactones (AHLs) produced in Gram-negative bacteria.[2] QS plays an important role in the production of virulence factors and pathogenicity in PAO1. Many researchers show that disrupt the QS system can decrease the secretion of virulence factors and biofilm formation, and it will be a hopeful pathway to inhibit bacterial infectious disease.[3]
Many researchers have explored QS inhibitors from natural organisms with the potential inhibition of the QS system of various pathogens. QS inhibitors were found from medicinal herbs, synthetic chemicals and microbes.[4] Okusa, et al. found that extracts of Cordia gilletii de wild (Boraginaceae) quench the QS of Pseudomonas aeruginosa PAO1. The extract from root barks was found to quench the production of pyocyanin and reduce biofilm formation. Moreover, this extract is able to inhibit the expression of several QS-regulated genes, such as lasB, rhlA, lasI, lasR, rhlI, and rhlR.[5] Mihalik et al.[6] results showed Camellia sinensis (green tea, GT) extracts inhibited the expression of QS-controlled virulence factors in P. aeruginosa. They tested the possible mechanism of inhibiting QS at the same time. In that study, extract of F. suspense was used to test for its activity to inhibit pigment production in C. violaceum 12472, biofilm formation, and virulent factors production in P. aeruginosa.
F. suspense (Thunb.) Vahl (Oleaceae.) is a notable herb that has long been used as antibacterial, antiviral, antipyretic, and anti-inflammatory agent in traditional Chinese medicine. It is widely distributed in Asia and Europe. F. suspense is widely grown mainly in Hebei, Shanxi, and Shanxi Province in China.[7] Dried fruit of F. suspense is the only drug source. However, little is known about its mechanism to prevent bacterial infection. Our results have suggested that the extract could obviously inhibit the purple pigment production in C. violaceum 12472 and could also inhibit QS-regulated virulence factors production and biofilm formation in P. aeruginosa. These results suggest that F. suspense extract, or some efficacious compounds, can be used as an alternative drug to control and cope with harmful infections caused by bacterial pathogens based on QS inhibition.
MATERIALS AND METHODS
Strains and culture conditions
C. violaceum ATCC 12472 was used as a report strain to detect QS inhibiting ability. The purple pigment, violacein production was underregulated by QS system, so it is used as a biosensor strain to detect possible QS inhibitors.[8] P. aeruginosa, a common Gram-negative rod-shaped opportunistic pathogen, has two QS systems, RhlI/R and LasI/R, which control the proteolytic activity, pyocyanin production, biofilm formation, and swimming motility.[9]
Both strains were grown in Luria-Bertani broth (LB, 0.5% yeast extract, 1.0% tryptone, 0.5% NaCl, pH 7.4) with or without antibiotics. P. aeruginosa and C. violaceum were grown at 37 and 30°C, respectively, with 120 rpm agitation in a shaking incubator overnight unless otherwise specified. The bacterial density was detected by recording OD 600 spectrophotometrically.
Crude extracts preparation
The extraction of F. suspense powder was carried out by the method of Perera et al.[10] Briefly, 100 g of F. suspense powder (the Tong Ren Tang Pharmaceutical Store. Nanjing, China) was decocted in water (2 L) for 2 h (two times). The extract was centrifuged at 10,000g for 10 min to remove all plant debris, and then the supernatant was filtered through a Whatman filter paper No. 1 paper filter. The filtrate was then evaporated in a rotary vacuum evaporator. The crude extract was re-dissolved into 50 mL water to a concentration of 2 g of raw herbal material per mL (2 g/mL). Filtration of the water extracts using a 0.22 μm filters and tested for microbial contamination. The extracts were stored at -20°C before application.
Effect of FSE on bacterial growth
The minimum inhibitory concentrations (MICs) of FSE for P. aeruginosa and C. violaceum 12472 were determined by two-fold macrodilution in LB broth with an inoculum of 106 CFU/ml. The technique as described by Chu et al.[11]. The MIC was defined as the lowest concentration at which there is no visible bacteria growth. A range of concentrations below MIC value (sub-MIC) of the drug was taken to evaluate their effect on the QS of P. aeruginosa.
Bioassay for QSI activity using C. violaceum 12472
C. violaceum biosensor method was used in this study.[11] Six milliliter of warm molten soft agar (LB with 0.7% agar) was seeded with 20 μL of 18-h cultured C. violaceum ATCC 12472 and mixed. Then mixed culture was poured over the surface of a solidified LB plate to form the overlay. Subsequently, 50-mm wells were punched through the agar after the overlay had solidified and 100 μL FSE at sub-MIC were loaded onto it. The assay plates were then cultured at 30°C for 18 h. The absence of the purple violacein pigmentation of the bacterial lawn surrounding the well indicated QS inhibition activity. Disks loaded with sterilized water were considered as controls.
Effect of FSE on C. violaceum 12472 violacein production
For quantitative QS inhibition assay, violacein was extracted from the wells by water-saturated butanol according to the method of Blosser and Gray (2000) and quantified spectrophotometrically at optical density OD 580 (UV-1800; Shimadzu).[12]
Quantification of secreted of virulence factors and static biofilms of PAO1
P. aeruginosa PAO1 was cultured in LB medium containing a series sub-MIC concentrations (0–0.25 g/mL) of extract for 18 h at 30°C and then centrifuged (10,000g, 5 min, 4°C); standard protocols were then used to detect the virulence factors in cell-free supernatants.
Pyocyanin assay
Pyocyanin was detected by growing the bacteria in LB medium for 18 h. Pyocyanin secreted by P. aeruginosa PAO1 in the supernatant was measured by methods as Reimmann et al.[13]. Two milliliter of cell-free supernatants was extracted with 1.5 mL chloroform and vortexed immediately. After centrifugation (10,000g, 3 min), the chloroform layer was transferred to a new tube and 1.5 mL 0.2 M HCl was added to the tube and the color of the liquid changed to pink. The absorbance was detected at OD 520.
Proteolytic assays
The proteolytic activity was tested by a skim milk plate assay.[14] Briefly, 50 μL of cell-free supernatant of FSE-treated and FSE-untreated P. aeruginosa was separately added in LB solid medium containing 5% skim milk have holes and incubated at 30°C for 18 h. The circle of casein hydrolysis was detected.
Biofilm formation assays
Biofilm formation experiments were performed as previously described by Agarwala et al.[15] in the presence or absence of extract. Briefly, 10 μL of 18 h cultured P. aeruginosa PAO1 (106 CFU/mL) was diluted with 2 mL of LB liquid medium containing a series of sub-MIC concentrations of FSE and then incubated for 18 h without shaking. The mixture was discarded after incubation, and the tube was washed thrice with phosphate buffered saline (PBS) and set in formaldehyde (10%) for 10 min. The solutions were removed and air dried at room temperature. Crystal violet (0.1% in ethanol) was added to stain the biofilm for 15 min. Deionized water was used to remove the unbound dye for three times and absorbed dye was eluted with ethanol. Then the OD 650 was recorded.
Swimming assay
For swimming assays,[16] PAO1 (106 CFU/mL) was point inoculated on LB plates containing 0.3% agar mixed with 0.25 g/mL FSE extract and incubated at 37°C for 18 h. The swimming diameter was detected by the ruler.
Analytical methods
Analytical Methods
All experiments were conducted in quadruplicates at least thrice. ANOVA was used to analyze the differences between the treatments using STATISTICA. P less than 0.01 was considered as significant unless otherwise specified and was represented by “***,” P less than 0.05 was represented by “**.”
RESULTS AND DISCUSSION
Biosensor assays
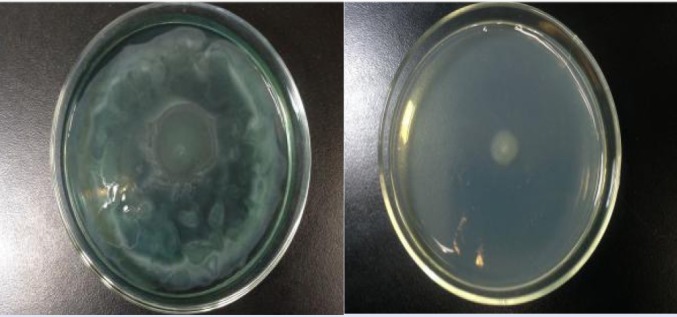
The FSE demonstrated QS inhibitory activity in C. violaceum 12472. At the concentration of 0.25 g/mL, a colorless muddy zone of purple pigment inhibition diameter was reached to 18 mm (6 mm hole) [Figure 1]. Similarly, Vasavi et al. used C. violaceum 12472 as a biosensor bioassay and found that Psidium guajava L. flavonoids inhibit violacein product.[17] Selected from the Traditional Chinese Herbs, Priya et al. found that Phyllanthus amarus showed a distinguished colorless area on the lawn of C. violaceum 026 indicating a QS inhibitory activity.[18]
Figure 1.
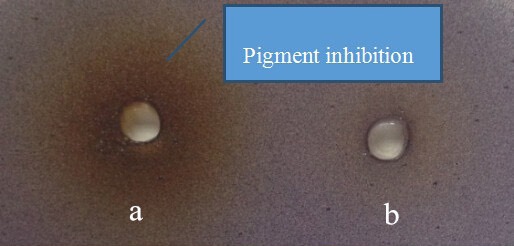
Anti-QS properties of F. suspense extract. (a) 0.25 g/mL F. suspense extract; (b) water. The bacteria around the positive control well was able to grow without producing violacein
MIC assays
Tests on antibacterial activity of the F. suspense extracts have revealed MIC values for P. aeruginosa relatively. For example, water extracts of F. suspense showed visible of 0.25 g/mL against P. aeruginosa, but invisible of 0.5 g/mL. So, MIC of FSE against P. aeruginosa was 0.5 g/mL. The MIC of FSE against C. violaceum 12472 was 0.5 g/mL.
Inhibition of violacein production in C. violaceum 12472 by FSE
Concentration-dependent inhibition of violacein production in C. violaceum 12472 by FSE was observed. Although all tested concentrations of FSE showed a significant drop in violacein content, the maximum QS inhibitory (69.28%) effect was seen at the highest concentration tested (0.25 g/mL) [Figure 2]. Bacterial cell count performed on LB agar plates at 24 h incubation showed no significant difference in the number of colony-forming units (CFUs) of the FSE concentrations tested (data not shown).
Figure 2.
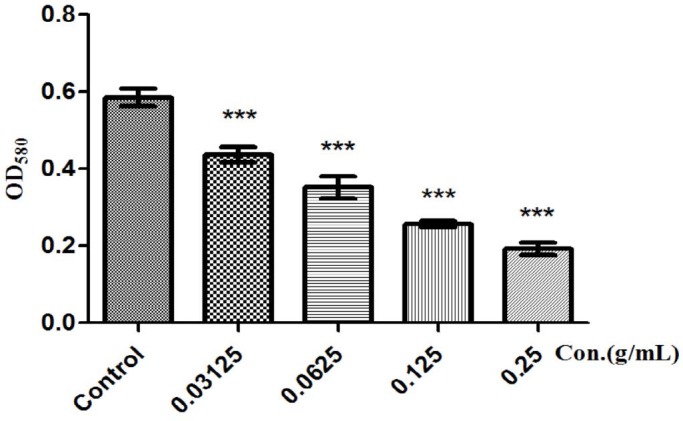
FSE inhibits C. violaceum 12472 violacein production in a concentration-dependent manner. The violacein production was measured spectrophotometrically and quantified by reading the OD values of the solution at 580 nm. Values are presented as mean ± SD, n = 3
Effect of FSE on P. aeruginosa PAO1 virulence factors production
In most cases, virulence is a required as a prior condition for infection, but it is not required for bacterial survival. Therefore, antivirulence strategies that demilitarize pathogens rather than killing them may apply moderate evolutionary pressure that does not lean toward the development of resistance.
Pyocyanin production and proteolytic activities
The synthesis of pyocyanin is controlled by QS-system.[19] Pyocyanin was produced when the cell of P. aeruginosa PAO1 reached high density, and pyocyanin was in limited production [Figure 3] when cultured with drugs that do not affect P. aeruginosa PAO1 growth but disrupt the QS systems. Similarly, significant decrease in proteases’ production was observed, with mostly inhibition at 0.25 g/mL [Figure 4]. The elastase activity and pyocyanin production was inhibited at a maximum of 40.97, 47.58% when P. aeruginosa was grown in the presence of 0.25 g/mL FSE.
Figure 3.
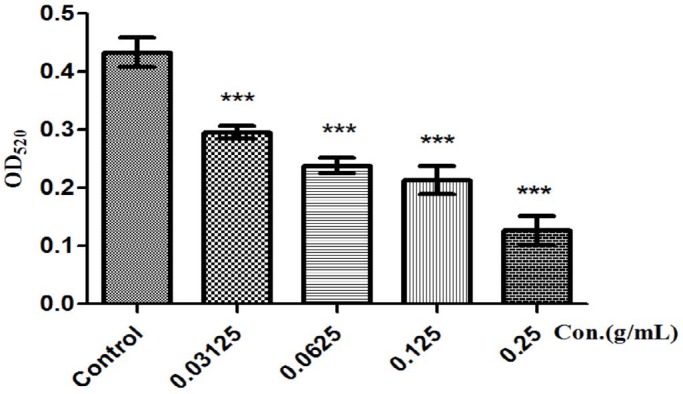
Pyocyanin inhibition was performed with P. aeruginosa PAO1 in triplicate with F. suspense. Error bars represent SD of three replicates
Figure 4.
Effect of F. suspense extract on P. aeruginosa PAO1 proteolytic activities. The total proteolytic activity on skim milk plate. Mean values of triplicate independent experiments and SD are shown
Biofilm formation
When bacteria-acquired biofilm may readily make incursions into the human body parts, it may cause many chronic infections; but the attempt to eliminate the biofilm is very arduous.[20] QS plays a significant role in the formation of biofilm. Research work indicates that disrupt bacterial QS may lead to the restricting of biofilm formation.[21] The ability of FSE in reducing QS-related biofilm formation activity was assessed. As shown in [Figure 5], a decrease of more than 72.45% in biofilm formation was observed when in PAO1 cultured with the 0.25 g/mL FSE.
Figure 5.
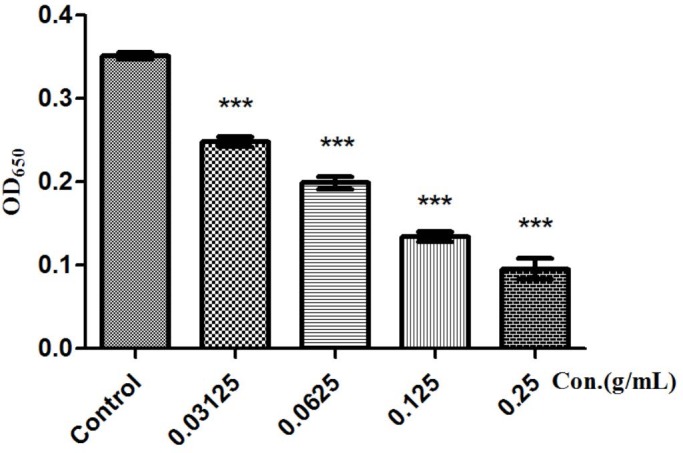
Biofilm formation of P. aeruginosa in the presence of F. suspense. Total biofilm amount at different F. suspense extract concentrations has distinguished difference. Values are presented as mean ± SD, n = 3
Swimming motility
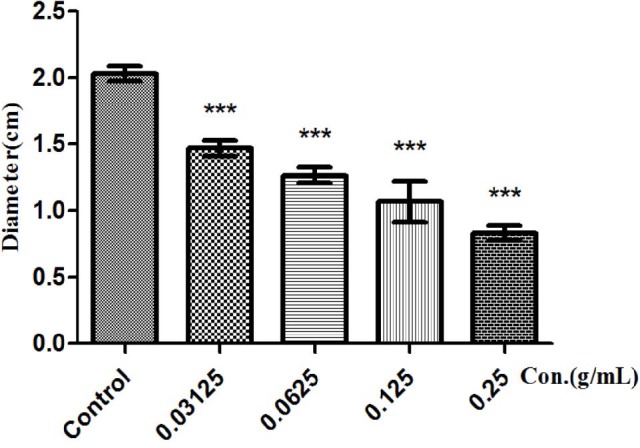
Swimming motility of P. aeruginosa was mediated by QS systems. Some results were shown that reduced and delayed swimming in rhlI/rhlR mutant strain, and the lasI/lasR mutants completely diminished swimming ability.[22] In this part, the extract of F. suspense inhibited swimming motility of P. aeruginosa, the swimming diameter was decreased as seen in [Figure 6] when FSE at 0.25 g/ml.
Figure 6.
Impairment of motility by a sub-MIC level of water extracts. swimming motility, the diameter of F. suspense-treated P. aeruginosa PAO1 cultures is significantly shorter than that of NC. The numbers associated with indicating concentration (0.25 g/mL for FSE) NC FSE
Our experimental data showed that F. suspense could be useful for weakening the virulence factors production in P. aeruginosa, thence more traditional Chinese herbal medicine should be screened for its anti-QS function, and its active ingredients might supply as hopeful antibacterial drugs. The active ingredients from herb might target the QS system by inhibiting the AHLs synthesis, degradation of the signal molecules, or inhibiting of the QS by targeting the AHLs receptor.[23] The appearance of multi-antibiotic resistance among pathogenic bacteria gives the researchers an opportunity to find out new strategies alternative to antibiotic against bacterial infection. The discoveries of the bacterial QS system provide a different treatment to conventional chemotherapy. Efforts to block the QS-based biofilms and virulence factors are potentially capable of attenuating the pathogenicity without killing the bacteria. This method makes its success, QS inhibitory substance may reduce the production of virulence factors in bacterial pathogen without killing bacterial cells as antibiotics. Therefore, improved understanding of QS systems in the pathogenicity of bacteria may provide more insight into combating bacterial infection by using either antibiotics or emerging methods such as regulated degradation of QS receptor or QS signals.[24]
In conclusion, F. suspense extract can inhibit the QS-controlled pigment production in C. violaceum and reduce biofilm formation and virulence factors production, which is crucial to the pathogenicity in P. aeruginosa. F. suspense has been used as anti-infection traditional Chinese medicinal herb for a long time and is hence considered as a safe medical herb. So far, F. suspense presents a possibility for use as a new substance for controlling P. aeruginosa infections in the future. This method could be an advantage for the use of anti-QS drugs that show superior results compared with traditional antimicrobial drugs by not exerting any selective pressure on the drug-resistant mutant strains.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We thank the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) for supported part of this study.
REFERENCES
- 1.Rattanaumpawan P, Sutha P, Thamlikitkul V. Effectiveness of drug use evaluation and antibiotic authorization on patients’ clinical outcomes, antibiotic consumption, and antibiotic expenditures. American J Infection Control. 2010;1:38–43. doi: 10.1016/j.ajic.2009.04.288. [DOI] [PubMed] [Google Scholar]
- 2.Shang ZQ, Wang HF, Zhou SX, Chu WH. Characterization of N-Acyl-homoserine lactones (AHLs)-deficient clinical isolates of Pseudomonas aeruginosa. Indian J Microbiol. 2014;2:158–62. doi: 10.1007/s12088-014-0449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalia VC, Wood TK, Kumar P. Evolution of resistance to quorum-sensing inhibitors. Microb Ecol. 2013 doi: 10.1007/s00248-013-0316-y. doi: 10.1007/s00248-013-0316-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sales EH, Sedeh FM, Rajabifar S. Effects of gamma irradiation and silver nano particles on microbiological characteristics of saffron, using hurdle technology. Indian J Microbiol. 2012;52:66–9. doi: 10.1007/s12088-011-0203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okusa PN, Rasamiravaka T, Vandeputte O, Stévigny C, Jaziri ME, Duez P. Extracts of Cordia gilletii de wild (Boraginaceae) quench the quorum sensing of Pseudomonas aeruginosa PAO1. J Intercult Ethnopharmacol. 2014;3:138–43. doi: 10.5455/jice.20140710031312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mihalik K, Chung DW, Crixell SH, McLean RJC, Vattem DA. Quorum sensing modulators of Pseudomonas aeruginosa characterized in Camellia sinensis. Asian Journal of Traditional Medicines. 2008;3:12–23. [Google Scholar]
- 7.Ge Y, Wang Y, Chen P, Wang Y, Hou C, Wu Y, et al. Polyhydroxytriterpenoids and Phenolic Constituents from Forsythia suspensa (Thunb.) Vahl Leaves. J. Agric Food Chem. 2016;64:125–31. doi: 10.1021/acs.jafc.5b04509. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh R, Tiwary BK, Kumar A, Chakraborty R. Guava leaf extract inhibits Quorumsensing and Chromobacterium violaceum induced lysis of human hepatoma cells: whole transcriptome analysis reveals differential gene expression. PLoS ONE. 2014;9:e107703. doi: 10.1371/journal.pone.0107703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhamodharan B, Chandran S, Shunmugiah KP. Anti-pathogenic potential of coral associated bacteria isolated from Gulf of Mannar against Pseudomonas aeruginosa. Indian J Microbiol. 2013;1:111–3. doi: 10.1007/s12088-012-0342-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perera MGAN, Soysa SSSBDP, Abeytunga DTU, Ramesha R. Antioxidant and cytotoxic properties of three traditional decoctions used for the treatment of cancer in Sri Lanka. Pharmacognosy Mag. 2008;4:172–81. [Google Scholar]
- 11.Chu W, Zhou S, Jiang Y, Zhu W, Zhuang X, Fu J. Effect of traditional chinese herbal medicine with antiquorum sensing activity on Pseudomonas aeruginosa. Evid Based Complement Alternat Med. 2013;11:648257. doi: 10.1155/2013/648257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blosser RS, Gray KM. Extraction of violacein from Chromobacterium violaceum provides a new quantitative bioassay for N-acyl homoserine lactone autoinducers. J Microbiol Methods. 2000;40:47–55. doi: 10.1016/s0167-7012(99)00136-0. [DOI] [PubMed] [Google Scholar]
- 13.Reimmann C, Ginet N, Michel L. Genetically programmed autoinducer destruction reduces virulence gene expression and swarming motility in Pseudomonas aeruginosa PAO1. Microbiology SGM. 2002;148:923–32. doi: 10.1099/00221287-148-4-923. [DOI] [PubMed] [Google Scholar]
- 14.Gutiérrez-Barranquero JA, Reen FJ, McCarthy RR, O’Gara F. Deciphering the role of coumarin as a novel quorum sensing inhibitor suppressing virulence phenotypes in bacterial pathogens. Appl Microbiol Biotechnol. 2015;99:3303–16. doi: 10.1007/s00253-015-6436-1. [DOI] [PubMed] [Google Scholar]
- 15.Agarwala M, Choudhury B, Yadav RNS. Comparative study of antibiofilm activity of copper oxide and iron oxide nanoparticles against multidrug resistant biofilm forming uropathogens. Indian J Microbiol. 2014;3:365–8. doi: 10.1007/s12088-014-0462-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Toole GA, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;2:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 17.Vasavi HS, Arun AB, Rekha PD. Anti-quorum sensing activity of Psidium guajava L flavonoids against Chromobacterium violaceum and Pseudomonas aeruginosa PAO1. Microbiol Immunol. 2014;58:286–93. doi: 10.1111/1348-0421.12150. [DOI] [PubMed] [Google Scholar]
- 18.Priya K, Yin WF, Chan KG. Anti-quorum sensing activity of the traditional Chinese herb, Phyllanthus amarus. Sensors. 2013;13:14558–69. doi: 10.3390/s131114558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang YY, Zhang YQ, Yang YX, Wang LH, Weng LX. Identification of a Pseudomonas sp that inhibits RHL system of quorum sensing. Indian J Microbiol. 2013;1:28–35. doi: 10.1007/s12088-012-0340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaur J, Niharika N, Lata P, Lal R. Biofilms: united we stand, divided we fall. Indian J Microbiol. 2014;2:246–7. doi: 10.1007/s12088-014-0460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalia VC, Kumar P. Potential applications of quorum sensing inhibitors in diverse fields. Quorum sensing vs quorum quenching: a battle with no end in sight. Springer India. 2015:359–70. [Google Scholar]
- 22.Köhler T, Curty LK, Barja F, Van Delden C, Pechère JC. Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J Bacteriol. 2000;182:5990–6. doi: 10.1128/jb.182.21.5990-5996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Husain FM, Ahmad I, Khan MS, Ahmad E, Tahseen Q, Khan MS, et al. Sub-MICs of Mentha piperita essential oil and menthol inhibits AHL mediated quorum sensing and biofilm of Gram-negative bacteria? Front Microbiol. 2015;6:420. doi: 10.3389/fmicb.2015.00420. doi: 10.3389/fmicb.201500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salini R, Sindhulakshmi M, Poongothai T, Pandian SK. Inhibition of quorum sensing mediated biofilm development and virulence in uropathogens by Hyptis suaveolens. 2015;107:1095. doi: 10.1007/s10482-015-0402-x. doi:10.1007/s10482-015-0402-x. [DOI] [PubMed] [Google Scholar]


